Radiofrequency Irradiation Mitigated UV-B-Induced Skin Pigmentation by Increasing Lymphangiogenesis
Abstract
:1. Introduction
2. Results
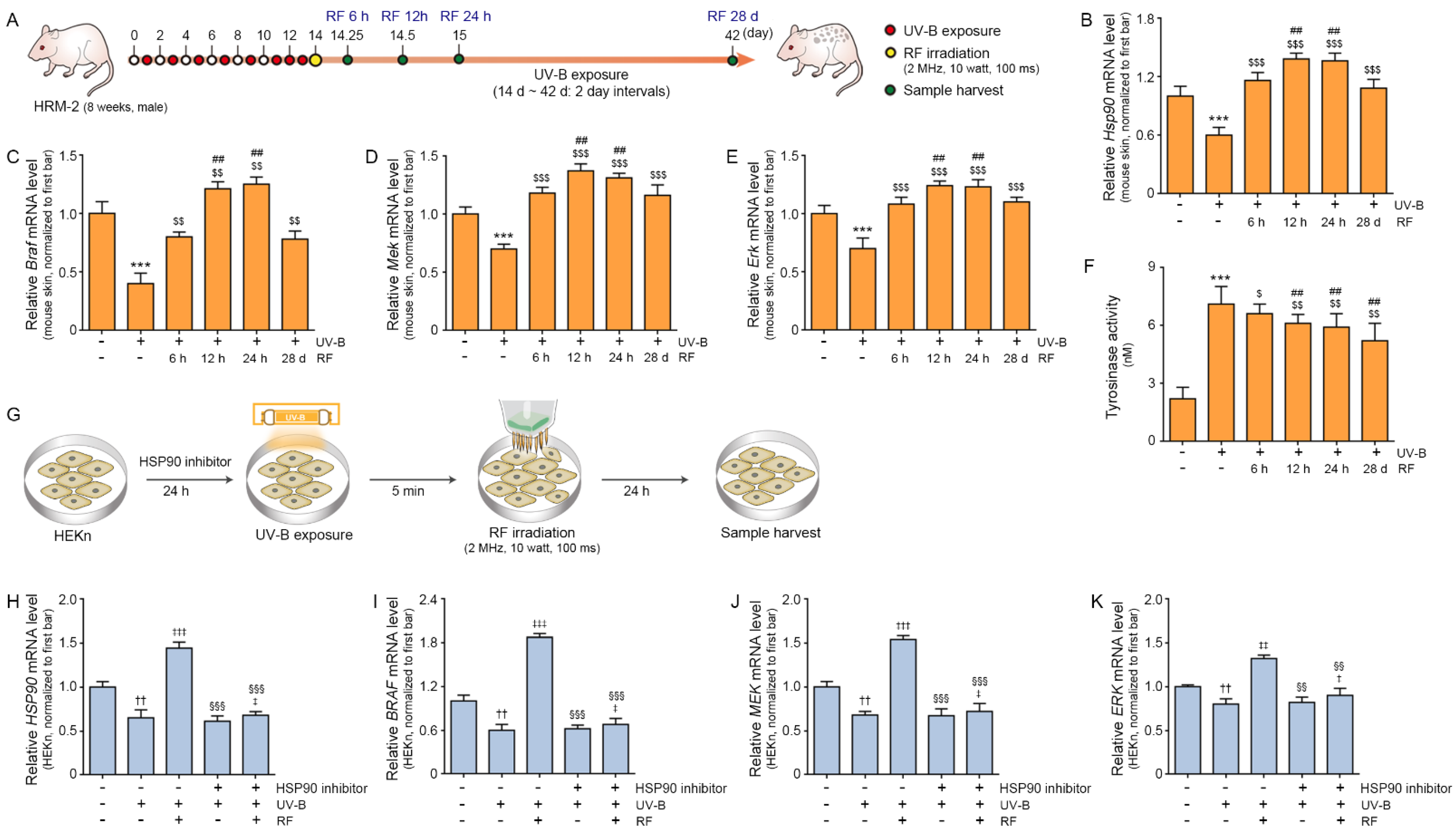
2.1. RF Irradiation Increased the Expression of HSP90, BRAF, MEK, and ERK
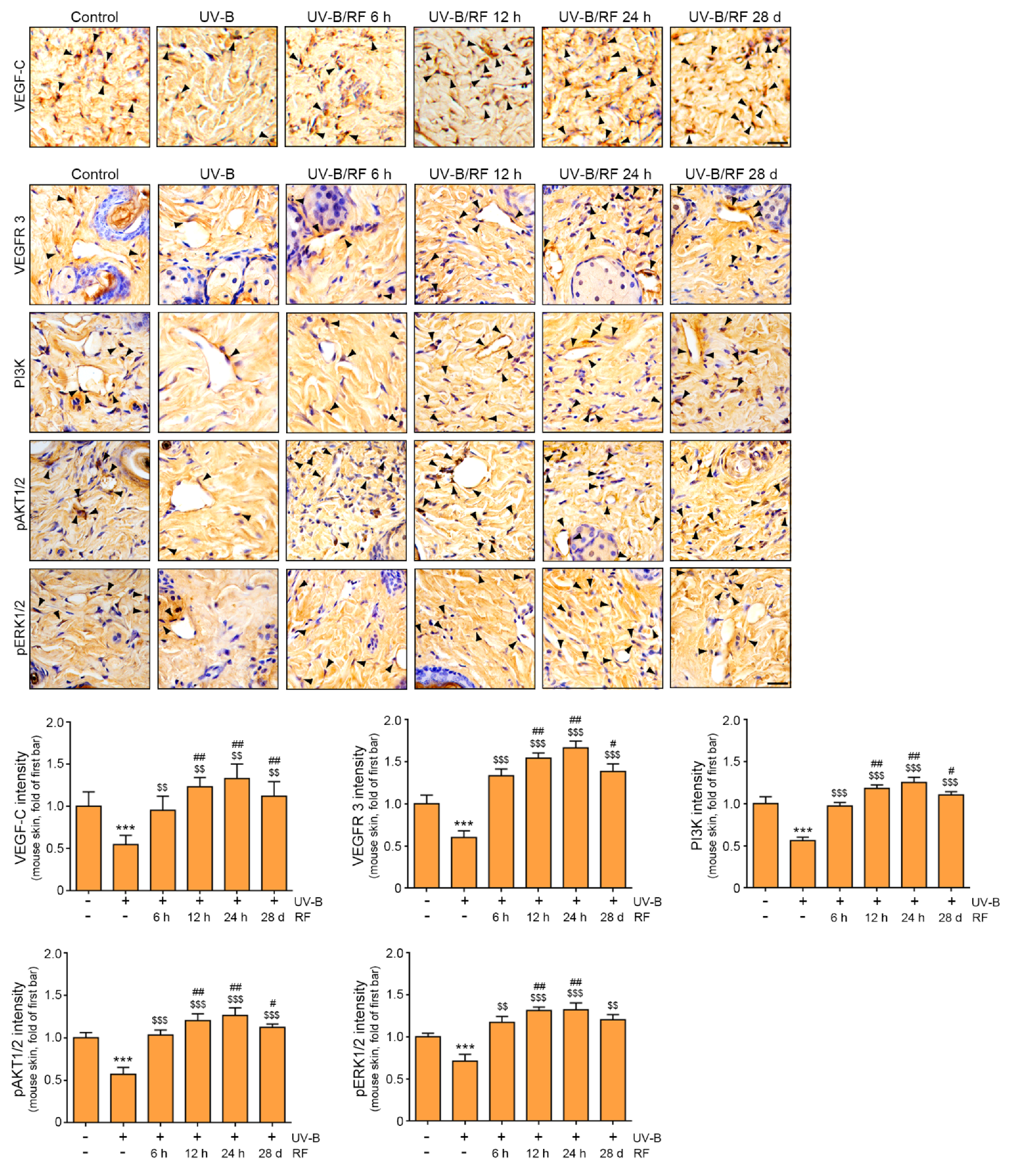
2.2. RF Upregulation of the VEGF-C/VEGFR 3/PI3K/pAKT/pERK Lymphangiogenesis Signaling Pathway in UV-B-Irradiated Skin
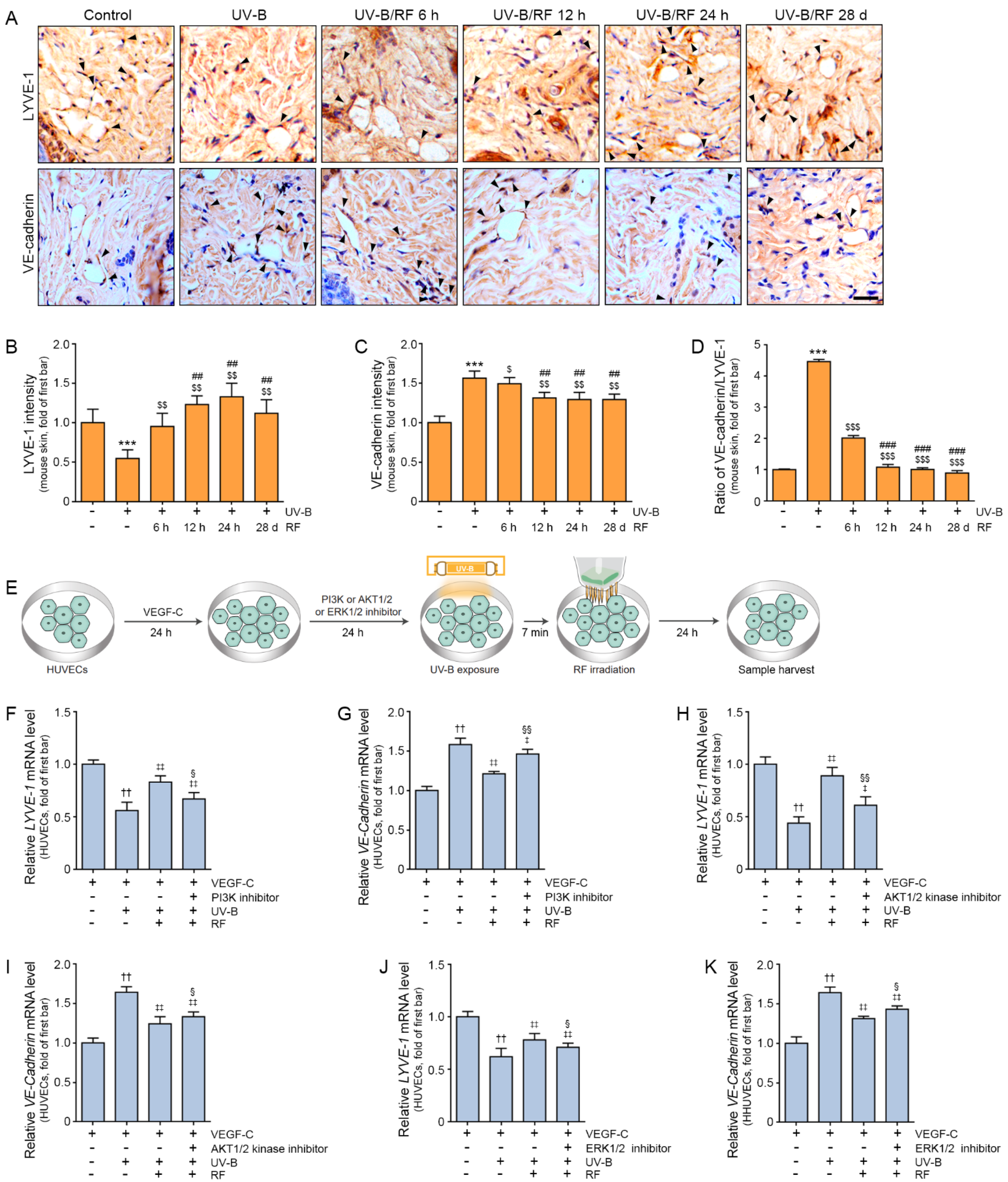
2.3. RF Irradiation Increased Lymphangiogenesis and Regulated the Permeability of Lymphatic Vessels
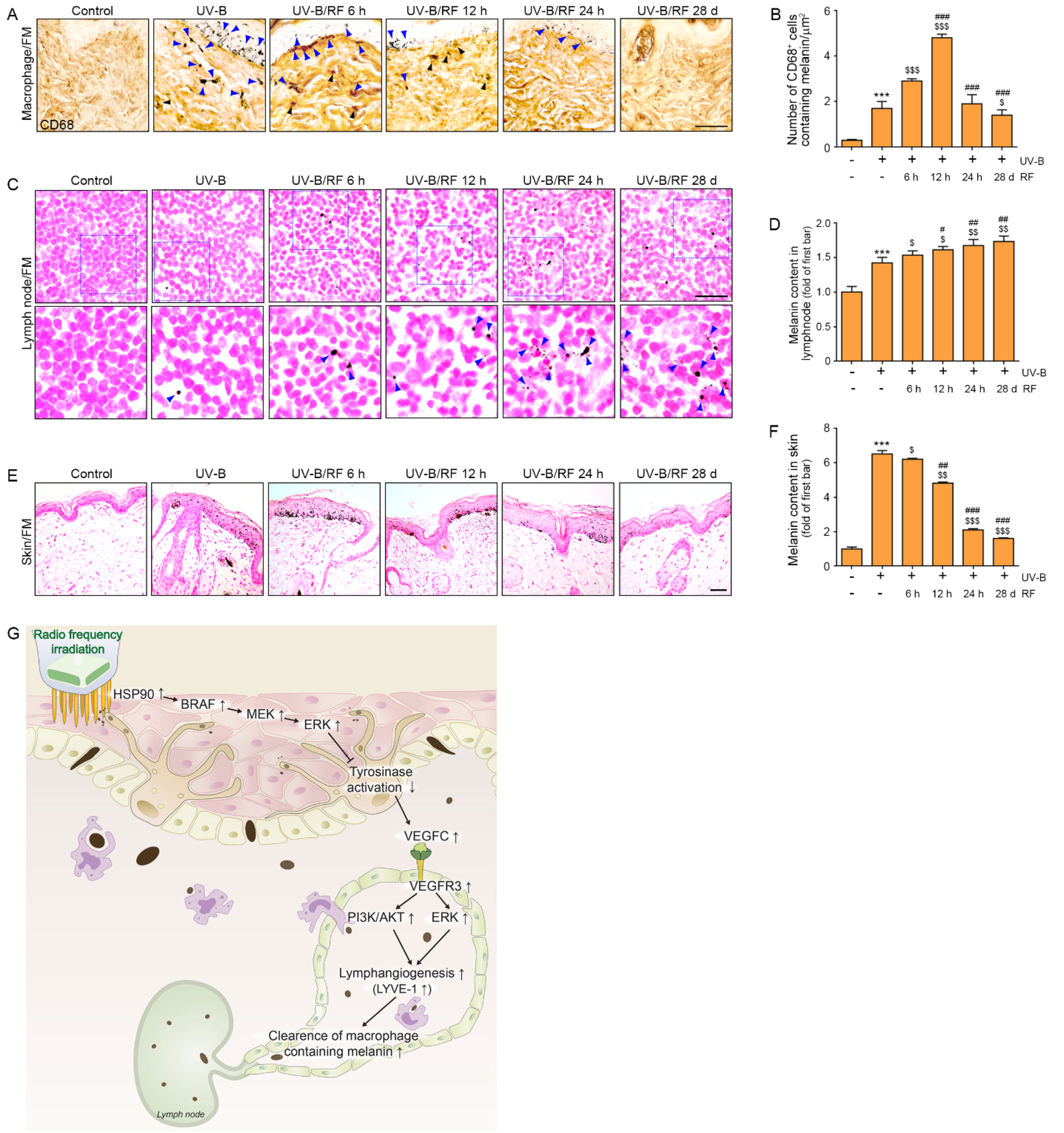
2.4. RF Irradiation of the Skin Decreased the Levels of Melanin-Containing Macrophages
2.5. RF Irradiation Increased the Melanin Content in Lymph Nodes and Decreased Skin Pigmentation
3. Discussion
4. Materials and Methods
4.1. In Vitro Model and RF Irradiation
4.2. Inhibition Study
4.2.1. HSP90 Inhibition Study on HEKn
4.2.2. PI3K, AKT, and ERK Inhibition Study on HUVECs
4.3. In Vivo Model and RF Irradiation
4.4. Sample Preparation
4.4.1. RNA Extraction and cDNA Synthesis
4.4.2. Paraffin-Embedded Tissue Sectioning
4.5. Quantitative Real-Time Polymerase Chain Reaction
4.6. Tyrosinase Activity Analysis
4.7. Immunohistochemistry Using 3,3-Diaminobenzidine
4.8. Fontana Masson Staining
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lambert, M.W.; Maddukuri, S.; Karanfilian, K.M.; Elias, M.L.; Lambert, W.C. The physiology of melanin deposition in health and disease. Clin. Dermatol. 2019, 37, 402–417. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Kohli, I.; Chaowattanapanit, S.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. J. Am. Acad. Dermatol. 2017, 4, 591–605. [Google Scholar] [CrossRef]
- Chang, M.W. Disorders of hyperpigmentation. In Dermatology, 4th ed.; Bolognia, J.L., Schaffer, J.V., Cerroni, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 1115–1143. [Google Scholar]
- Rodrigues, M.; Pandya, A.G. Hypermelanoses. In Fitzpatrick’s Dermatology, 9th ed.; Kang, S., Amagi, M., Bruckner, A.L., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw Hill: New York, NY, USA, 2019; Volume 2, pp. 1351–1389. [Google Scholar]
- Tomita, Y.; Maeda, K.; Tagami, H. Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment. Cell Res. 1992, 5, 357–361. [Google Scholar] [CrossRef]
- Taylor, S.; Grimes, P.; Lim, J.; Im, S.; Lui, H. Postinflammatory hyperpigmentation. J. Cutan. Med. Surg. 2009, 13, 183–191. [Google Scholar] [CrossRef]
- Yoshino, M.; Yamazaki, H.; Hayashi, S. Analysis of capturing skin antigens in the steady state using milk fat globule EGF factor 8-deficient skin-hyperpigmented mice. Immunol. Lett. 2008, 115, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Yamazaki, H.; Shultz, L.D.; Hayashi, S. Constant rate of steady-state self-antigen trafficking from skin to regional lymph nodes. Int. Immunol. 2006, 18, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Okuma, M.; Seiji, M. Lymphatic transport of melanosomes to the lymph node. Tohoku J. Exp. Med. 1973, 111, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossi, A.B.; Hyttel, P.; Jensen, H.E.; Leifsson, P.S. Porcine melanotic cutaneous lesions and lymph nodes:Immunohistochemical dierentiation of melanocytes and melanophages. Vet. Pathol. 2015, 52, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, R.; Ullmann, S.; Proulx, S.T.; Pytowski, B.; Alitalo, K.; Detmar, M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J. Exp. Med. 2010, 207, 2255–2269. [Google Scholar] [CrossRef] [Green Version]
- Huggenberger, R.; Siddiqui, S.S.; Brander, D.; Ullmann, S.; Zimmermann, K.; Antsiferova, M.; Werner, S.; Alitalo, K.; Detmar, M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 2011, 117, 4667–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataru, R.P.; Jung, K.; Jang, C.; Yang, H.; Schwendener, R.A.; Baik, J.E.; Han, S.H.; Alitalo, K.; Koh, G.Y. Critical role of CD11b1 macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 2009, 113, 5650–5659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halin, C.; Tobler, N.E.; Vigl, B.; Brown, L.F.; Detmar, M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood 2007, 110, 3158–3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuest, T.R.; Carr, D.J. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J. Exp. Med. 2010, 207, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.E.; Koh, Y.J.; Jeon, B.H.; Jang, C.; Han, J.; Kataru, R.P.; Schwendener, R.A.; Kim, J.M.; Koh, G.Y. Role of CD11b1 macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am. J. Pathol. 2009, 175, 1733–1745. [Google Scholar] [CrossRef] [Green Version]
- Baluk, P.; Tammela, T.; Ator, E.; Lyubynska, N.; Achen, M.G.; Hicklin, D.J.; Jeltsch, M.; Petrova, T.V.; Pytowski, B.; Stacker, S.A.; et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Investig. 2005, 115, 247–257. [Google Scholar] [PubMed]
- Kubo, H.; Cao, R.; Brakenhielm, E.; Mäkinen, T.; Cao, Y.; Alitalo, K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc. Natl. Acad. Sci. USA 2002, 99, 8868–8873. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.W.; Chong, S.Z.; Wong, F.H.; Evrard, M.; Tan, S.M.; Keeble, J.; Kemeny, D.M.; Ng, S.G.; Abastado, J.P.; Angeli, V. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood 2013, 122, 3666–3677. [Google Scholar] [CrossRef] [Green Version]
- Sarangarajan, R.; Apte, S.P. Melanization and phagocytosis: Implications for age related macular degeneration. Mol. Vis. 2005, 11, 482–490. [Google Scholar]
- Higa, K.; Shimmura, S.; Miyashita, H.; Shimazaki, J.; Tsubota, K. Melanocytes in the corneal limbus interact with K19-positive basal epithelial cells. Exp. Eye Res. 2005, 81, 218–223. [Google Scholar] [CrossRef]
- Büttner, C.; Clahsen, T.; Regenfuss, B.; Dreisow, M.L.; Steiber, Z.; Bock, F.; Reis, A.; Cursiefen, C. Tyrosinase is a novel endogenous regulator of developmental and inflammatory lymphangiogenesis. Am. J. Pathol. 2019, 189, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.S.; Adini, I.; McBride, A.F.; Birsner, A.E.; D’Amato, R.J. The albino mutation of tyrosinase alters ocular angiogenic responsiveness. Angiogenesis 2013, 16, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Wilczyński, S.; Stolecka-Warzecha, A.; Deda, A.; Koprowski, R.; Flasz, K.; Błoński, B.; Musioł, M. In vivo dynamic thermal imaging of skin radiofrequency treatment. J. Cosmet. Dermatol. 2018, 18, 1307–1316. [Google Scholar] [CrossRef]
- Belenky, I.; Margulis, A.; Elman, M.; Bar-Yosef, U.; Paun, S.D. Exploring channeling optimized radiofrequency energy: A review of radiofrequency history and applications in esthetic fields. Adv. Ther. 2012, 29, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.F.; de Oliveira, P.; Silva, F.K.B.A.; da Costa, A.C.S.; Pereira, C.R.A.; Casenave, S.; Valentim Silva, R.M.; Araújo-Neto, L.G.; Santos-Filho, S.D.; Aizamaque, E.; et al. Radiofrequency treatment induces fibroblast growth factor 2 expression and subsequently promotes neocollagenesis and neoangiogenesis in the skin tissue. Laser Med. Sci. 2017, 32, 1727–1736. [Google Scholar] [CrossRef]
- Edwards, M.J.; Marks, R.; Dykes, P.J.; Merrett, V.R.; Morgan, H.E.; O’Donovan, M.R. Heat shock proteins in cultured human keratinocytes and fibroblasts. J. Investig. Dermatol. 1991, 96, 392–396. [Google Scholar] [CrossRef] [Green Version]
- Trautinger, F.; Kindas-Mügge, I.; Dekrout, B.; Knobler, R.M.; Metze, D. Expression of the 27-kDa heat shock protein in human epidermis and in epidermal neoplasms: An immunohistological study. Br. J. Dermatol. 1995, 133, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Emilia del Pino, M.; Rosado, R.H.; Azuela, A.; Graciela Guzmán, M.; Argüelles, D.; Rodríguez, C.; Rosado, G.M. Effect of controlled volumetric tissue heating with radiofrequency on cellulite and the subcutaneous tissue of the buttocks and thighs. J. Drugs Dermatol. 2006, 5, 714–722. [Google Scholar]
- Tokalov, S.V.; Gutzeit, H.O. Weak electromagnetic fields (50 Hz) elicit a stress response in human cells. Environ. Res. 2004, 94, 145–151. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Wong, D.S.; Jay, D.G. Emerging Roles of Extracellular Hsp90 in Cancer. Adv. Cancer Res. 2016, 129, 41–163. [Google Scholar]
- Mäkinen, T.; Veikkola, T.; Mustjoki, S.; Karpanen, T.; Catimel, B.; Nice, E.C.; Wise, L.; Mercer, A.; Kowalski, H.; Kerjaschki, D.; et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001, 20, 4762–4773. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Chen, S.; An, Q.; Li, B.; Fu, Y.; Luo, Y. Extracellular Hsp90α promotes tumor lymphangiogenesis and lymph node metastasis in breast cancer. Int. J. Mol. Sci. 2021, 22, 7747. [Google Scholar] [CrossRef]
- Shah, A.; Delgado-Goni, T.; Casals Galobart, T.; Wantuch, S.; Jamin, Y.; Leach, M.O.; Robinson, S.P.; Bamber, J.; Beloueche-Babari, M. Detecting human melanoma cell redifferentiation following BRAF or heat shock protein 90 inhibition using photoacoustic and magnetic resonance imaging. Sci. Rep. 2017, 7, 8215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alesiani, D.; Cicconi, R.; Mattei, M.; Bei, R.; Canini, A. Inhibition of Mek 1/2 kinase activity and stimulation of melanogenesis by 5,7-dimethoxycoumarin treatment of melanoma cells. Int. J. Oncol. 2009, 34, 1727–1735. [Google Scholar] [PubMed]
- Li, H.; Kim, J.; Hahn, H.G.; Yun, J.; Jeong, H.S.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Min, Y.S.; Park, K.C.; et al. KHG26792 inhibits melanin synthesis in Mel-Ab cells and a skin Eequivalent model. Korean J. Physiol. Pharm. 2014, 18, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, J.J.; Fisher, D.E. The roles of microphthalmia-associated transcription factor and pigmentation in melanoma. Arch. Biochem. Biophys. 2014, 563, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Park, S.H.; Kwon, S.B.; Park, E.S.; Huh, C.H.; Youn, S.W.; Park, K.C. Sphingosylphosphorylcholine-induced ERK activation inhibits melanin synthesis in human melanocytes. Pigment. Cell Res. 2006, 19, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Hwang, E.S.; Lee, J.E.; Kim, S.Y.; Kwon, S.B.; Park, K.C. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell Sci. 2003, 116, 1699–1706. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.W.; Jeong, H.O.; Jang, E.J.; Choi, Y.J.; Kim, D.H.; Kim, S.R.; Lee, K.J.; Lee, H.J.; Chun, P.; Byun, Y.; et al. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim. Biophys. Acta 2013, 1830, 4752–4761. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.N.; Vestweber, D. Mechanisms Ensuring Endothelial Junction Integrity Beyond VE-Cadherin. Front. Physiol. 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Burchill, M.A.; Finlon, J.M.; Goldberg, A.R.; Gillen, A.E.; Dahms, P.A.; McMahan, R.H.; Tye, A.; Winter, A.B.; Reisz, J.A.; Bohrnsen, E.; et al. Oxidized low-density lipoprotein drives dysfunction of the liver lymphatic system. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 573–595. [Google Scholar] [CrossRef]
- Nakano, S.; Abe, Y.; Nakajima, K.; Sano, S.; Yamamoto, O.; Wakamatsu, K.; Ito, S.; Hayashi, M.; Suzuki, T. Establishment of a mouse model for post-inflammatory hyperpigmentation. Pigment. Cell Melanoma Res. 2021, 34, 101–110. [Google Scholar] [CrossRef]
- Hos, D.; Schlereth, S.L.; Bock, F.; Heindl, L.M.; Cursiefen, C. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis: What can we learn from the eye? Semin. Cell Dev. Biol. 2015, 38, 117–130. [Google Scholar] [CrossRef]
- Streilein, J.W. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003, 3, 879–889. [Google Scholar] [CrossRef]
- Jones, D.; Min, W. An overview of lymphatic vessels and their emerging role in cardiovascular disease. J. Cardiovasc. Dis. Res. 2011, 2, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Oh, S.; Byun, K.-A.; Yang, J.Y.; Sun, H.J.; Kang, D.; Son, K.H.; Byun, K. Radiofrequency Irradiation Mitigated UV-B-Induced Skin Pigmentation by Increasing Lymphangiogenesis. Molecules 2022, 27, 454. https://doi.org/10.3390/molecules27020454
Kim HM, Oh S, Byun K-A, Yang JY, Sun HJ, Kang D, Son KH, Byun K. Radiofrequency Irradiation Mitigated UV-B-Induced Skin Pigmentation by Increasing Lymphangiogenesis. Molecules. 2022; 27(2):454. https://doi.org/10.3390/molecules27020454
Chicago/Turabian StyleKim, Hyoung Moon, Seyeon Oh, Kyung-A Byun, Jin Young Yang, Hye Jin Sun, Donghwan Kang, Kuk Hui Son, and Kyunghee Byun. 2022. "Radiofrequency Irradiation Mitigated UV-B-Induced Skin Pigmentation by Increasing Lymphangiogenesis" Molecules 27, no. 2: 454. https://doi.org/10.3390/molecules27020454
APA StyleKim, H. M., Oh, S., Byun, K.-A., Yang, J. Y., Sun, H. J., Kang, D., Son, K. H., & Byun, K. (2022). Radiofrequency Irradiation Mitigated UV-B-Induced Skin Pigmentation by Increasing Lymphangiogenesis. Molecules, 27(2), 454. https://doi.org/10.3390/molecules27020454






