Investigations into the Antifungal, Photocatalytic, and Physicochemical Properties of Sol-Gel-Produced Tin Dioxide Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. XRD Analysis
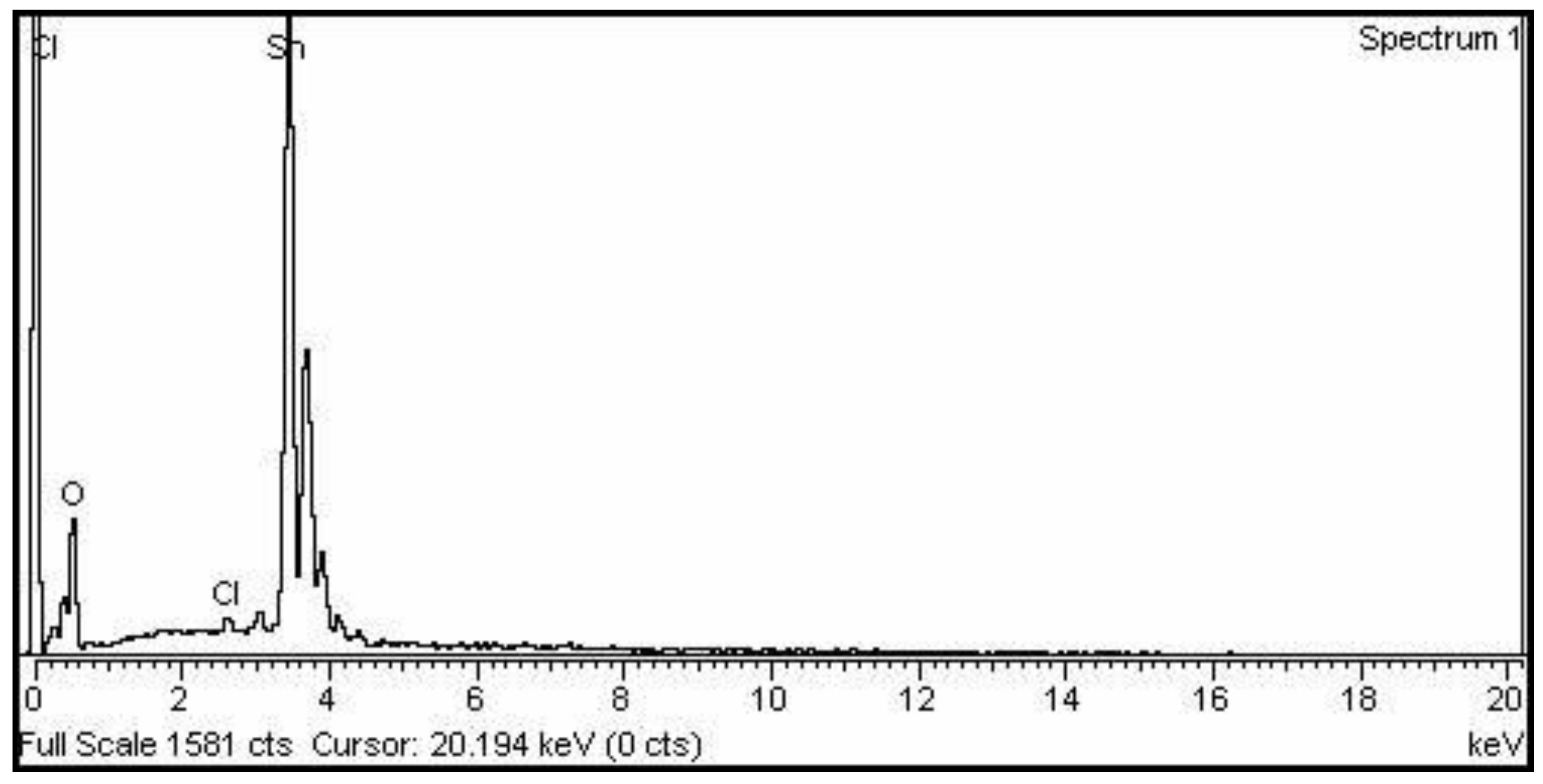
2.2. EDX Analysis
2.3. SEM Analysis
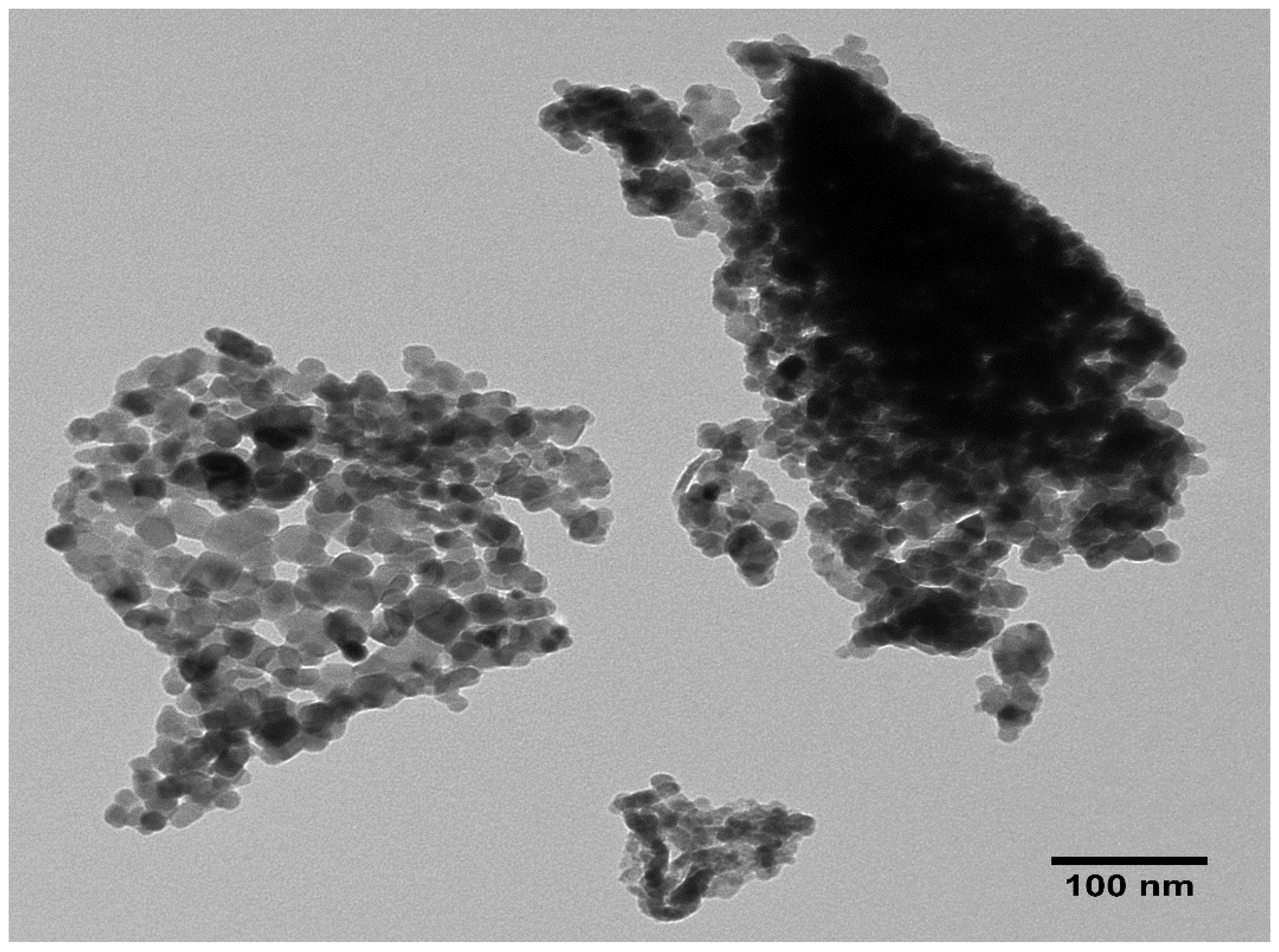
2.4. TEM Analysis
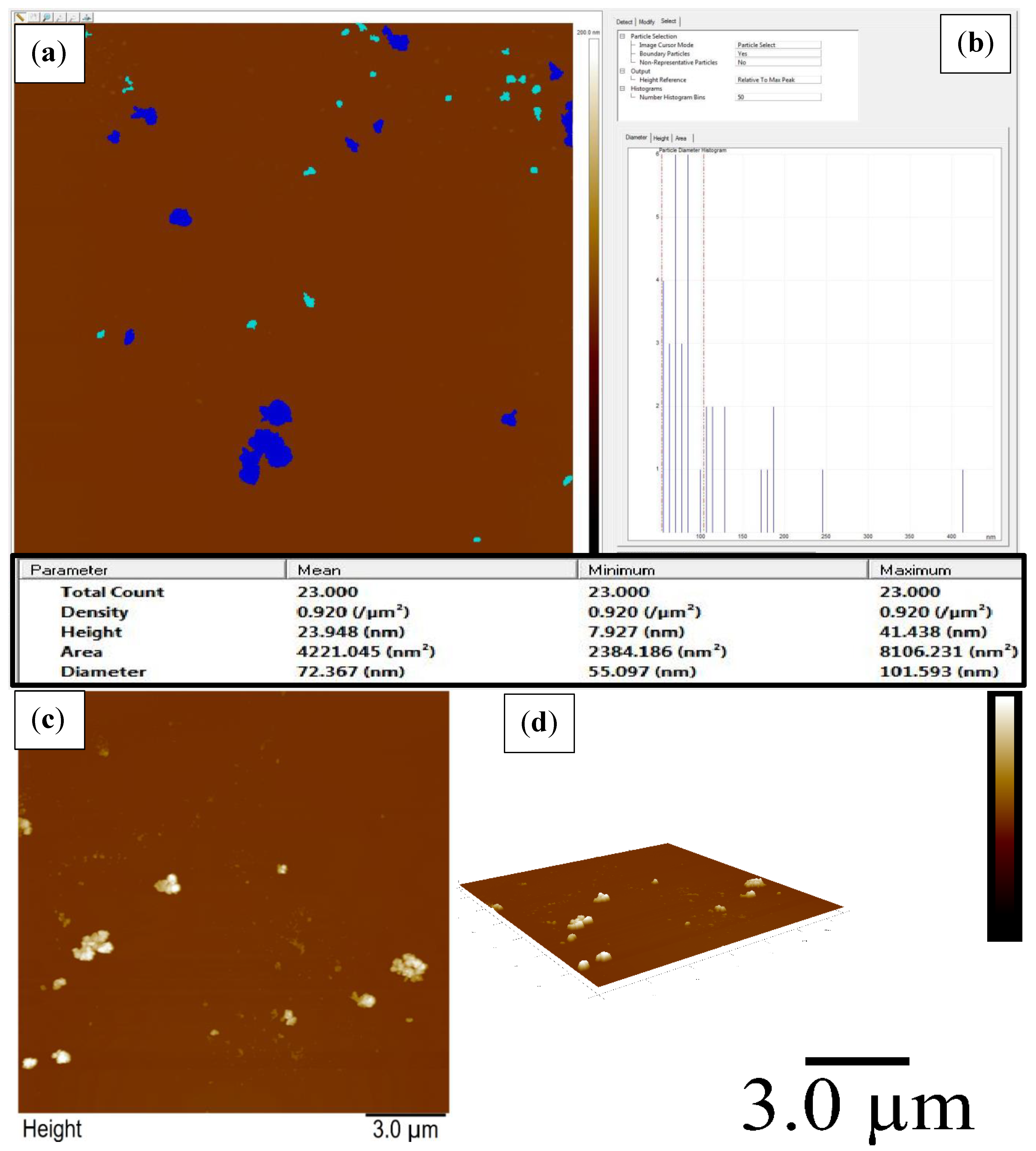
2.5. AFM Analysis
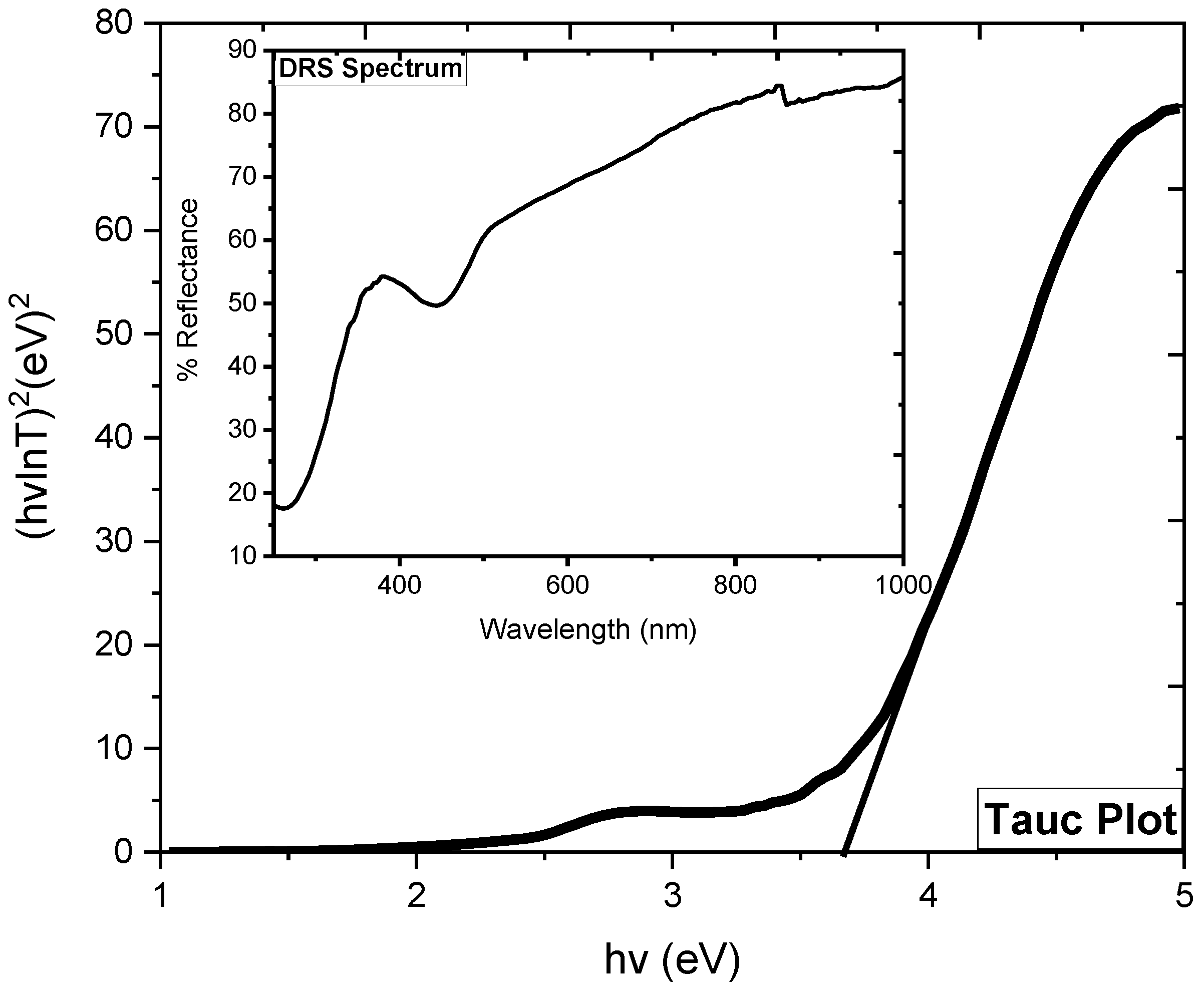
2.6. DRS Analysis
2.7. FTIR Analysis
2.8. Antifungal Study
2.9. Photocatalytic Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis of SnO2 NPs
3.3. Characterization
3.4. Antifungal Assay
3.5. Photocatalytic Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Alananbeh, K.M.; Al-Refaee, W.J.; Al-Qodah, Z. Antifungal Effect of Silver Nanoparticles on Selected Fungi Isolated from Raw and Waste Water. Indian J. Pharm. Sci. 2017, 79, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, B.M. Review of Fungal Infection in Human Beings and Role of COVID-19 Pandemic. Indian J. Forensic Med. Toxicol. 2021, 15, 887–897. [Google Scholar]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.A.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against rhizoctonia solani in faba bean plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Wani, I.A.; Lone, I.H.; Ganguly, A.; Manzoor, N.; Ahmad, A.; Ahmed, J.; Al-Shihri, A.S. Antifungal activity of gold nanoparticles prepared by solvothermal method. Mater. Res. Bull. 2013, 48, 12–20. [Google Scholar] [CrossRef]
- Abomuti, M.A.; Danish, E.Y.; Firoz, A.; Hasan, N.; Malik, M.A. Green synthesis of zinc oxide nanoparticles using salvia officinalis leaf extract and their photocatalytic and antifungal activities. Biology 2021, 10, 1075. [Google Scholar] [CrossRef]
- Pino, E. Photocatalytic Degradation of Aqueous Rhodamine 6G Using Supported TiO2 Catalysts. A Model for the Removal of Organic Contaminants from Aqueous samples. Front. Chem. 2020, 8, 365. [Google Scholar] [CrossRef]
- Suwunwong, T.; Patho, P.; Choto, P.; Phoungthong, K. Enhancement the rhodamine 6G adsorption property on Fe3O4-composited biochar derived from rice husk. Mater. Res. Express 2020, 7, 025511. [Google Scholar] [CrossRef]
- Silva, L.C.; Barrocas, B.; Jorge, M.E.M. Photocatalytic Degradation of Rhodamine 6G using TiO2/WO3 Bilayered Films Produced by Reactive Sputtering. In Proceedings of the 6th International Conference on Photonics, Optics and Laser Technology, Funchal, Portugal, 25–27 January 2018; pp. 334–340. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Shah, A.; Khan, A.R. Green synthesis and characterization of tin dioxide nanoparticles for photocatalytic and antimicrobial studies. Mater. Res. Express 2020, 7, 025012. [Google Scholar] [CrossRef]
- Surendhiran, K.C.S.S.; Kumar, P.M.; Kumar, E.R.; Khadar, Y.A.S. Green synthesis of SnO2 nanoparticles using Delonix elata leaf extract: Evaluation of its structural, optical, morphological and photocatalytic properties. SN Appl. Sci. 2020, 2, 1735. [Google Scholar] [CrossRef]
- Garrafa-galvez, H.E.; Nava, O.; Soto-robles, C.A.; Vilchis-nestor, A.R. Green synthesis of SnO2 nanoparticle using Lycopersicon esculentum peel extract. J. Mol. Struct. 2019, 1197, 354–360. [Google Scholar] [CrossRef]
- Adnan, R.; Razana, N.A.; Rahman, I.A.; Farrukh, M.A. Synthesis and Characterization of High Surface Area Tin Oxide Nanoparticles via the Sol-Gel Method as a Catalyst for the Hydrogenation of Styrene Synthesis and Characterization of High Surface Area Tin Oxide Nanoparticles via the Sol-Gel Method as a Catal. J. Chin. Chem. Soc. 2010, 57, 222–229. [Google Scholar] [CrossRef]
- Viet, P.V.; Thi, C.M.; Hieu, L.V. The High Photocatalytic Activity of SnO2 Nanoparticles Synthesized by Hydrothermal Method. J. Nanomater. 2016, 2016, 4231046. [Google Scholar] [CrossRef] [Green Version]
- Suvaitha, P.; Selvam, S.; Ganesan, D.; Rajangam, V.; Raji, A. Green Synthesis of SnO2 Nanoparticles for Catalytic Degradation of Rhodamine B. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 661–676. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Rehman, M. Modeling, Thermodynamic Study and Sorption Mechanism of Cadmium Ions onto Isopropyl Alcohol Mediated Tin Dioxide Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1197–1205. [Google Scholar] [CrossRef]
- Gajendiran, J.; Rajendran, V. Synthesis and Characterization of Ultrafine SnO2 Nanoparticles via Solvothermal Process. Int. J. Phys. Appl. 2010, 2, 45–50. [Google Scholar]
- Yao, W.; Wu, S.; Zhan, L.; Wang, Y. Two-dimensional porous carbon-coated sandwich-like mesoporous SnO2/graphene/mesoporous SnO2 nanosheets towards high-rate and long cycle life lithium-ion batteries. Chem. Eng. J. 2019, 361, 329–341. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Rehman, M.U.; Khan, B. Adsorption of Cd2+ ions onto SnO2 nanoparticles synthesized via sol-gel method: Physiochemical study. Mater. Res. Express 2019, 6, 105035. [Google Scholar] [CrossRef]
- Anjaneyulu, Y.; Rao, R.P. Preparation, characterization and antimicrobial activity studies on some ternary complexes of Cu(II) with acetylacetone and various salicylic acids. Synth. React. Inorg. Met.-Org. Chem. 1986, 16, 257–272. [Google Scholar] [CrossRef]
- Manjula, N.; Selvan, G.; Balu, A.R. Photocatalytic Performance of SnO2:Mo Nanopowders Against the Degradation of Methyl Orange and Rhodamine B Dyes Under Visible Light Irradiation. J. Electron. Mater. 2019, 48, 401–408. [Google Scholar] [CrossRef]
- Shah, A.; Haq, S.; Rehman, W.; Muhammad, W.; Shoukat, S.; Rehman, M. Photocatalytic and antibacterial activities of Paeonia emodi mediated silver oxide nanoparticles. Mater. Res. Express 2019, 6, 045045. [Google Scholar] [CrossRef]
- Haq, S.; Shoukat, S.; Rehman, W.; Waseem, M.; Shah, A. Green fabrication and physicochemical investigations of zinc-cobalt oxide nanocomposite for wastewater treatment. J. Mol. Liq. 2020, 318, 114260. [Google Scholar] [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]





| Species | Concentration (μg/mL) | Inhibition Zone (mm) | PS | NC | Variance (S2) | Standard Deviation (S) | Pearson Constant (<0.05) |
|---|---|---|---|---|---|---|---|
| C. Albicans | 40 | 0 | 6.1 | 0 | 1.72 | 1.3 | 0.0054 |
| 60 | 2 | ||||||
| 80 | 3.4 | ||||||
| 100 | 4.9 | ||||||
| A. Niger | 40 | 0 | 6.3 | 0 | 1.5 | 1.2 | 0.0055 |
| 60 | 2.2 | ||||||
| 80 | 3.1 | ||||||
| 100 | 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, S.; Shahzad, N.; Shahzad, M.I.; Elmnasri, K.; Ali, M.B.; Baazeem, A.; Hedfi, A.; Ehsan, R. Investigations into the Antifungal, Photocatalytic, and Physicochemical Properties of Sol-Gel-Produced Tin Dioxide Nanoparticles. Molecules 2022, 27, 6750. https://doi.org/10.3390/molecules27196750
Haq S, Shahzad N, Shahzad MI, Elmnasri K, Ali MB, Baazeem A, Hedfi A, Ehsan R. Investigations into the Antifungal, Photocatalytic, and Physicochemical Properties of Sol-Gel-Produced Tin Dioxide Nanoparticles. Molecules. 2022; 27(19):6750. https://doi.org/10.3390/molecules27196750
Chicago/Turabian StyleHaq, Sirajul, Nadia Shahzad, Muhammad Imran Shahzad, Khaled Elmnasri, Manel Ben Ali, Alaa Baazeem, Amor Hedfi, and Rimsha Ehsan. 2022. "Investigations into the Antifungal, Photocatalytic, and Physicochemical Properties of Sol-Gel-Produced Tin Dioxide Nanoparticles" Molecules 27, no. 19: 6750. https://doi.org/10.3390/molecules27196750
APA StyleHaq, S., Shahzad, N., Shahzad, M. I., Elmnasri, K., Ali, M. B., Baazeem, A., Hedfi, A., & Ehsan, R. (2022). Investigations into the Antifungal, Photocatalytic, and Physicochemical Properties of Sol-Gel-Produced Tin Dioxide Nanoparticles. Molecules, 27(19), 6750. https://doi.org/10.3390/molecules27196750








