Development and Validation of an Analytical HPLC Method to Assess Chemical and Radiochemical Purity of [68Ga]Ga-NODAGA-Exendin-4 Produced by a Fully Automated Method
Abstract
:1. Introduction
2. Results
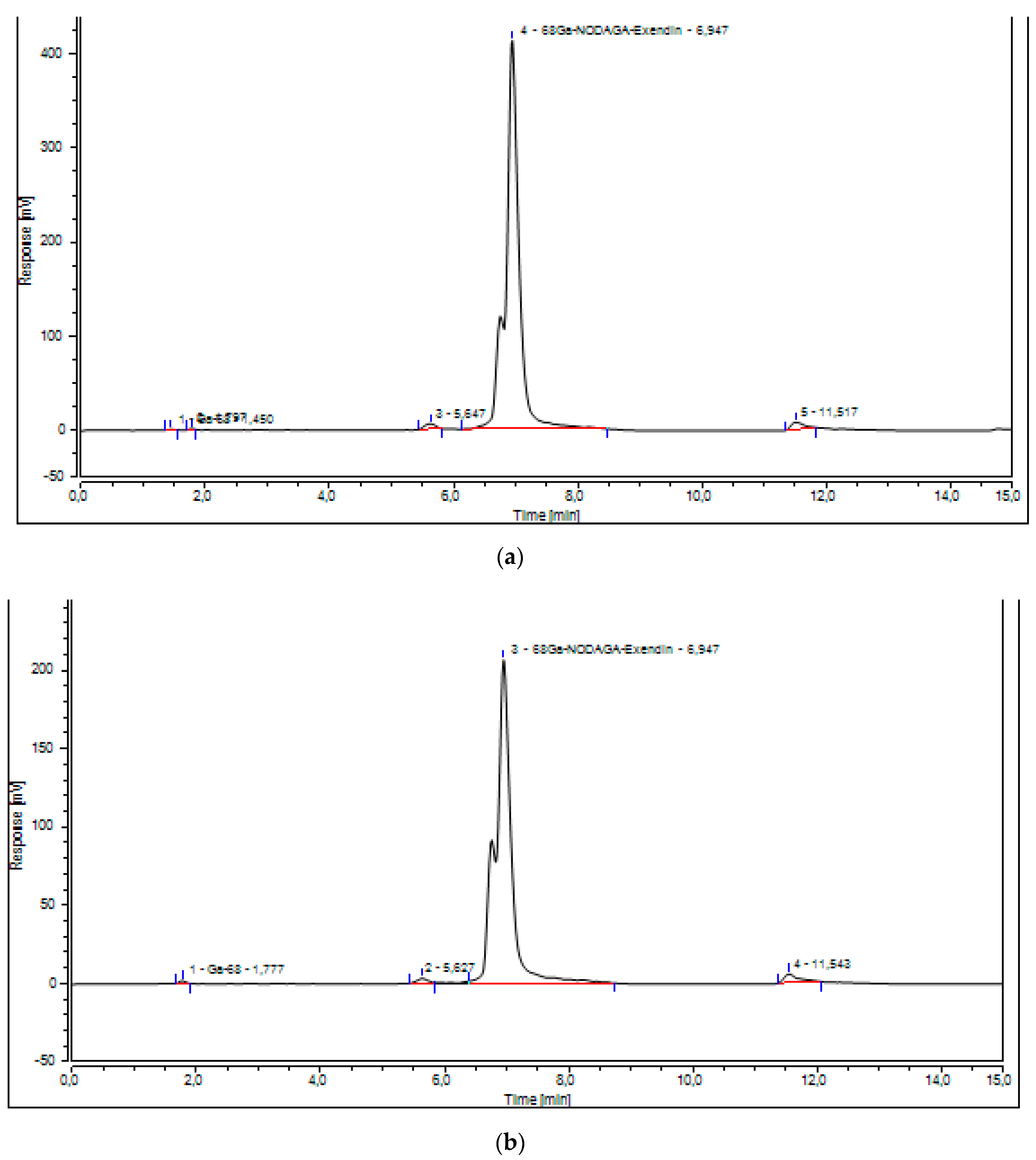
2.1. Validation of the UV-Radio-HPLC Method
2.2. Validation of the Process for Producing and Controlling [68Ga]Ga-NODAGA-Exendin-4
3. Discussion
4. Materials and Methods
4.1. Reagents, Radionuclides
4.2. Automated Synthesis of [68Ga]Ga-NODAGA-Exendin-4
4.3. Quality Controls and Process Validation
4.3.1. Appearance
4.3.2. Instant Thin Layer Chromatography
4.3.3. High-Pressure Liquid Chromatography
4.3.4. Ge-68 Breakthrough
4.3.5. Radionuclide Identification and Activity Measurements
4.3.6. pH Evaluation
4.3.7. Endotoxin and Sterility
4.3.8. Residual Solvents and HEPES
4.4. Validation of UV-Radio-HPLC Method to Determine the Chemical Purity
4.4.1. Specificity
4.4.2. Linearity
4.4.3. Precision and Accuracy
4.4.4. Limit of Quantitation (LOQ)
4.5. Validation of UV-Radio-HPLC Method to Determine the Radiochemical Purity
4.5.1. Linearity
4.5.2. Precision
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksson, O.; Velikyan, I.; Selvaraju, R.K.; Kandeel, F.; Johansson, L.; Antoni, G.; Eriksson, B.; Sörensen, J.; Korsgren, O. Detection of Metastatic Insulinoma by Positron Emission Tomography With [68Ga]Exendin-4—A Case Report. J. Clin. Endocrinol. Metab. 2014, 99, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Mäcke, H.; Christ, E.; Gloor, B.; Reubi, J.C. Glucagon-like Peptide 1–Receptor Scans to Localize Occult Insulinomas. N. Engl. J. Med. 2008, 359, 766–768. [Google Scholar] [CrossRef]
- Christ, E.; Wild, D.; Forrer, F.; Brändle, M.; Sahli, R.; Clerici, T.; Gloor, B.; Martius, F.; Maecke, H.; Reubi, J.C. Glucagon-Like Peptide-1 Receptor Imaging for Localization of Insulinomas. J. Clin. Endocrinol. Metab. 2009, 94, 4398–4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowa-Staszczak, A.; Pach, D.; Mikolajczak, R.; Macke, H.; Jabrocka-Hybel, A.; Stefanska, A.; Tomaszuk, M.; Janota, B.; Gilis-Januszewska, A.; Malecki, M.; et al. Glucagon-like peptide-1 receptor imaging with [Lys40(Ahx-HYNIC-99mTc/EDDA)NH2]-exendin-4 for the detection of insulinoma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Yu, M.; Pan, Q.; Wu, W.; Zhang, T.; Kiesewetter, D.; Zhu, Z.; Li, F.; Chen, X.; Zhao, Y. 68GaNOTA-exendin-4 PET/CT in detection of occult insulinoma and evaluation of physiological uptake. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 531–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antwi, K.; Fani, M.; Nicolas, G.; Rottenburger, C.; Heye, T.; Reubi, J.C.; Gloor, B.; Christ, E.; Wild, D. Localization of hidden insulinomas with 68GaDOTA-exendin-4 PET/CT: A pilot study. J. Nucl. Med. 2015, 56, 1075–1078. [Google Scholar] [CrossRef] [Green Version]
- Velikyan, I.; Bulenga, T.N.; Selvaraju, R.; Lubberink, M.; Espes, D.; Rosenström, U.; Eriksson, O. Dosimetry of [177Lu]-DO3A-VS-Cys40-Exendin-4 – impact on the feasibility of insulinoma internal radiotherapy. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 109–126. [Google Scholar]
- Wild, D.; Christ, E.; Caplin, M.E.; Kurzawinski, T.R.; Forrer, F.; Brändle, M.; Seufert, J.; Weber, W.A.; Bomanji, J.; Perren, A.; et al. Glucagon-Like Peptide-1 Versus Somatostatin Receptor Targeting Reveals 2 Distinct Forms of Malignant Insulinomas. J. Nucl. Med. 2011, 52, 1073–1078. [Google Scholar] [CrossRef] [Green Version]
- Christ, E.; Wild, D.; Ederer, S.; Béhé, M.; Nicolas, G.E.; Caplin, M.; Brändle, M.; Clerici, T.; Fischli, S.; Stettler, C.; et al. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: A prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013, 1, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Sowa-Staszczak, A.; Trofimiuk-Müldner, M.; Stefanska, A.; Tomaszuk, M.; Buziak-Bereza, M.; Gilis-Januszewska, A.; Jabrocka-Hybel, A.; Głowa, B.; Małecki, M.; Bednarczuk, T.; et al. 99mTc Labeled Glucagon-Like Peptide-1-Analogue (99mTc-GLP1) Scintigraphy in the Management of Patients with Occult Insulinoma. PLoS ONE 2016, 11, e0160714. [Google Scholar] [CrossRef]
- Schottelius, M.; Wester, H.J. Molecular imaging targeting peptide receptors. Methods 2009, 48, 161–177. [Google Scholar] [CrossRef]
- Laverman, P.; Sosabowski, J.K.; Boerman, O.C.; Oyen, W.J.G. Radiolabelled peptides for oncological diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 78–92. [Google Scholar] [CrossRef] [Green Version]
- Reubi, J.C. Old and New Peptide Receptor Targets in Cancer: Future Directions. Adv. Struct. Saf. Stud. 2012, 194, 567–576. [Google Scholar] [CrossRef]
- Brom, M.; Der Weg, W.W.-V.; Joosten, L.; Frielink, C.; Bouckenooghe, T.; Rijken, P.; Andralojc, K.; Göke, B.J.; De Jong, M.; Eizirik, D.L.; et al. Non-invasive quantification of the beta cell mass by SPECT with 111In-labelled exendin. Diabetologia 2014, 57, 950–959. [Google Scholar] [CrossRef] [Green Version]
- Pach, D.; Sowa-Staszczak, A.; Jabrocka-Hybel, A.; Stefańska, A.; Tomaszuk, M.; Mikołajczak, R.; Janota, B.; Trofimiuk-Müldner, M.; Przybylik-Mazurek, E.; Hubalewska-Dydejczyk, A. Glucagon-Like Peptide-1 Receptor Imaging with [Lys40(Ahx-HYNIC-99mTc/EDDA)NH2]-Exendin-4 for the Diagnosis of Recurrence or Dissemination of Medullary Thyroid Cancer: A Preliminary Report. Int. J. Endocrinol. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Velikyan, I. Prospective of 68Ga-Radiopharmaceutical Development. Theranostics 2014, 4, 47–80. [Google Scholar] [CrossRef] [Green Version]
- Decristoforo, C.; Penuelas, I.; Patt, M.; Todde, S. European regulations for the introduction of novel radiopharmaceuticals in the clinical setting. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Elsinga, P.; Gillings, N.; Hjelstuen, O.; Ballinger, J.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Kolenc Peitl, P.; Koziorowski, J.; et al. Guidance on current good radiopharmacy practice (cGRPP) for the small-scale preparation of radiopharmaceuticals. Radiopharmacy Committee of the EANM. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1049–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todde, S.; Peitl, P.K.; Elsinga, P.; Koziorowski, J.; Ferrari, V.; Ocak, E.M.; Hjelstuen, O.; Patt, M.; Mindt, T.L.; Behe, M. Guidance on validation and qualification of processes and operations involving radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2017, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annex 15 (EU) of Good Manufacturing Practice (GMP) Guidelines: Qualification and Validation. 2015. Available online: https://www.gmp-compliance.org/files/guidemgr/2015-10_annex15.pdf (accessed on 8 December 2021).
- EMA. ICH Guideline Q2(R1) Validation of Analytical Procedure: Text and Methodology; European Medicines Agency: London, UK, 1995. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 8 December 2021).
- EMA. ICH Q8 (R2): Pharmaceutical Development (ICH Guideline); European Medicines Agency (EMA): London, UK, 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-11.pdf (accessed on 8 December 2021).
- EDQM. EDQM Guidelines: Guide for the Elaboration of Monographs on Radiopharmaceutical Preparations European Pharmacopoeia Edition; EDQM: Strasbourg, France, 2018; Available online: https://www.edqm.eu/sites/default/files/guide_-_guide_for_the_elaboration_of_monographs_on_radio-pharmaceutical_preparations_-_october_2018.pdf (accessed on 8 December 2021).
- Gillings, N.; Todde, S.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; Hjelstuen, O.; Peitl, P.K.; Koziorowski, J.; Laverman, P.; et al. EANM guideline on the validation of analytical methods for radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2020, 5, 7–29. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). World Health Organization (WHO) Guideline on Good Manufacturing Practices for Investigational Radiopharmaceutical Products; Draft Working Document 2021; IAEA: Vienna, Austria, 2021. [Google Scholar]
- WHO. Annex 2 International Atomic Energy Agency and World Health Organization (WHO) Guideline on Good Manufacturing Practices for Radiopharmaceutical Products; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- De Blois, E.; De Zanger, R.M.S.; Chan, H.S.; Konijnenberg, M.; Breeman, W.A.P. Radiochemical and analytical aspects of inter-institutional quality control measurements on radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2019, 4, 3. [Google Scholar] [CrossRef]
- Levin, S. High Performance Liquid Chromatography (HPLC) in the Pharmaceutical Analysis. Pharm. Sci. Encycl. 2010, 1–34. [Google Scholar] [CrossRef]
- Zarghi, A.; Foroutan, S.; Shafaati, A.; Khoddam, A. A Rapid HPLC Method for the Determination of Losartan in Human Plasma Using a Monolithic Column. Arzneimittelforschung 2005, 55, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Gillings, N.M.; Elsinga, P.H. Spotlight on: Guideline on current good radiopharmacy practice (cGRPP) for the small-scale preparation of radiopharmaceuticals published in EJNMMI Radiopharmacy and Chemistry (2021)6:8. Clin. Transl. Imaging 2021, 9, 281–282. [Google Scholar] [CrossRef]
- Boss, M.; Buitinga, M.; Jansen, T.; Brom, M.; Visser, E.P.; Gotthardt, M. PET-Based Human Dosimetry of 68Ga-NODAGA-Exendin-4, a Tracer for β-Cell Imaging. J. Nucl. Med. 2020, 61, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Velikyan, I.; Rosenstrom, U.; Eriksson, O. Fully automated GMP production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 for clinical use. Am. J. Nucl. Med. Imaging 2017, 7, 111–125. [Google Scholar]
- Migliari, S.; Sammartano, A.; Scarlattei, M.; Baldari, G.; Janota, B.; Bonadonna, R.C.; Ruffini, L. Feasibility of a scale-down production of [68Ga]Ga-NODAGA-Exendin-4 in a hospital based radiopharmacy. Curr. Radiopharm. 2021, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Poster EANM 2020, EP-331: “Scale-Down Production of [68Ga]Ga-NODAGA-Exendin-4 PET Tracer”. Available online: https://eanm21.eanm.org/content/uploads/2021/10/EANM21_Final_Programme.pdf (accessed on 8 December 2021).
- Poster EANM 2018, EP-0911: “Comparison of Two Different Synthesis Methods to Label [Lys40,Nle14(Ahx-NODAGA)NH2]exendin-4 with Ga-68”. Available online: https://eanm19.eanm.org/content/uploads/2019/10/Final-Programme-EANM19.pdf (accessed on 8 December 2021).
- Brom, M.; Franssen, G.M.; Joosten, L.; Gotthardt, M.; Boerman, O.C. The effect of purification of Ga-68-labeled exendin on in vivo distribution. EJNMMI Res. 2016, 6, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallium (68Ga) Edotreotide (monograph 2482). In European Pharmacopoeia; European Directorate for the Quality of Medicines: Strasbourg, France, 2011; Volume 1, pp. 1062–1064.
- Gallium Chloride (68Ga) solution for labeling (Monograph 2464). In European Pharmacopoeia; European Pharmacopea: Strasbourg, France, 2011.
- Christ, E.; Wild, D.; Antwi, K.; Waser, B.; Fani, M.; Schwanda, S.; Heye, T.; Schmid, C.; Baer, H.U.; Perren, A.; et al. Preoperative localization of adult nesidioblastosis using 68Ga-DOTA-exendin-4-PET/CT. Endocrine 2015, 50, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I.; Sundin, A.; Eriksson, B.; Sörensen, H.L.J.; Bergström, M.; Långström, B. In vivo binding of [68Ga] -DOTATOC to somatostatin receptors in neuroendocrine tumours--impact of peptide mass. Nucl. Med. Biol. 2010, 37, 265–275. [Google Scholar] [CrossRef]
- Velikyan, I.; Wennborg, A.; Feldwisch, J.; Lindman, H.; Carlsson, J.; Sörensen, J. Good manufacturing practice production of [(68)Ga] Ga-ABY-025 for HER2 specific breast cancer imaging. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 135–153. [Google Scholar]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-Receptor Expression in Metastatic Breast Cancer Using [68Ga] ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef]
- Langille, S.E. Particulate matter in injectable drug products. PDA J. Pharm. Sci. Technol. 2013, 67, 186–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ICH Topic Q4B Annex 8 Sterility Test General Chapter; European Medicines Agency (EMA): London, UK, 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q4b-annex-8-evaluation-recommendation-pharmacopoeial-texts-use-ich-regions-sterility_en.pdf (accessed on 8 December 2021).
- Velikyan, I. 68Ga-Based Radiopharmaceuticals: Production and Application Relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [Green Version]
- Mu, L.; Hesselmann, R.; Oezdemir, U.; Bertschi, L.; Blanc, A.; Dragic, M.; Löffler, D.; Smuda, C.; Johayem, A.; Schibli, R. Identification, characterization and suppression of side-products formed during the synthesis of high dose 68Ga-DOTA-TATE. Appl. Radiat. Isot. 2013, 76, 63–69. [Google Scholar] [CrossRef]
- Eppard, E.; Pèrez-Malo, M.; Rösch, F. Improved radiolabeling of DOTATOC with trivalent radiometals for clinical application by addition of ethanol. EJNMMI Radiopharm. Chem. 2016, 1, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helm, L.; Merbach, A.E. Inorganic and Bioinorganic Solvent Exchange Mechanisms. Chem. Rev. 2005, 105, 1923–1960. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Malo, M.; Szabó, G.; Eppard, E.; Vagner, A.; Brücher, E.; Tóth, I.; Maiocchi, A.; Suh, E.H.; Kovács, Z.; Baranyai, Z.; et al. Improved Efficacy of Synthesizing *MIII-Labeled DOTA Complexes in Binary Mixtures of Water and Organic Solvents. A Combined Radio- and Physicochemical Study. Inorg. Chem. 2018, 57, 6107–6117. [Google Scholar] [CrossRef] [PubMed]









| Test | Acceptance Criteria |
|---|---|
| Specificity | ≥2.5 |
| Linearity | R2 ≥ 0.99 |
| Repeatability | CV% < 2% |
| Quantification limit (LOQ) | CV% < 5% |
| Accuracy | bias% > 95% |
| Test | Acceptance Criteria |
|---|---|
| Specificity | Not applicable |
| Linearity | R2 ≥ 0.99 |
| Repeatability | CV% < 2% |
| Quantification limit (LOQ) | Not applicable |
| Accuracy | Not applicable |
| Test | Acceptance Criteria | Batch 1 | Batch 2 | Batch 3 |
|---|---|---|---|---|
| Radiochemical purity (UV-Radio-HPLC) | [68Ga]Ga-NODAGA-exendin-4 ≥ 90% 68Ga3+ and impurities < 10% | 97.05% / | 95.75% / | 96.15% / |
| Radiochemical purity (Radio-TLC) | [68Ga]Ga-NODAGA-exendin-4 ≥ 90% 68Ga-colloids < 3% | 100% / | 100% / | 100% / |
| pH | 4–8.5 | 7 | 7 | 7 |
| Radioactivity | 50–500 MBq | 331 MBq | 333 MBq | 336 MBq |
| Volume | 2–16 mL | 16 mL | 16 mL | 16 mL |
| Color | colorless | Colorless | Colorless | Colorless |
| Molar activity | 1–150 GBq/µmol | 33.10 GBq/µmol | 33.30 GBq/µmol | 33.60 GBq/µmol |
| Ge-68 breakthrough | <0.001% | 0.00000035% | 0.00000033% | 0.00000035% |
| Ga-68 half life | T1/2 Ga-68: 62–74 min | 66.1 min | 64 min | 62.5 min |
| Stability | RCP% > 90% within 240 min | RCP% > 90% | RCP% > 90% | RCP% > 90% |
| Ethanol content | <10% (v/v) (<2.5 g) | 5.23% | 6.55% | 5.68% |
| HEPES content | Less than 200 µg/V of HEPES in test solution | Conformed | Conformed | Conformed |
| Endotoxins | <17.5 IU/mL | 4.85 EU/mL | 4.80 EU/mL | 4.95 EU/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliari, S.; Sammartano, A.; Boss, M.; Gotthardt, M.; Scarlattei, M.; Baldari, G.; Silva, C.; Bonadonna, R.C.; Ruffini, L. Development and Validation of an Analytical HPLC Method to Assess Chemical and Radiochemical Purity of [68Ga]Ga-NODAGA-Exendin-4 Produced by a Fully Automated Method. Molecules 2022, 27, 543. https://doi.org/10.3390/molecules27020543
Migliari S, Sammartano A, Boss M, Gotthardt M, Scarlattei M, Baldari G, Silva C, Bonadonna RC, Ruffini L. Development and Validation of an Analytical HPLC Method to Assess Chemical and Radiochemical Purity of [68Ga]Ga-NODAGA-Exendin-4 Produced by a Fully Automated Method. Molecules. 2022; 27(2):543. https://doi.org/10.3390/molecules27020543
Chicago/Turabian StyleMigliari, Silvia, Antonino Sammartano, Marti Boss, Martin Gotthardt, Maura Scarlattei, Giorgio Baldari, Claudia Silva, Riccardo C. Bonadonna, and Livia Ruffini. 2022. "Development and Validation of an Analytical HPLC Method to Assess Chemical and Radiochemical Purity of [68Ga]Ga-NODAGA-Exendin-4 Produced by a Fully Automated Method" Molecules 27, no. 2: 543. https://doi.org/10.3390/molecules27020543







