Comparative Study of the Petal Structure and Fragrance Components of the Nymphaea hybrid, a Precious Water Lily
Abstract
1. Introduction
2. Results
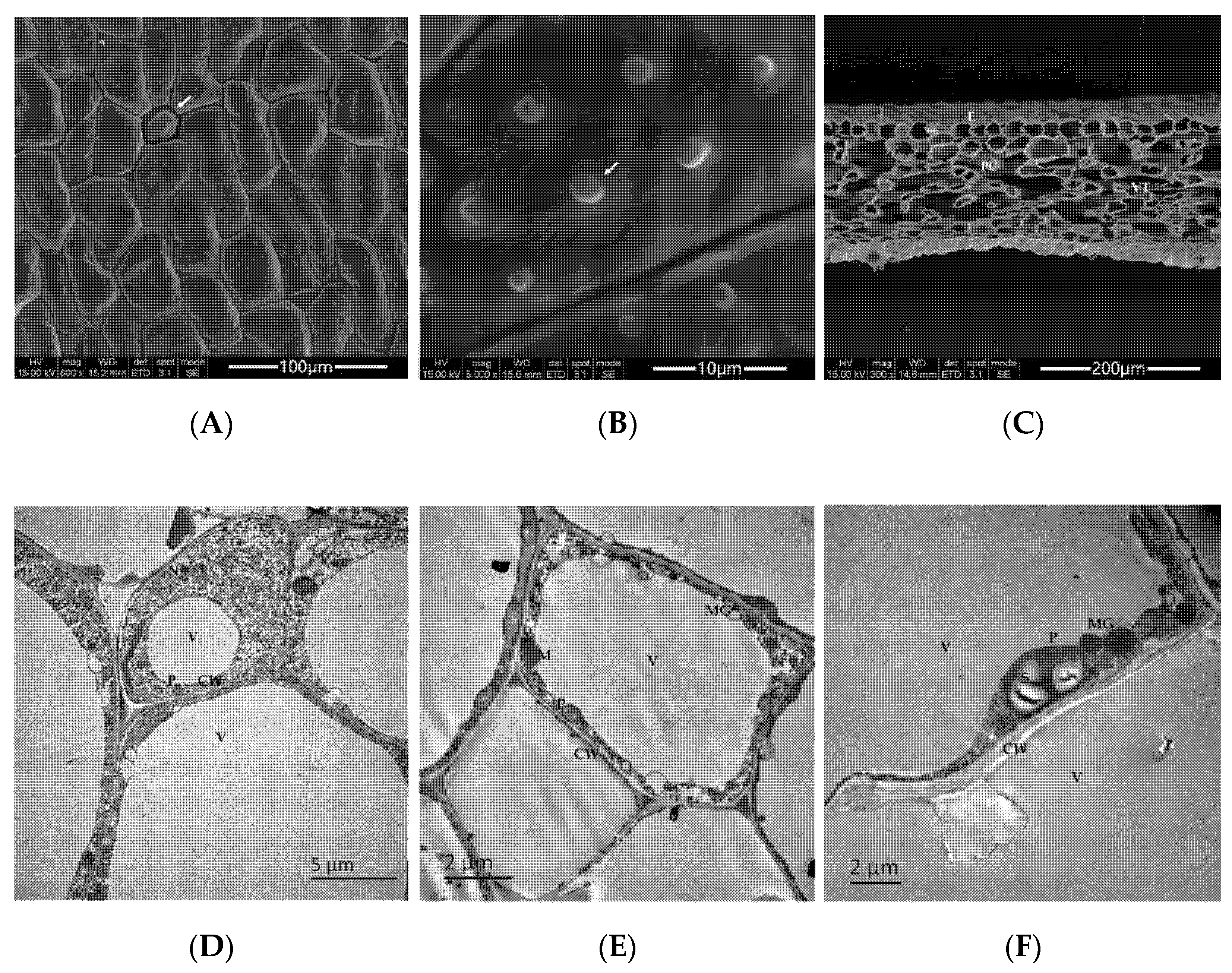
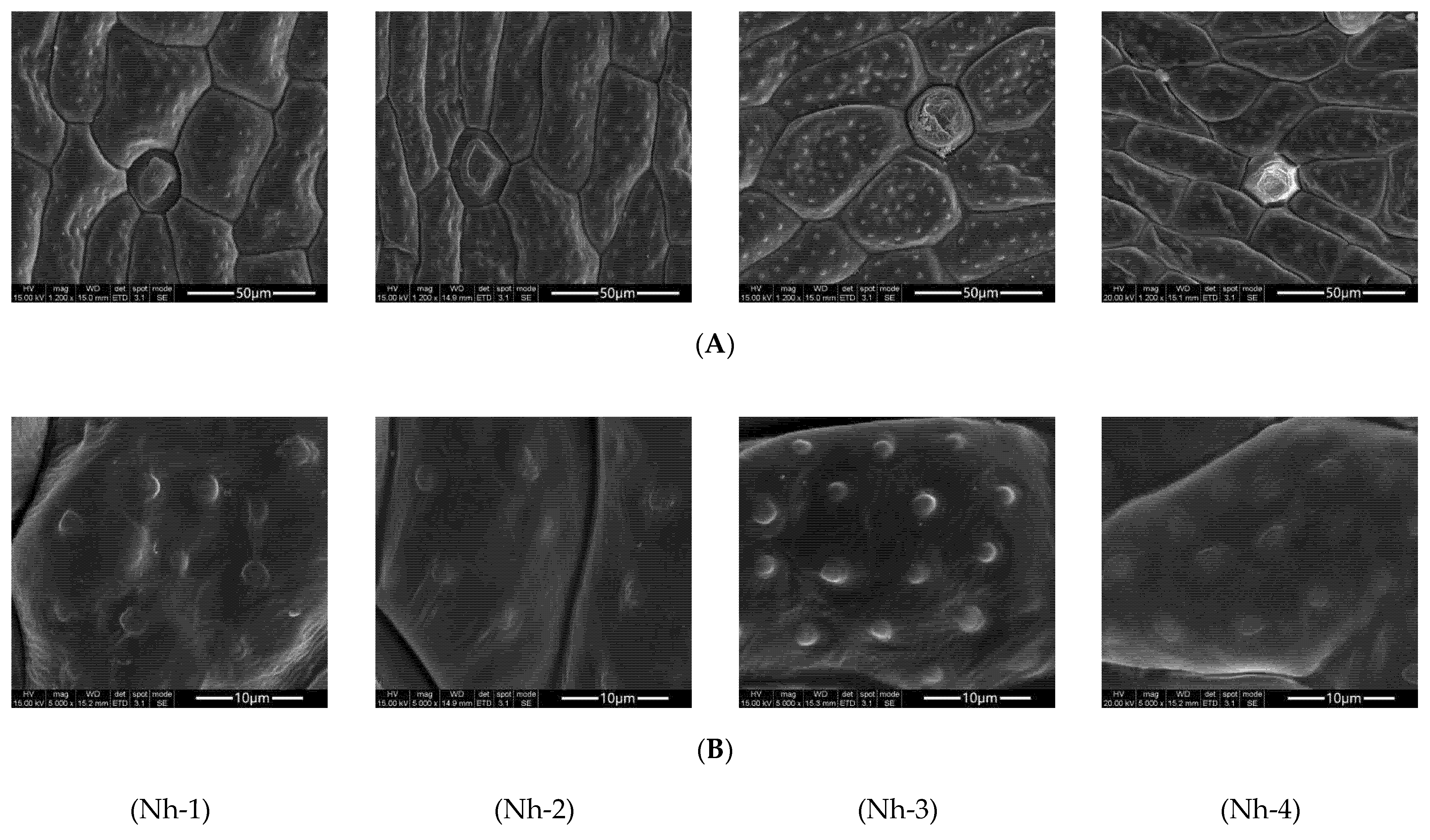
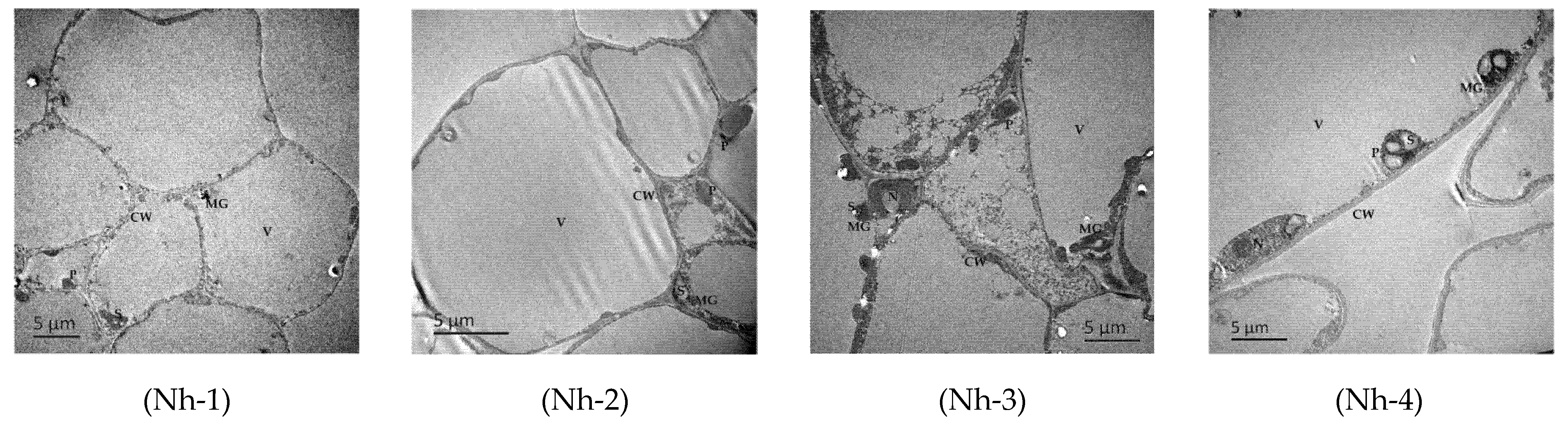
2.1. Observation of the Microstructure of the Flower Petals of N. hybrid
2.1.1. Basic Structures and Characteristics of Flower Petals
2.1.2. Differences in Cell Structures of N. hybrid
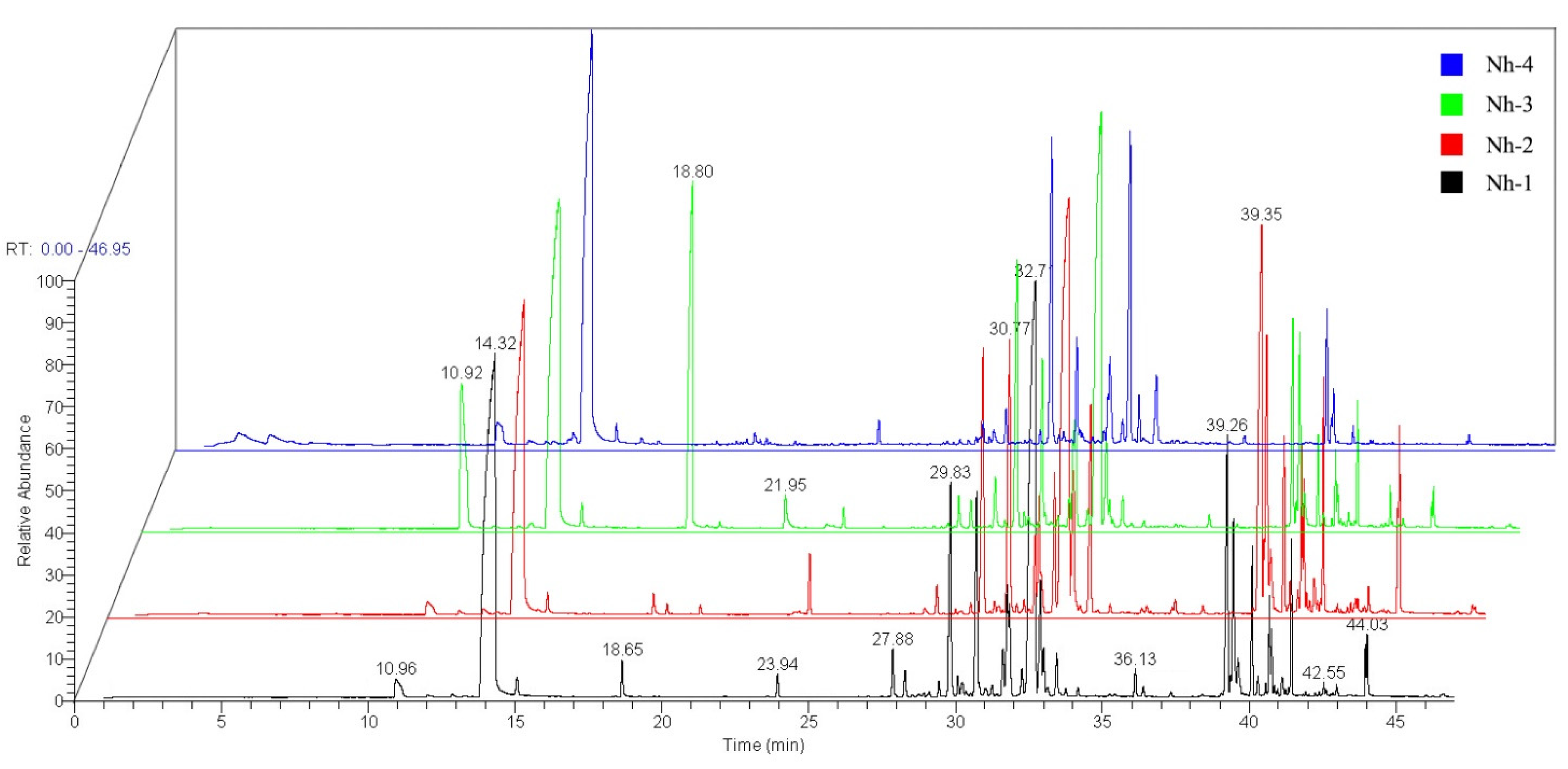
2.2. Volatile Compounds of the Flower Petals of N. hybrid
2.2.1. Identification and Concentration of the Volatile Compounds in N. hybrid
2.2.2. Comparison of the Main Volatile Compounds
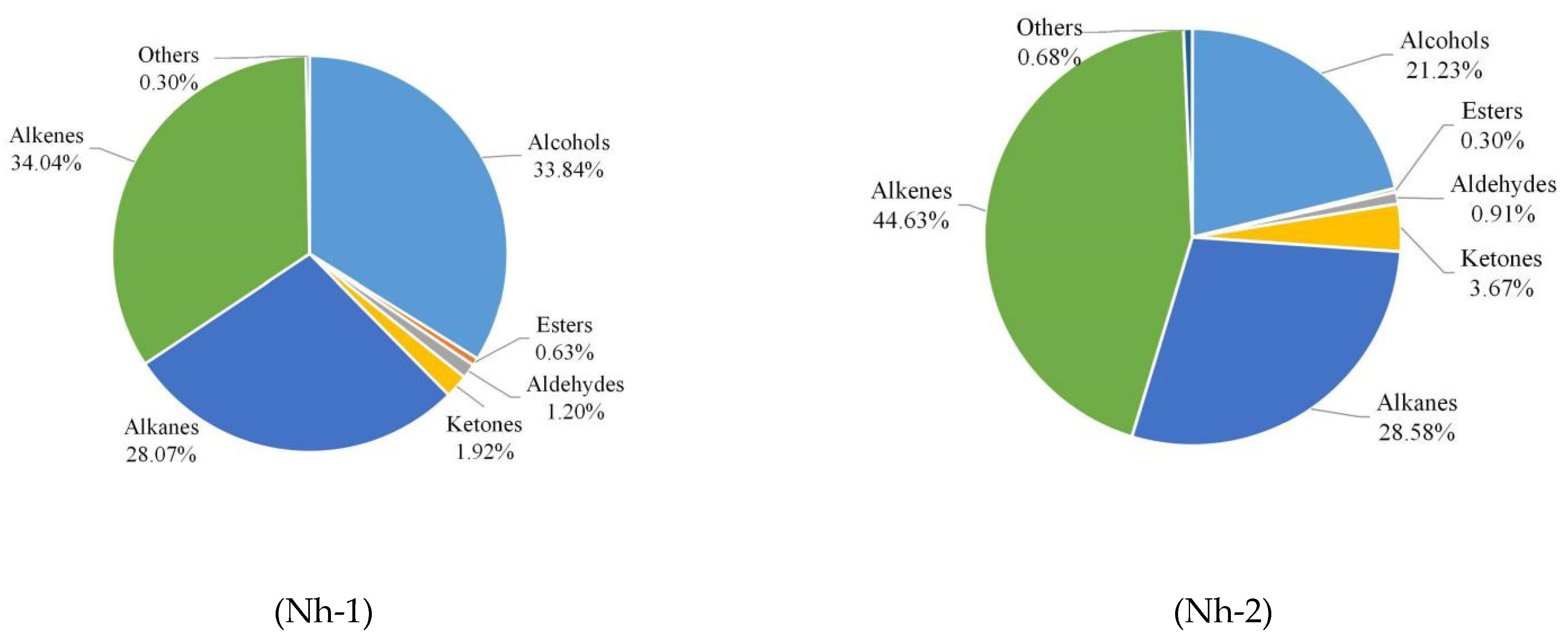
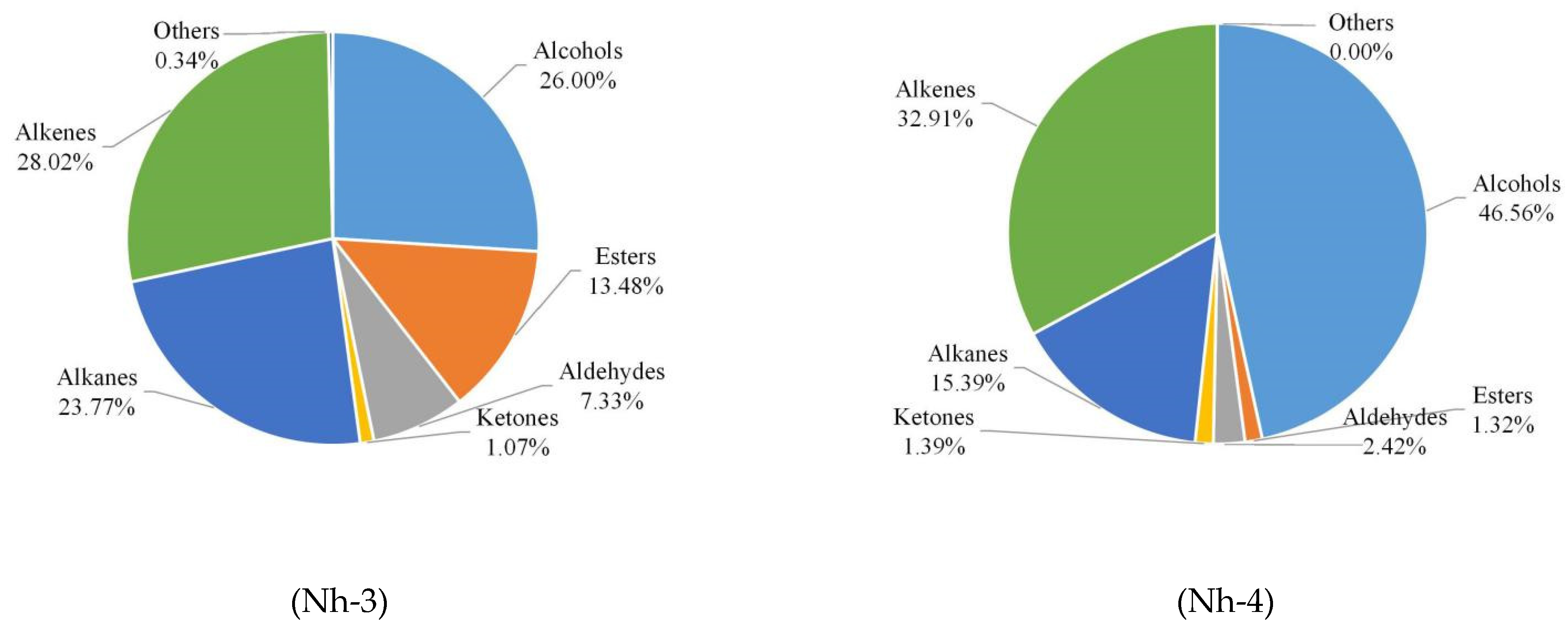
2.2.3. Relative Abundances of Different Classes of Volatile Compounds
2.2.4. Relationships among Different Classes of Volatile Compounds
2.3. Multivariate Analysis
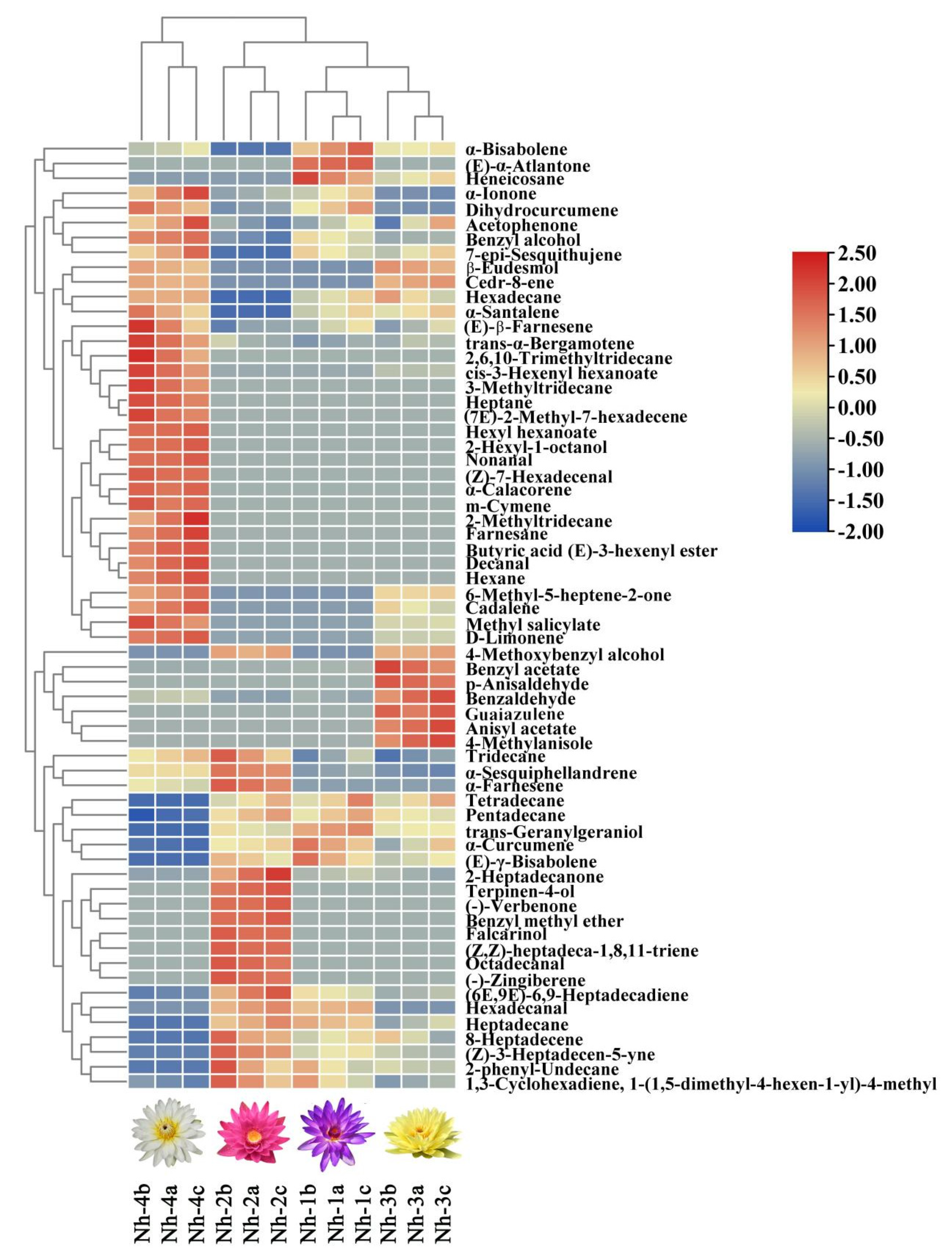
2.3.1. Hierarchical Cluster Analysis of Volatile Compounds
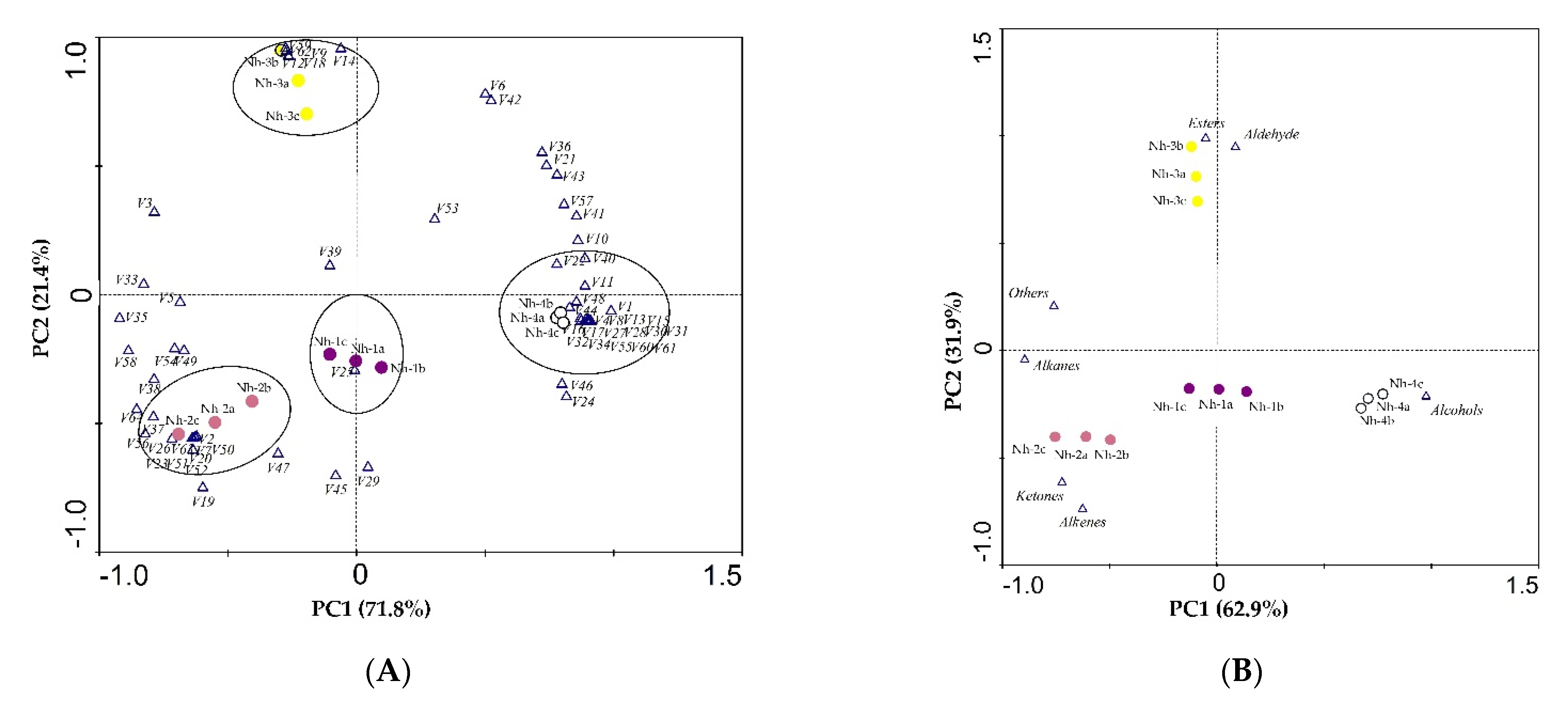
2.3.2. Principal Component Analysis of Volatile Compounds
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Microstructural Observations of Petals
4.2.1. Analyses with Scanning Electron Microscopy (SEM)
4.2.2. Analyses with Transmission Electron Microscopy (TEM)
4.3. Volatiles Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef]
- Zidi, K.; Kati, D.E.; Bachir-bey, M.; Genva, M.; Fauconnier, M.L. Comparative study of fig volatile compounds using headspace solid-phase microextraction-gas chromatography/mass spectrometry: Effects of cultivars and ripening stages. Front. Plant Sci. 2021, 12, 667809. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.D.; Herrera, C.M.; Tterl, S.D. Floral volatiles play a key role in specialized ant pollination. Perspect. Plant Ecol. 2014, 16, 32–42. [Google Scholar] [CrossRef]
- Rosati, L.; Romano, V.A.; Cerone, L.; Fascetti, S.; Farris, E. Pollination features and floral volatiles of Gymnospermium scipetarum (barberidaceae). J. Plant Res. 2019, 132, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Junker, R.R. Floral scents repel facultative flower visitors, but attract obligate ones. Ann. Bot. 2010, 105, 777–782. [Google Scholar] [CrossRef]
- Kong, C.; Hu, F.; Xu, X. Allelopathic potential and chemical constituents of volatiles from Ageratum conyzoides under stress. J. Chem. Ecol. 2002, 28, 1173–1182. [Google Scholar] [CrossRef]
- Giusto, B.D.; Bessière, J.M.; Guéroult, M.; Lim, L.; Marshall, D.J.; Hossaert-Mckey, M.; Gaumel, L. Flower-scent mimicry masks a deadly trap in the carnivorous plant Nepenthes rafflesiana. J. Ecol. 2010, 98, 845–856. [Google Scholar] [CrossRef]
- Ibrahim, M.; Agarwal, M.; Hardy, G.; Ren, Y. Optimized method to analyze rose plant volatile organic compounds by HS-SPME-GC-FID/MSD. J. Biosci. Med. 2017, 5, 13–31. [Google Scholar] [CrossRef]
- Dong, X.; Gao, X.; Liu, L.; Chen, L.; Zhang, D. Function-specific volatiles and volatilization characteristics of Dendrobium officinale. J. King Saud Univ. Sci. 2020, 32, 2020–2028. [Google Scholar] [CrossRef]
- Kilic, A.; Hafizoglu, H.; Kollmannsberger, H.; Nitz, S. Volatile constituents and key odorants in leaves, buds, flowers, and fruits of Laurus nobilis L. J. Agric. Food Chem. 2004, 52, 1601–1606. [Google Scholar] [CrossRef]
- Andrew, M.; Peter, K.; Les, S.; Shalin, K.; Brian, M.G. The impact of greenhouse tomato (Solanales: Solanaceae) floral volatiles on bumble bee (Hymenoptera: Apidae) pollination. Environ. Entomol. 2012, 4, 855–864. [Google Scholar] [CrossRef]
- Verdonk, J.C.; Haring, M.A.; Tunen, V.; Schuurink, R.C. ODORANT1 regulates fragrance biosynthesis in Petunia flowers. Plant Cell 2005, 17, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Oyama-Okubo, N.; Yamagishi, M. An R2R3-MYB transcription factor ODORANT1 regulates fragrance biosynthesis in lilies (Lilium spp.). Mol. Breed. 2018, 38, 144. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, W.; Zhou, Y.; Zhang, J.; Li, Y.; Sagheer, A.; Zhang, Q.X. Comparative transcriptome reveals benzenoid biosynthesis regulation as inducer of floral scent in the woody plant Prunus mume. Front. Plant Sci. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Rudall, P.J.; Bateman, R.M.; Eastman, F.A. Floral anatomy and systematics of Alliaceae with particular reference to Gilliesia, a presumed insect mimic with strongly zygomorphic flowers. Am. J. Bot. 2002, 89, 1867–1883. [Google Scholar] [CrossRef] [PubMed]
- Plachno, B.J.; Swiatek, P.; Szymczak, G. Can a stench be beautiful? Osmophores in stem-succulent stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae-Stapeliinae). Flora 2010, 205, 101–105. [Google Scholar] [CrossRef]
- Teixeira, S.D.P.; Borba, E.L.; Semir, J. Lip anatomy and its implications for the pollination mechanisms of Bulbophyllum species (Orchidaceae). Ann. Bot. 2004, 93, 499–505. [Google Scholar] [CrossRef][Green Version]
- Cui, C.J.; Zou, P.; Kuang, Y.F.; Liu, H.F.; Liao, J.P. Research advances on the osmophores in plants. J. Trop. Subtrop. Bot. 2015, 23, 710–719. [Google Scholar] [CrossRef]
- Tahir, S.S.; Rajput, M.T.M. Sem studies of petal structure of corolla of the species Sibbaldia L. (Rosaceae). Pak. J. Bot. 2010, 42, 1443–1449. [Google Scholar] [CrossRef]
- Tlke, E.D.; Bachelier, J.B.; Lima, E.; Ferreira, M.; Demarco, D.; Carmello-Guerreiro, S.M. Osmophores and floral fragrance in Anacardium humile and Mangifera indica (anacardiaceae): An overlooked secretory structure in sapindales. AoB Plants 2018, 10, 62. [Google Scholar] [CrossRef]
- Guo, S.Z.; Qiu, D.L.; Zhang, M.H. Variation in perianth structure during the blooming of Michelia alba DC. and its aroma emission mechanism. Chin. J. Trop. Crop. 2006, 27, 34–40. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Chen, R.B.; Huang, F.P.; You, X.M. Aromatic constituents of Chunlan and its maternal variety Tieguanying. Fujian J. Agricul. Sci. 1999, 14, 33–37. [Google Scholar] [CrossRef]
- Figuieredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Fu, J.; Dan, H.; Chao, Z.; Bao, Z.; Zhao, H.; Hu, S. The emission of the floral scent of four Osmanthus fragrans cultivars in response to different temperatures. Molecules 2017, 22, 430. [Google Scholar] [CrossRef]
- Yuan, R.; Li, S.; Zheng, X.; Wu, Q.; Zhang, H.; Wang, L. Determination of volatiles in water lily flowers using gas chromatography–mass spectrometry. Anal. Lett. 2014, 47, 1541–1551. [Google Scholar] [CrossRef]
- Povilus, R.A.; Losada, J.M.; Friedman, W.E. Floral biology and ovule and seed ontogeny of Nymphaea thermarum, water lily at the brink of extinction with potential as a model system for basal angiosperms. Ann. Bot. 2015, 115, 211–226. [Google Scholar] [CrossRef]
- Henry, S.C. The Waterlilies; The Carnegie Institution of Washington: Washington, DC, USA, 1905; pp. 125–211. [Google Scholar]
- Zhou, Q.; Zeng, Y.; Zhu, Z.L. Extraction optimization through response surface methodology and antioxidant activity of polyphenols of Nymphaea Hybrid. Mod. Food Sci. Technol. 2018, 34, 200–207. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.M.; Jiang, H.F.; Jin, J.H.; Shan, C.Y.; Zhang, J. Analysis of essential oil from the flowers of Nymphaea hybrida by GC-MS. Food Res. Dev. 2008, 29, 101–103. [Google Scholar] [CrossRef]
- Jiang, H.F.; Zhou, H.Y.; Shi, B.J.; Shan, C.Y.; Xu, H.; Zhang, J.; Zhang, W.M. Extraction, isolation and structural determination of the polysaccharides from Nymphaea hybrid. Chin. Wild Plant Res. 2017, 36, 19–22. [Google Scholar] [CrossRef]
- Jin, J.H.; Zhang, W.M. Acute and chronic toxicity of Nymphaea hybrida. Chin. Wild Plant Res. 2015, 34, 5–9. [Google Scholar] [CrossRef]
- Che, L.; Wu, X.Q.; Zheng, Q.Q.; Lu, F.; Shen, J.F. Effect of Nymphaea hybrid on prostatic hyperplasia in rats. J. Chin. Inst. Food Sci. Technol. 2015, 15, 28–33. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, Z.Y.; Hou, B.W.; Zhang, W.M.; Sun, L.J. Inhibitory effect of extracts from Nymphaea hybrid flowers on pancreatic lipase. Food Sci. Technol. 2017, 42, 227–231. [Google Scholar] [CrossRef]
- Sun, Y.J.; Li, C.S.; Lu, F.; Wu, X.Q.; Shen, J.F. Study on whitening effect of Nymphaea hybrid extracts. J. Food Sci. Biotechnol. 2018, 37, 776–783. [Google Scholar] [CrossRef]
- Ren, H.R.; Zhang, W.M.; Shan, C.Y.; Jiang, H.F.; Ma, S.H.; Xu, H.; Wang, S.X. Study on inhibition of ethanol extract from Nymphaea hybrid on the activity of tyrosinase. Deterg. Cosmet. 2009, 12, 19–21. [Google Scholar] [CrossRef]
- Zhang, H.H.; Wu, H.Y.; Zhou, Q.; Zhao, R.N.; Zhu, Z.L. Flowering characteristics and reproductive biology of Nymphaea hybrid, a precious water lily. Sci. Hortic. 2021, 287, 110268. [Google Scholar] [CrossRef]
- Wiemer, A.P.; More, M.; Benitez-Vieyra, S.; Cocucci, A.A.; Raguso, R.A.; Sérsic, A.N. A simple floral fragrance and unusual osmophore structure in Cyclopogon elatus (Orchidaceae). Plant Biol. 2010, 11, 506–514. [Google Scholar] [CrossRef]
- Gagliardi, K.B.; Cordeiro, I.; Demarco, D. Protection and attraction: Bracts and secretory structures in reduced inflorescences of Malpighiales. Flora 2016, 220, 52–62. [Google Scholar] [CrossRef]
- Marinho, C.R.; Souza, C.D.; Barros, T.C.; Teixeira, S.P. Scent glands in legume flowers. Plant Biol. 2014, 16, 215–226. [Google Scholar] [CrossRef]
- Paucar, J.; Isaias, R.; Stehmann, J.R. Unravelling the structure and function of the petal appendages in the tribe Schwenckieae (Solanaceae). Plant Biol. 2020, 22, 146–156. [Google Scholar] [CrossRef]
- Xu, L.; Yu, F. Corolla structure and fragrance components in Styrax tonkinensis. Trees 2015, 29, 1127–1134. [Google Scholar] [CrossRef]
- Marinho, C.R.; Martucci, M.E.P.; Gobbo-Neto, L.; Teixeira, S.P. Chemical composition and secretion biology of the floral bouquet in legume trees (Fabaceae). Bot. J. Linn. Soc. 2018, 187, 5–25. [Google Scholar] [CrossRef]
- Borghi, M.; Fernie, A.R.; Schiestl, F.P.; Bouwmeester, H.J. The sexual advantage of looking, smelling, and tasting good: The metabolic network that produces signals for pollinators. Trends Plant Sci. 2017, 22, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.T.; Yang, X.L.; Wang, L.G. Study on the aroma component emission pattern of Osmanthus fragrans ‘Boye Jingui’. J. Nanjing For. Univ. 2018, 42, 97–104. [Google Scholar] [CrossRef]
- Custódio, L.; Serra, H.; Nogueira, J.; Gonalves, S.; Romano, A. Analysis of the volatiles emitted by whole flowers and isolated flower organs of the carob tree using HS-SPME-GC/MS. J. Chem. Ecol. 2006, 32, 929–942. [Google Scholar] [CrossRef]
- Yang, B.C.; Yin, Z.H.; Kang, W. Study of volatiles in Lysimachia parvifolia flower using HS-SPME-GC-MS. Chem. Nat. Compd. 2014, 50, 1130–1131. [Google Scholar] [CrossRef]
- Ma, X.K.; Li, X.F.; Zhang, J.Y.; Lei, J.; Li, W.W.; Wang, G. Analysis of the volatile components in Selaginella doederleinii by headspace solid phase microextraction-gas chromatography-mass spectrometry. Molecules 2020, 25, 115. [Google Scholar] [CrossRef]
- Tsai, F.J.; Liu, H.J.; Lee, M.Y.; Lin, C.C. Determination of volatile components from live water lily flowers by an orthogonal-array-design-assisted trapping cell. Appl. Sci. 2019, 9, 1269. [Google Scholar] [CrossRef]
- Shi, N.; Liu, X.J.; Du, F.F.; Chang, Y.J.; Li, N.W.; Yao, D.R. GC-MS analysis on components of essential oil from fresh flowers of tropical water lily. J. Plant Res. Environ. 2017, 26, 104–106. [Google Scholar] [CrossRef]
- Zhang, L.X.; Shi, Z.P. Research on the cell microstructure of Jasminum sambac’s petal. J. Hunan Agric. Univ. 1999, 25, 108–111. [Google Scholar] [CrossRef]
- Mookherjee, B.D.; Trenkle, R.W.; Wilson, R.A. The chemistry of flowers, fruits and spices: Live vs. dead—A new dimension in fragrance research. Pure Appl. Chem. 1990, 62, 1357–1364. [Google Scholar] [CrossRef]
- Jirapong, C.; Inplub, K.; Wongs-Aree, C. Volatile compounds in four species of thai waterlily (Nymphaea spp.). Acta Hortic. 2012, 943, 117–122. [Google Scholar] [CrossRef]
- Francesco, S.R.D.C.; Jacopo, C.; Elia, B.; Annalisa, G.; Cinzia, B.; Aldo, T. Characterization and antioxidant activity of essential oil of four sympatric orchid species. Molecules 2019, 24, 3878. [Google Scholar] [CrossRef]
- Russo, F.; Caporaso, N.; Paduano, A.; Sacchi, R. Characterisation of volatile compounds in Cilento (Italy) figs (Ficus carica L.) cv. Dottato as affected by the drying process. Int. J. Food Prop. 2017, 20, 1366–1376. [Google Scholar] [CrossRef][Green Version]
- Loch, C.; Reusch, H.; Ruge, I.; Godelmann, R.; Pflaum, T.; Kuballa, T.; Schumacher, S.; Lachenmeier, D.W. Benzaldehyde in cherry flavour as a precursor of benzene formation in beverages. Food Chem. 2016, 206, 74–77. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Strehmel, N.; Hummel, J.; Erban, A.; Strassburg, K.; Kopka, J. Retention index thresholds for compound matching in GC-MS metabolite profiling. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 871, 182–190. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Li, J.; Luo, J.; Chen, W.; Li, X. Comparative study of volatile compounds in the fruit of two banana cultivars at different ripening stages. Molecules 2018, 23, 2456. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.D.; Yuan, R.Y.; Wu, Q.; Li, S.S.; Shao, S.; Xu, Y.J.; Hao, X.H.; Wang, L.S. Assessment of flavonoids and volatile compounds in tea infusions of water lily flowers and their antioxidant activities. Food Chem. 2015, 187, 20–28. [Google Scholar] [CrossRef] [PubMed]

| Categories | RI (exp.) | RI (lit.) | Volatile Compounds | Molecular Formula | Aroma Description 1 | Relative Content (%) 2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Nh-1 | Nh-2 | Nh-3 | Nh-4 | ||||||
| Alcohols | |||||||||
| 1 | 1035 | 1037 | Benzyl alcohol | C7H8O | Sweet, flower | 32.57 b | 20.25 d | 25.27 c | 44.55 a |
| 2 | 1160 | 1164 | Terpinen-4-ol | C10H18O | Turpentine, nutmeg | nd | 0.12 a | nd | nd |
| 3 | 1245 | 1247 | 4-Methoxybenzyl alcohol | C8H10O2 | Sweet, powdery creamy | nd | 0.12 a | 0.11 a | nd |
| 4 | 1651 | 1650 | β-Eudesmol | C15H26O | Wood, green | nd | nd | 0.12 a | 0.11 a |
| 5 | 2024 | 2000 | Falcarinol | C17H24O | - | nd | 0.12 a | nd | nd |
| 6 | 2060 | 2069 | 2-Hexyl-1-octanol | C14H30O | - | nd | nd | nd | 0.11 a |
| 7 | 2201 | 2201 | trans-Geranylgeraniol | C20H34O | - | 0.18 a | 0.11 b | 0.12 b | nd |
| Total | 32.75 b | 20.73 d | 25.62 c | 44.77 a | |||||
| Esters | |||||||||
| 8 | 1165 | 1169 | Butyric acid (E)-3-hexenyl ester | C10H18O2 | - | nd | nd | nd | 0.14 a |
| 9 | 1172 | 1166 | Benzyl acetate | C9H10O2 | Fresh, boiled vegetable | 0.61 b | 0.29 b | 12.18 a | nd |
| 10 | 1195 | 1193 | Methyl salicylate | C8H8O3 | Peppermint | nd | nd | 0.11 b | 0.36 a |
| 11 | 1380 | 1370 | cis-3-Hexenyl hexanoate | C12H22O2 | Fruit, prune | nd | nd | 0.11 b | 0.77 a |
| 12 | 1386 | 1379 | Hexyl hexanoate | C12H24O2 | Apple peel, peach | nd | nd | nd | 0.14 a |
| 13 | 1415 | 1413 | Anisyl acetate | C10H12O3 | Floral, anisic, fruity | nd | nd | 0.88 a | nd |
| Total | 0.61 b | 0.29 b | 13.28 a | 1.27 b | |||||
| Aldehydes | |||||||||
| 14 | 960 | 963 | Benzaldehyde | C7H6O | Cherry, fruity | 0.98 b | 0.56 c | 6.62 a | 1.94 b |
| 15 | 1081 | 1083 | Nonanal | C9H18O | Fat, citrus, green | nd | nd | nd | 0.11 a |
| 16 | 1188 | 1205 | Decanal | C10H20O | Soap, orange peel | nd | nd | nd | 0.17 a |
| 17 | 1255 | 1252 | p-Anisaldehyde | C8H8O2 | Mint, sweet | nd | nd | 0.60 a | nd |
| 18 | 1785 | 1797 | Hexadecanal | C16H32O | Cardboard | 0.18 b | 0.21 a | nd | nd |
| 19 | 1798 | 1798 | (Z)-7-Hexadecenal | C16H30O | - | nd | nd | nd | 0.11 a |
| 20 | 2021 | 2012 | Octadecanal | C18H36O | - | nd | 0.12 a | nd | nd |
| Total | 1.16 c | 0.89 c | 7.22 a | 2.33 b | |||||
| Ketones | |||||||||
| 21 | 988 | 975 | 6-Methyl-5-heptene-2-one | C8H14O | Citrus | nd | nd | 0.12 b | 0.19 a |
| 22 | 1070 | 1067 | Acetophenone | C8H8O | Must, flower, almond | 0.36 b | 0.29 b | 0.38 b | 0.51 a |
| 23 | 1204 | 1206 | (-)-Verbenone | C10H14O | Spicy, mint | nd | 0.12 a | nd | nd |
| 24 | 1425 | 1426 | α-Ionone | C13H20O | Wood, violet | 0.27 b | 0.17 c | 0.12 c | 0.41 a |
| 25 | 1775 | 1772 | (E)-α-Atlantone | C15H22O | - | 0.51 a | nd | nd | nd |
| 26 | 1882 | 1883 | 2-Heptadecanone | C17H34O | - | 0.71 b | 3.00 a | 0.44 b | 0.22 b |
| Total | 1.85 b | 3.59 a | 1.06 c | 1.34 b | |||||
| Alkanes | |||||||||
| 27 | 600 | 600 | Hexane | C6H14 | Alkane | nd | nd | nd | 0.79 a |
| 28 | 700 | 700 | Heptane | C7H16 | Alkane | nd | nd | nd | 0.79 a |
| 29 | 1300 | 1300 | Tridecane | C13H28 | Alkane | 0.40 b | 0.86 a | 0.31 b | 0.71 a |
| 30 | 1364 | 1366 | 2-Methyltridecane | C14H30 | - | nd | nd | nd | 0.17 a |
| 31 | 1371 | 1373 | 3-Methyltridecane | C14H30 | - | nd | nd | nd | 0.16 a |
| 32 | 1378 | 1381 | Farnesane | C15H32 | - | nd | nd | nd | 0.13 a |
| 33 | 1405 | 1400 | Tetradecane | C14H30 | Alkane | 0.49 a | 0.43 a | 0.44 a | nd |
| 34 | 1446 | 1450 | 2,6,10-Trimethyltridecane | C16H34 | - | nd | nd | nd | 0.23 a |
| 35 | 1505 | 1500 | Pentadecane | C15H32 | Alkane | 22.43 a | 23.09 a | 20.49 a | 11.15 b |
| 36 | 1600 | 1600 | Hexadecane | C16H34 | Alkane | 0.18 b | nd | 0.21 a | 0.26 a |
| 37 | 1706 | 1700 | Heptadecane | C17H36 | Alkane | 2.64 a | 2.85 a | 1.41 b | 0.39 c |
| 38 | 1712 | 1715 | 2-phenyl-Undecane | C17H28 | - | 0.46 b | 0.67 a | 0.31 b | nd |
| 39 | 2106 | 2100 | Heneicosane | C21H44 | Alkane | 0.57 a | nd | 0.25 b | nd |
| Total | 27.17 a | 27.91 a | 23.43 b | 14.80 c | |||||
| Alkenes | |||||||||
| 40 | 1020 | 1198 | D-Limonene | C10H16 | Citrus, mint | nd | nd | 0.11 b | 0.45 a |
| 41 | 1035 | 1022 | m-Cymene | C10H14 | Solvent, gasoline, citrus | nd | nd | nd | 0.11 a |
| 42 | 1392 | 1393 | 7-epi-Sesquithujene | C15H24 | - | 0.88 b | 0.12 c | 0.83 b | 1.26 a |
| 43 | 1411 | 1415 | Cedr-8-ene | C15H24 | - | nd | nd | 0.12 a | 0.11 a |
| 44 | 1428 | 1421 | α-Santalene | C15H24 | - | 0.11 b | nd | 0.13 b | 0.17 a |
| 45 | 1440 | 1434 | trans-α-Bergamotene | C15H24 | Wood, warm, tea | 4.49 b | 5.40 b | 5.34 b | 10.68 a |
| 46 | 1448 | 1448 | Dihydrocurcumene | C15H24 | - | 0.29 a | 0.14 b | 0.12 b | 0.34 a |
| 47 | 1455 | 1456 | α-Farnesene | C15H24 | Wood, sweet | nd | 2.28 a | nd | 0.83 b |
| 48 | 1476 | 1480 | (E)-β-Farnesene | C15H24 | Citrus, green | 5.51 b | 4.59 b | 5.13 b | 7.26 a |
| 49 | 1480 | 1482 | α-Curcumene | C15H22 | - | 2.96 a | 2.26 a | 1.82 b | 0.32 c |
| 50 | 1483 | 1478 | 1,3-Cyclohexadiene, 1-(1,5-dimethyl-4-hexen-1-yl)-4-methyl | C15H24 | - | 0.76 a | 0.98 a | 0.42 b | 0.30 b |
| 51 | 1495 | 1495 | (-)-Zingiberene | C15H24 | Spice, fresh, sharp | nd | 2.15 a | nd | nd |
| 52 | 1509 | 1503 | α-Bisabolene | C15H24 | Balsamic | 2.69 a | 0.13 c | 1.73 b | 1.39 b |
| 53 | 1515 | 1511 | (E)-γ-Bisabolene | C15H24 | Balsamic | 0.22 a | 0.18 b | 0.13 b | nd |
| 54 | 1520 | 1523 | α-Sesquiphellandrene | C15H24 | Sweet, fruit, herb | 1.18 c | 3.77 a | 0.80 d | 2.62 b |
| 55 | 1542 | 1540 | α-Calacorene | C15H20 | Wood | nd | nd | nd | 0.11 a |
| 56 | 1665 | 1655 | (Z,Z)-heptadeca-1,8,11-triene | C17H30 | - | nd | 0.15 a | nd | nd |
| 57 | 1675 | 1667 | (6E,9E)-6,9-Heptadecadiene | C17H32 | - | 9.96 b | 15.76 a | 6.88 c | 3.67 d |
| 58 | 1680 | 1671 | Cadalene | C15H18 | - | nd | nd | 0.23 b | 0.52 a |
| 59 | 1695 | 1705 | 8-Heptadecene | C17H34 | - | 3.87 b | 5.66 a | 3.60 b | 1.28 c |
| 60 | 1756 | 1760 | (7E)-2-Methyl-7-hexadecene | C17H34 | - | nd | nd | nd | 0.21 a |
| 61 | 1772 | 1770 | Guaiazulene | C15H18 | - | nd | nd | 0.23 a | nd |
| Total | 32.94 b | 43.57 a | 27.62 c | 31.64 b | |||||
| Others | |||||||||
| 62 | 1021 | 1016 | 4-Methylanisole | C8H10O | Naphthyl | nd | nd | 0.15 a | nd |
| 63 | 1391 | 1396 | Benzyl methyl ether | C8H10O | Fruity | nd | 0.11 a | nd | nd |
| 64 | 1838 | 1840 | (Z)-3-Heptadecen-5-yne | C17H30 | - | 0.29 b | 0.55 a | 0.19 c | nd |
| Total | 0.29 c | 0.67 a | 0.33 b | 0.00 d | |||||
| Categories | Volatile Compounds | Relative Content (%) 1 | |||
|---|---|---|---|---|---|
| Nh-1 | Nh-2 | Nh-3 | Nh-4 | ||
| Alcohols | Benzyl alcohol | 32.57 b | 20.25 d | 25.27 c | 44.55 a |
| Esters | Benzyl acetate | 0.61 b | 0.29 b | 12.18 a | nd |
| Aldehydes | Benzaldehyde | 0.98 b | 0.56 c | 6.62 a | 1.94 b |
| Ketones | 2-Heptadecanone | 0.71 b | 3.00 a | 0.44 b | 0.22 b |
| Alkanes | Pentadecane | 22.43 a | 23.09 a | 20.49 a | 11.15 b |
| Heptadecane | 2.64 a | 2.85 a | 1.41 b | 0.39 c | |
| Alkenes | trans-α-Bergamotene | 4.49 b | 5.40 b | 5.34 b | 10.68 a |
| α-Sesquiphellandrene | 1.18 c | 3.77 a | 0.80 d | 2.62 b | |
| (E)-β-Farnesene | 5.51 b | 4.59 b | 5.13 b | 7.26 a | |
| α-Curcumene | 2.96 a | 2.26 a | 1.82 b | 0.32 c | |
| α-Bisabolene | 2.69 a | 0.13 c | 1.73 b | 1.39 b | |
| (6E,9E)-6,9-Heptadecadiene | 9.96 b | 15.76 a | 6.88 c | 3.67 d | |
| 8-Heptadecene | 3.87 b | 5.66 a | 3.60 b | 1.28 c | |
| Total | 90.61 a | 87.62 b | 91.70 a | 85.48 b | |
| Alcohols | Esters | Aldehydes | Ketones | Alkanes | Alkenes | Others | |
|---|---|---|---|---|---|---|---|
| Alcohols | 1 | ||||||
| Esters | −0.272 | ||||||
| Aldehydes | −0.131 | 0.945 ** | 1 | ||||
| Ketones | −0.579 * | −0.545 | −0.629 * | 1 | |||
| Alkanes | −0.835 ** | −0.056 | −0.199 | 0.618 * | 1 | ||
| Alkenes | −0.461 | −0.660 * | −0.699 * | 0.934 ** | 0.465 | 1 | |
| Others | −0.927 ** | −0.043 | −0.168 | 0.005 | 0.823 ** | 0.705 ** | 1 |
| Categories | Volatile Compounds | Relative Content (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nh-1 | Nh-2 | Nh-3 | Nh-4 | N1 | N2 | N3 | N4 | N5 | N6 | N7 | N8 | N9 | N10 | N11 | N12 | ||
| Alcohols | Benzyl alcohol | 32.57 | 20.25 | 25.27 | 44.55 | 13.0 | 1.0 | 4.0 | - | 17.0 | - | - | 0.19 | 7.19 | - | - | 4.42 |
| 4-Methoxy- benzenemethanol | - | - | - | - | - | - | - | - | - | 7.23 | - | - | - | - | - | - | |
| Phytol | - | - | - | - | - | - | - | - | - | 13.82 | 1.34 | 4.96 | 5.01 | 1.40 | 1.82 | - | |
| Stigmasterol | - | - | - | - | - | - | - | - | - | 3.29 | - | - | - | - | - | - | |
| Gamma.-sitosterol | - | - | - | - | - | - | - | - | - | 13.19 | - | 2.49 | - | - | - | - | |
| Stigmasta-5,22-dien-3-ol | - | - | - | - | - | - | - | - | - | - | - | - | 2.55 | - | - | - | |
| (Z,E)-Farnesol | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 10.81 | - | |
| Esters | Benzyl acetate | 0.61 | 0.29 | 12.18 | - | 9.0 | 2.0 | 3.0 | - | 4.0 | - | - | - | - | - | - | 10.42 |
| 2-Methyl-1-butanol acetate | - | - | - | - | - | - | - | - | - | 1.84 | 1.17 | 1.25 | 0.42 | - | - | - | |
| n-Dodecyl acetate | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Methyl α-linolenate | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.01 | - | |
| Anisyl acetate | - | - | - | - | 3.0 | 0.4 | 2.3 | - | 0.1 | - | - | - | - | - | - | - | |
| Aldehydes | Benzaldehyde | 0.98 | 0.56 | 6.62 | 1.94 | - | - | - | - | - | - | - | - | - | 1.01 | - | - |
| Hexadecanal | - | - | - | - | - | - | - | - | - | 5.57 | - | 0.90 | - | - | - | - | |
| Pentadecanal | - | - | - | - | - | - | - | - | - | - | - | - | - | 2.21 | - | - | |
| Anisic aldehyde | - | - | - | - | 2.0 | - | 0.1 | 3.0 | - | - | - | - | - | - | - | - | |
| Ketones | 2-Heptadecanone | 0.71 | 3.00 | 0.44 | 0.22 | - | - | - | - | - | - | - | 4.29 | 3.88 | - | - | 2.21 |
| 2-Nonadecanone | - | - | - | - | - | - | - | - | - | - | - | - | - | 8.24 | - | - | |
| Alkanes | Tridecane | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.66 | - |
| Pentadecane | 22.43 | 23.09 | 20.49 | 11.15 | 25.0 | 40.0 | 32.0 | 18.0 | 41.0 | - | - | 3.11 | 2.95 | 11.09 | 9.73 | 15.49 | |
| Heptadecane | 2.64 | 2.85 | 1.41 | 0.39 | - | - | - | - | - | - | 1.89 | 1.42 | 1.53 | - | - | 2.23 | |
| Nonane | - | - | - | - | - | - | - | - | - | 2.58 | 1.66 | 1.78 | 0.52 | - | - | - | |
| Tetradecane | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 10.64 | - | |
| Cyclotetradecane | - | - | - | - | - | - | - | - | - | - | - | 2.80 | 1.71 | - | - | - | |
| Hexadecane | - | - | - | - | - | - | - | - | - | - | - | - | - | 2.10 | - | - | |
| 2,6,10-Trimethyl-dodecane | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.72 | - | - | |
| 2-Methyl- bicyclo[2.2.2]octane | - | - | - | - | - | - | - | - | - | - | - | 8.66 | 9.99 | - | - | - | |
| Octadecane | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.17 | - | - | |
| Heneicosane | - | - | - | - | - | - | - | - | - | 6.60 | 12.66 | 7.62 | 5.00 | - | 6.67 | - | |
| Tricosane | - | - | - | - | - | - | - | - | - | 12.04 | - | 16.75 | - | - | - | - | |
| Nonadecane | - | - | - | - | - | - | - | - | - | 1.04 | 23.85 | 2.50 | 9.54 | - | - | - | |
| Eicosane | - | - | - | - | - | - | - | - | - | 2.25 | 6.86 | - | 4.10 | - | - | - | |
| Pentacosane | - | - | - | - | - | - | - | - | - | 3.33 | 1.34 | 6.35 | 0.07 | - | - | - | |
| Heptacosane | - | - | - | - | - | - | - | - | - | - | 5.34 | 2.99 | 2.61 | - | - | - | |
| Pentatriacontane | - | - | - | - | - | - | - | - | - | - | 1.50 | - | - | - | - | - | |
| Alkenes | trans-α-Bergamotene | 4.49 | 5.40 | 5.34 | 10.68 | - | - | - | - | - | - | - | - | - | 1.73 | 0.46 | - |
| α-Sesquiphellandrene | 1.18 | 3.77 | 0.80 | 2.62 | - | - | - | - | - | - | - | - | - | - | - | - | |
| (E)-β-Farnesene | 5.51 | 4.59 | 5.13 | 7.26 | - | - | - | - | - | - | - | - | - | 5.22 | 9.25 | 1.55 | |
| α-Curcumene | 2.96 | 2.26 | 1.82 | 0.32 | - | - | - | - | - | - | - | - | - | - | - | - | |
| α-Bisabolene | 2.69 | 0.13 | 1.73 | 1.39 | - | - | - | - | - | - | - | - | - | - | - | - | |
| (6E,9E)-6,9-Heptadecadiene | 9.96 | 15.76 | 6.88 | 3.67 | 16.0 | 21.0 | 26.0 | 35.0 | 4.5 | - | - | - | - | - | - | 40.1 | |
| 8-Heptadecene | 3.87 | 5.66 | 3.60 | 1.28 | - | - | - | - | - | - | 2.96 | 5.78 | 6.38 | 7.95 | 4.20 | 15.27 | |
| 9-nonadecene | - | - | - | - | - | - | - | - | - | - | 0.49 | - | 2.40 | - | - | - | |
| 1,15-Hexadecadiene | - | - | - | - | - | - | - | - | - | - | - | 3.16 | - | - | - | - | |
| Squalene | - | - | - | - | - | - | - | - | - | 3.34 | 3.86 | 4.16 | 6.05 | - | - | - | |
| Others | 8-Hexadecyne | - | - | - | - | - | - | - | - | - | - | - | - | - | 33.62 | 30.20 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Shi, M.; Zhang, H.; Zhu, Z. Comparative Study of the Petal Structure and Fragrance Components of the Nymphaea hybrid, a Precious Water Lily. Molecules 2022, 27, 408. https://doi.org/10.3390/molecules27020408
Zhou Q, Shi M, Zhang H, Zhu Z. Comparative Study of the Petal Structure and Fragrance Components of the Nymphaea hybrid, a Precious Water Lily. Molecules. 2022; 27(2):408. https://doi.org/10.3390/molecules27020408
Chicago/Turabian StyleZhou, Qi, Man Shi, Huihui Zhang, and Zunling Zhu. 2022. "Comparative Study of the Petal Structure and Fragrance Components of the Nymphaea hybrid, a Precious Water Lily" Molecules 27, no. 2: 408. https://doi.org/10.3390/molecules27020408
APA StyleZhou, Q., Shi, M., Zhang, H., & Zhu, Z. (2022). Comparative Study of the Petal Structure and Fragrance Components of the Nymphaea hybrid, a Precious Water Lily. Molecules, 27(2), 408. https://doi.org/10.3390/molecules27020408






