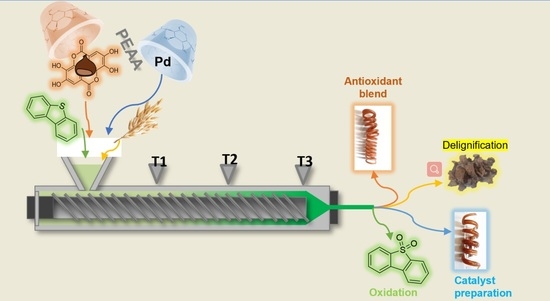
Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials
Abstract
Share and Cite
Calcio Gaudino, E.; Grillo, G.; Manzoli, M.; Tabasso, S.; Maccagnan, S.; Cravotto, G. Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules 2022, 27, 449. https://doi.org/10.3390/molecules27020449
Calcio Gaudino E, Grillo G, Manzoli M, Tabasso S, Maccagnan S, Cravotto G. Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules. 2022; 27(2):449. https://doi.org/10.3390/molecules27020449
Chicago/Turabian StyleCalcio Gaudino, Emanuela, Giorgio Grillo, Maela Manzoli, Silvia Tabasso, Simone Maccagnan, and Giancarlo Cravotto. 2022. "Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials" Molecules 27, no. 2: 449. https://doi.org/10.3390/molecules27020449
APA StyleCalcio Gaudino, E., Grillo, G., Manzoli, M., Tabasso, S., Maccagnan, S., & Cravotto, G. (2022). Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules, 27(2), 449. https://doi.org/10.3390/molecules27020449