Preparation of Hollow Core–Shell Fe3O4/Nitrogen-Doped Carbon Nanocomposites for Lithium-Ion Batteries
Abstract
:1. Introduction
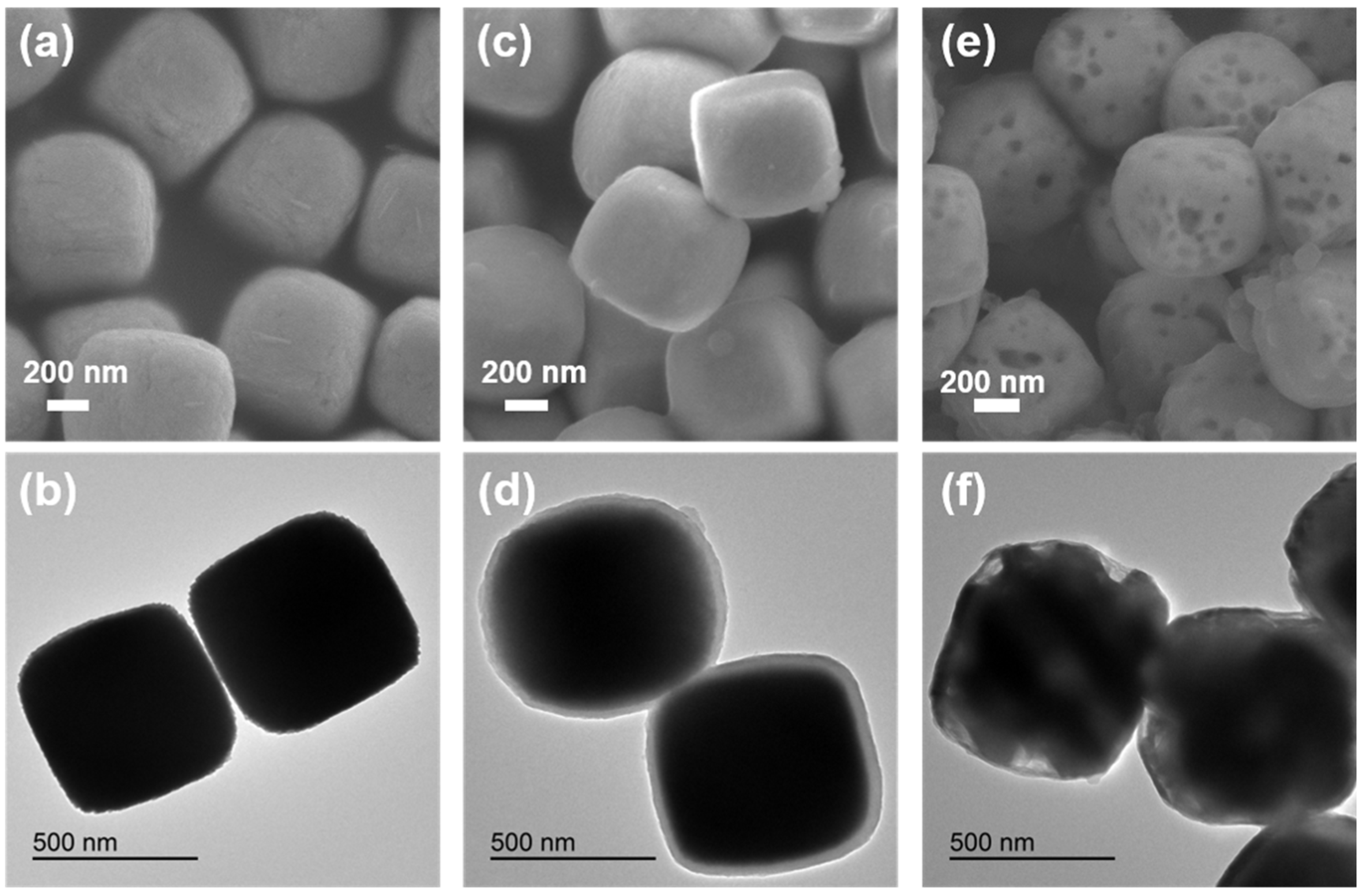
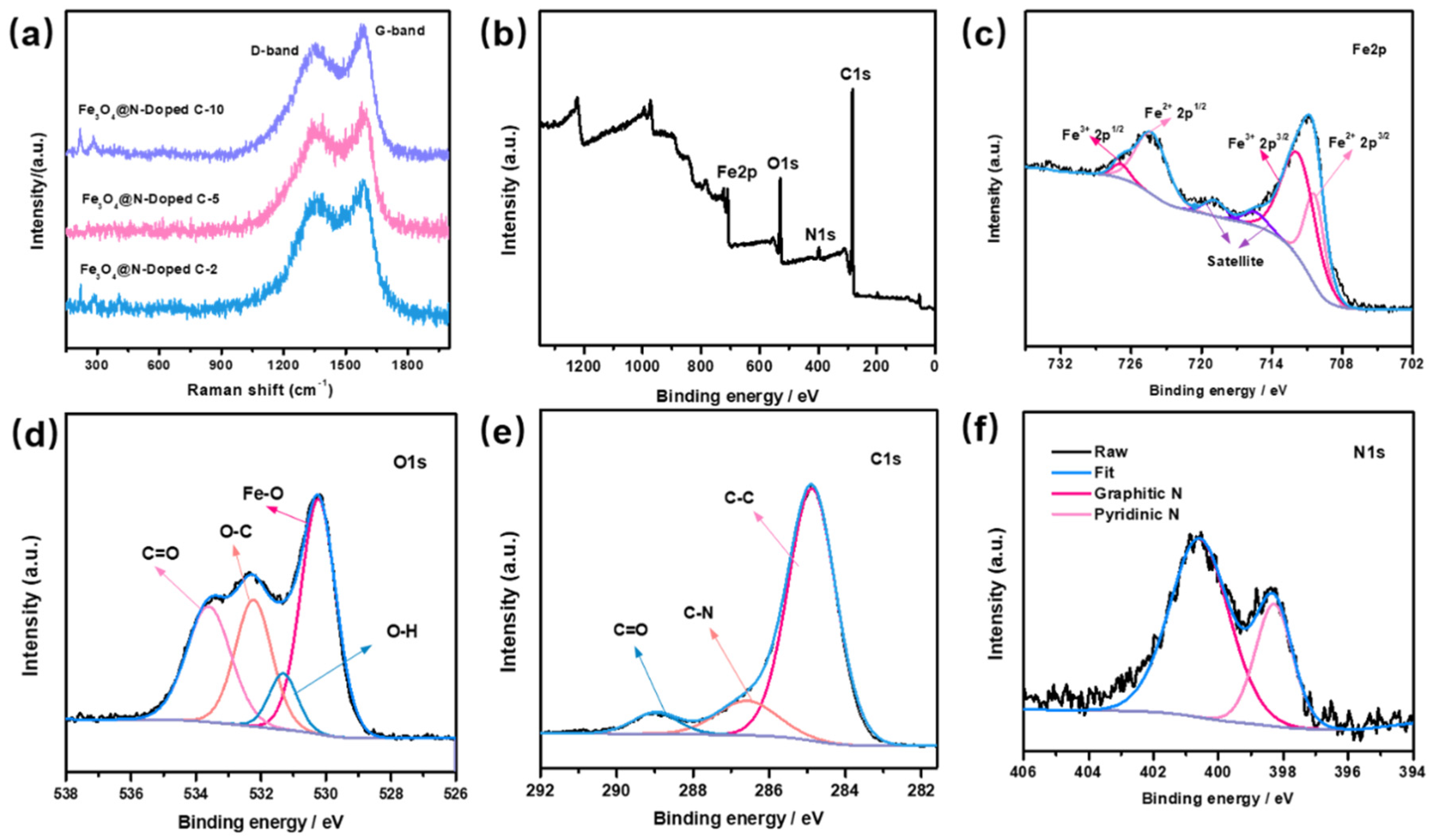
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. 3D-0D Graphene-Fe3O4 Quantum Dot Hybrids as High-Performance Anode Materials for Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Hu, W.; Zheng, M.; Xu, B.; Wei, Y.; Zhu, W.; Li, Q.; Pang, H. Design of Hollow Carbon-based Materials Derived from Metal–Organic Frameworks for Electrocatalysis and Electrochemical Energy Storage. J. Mater. Chem. A 2021, 9, 3880–3917. [Google Scholar] [CrossRef]
- Du, M.; Li, Q.; Zhao, Y.; Liu, C.S.; Pang, H. A Review of Electrochemical Energy Storage Behaviors Based on Pristine Metal–Organic Frameworks and Their Composites. Coord. Chem. Rev. 2020, 416, 213341. [Google Scholar] [CrossRef]
- Singh, G.K. Solar Power Generation by PV (Photovoltaic) Technology: A Review. Energy 2013, 53, 1–13. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y.; Wang, Z.L. Scavenging Wind Energy by Triboelectric Nanogenerators. Adv. Energy Mater. 2018, 8, 1702649. [Google Scholar] [CrossRef]
- Rourke, F.O.; Boyle, F.; Reynolds, A. Tidal Energy Update 2009. Appl. Energy 2010, 87, 398–409. [Google Scholar] [CrossRef]
- Liu, H.; Jia, M.; Zhu, Q.; Cao, B.; Chen, R.; Wang, Y.; Wu, F.; Xu, B. 3D-0D Graphene-Fe3O4 Quantum Dot Hybrids as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 26878–26885. [Google Scholar] [CrossRef]
- Guo, M.; Balamurugan, J.; Li, X.; Kim, N.H.; Lee, J.H. Hierarchical 3D Cobalt-Doped Fe3O4 Nanospheres@NG Hybrid as an Advanced Anode Material for High-Performance Asymmetric Supercapacitors. Small 2017, 13, 1701275. [Google Scholar] [CrossRef]
- Cao, X.; Tan, C.; Zhang, X.; Zhao, W.; Zhang, H. Solution-Processed Two-Dimensional Metal Dichalcogenide-Based Nanomaterials for Energy Storage and Conversion. Adv. Mater. 2016, 28, 6167–6196. [Google Scholar] [CrossRef]
- Lv, T.-T.; Liu, Y.-Y.; Wang, H.; Yang, S.-Y.; Liu, C.-S.; Pang, H. Crystal Water Enlarging The Interlayer Spacing of Ultrathin V2O5·4VO2·2.72H2O Nanobelts for High-Performance Aqueous Zinc-Ion Battery. Chem. Eng. J. 2021, 411, 128533. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, M.Y.; Bai, Y.; Du, M.; Guo, X.; Pang, H. Design and Synthesis of Transition Metal Oxide/Zeolitic Imidazolate Framework-67 Composites. Chem. Eng. J. 2022, 429, 132146. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, Y.; Hou, Y.; Liu, L.; Wu, Y.; Loh, K.P.; Zhang, H.; Zhu, K. Aqueous Rechargeable Lithium Batteries as An Energy Storage System of Superfast Charging. Energy Environ. Sci. 2013, 6, 2093–2104. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Croguennec, L.; Palacin, M.R. Recent Achievements on Inorganic Electrode Materials for Lithium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 3140–3156. [Google Scholar] [CrossRef]
- Li, F.-S.; Wu, Y.-S.; Chou, J.; Winter, M.; Wu, N.-L. A Mechanically Robust and Highly Ion-Conductive Polymer-Blend Coating for High-Power and Long-Life Lithium-Ion Battery Anodes. Adv. Mater. 2015, 27, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; He, C.; Zhao, N.; Wang, Z.; Shi, C.; Liu, E.-Z.; Li, J. Graphene Networks Anchored with Sn@Graphene as Lithium Ion Battery Anode. ACS Nano 2014, 8, 1728–1738. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, N.; Jiao, L.; Tao, Z.; Chen, J. Ultrasmall Sn Nanoparticles Embedded in Carbon as High-Performance Anode for Sodium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 214–220. [Google Scholar] [CrossRef]
- Wu, S.; Han, C.; Iocozzia, J.; Lu, M.; Ge, R.; Xu, R.; Lin, Z. Germanium-Based Nanomaterials for Rechargeable Batteries. Angew. Chem. Int. Ed. 2016, 55, 7898–7922. [Google Scholar] [CrossRef]
- Kwon, H.-T.; Lee, C.K.; Jeon, K.-J.; Park, C.-M. Silicon Diphosphide: A Si-Based Three-Dimensional Crystalline Framework as a High-Performance Li-Ion Battery Anode. ACS Nano 2016, 10, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, J.-Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- Xu, X.; Shen, J.; Li, F.; Wang, Z.; Zhang, D.; Zuo, S.; Liu, J. Fe3O4@C Nanotubes Grown on Carbon Fabric as a Free-Standing Anode for High-Performance Li-Ion Batteries. Chem. A Eur. J. 2020, 26, 14708–14717. [Google Scholar] [CrossRef]
- Liu, J.; Xu, X.; Hu, R.; Yang, L.; Zhu, M. Uniform Hierarchical Fe3O4@Polypyrrole Nanocages for Superior Lithium Ion Battery Anodes. Adv. Energy Mater. 2016, 6, 1600256. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Huang, R.; Yao, J. Ternary Fe2O3/Fe3O4/FeCO3 Composite as a High-Performance Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2019, 123, 12614–12622. [Google Scholar] [CrossRef]
- Zheng, M.; Tang, H.; Li, L.; Hu, Q.; Zhang, L.; Xue, H.; Pang, H. Hierarchically Nanostructured Transition Metal Oxides for Lithium-Ion Batteries. Adv. Sci. 2018, 5, 1700592. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.-H.; Lee, S.H.; Lee, D.J.; Sung, Y.-E.; Hyeon, T. Conversion Reaction-Based Oxide Nanomaterials for Lithium Ion Battery Anodes. Small 2016, 12, 2146–2172. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Xia, Q.; Hu, Z.; Zhu, Y.; Yan, S.; Ge, C.; Zhang, Q.; Wang, X.; Shang, X.; et al. Extra Storage Capacity in Transition Metal Oxide Lithium-Ion Batteries Revealed by in Situ Magnetometry. Nat. Mater. 2021, 20, 76–83. [Google Scholar]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Cho, J.S.; Hong, Y.J.; Kang, Y.C. Design and Synthesis of Bubble-Nanorod-Structured Fe2O3–Carbon Nanofibers as Advanced Anode Material for Li-Ion Batteries. ACS Nano 2015, 9, 4026–4035. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-Based Supercapacitor Electrodes: Advances and Challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Ma, J.; Guo, X.; Yan, Y.; Xue, H.; Pang, H. FeOx-Based Materials for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700986. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, Z.; Xiong, G.; Guo, R.; Liu, Z.; Luo, H. Anchoring Fe3O4 Nanoparticles on Three-Dimensional Carbon Nanofibers toward Flexible High-Performance Anodes for Lithium-Ion Batteries. J. Power Sources 2015, 294, 414–419. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Pan, H.; Liu, Y.; Zhang, Z.; Li, J.; Su, Q.; Du, G.; Zhu, M.; Ouyang, L.; et al. Mesoporous Fe2O3 Flakes of High Aspect Ratio Encased within Thin Carbon Skeleton for Superior Lithium-Ion Battery Anodes. J. Mater. Chem. A 2015, 3, 14178–14187. [Google Scholar] [CrossRef]
- Fu, C.; Mahadevegowda, A.; Grant, P.S. Fe3O4/Carbon Nanofibres with Necklace Architecture for Enhanced Electrochemical Energy Storage. J. Mater. Chem. A 2015, 3, 14245–14253. [Google Scholar] [CrossRef]
- Li, X.; Ma, Y.; Cao, G.; Qu, Y. FeOx@Carbon Yolk/Shell Nanowires with Tailored Void Spaces as Stable and High-Capacity Anodes for Lithium Ion Batteries. J. Mater. Chem. A 2016, 4, 12487–12496. [Google Scholar] [CrossRef]
- Xu, Z.L.; Yao, S.; Cui, J.; Zhou, L.; Kim, J.K. Atomic Scale, Amorphous FeOx/Carbon Nanofiber Anodes for Li-Ion and Na-Ion Batteries. Energy Storage Mater. 2017, 8, 10–19. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Xiong, D.; Li, D.; Lawes, S.; Sun, X. Recent Developments and Understanding of Novel Mixed Transition-Metal Oxides as Anodes in Lithium Ion Batteries. Adv. Energy Mater. 2016, 6, 1502175. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Li, J.; Ma, Y.; Qu, Y. Structural Influence of Porous FeOx@C Nanorods on Their Performance as Anodes of Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 18649–18656. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Noonan, O.; Martin, D.J.; Whittaker, A.K.; Yu, C. Tailoring the Void Size of Iron Oxide@Carbon Yolk–Shell Structure for Optimized Lithium Storage. Adv. Funct. Mater. 2014, 24, 4337–4342. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, R.; Liu, W.; Zhang, X.; Shen, X.; Li, W.; Diao, G.; Chen, M. In-Depth Nanocrystallization Enhanced Li-Ions Batteries Performance with Nitrogen-Doped Carbon Coated Fe3O4 Yolk−Shell Nanocapsules. J. Power Sources 2017, 344, 74–84. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, Y.C. Synthesis of Uniquely Structured Yolk–Shell Metal Oxide Microspheres Filled with Nitrogen-Doped Graphitic Carbon with Excellent Li–Ion Storage Performance. Small 2017, 13, 1701585. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, C.; Dou, S.; Wang, S.; Zhang, J.; Gao, X.; Ma, J.; Yu, Y. Nitrogen-Doped Hierarchically Porous Carbon Networks: Synthesis and Applications in Lithium-Ion Battery, Sodium-Ion Battery and Zinc-Air Battery. Electrochim. Acta 2016, 219, 592–603. [Google Scholar] [CrossRef]
- Yang, L.; Guo, G.; Sun, H.; Shen, X.; Hu, J.; Dong, A.; Yang, D. Ionic Liquid as the C and N Sources to Prepare Yolk-shell Fe3O4@N-doped Carbon Nanoparticles and its High Performance in Lithium-ion Battery. Electrochim. Acta 2016, 190, 797–803. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, G.; Liu, H.; Liu, Y.; Li, J. α-and γ-Fe2O3 Nanoparticle/Nitrogen Doped Carbon Nanotube Catalysts for High-Performance Oxygen Reduction Reaction. Sci. China Mater. 2015, 58, 683–692. [Google Scholar] [CrossRef] [Green Version]
- Lei, C.; Han, F.; Li, D.; Li, W.-C.; Sun, Q.; Zhang, X.-Q.; Lu, A.-H. Dopamine as The Coating Agent and Carbon Precursor for The Fabrication of N-Doped Carbon Coated Fe3O4 Composites as Superior Lithium Ion Anodes. Nanoscale 2013, 5, 1168–1175. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Peng, Z.; Liu, X.; Qu, C.; Huang, Z.; Cai, Z.; Fan, H.; Zhang, Y. Nanocavity-Enriched Co3O4@ZnCo2O4@NC Porous Nanowires Derived from 1D Metal Coordination Polymers for Fast Li+ Diffusion Kinetics and Super Li+ Storage. Dalt. Trans. 2021, 50, 7277–7283. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, L.; Li, D.; Dong, Y.; Yuan, Q.; Huang, S.; Yang, H.Y.; Wei, X.; Zhuang, Q.; Ju, Z.; et al. Undercooling-Directed NaCl Crystallization: An Approach towards Nanocavity-Linked Graphene Networks for Fast Lithium and Sodium Storage. Nanoscale 2020, 12, 7622–7630. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, J.; Jiang, J.; Zhou, W.; Yang, H.; Qi, X.; Zhang, H.; Fan, H.J.; Yu, D.Y.W.; Li, C.M.; et al. Seed-Assisted Synthesis of Highly Ordered TiO2@α-Fe2O3 Core/Shell Arrays on Carbon Textiles for Lithium-Ion Battery Applications. Energy Environ. Sci. 2012, 5, 6559–6566. [Google Scholar] [CrossRef]
- Zheng, F.; He, M.; Yang, Y.; Chen, Q. Nano Electrochemical Reactors of Fe2O3 Nanoparticles Embedded in Shells of Nitrogen-Doped Hollow Carbon Spheres as High-Performance Anodes for Lithium-Ion Batteries. Nanoscale 2015, 7, 3410–3417. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Gao, Y.J.; Jeon, I.; Yang, H.; Kim, J.P.; Jeong, S.Y.; Cho, C.R. Rice-Panicle-Like γ-Fe2O3@C Nanofibers as High-Rate Anodes for Superior Lithium-Ion Batteries. Chem. Eng. J. 2019, 356, 60–68. [Google Scholar] [CrossRef]
- Na, Z.; Yao, R.; Yan, Q.; Wang, X.; Sun, X.; Wang, X. A General Strategy for Enabling Fe3O4 with Enhanced Lithium Storage Performance: Synergy between Yolk-Shell Nanostructures and Doping-Free Carbon. Electrochim. Acta 2021, 367, 137464. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, K.; Xu, Q.; Zhou, Y.; Cheng, F.; Guo, S. Beyond Yolk–Shell Nanoparticles: Fe3O4@Fe3C Core@Shell Nanoparticles as Yolks and Carbon Nanospindles as Shells for Efficient Lithium Ion Storage. ACS Nano 2015, 9, 3369–3376. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hu, Q.; Hu, W.; Zhu, W.; Wei, Y.; Pan, K.; Zheng, M.; Pang, H. Preparation of Hollow Core–Shell Fe3O4/Nitrogen-Doped Carbon Nanocomposites for Lithium-Ion Batteries. Molecules 2022, 27, 396. https://doi.org/10.3390/molecules27020396
Wang J, Hu Q, Hu W, Zhu W, Wei Y, Pan K, Zheng M, Pang H. Preparation of Hollow Core–Shell Fe3O4/Nitrogen-Doped Carbon Nanocomposites for Lithium-Ion Batteries. Molecules. 2022; 27(2):396. https://doi.org/10.3390/molecules27020396
Chicago/Turabian StyleWang, Jie, Qin Hu, Wenhui Hu, Wei Zhu, Ying Wei, Kunming Pan, Mingbo Zheng, and Huan Pang. 2022. "Preparation of Hollow Core–Shell Fe3O4/Nitrogen-Doped Carbon Nanocomposites for Lithium-Ion Batteries" Molecules 27, no. 2: 396. https://doi.org/10.3390/molecules27020396
APA StyleWang, J., Hu, Q., Hu, W., Zhu, W., Wei, Y., Pan, K., Zheng, M., & Pang, H. (2022). Preparation of Hollow Core–Shell Fe3O4/Nitrogen-Doped Carbon Nanocomposites for Lithium-Ion Batteries. Molecules, 27(2), 396. https://doi.org/10.3390/molecules27020396





