Preservation of Mimosa tenuiflora Antiaflatoxigenic Activity Using Microencapsulation by Spray-Drying
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biological Activities of the MAE and Evolution after One-Year Storage (sMAE)
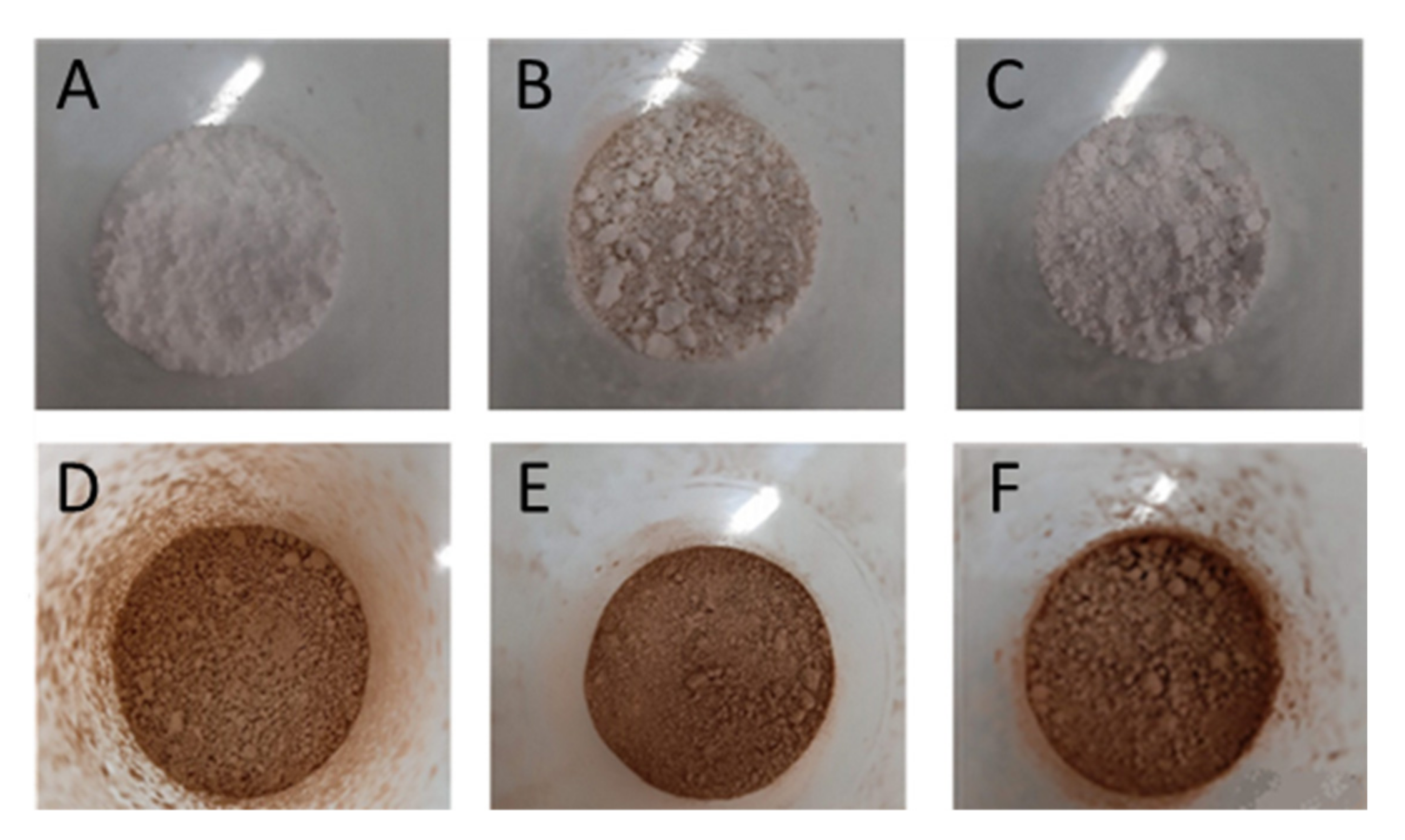
2.2. MAE Spray-Drying Encapsulation
2.3. Particles Characterization
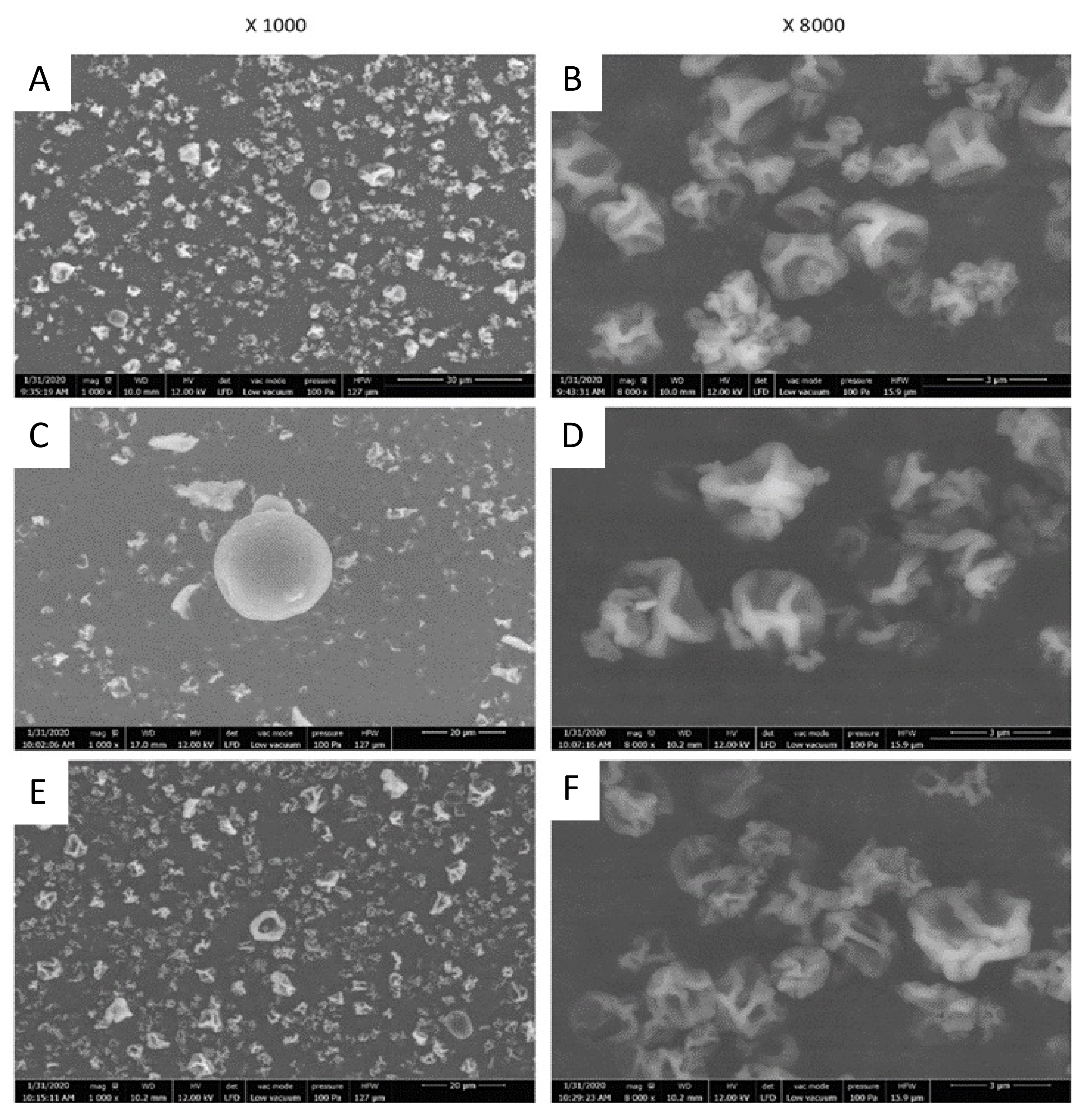
2.3.1. Particles Morphology and Size
2.3.2. Total Polyphenol Content and Antioxidant Activity of Wall Material Microparticles
2.3.3. Total Polyphenol Content and Antioxidant Capacity of Microparticles with MAE and Evolution after 1 Year of Storage
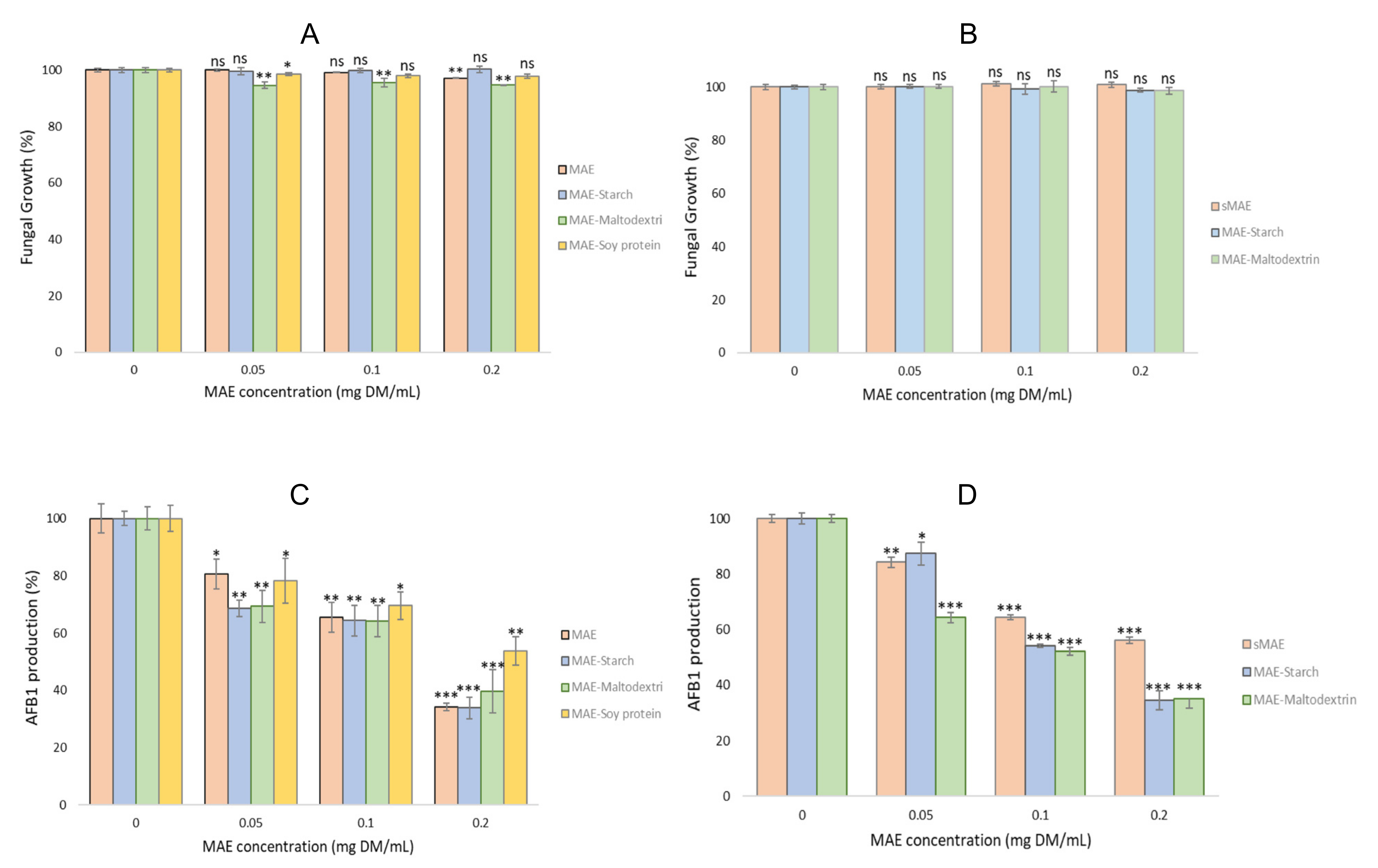
2.4. Antifungal and Antiaflatoxin Activities
2.4.1. Antifungal and Antiaflatoxin Activities of the Wall Material Microparticles
2.4.2. Antifungal and Antiaflatoxin Activities of the Microparticles with MAE and Evolution after One Year pf Storage
3. Materials and Methods
3.1. Chemicals, Reagents and Encapsulating Agents
3.2. Plant Material
3.3. Preparation of M. tenuiflora Aqueous Extract (MAE)
3.4. Dry Matter Content
3.5. MAE Encapsulation by Spray-Drying
3.6. Polyphenol Encapsulation Efficiency (PEE)
3.7. Scanning Electron Microscopy (SEM) Analysis
3.8. Solubilization of Microparticles for Analyses
3.9. Determination of Total Polyphenol Content
3.10. Determination of Antioxidant Activity
3.11. Effect of MAE and Microparticles on Aspergillus Flavus Growth and Aflatoxin B1 Synthesis
3.11.1. Fungal Strain and Culture Conditions
3.11.2. Extraction and Quantification of AFB1 by HPLC
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- IARC. Fungi Producing Significant Mycotoxins. IARC Sci. Publ. 2012, 158, 1–30. [Google Scholar]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.-B.; Nováková, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus Section Flavi and Their Production of Aflatoxins, Ochratoxins and Other Mycotoxins. Stud. Mycol. 2018, 91, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Gibb, H.; Devleesschauwer, B.; Bolger, P.M.; Wu, F.; Ezendam, J.; Cliff, J.; Zeilmaker, M.; Verger, P.; Pitt, J.; Baines, J.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of Four Foodborne Chemical Toxins, 2010: A Data Synthesis. F1000Research 2015, 4, 1393. [Google Scholar] [CrossRef]
- Bailly, S.; Mahgubi, A.; Carvajal-Campos, A.; Lorber, S.; Puel, O.; Oswald, I.; Bailly, J.-D.; Orlando, B. Occurrence and Identification of Aspergillus Section Flavi in the Context of the Emergence of Aflatoxins in French Maize. Toxins 2018, 10, 525. [Google Scholar] [CrossRef] [Green Version]
- Waliyar, F.; Osiru, M.; Sudini, H.; Njoroge, S. Reducing Aflatoxins in Groundnuts through Integrated Management and Biocontrol. In Aflatoxins-Finding Solutions for Improved Food Safety; International Food Policy Research Institute: Washington, DC, USA, 2013; pp. 1–2. [Google Scholar]
- Sipos, P.; Peles, F.; Brassó, D.L.; Béri, B.; Pusztahelyi, T.; Pócsi, I.; Győri, Z. Physical and Chemical Methods for Reduction in Aflatoxin Content of Feed and Food. Toxins 2021, 13, 204. [Google Scholar] [CrossRef]
- FAO. Worldwide Regulations for Mycotoxins in Food and Feed in 2003; FAO: Rome, Italy, 2004. [Google Scholar]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Biocontrol of Aflatoxins Using Non-Aflatoxigenic Aspergillus flavus: A Literature Review. J. Fungi 2021, 7, 381. [Google Scholar] [CrossRef]
- Moore, G.G. Sex and recombination in aflatoxigenic Aspergilli: Global implications. Front. Microbiol. 2014, 5, 32. [Google Scholar] [CrossRef]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mulè, G. Plant Bioactive Compounds in Pre- and Postharvest Management for Aflatoxins Reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef]
- Peles, F.; Sipos, P.; Kovacs, S.; Gyori, Z.; Pocsi, I.; Pusztahelyi, T. Biological control and mitigation of Aflatoxin contamination in commodities. Toxins 2021, 13, 104. [Google Scholar] [CrossRef]
- Gomez, J.V.; Tarazona, A.; Mateo-Castro, R.; Gimeno-Adelantado, J.V.; Jimenez, M.; Mateo, E.M. Selected plant essential oils and their main active components, a promising approach to inhibit aflatoxigenic fungi and aflatoxin production in food. Food Addit. Contam. Part A 2018, 35, 1581–1595. [Google Scholar] [CrossRef]
- Hernandez, C.; Cadenillas, L.; Maghubi, A.E.; Caceres, I.; Durrieu, V.; Mathieu, C.; Bailly, J.-D. Mimosa Tenuiflora Aqueous Extract: Role of Condensed Tannins in Anti-Aflatoxin B1 Activity in Aspergillus flavus. Toxins 2021, 13, 391. [Google Scholar] [CrossRef]
- Bastos, R.W.; Rossato, L.; Goldman, G.H.; Santos, D.A. Fungicide effects on human fungal pathogens: Cross resistance to medical drugs and beyond. PLoS Pathog. 2021, 17, e1010073. [Google Scholar] [CrossRef]
- Montibus, M.; Vitrac, X.; Coma, V.; Loron, A.; Pinson-Gadais, L.; Ferrer, N.; Verdal-Bonnin, M.-N.; Gabaston, J.; Waffo-Téguo, P.; Richard-Forget, F.; et al. Screening of Wood/Forest and Vine By-Products as Sources of New Drugs for Sustainable Strategies to Control Fusarium graminearum and the Production of Mycotoxins. Molecules 2021, 26, 405. [Google Scholar] [CrossRef]
- Holmes, R.A.; Boston, R.S.; Payne, G.A. Diverse Inhibitors of Aflatoxin Biosynthesis. Appl. Microbiol. Biotechnol. 2008, 78, 559–572. [Google Scholar] [CrossRef]
- Caceres, I.; El Khoury, R.; Bailly, S.; Oswald, I.P.; Puel, O.; Bailly, J.-D. Piperine Inhibits Aflatoxin B1 Production in Aspergillus Flavus by Modulating Fungal Oxidative Stress Response. Fungal Genet. Biol. 2017, 107, 77–85. [Google Scholar] [CrossRef]
- Jimenez-Zamora, A.; Delgado-Andrade, C.; Rufian-Hneras, J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016, 199, 339–346. [Google Scholar] [CrossRef]
- Zilic, S.; Kocadagli, T.; Vancetovic, J.; Gokmen, V. Effects of baking conditions and dought formulations on phenolic compounds stability, antioxidant capacity and color of cookies made from anthocyanin-rich corn flour. Food Sci. Technol. 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, L.; Li, J.; Oliveira, H.; Yang, N.; Jin, W.; Zhu, Z.; Li, S.; He, J. Microencapsulation of Anthocyanins Extracted from Grape Skin by Emulsification/Internal Gelation Followed by Spray/Freeze-Drying Techniques: Characterization, Stability and Bioaccessibility. LWT-Food Sci. Technol. 2020, 123, 109097. [Google Scholar] [CrossRef]
- Tomsone, L.; Galoburda, R.; Kruma, Z.; Durrieu, V.; Cinkmanis, I. Microencapsulation of Horseradish (Armoracia Rusticana L.) Juice Using Spray-Drying. Foods 2020, 9, 1332. [Google Scholar] [CrossRef]
- Dumitrascu, L.; Nicoleta, S.; Borda, D.; Corina, N.; Enachi, E.; Barbu, V.; Aprodu, I. Microencapsulation of Bioactive Compounds from Cornelian Cherry Fruits Using Different Biopolymers with Soy Proteins. Food Biosci. 2021, 41, 101032. [Google Scholar] [CrossRef]
- Pereira Souza, A.C.; Deyse Gurak, P.; Damasceno Ferreira Marczak, L. Maltodextrin, Pectin and Soy Protein Isolate as Carrier Agents in the Encapsulation of Anthocyanins-Rich Extract from Jaboticaba pomace. Food Bioprod. Processing 2017, 102, 186–194. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.; Stathopoulos, C.; Roach, P.D. Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying. Foods 2015, 4, 400–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacerda, E.C.Q.; Calado, V.M.D.A.; Monteiro, M.; Finotelli, P.V.; Torres, A.G.; Perrone, D. Starch, Inulin and Maltodextrin as Encapsulating Agents Affect the Quality and Stability of Jussara Pulp Microparticles. Carbohydr. Polym. 2016, 151, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Simon-Brown, K.; Solval, K.M.; Chotiko, A.; Alfaro, L.; Reyes, V.; Liu, C.; Dzandu, B.; Kyereh, E.; Goldson Barnaby, A.; Thompson, I.; et al. Microencapsulation of Ginger (Zingiber officinale) Extract by Spray Drying Technology. LWT-Food Sci. Technol. 2016, 70, 119–125. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Violleau, F.; Silvestre, F.; Durrieu, V. A New Way of Valorizing Biomaterials: The Use of Sunflower Protein for α-Tocopherol Microencapsulation. Food Res. Int. 2013, 53, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Marisa Ribeiro, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of Polyphenols—The Specific Case of the Microencapsulation of Sambucus Nigra L. Extracts—A Review. Trends Food Sci. Technol. 2020, 105, 454–467. [Google Scholar] [CrossRef]
- Shen, C.-H. Quantification and Analysis of Proteins. In Diagnostic Molecular Biology; Shen, C.-H., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 187–214. ISBN 978-0-12-802823-0. [Google Scholar]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of Polyphenols and Anthocyanins from Pomegranate (Punica Granatum) by Spray Drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Ferreira, L.M.D.M.C.; Pereira, R.R.; Carvalho, F.B.D.; Silva Santos, A.; Ribeiro-Costa, R.M.; Carréra Silva Júnior, J.O. Green Extraction by Ultrasound, Microencapsulation by Spray Drying and Antioxidant Activity of the Tucuma Coproduct (Astrocaryum Vulgare Mart.) Almonds. Biomolecules 2021, 11, 545. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- El Mahgubi, A.; Puel, O.; Bailly, S.; Tadrist, S.; Querin, A.; Ouadia, A.; Oswald, I.P.; Bailly, J.D. Distribution and Toxigenicity of Aspergillus Section Flavi in Spices Marketed in Morocco. Food Control 2013, 32, 143–148. [Google Scholar] [CrossRef]

| Dry Mass (%) | Polyphenol Content (mg GAE/g DM Extract) | Antioxidant Activity b IC50 (mg/L) | Maximum AFB1 Inhibition c (%) | Maximum Fungal Inhibition c (%) | |
|---|---|---|---|---|---|
| MAE | 11.2 | 397 ± 22 | 10 | 65.7 | 3 |
| sMAE a | 11.1 | 376 ± 2 ns | 12 | 43.8 * | 1 ns |
| Composition of the Microparticles | Wall Material/Extract Ratio (w/w Dry Matter) | Spray-Drying Yield (%) |
|---|---|---|
| Maltodextrin | 100/0 | 59 |
| Maltodextrin + MAE | 50/50 | 63 |
| Soy protein | 100/0 | 57 |
| Soy protein + MAE | 50/50 | 54 |
| Starch | 100/0 | 58 |
| Starch + MAE | 50/50 | 59 |
| Wall Material | Polyphenol Content (mg GAE/g DM) | Antioxidant Activity on DPPH IC50 (mg DM/L) |
|---|---|---|
| Maltodextrin | 0.7 ± 0.3 | >1000 |
| Soy protein | 17.0 ± 0.4 | >1000 |
| Starch | 1.9 ± 0.4 | >1000 |
| Wall Material Used | Theoretical Dry Mass of the Extract in the Microparticles (g) | Theoretical Polyphenol Content in Microparticles (mg GAE/g Microparticles) | Time 0 | After One-Year Storage c | |||
|---|---|---|---|---|---|---|---|
| Experimental Polyphenol Content in Microparticles (mg GAE/g Microparticles) | PEE a (%) | Antioxidant Activity IC50 b (mg/L) | Experimental Polyphenol Content in Microparticles (mg GAE/g Microparticles) | Antioxidant Activity IC50 b (mg/L) | |||
| Maltodextrin | 0.65 | 258 | 168 ± 7 | 65 | 23 | 188 ± 3 | 27 |
| Starch | 0.62 | 246 | 214 ± 8 | 86 | 25 | 211 ± 3 | 27 |
| Soy protein | 0.62 | 246 | 142 ± 9 | 58 | 48 | 149 ± 3 | 79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, C.; Cadenillas, L.; Mathieu, C.; Bailly, J.-D.; Durrieu, V. Preservation of Mimosa tenuiflora Antiaflatoxigenic Activity Using Microencapsulation by Spray-Drying. Molecules 2022, 27, 496. https://doi.org/10.3390/molecules27020496
Hernandez C, Cadenillas L, Mathieu C, Bailly J-D, Durrieu V. Preservation of Mimosa tenuiflora Antiaflatoxigenic Activity Using Microencapsulation by Spray-Drying. Molecules. 2022; 27(2):496. https://doi.org/10.3390/molecules27020496
Chicago/Turabian StyleHernandez, Christopher, Laura Cadenillas, Céline Mathieu, Jean-Denis Bailly, and Vanessa Durrieu. 2022. "Preservation of Mimosa tenuiflora Antiaflatoxigenic Activity Using Microencapsulation by Spray-Drying" Molecules 27, no. 2: 496. https://doi.org/10.3390/molecules27020496
APA StyleHernandez, C., Cadenillas, L., Mathieu, C., Bailly, J.-D., & Durrieu, V. (2022). Preservation of Mimosa tenuiflora Antiaflatoxigenic Activity Using Microencapsulation by Spray-Drying. Molecules, 27(2), 496. https://doi.org/10.3390/molecules27020496








