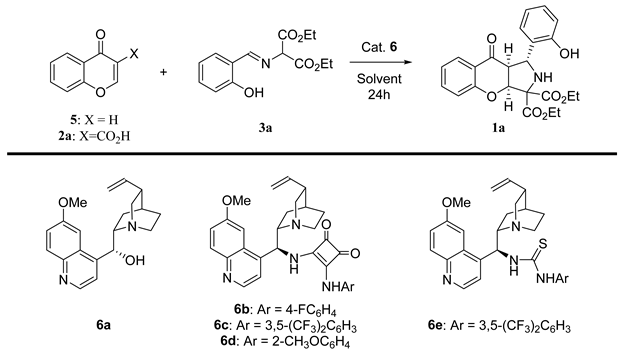
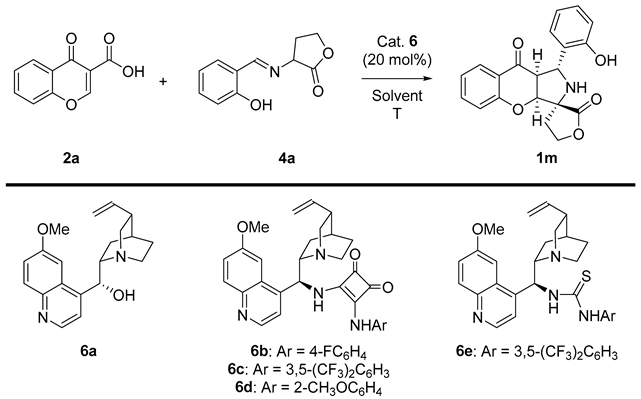
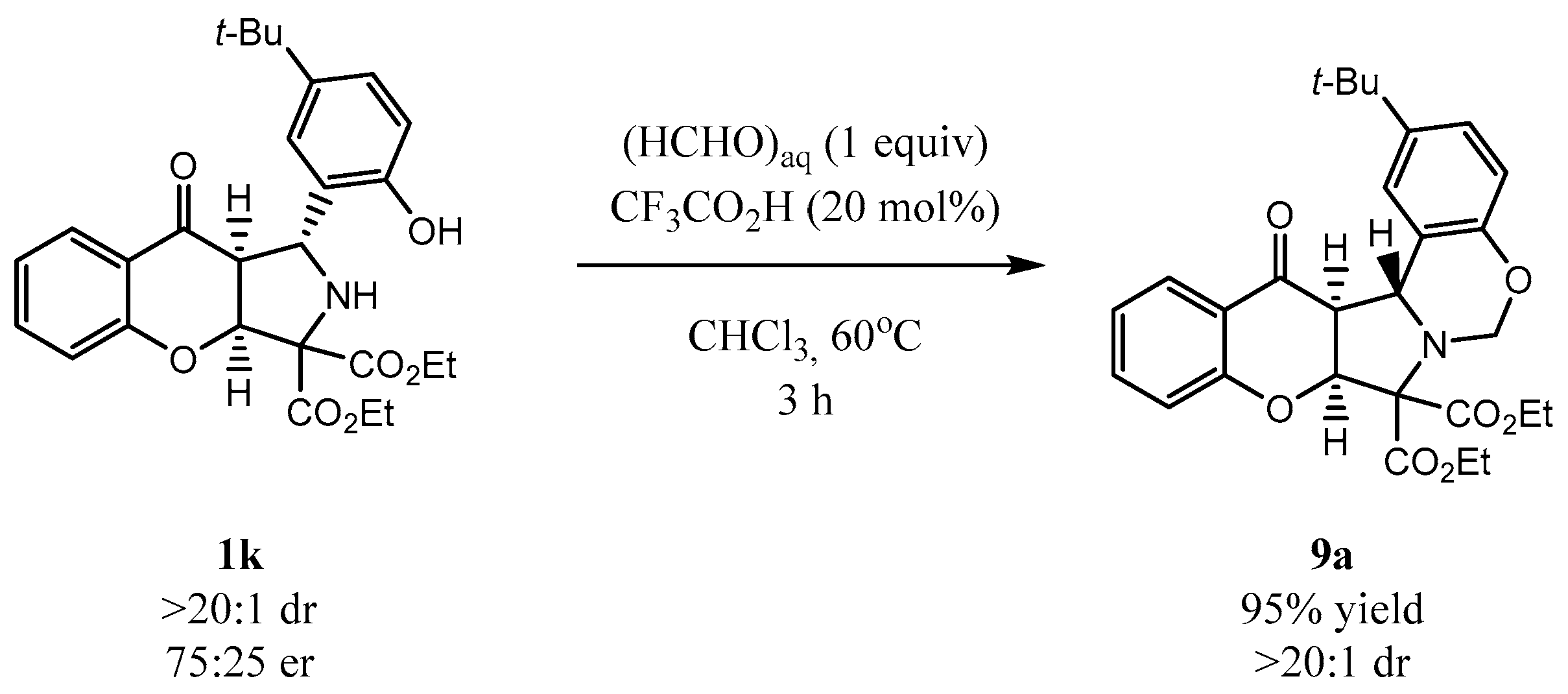Abstract
Herein, we describe the synthesis of a variety of chiral hybrid pyrrolidine-chromanone polycyclic derivatives. A convenient (3+2)-annulation of azomethine ylides with chromone-3-carboxylic acid realized under Brønsted base catalysis produced highly functionalized products in high yields with good stereoselectivities through asymmetric, intermolecular, and decarboxylative (3+2)-cyclization.
1. Introduction
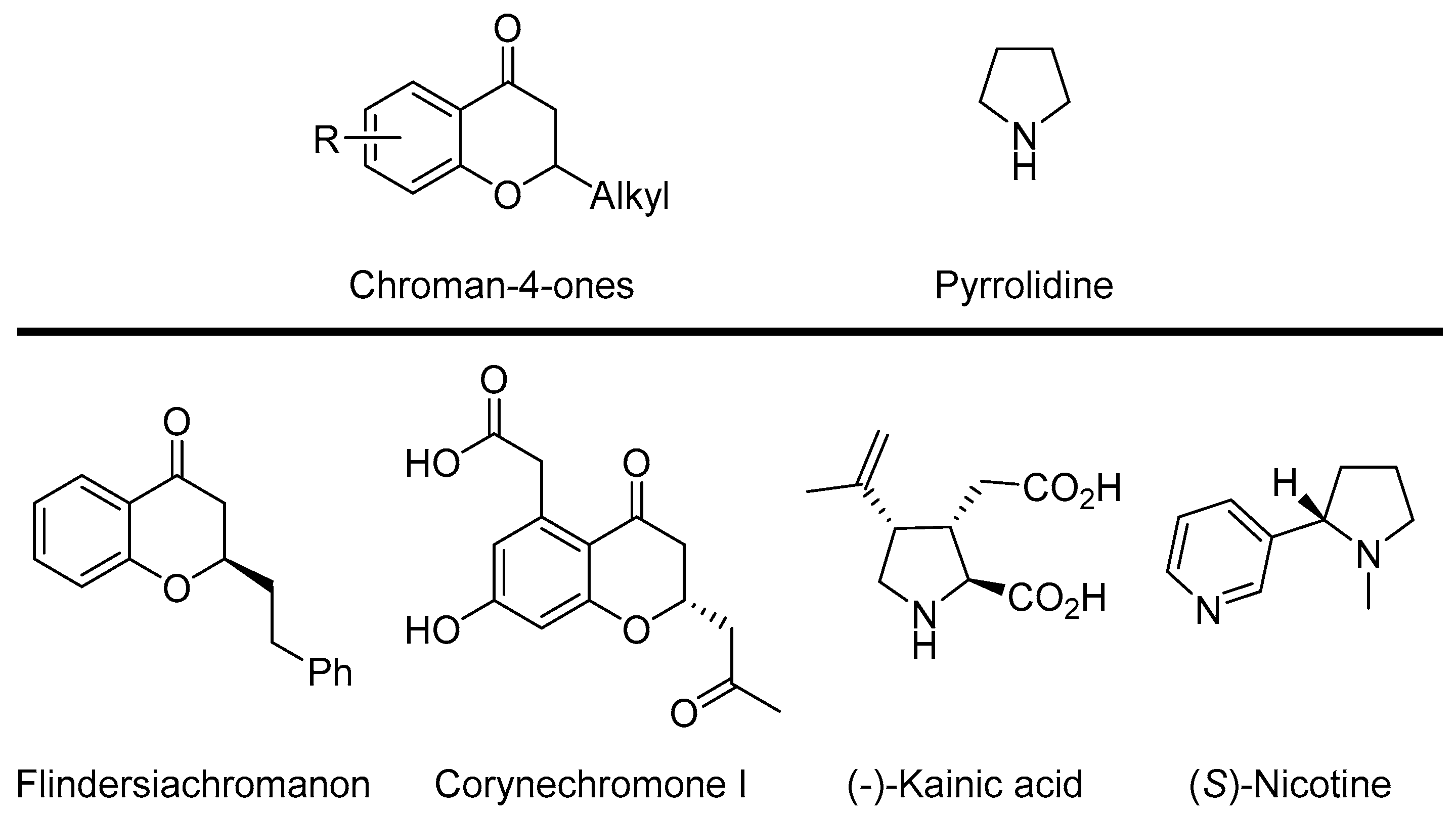
Chroman-4-ones and related compounds are found in different bioactive molecules relevant to the life-science industry [1,2,3,4,5,6,7]. Although these compounds are abundant in nature, the synthetic methods for their preparation are not very common. The main representatives of this class of compounds are two natural products: Flidersiachromon [4] isolated from the bark of Flindersia laevicarpa and Corynechromone I derived from the fungus Corynespora cassiicola [5] (Scheme 1).
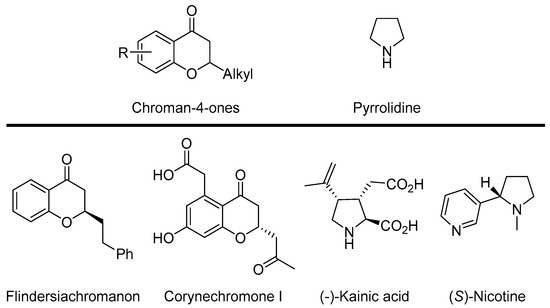
Scheme 1.
Representative examples of biologically relevant chroman-4-ones or pyrrolidine derivatives.
On the other hand, pyrrolidine derivatives have been extensively exploited because of their applications as bioactive natural products, pharmaceuticals, and potential drug candidates, [8,9,10,11,12,13,14,15,16,17] their wide applications as chiral ligands, [18,19] and their use as organocatalysts [20,21,22]. A pyrrolidine ring is the main constituent of Kainic acid, a natural product that can be found in marine algae, well recognized for its anthelmintic activity [14,15]. Another type of natural product with a pyrrolidine moiety is nicotine. It possesses antioxidant, anti-inflammatory, and antihyperglycemic properties [17,23].
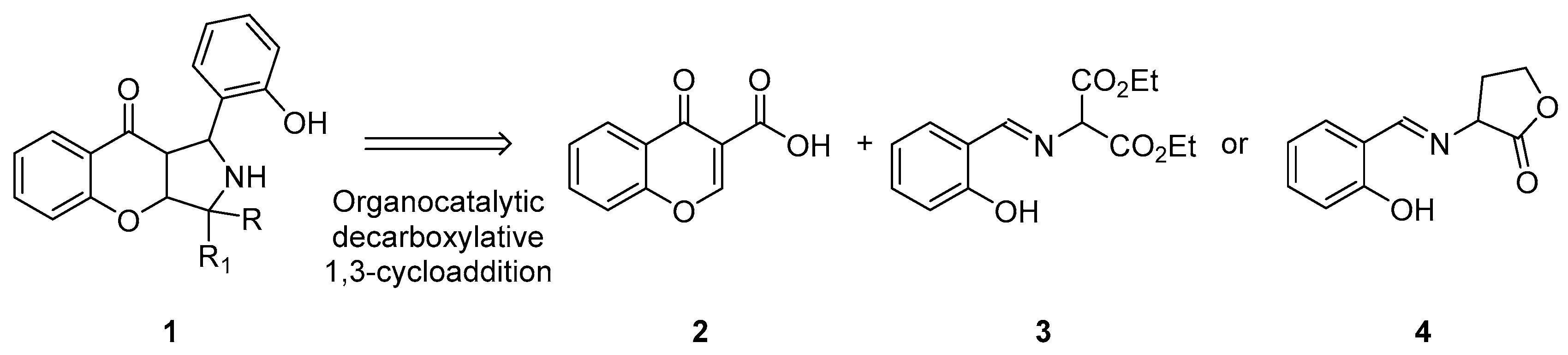
Due to the high significance of these two classes of compounds in drug discovery, the synthesis of compounds containing both of these two moieties is highly desirable [24,25,26,27,28,29]. To achieve this goal, decarboxylative 1,3-dipolar cycloaddition (1,3-DC) between carboxylic-acid-group-activated olefins and azomethine ylides was devised [23,30,31,32,33,34,35,36,37,38] (Scheme 2). Decarboxylative reactions constitute a very useful strategy in organic synthesis including stereoselective approaches [39,40,41,42]. They rely on the application of carboxylic-acid-activated Michael acceptors or donors. In such a setup, a carboxylate moiety serves a double purpose. It enhances the electrophilic or nucleophilic properties of the starting material and creates the opportunity for its facile removal via decarboxylation.
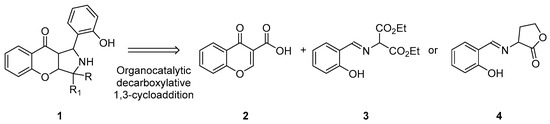
Scheme 2.
Synthetic objective of the present work.
Herein, we present our studies on decarboxylative (3+2)-cycloaddition between chromone-3-carboxylic acids 2 (acting as electron-poor dipolarophiles) and azomethine ylides 3 or 4 derived from salicylaldehydes and appropriate amines (acting as a dipol) proceeding under mild, basic conditions [43,44]. Our studies demonstrate that the presence of a carboxylic acid group is beneficial for the process, providing an alternative method for the preparation of hybrid molecules 1 containing chromenopyrrolidine units with a quaternary stereogenic center, in some cases.
2. Results
Initially, the quinine-catalyzed cycloaddition between chromen-4-one 5 and imine 3a was attempted (Table 1, entry 1). Disappointingly, no reaction was observed. Therefore, the activation of 2a through the introduction of the carboxylic acid moiety was attempted. We were pleased to observe that the devised decarboxylative cycloaddition with chromone-3-carboxylic acid 2a proceeded efficiently and with concomitant decarboxylation (Table 1, entry 2). Importantly, both the efficiency and the diastereoselectivity of the process were excellent. However, the enantioselectivity required further optimization. Consequently, in the first step, five different catalysts were tested (Table 1, entries 2–6). Optimization studies indicated that bifunctional cinchona alkaloid derivatives 6b-e (Table 1, entries 3–6) were better when compared to simple alkaloids such as quinine 6a (Table 1, entry 2). It was found that the presence of a strong H-bonding unit in the catalyst structure was beneficial for the enantiomeric ratio. Unfortunately, a significant decrease in the diastereoselectivity of the cycloaddition was noted. Subsequently, solvent screening using 6c as the catalyst was performed (Table 1, entries 7–10). As a consequence, CHCl3 was identified as the best solvent for the optimized cascade (Table 1, entry 8). The temperature screening (Table 1, entries 8, 11, 12) indicated that the enantioselectivity of the process can be slightly enhanced by lowering the reaction temperature to 0 °C (Table 1, entry 11). Further reduction in the temperature to −20 °C did not affect the enantiomeric excess (Table 1, entry 12). Importantly, both the amount of solvent used and the catalyst loading can be reduced without any effect on the reaction outcome (Table 1, entries 12–16).

Table 1.
Enantioselective (3+2)-cycloaddition of chromone-3-carboxylic acids 2 and diethyl iminomalonates 3—optimization studies a.
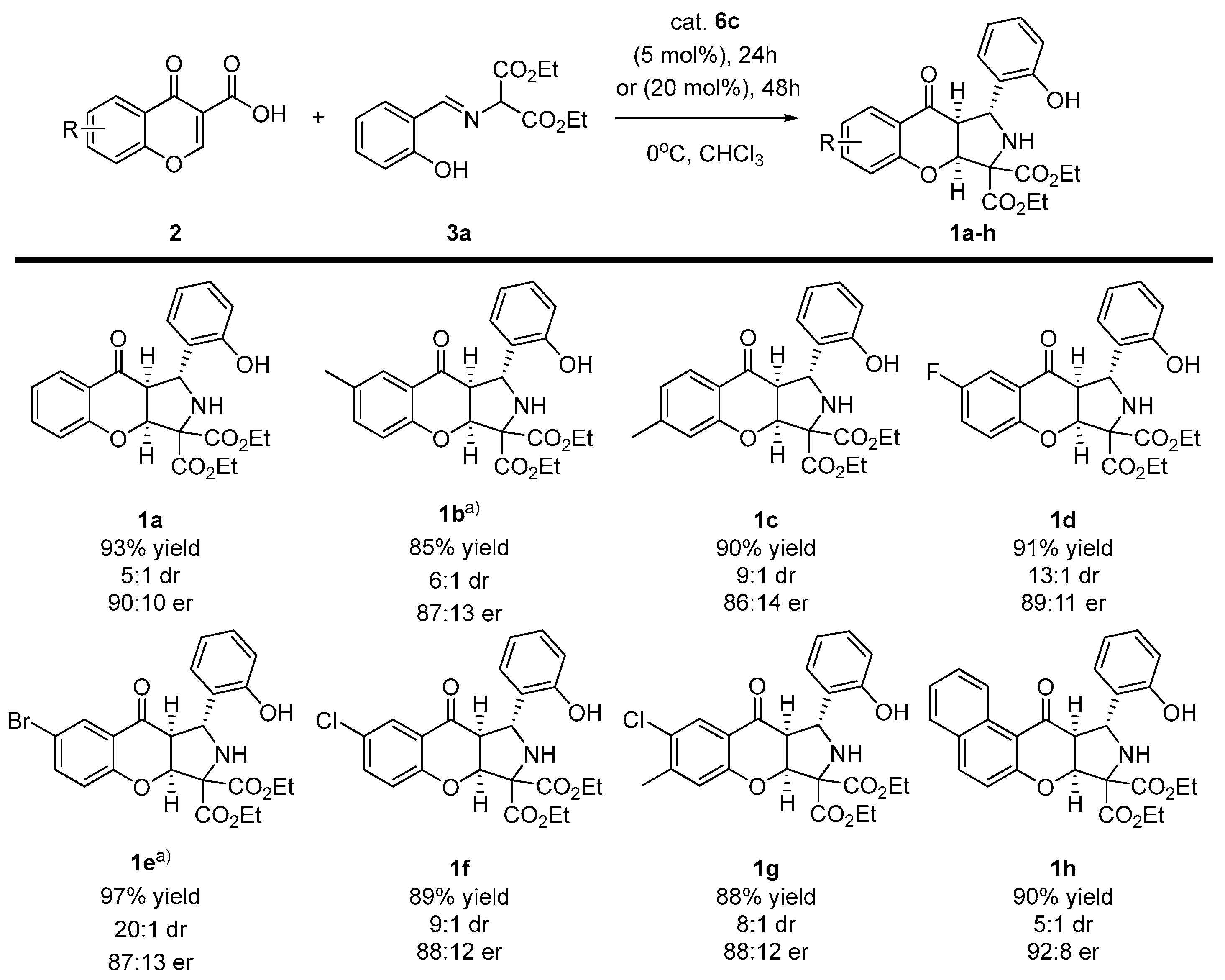
Having accomplished the optimization studies, the scope of the methodology with regard to both reaction partners was studied (Scheme 3 and Scheme 4). Initially, various chromone-3-carboxylic acids 2 were reacted with the imine 3a under optimized reaction conditions (Scheme 3). In some of the cases (Scheme 3, products 1b,f) a longer reaction time and higher amounts of catalyst were required in order to achieve full conversion. To our delight, the decarboxylative (3+2)-cycloaddition proceeded efficiently, providing chromenopyrroles 1a-h in good to high yields. Moreover, the diastereoselectivity of the developed reaction increased in all of the cases. In terms of enantioselectivity, the cycloaddition was found to be unbiased towards the electronic properties of substituents on the aromatic ring in acids 2a-h, and it remained at a similar level to the model reaction.
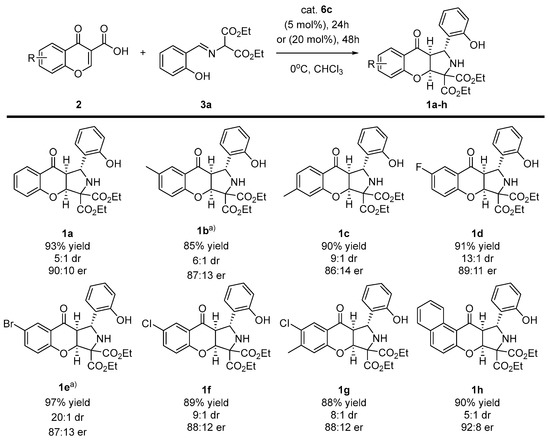
Scheme 3.
Enantioselective, decarboxylative (3+2)-cycloaddition of chromone-3-carboxylic acids 2 and diethyl iminomalonates 3a—scope of chromone-3-carboxylic acid 2a-h. a) Reactions performed for 48 h using 20 mol% of catalyst 6c.
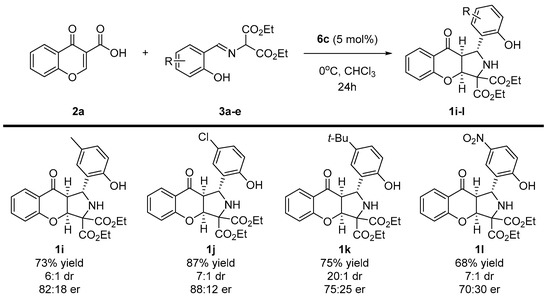
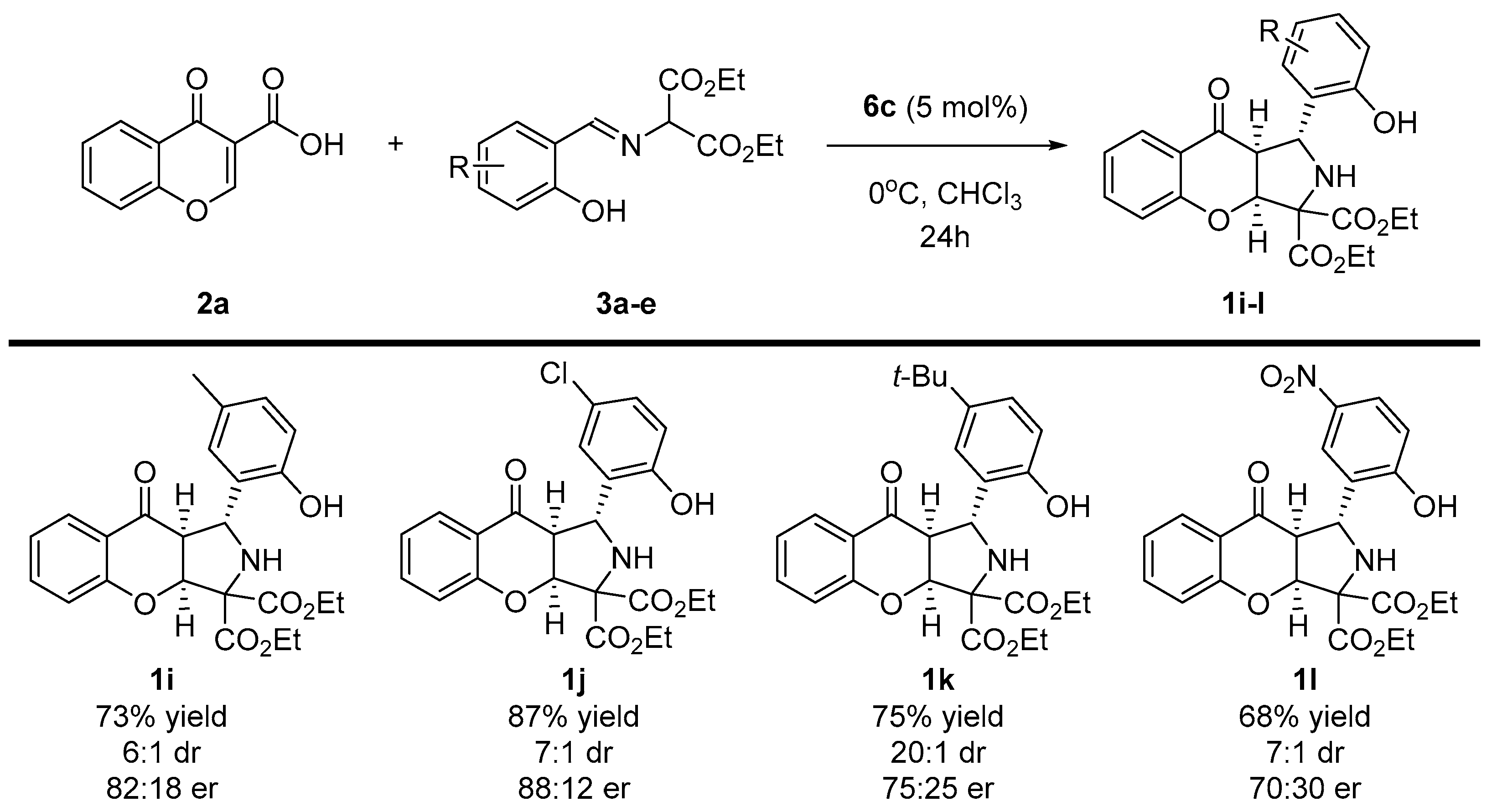
Scheme 4.
Enantioselective, decarboxylative (3+2)-cycloaddition of chromone-3-carboxylic acids 2a and diethyl iminomalonates 3 to 1i-l—scope diethyl iminomalonates 3a-e.
In the second part of the scope studies, the possibility of employing various diethyl iminomalonates 3a-e in the devised strategy was tested (Scheme 4). It turned out that the application of imines 3a-e had significant influences on both the yield and the enantioselectivity of the methodology. The reaction efficiency decreased compared to that shown in the first part of the optimization studies, and instead of obtaining the product quantitatively, yields in the order of 70–80% were obtained. Moreover, when the branching of the alkyl chain in 3d was introduced, enantioselectivity lowered to 75:25 er. Gratifyingly, strongly electron-withdrawing substituent (NO2) 3e was tolerated in this reaction, and the desired chromenopyrrole 1l was afforded with good yield. The diastereoselectivity of the methodology remained at a good level; in addition for the reaction with the tert-butyl substituent, it was as high as 20:1 dr.
The possibility to replace the diethyl iminomalonates 3 scaffold with imines 4 bearing γ-lactone rings was also evaluated, and studies were initiated with the goal of finding optimal reaction conditions. In the first step, catalyst screening was performed, and gratifyingly, it was found that cinchona alkaloids 6a promote cycloaddition (Table 2, entries 1–5). The reaction was terminated within 24 h, and chromenopyrrole 1 was obtained as a mixture of two diastereoisomers which differed in configuration on C-3 stereogenic centers with yields within the range of 70–90%. In particular, the application of bifunctional catalysts 6b-e, bearing either a thiourea or squaramide moiety, led to a significant increase in the reaction enantioselectivity; however, its diastereoselectivity remained low. Among all catalysts tested, derivative 6c proved optimal. With the best catalyst identified, solvent screening was initiated (Table 2, entries 7–10); however, none of the tested solvents were found to improve the reaction outcome, and therefore, further optimization was carried out using CH2Cl2 (Table 2, entry 5). In order to obtain better diastereoselectivity, the temperature was lowered to 0 °C (Table 2, entry 11). Carrying out the reaction at −20 °C did not provide any product. In the next part of the optimization studies, the influence of the catalyst amount and the concentration of the reaction were evaluated (Table 2, entries 13–16), leading to the identification of the optimal reaction parameters (Table 2, entry 13). Finally, the reaction time was extended from 24 to 48 h, which increased the reaction yield from 71 to 87%.

Table 2.
Enantioselective, decarboxylative (3+2)-cycloaddition of chromone-3-carboxylic acids 2 and iminodihydrofuran-2-one 4—optimization studies a.
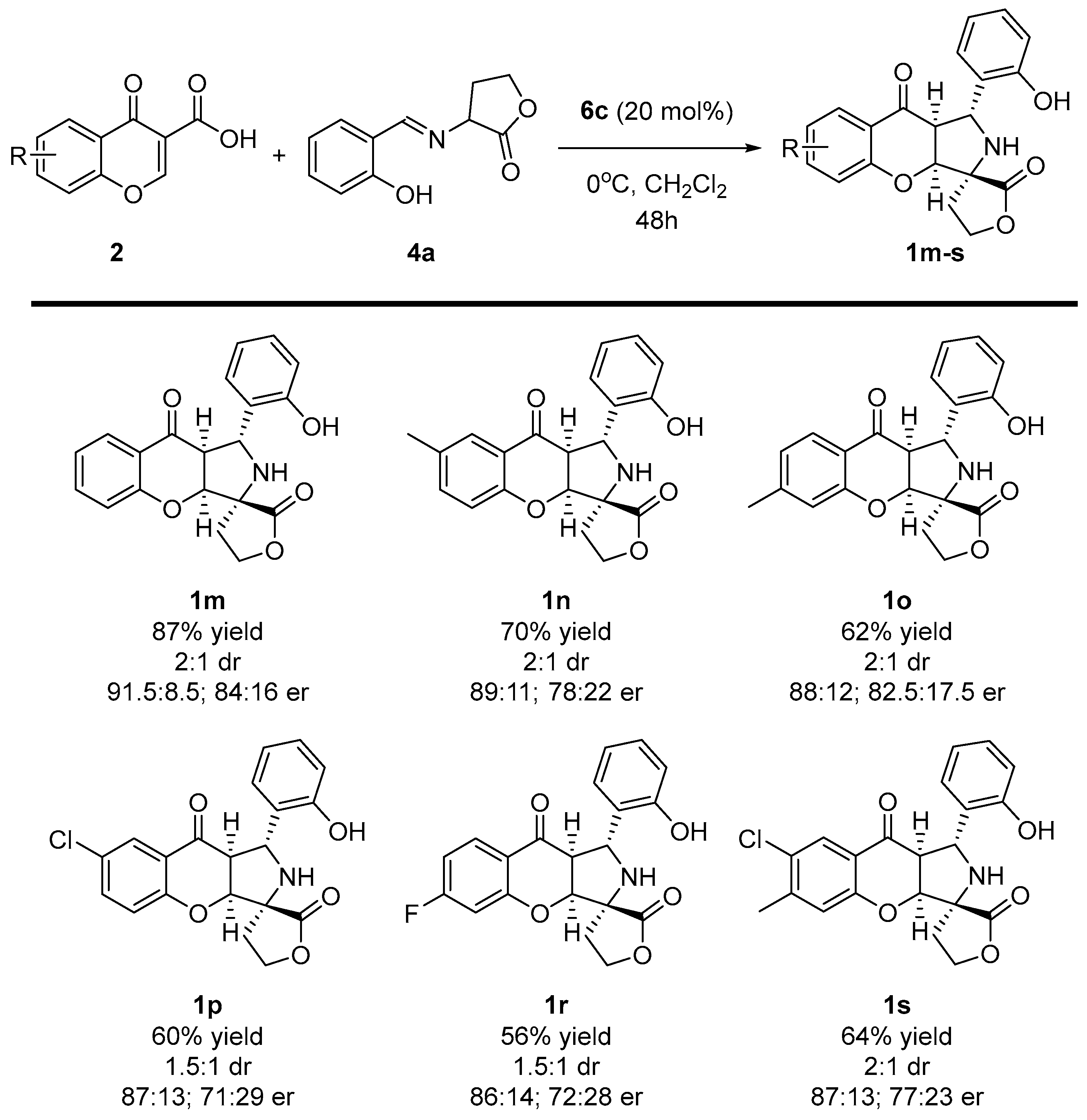
Having established the best reaction conditions, the scope of the methodology was studied (Scheme 5 and Scheme 6). At the beginning, various chromone-3-carboxylic acids 2 containing either electron-withdrawing or donating substituents on the aromatic ring were tested in the reaction (Scheme 5). It was found that the enantioselectivity of the cascade remained at a similar level as for the model reaction; however, its efficiency decreased. Target products 1m-s were obtained in yields within the range of 56–70% as a mixture of two diastereoisomers which differed in their configuration on the C-3 stereogenic center (only the main isomer was presented on Scheme 5 and Scheme 6). In this section, the studies also indicated that the position of the substituent in chromone-3-carboxylic acids 2 had no pronounced influence on the stereochemical reaction outcome, and the introduction of two substituents on the aromatic ring was also possible (Scheme 5, product 1s).
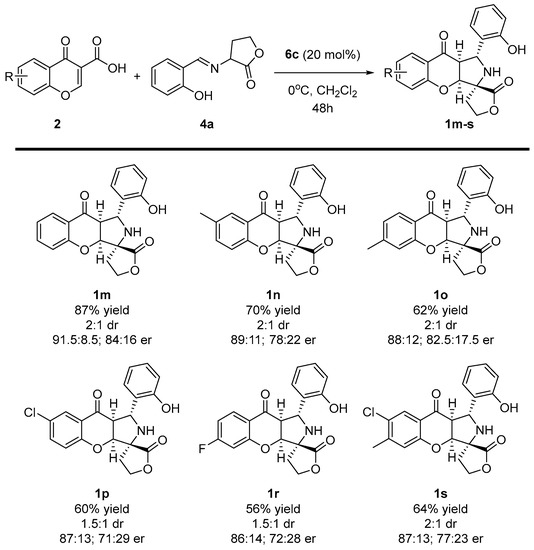
Scheme 5.
Enantioselective cycloaddition of chromone-3-carboxylic acids 2 and iminodihydrofuran-2-one 4a—scope of chromone-3-carboxylic acids 2 to 1m-s.
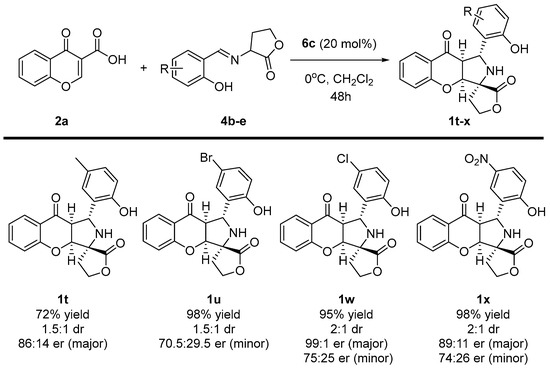
Scheme 6.
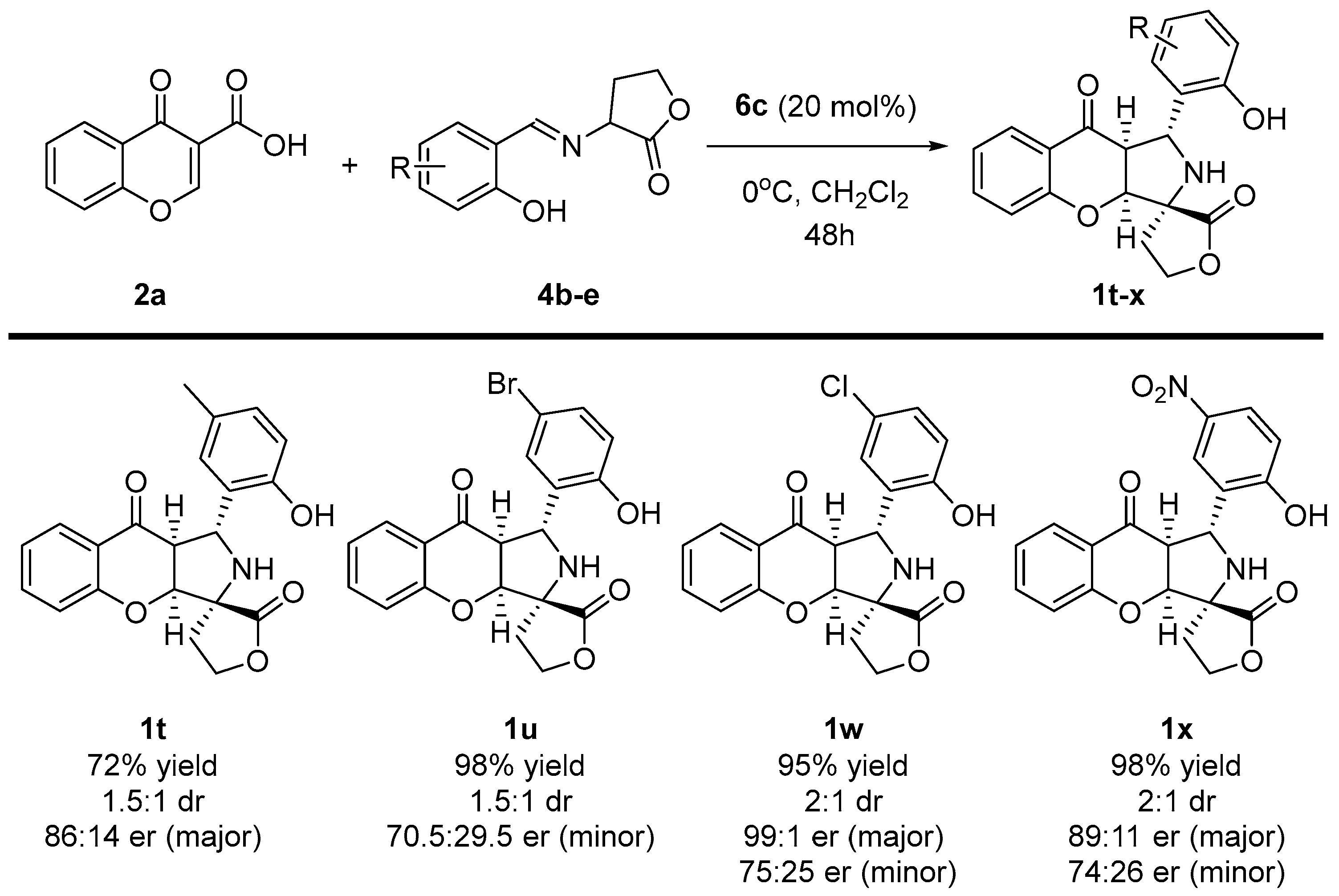
Enantioselective cycloaddition of chromone-3-carboxylic acids 2a and iminodihydrofuran-2-one 4b-e—scope of iminodihydrofuran-2-one 4 to 1t-x.
Importantly, the incorporation of different functional groups on the aromatic ring of iminodihydrofuran-2-ones 3 was also performed (Scheme 6). To our delight, apart from the example with methyl group 4b, the annulative strategy took place with excellent yields. Moreover, target products 1t-x were afforded with no significant influences either on the diastereoselectivity or the enantioselectivity of the reaction. In all cases, the desired chromenopyrroles 1t-x were obtained as two diastereoisomers at a ratio of around 2:1. For the major diastereoisomer, the enantiomeric ratio was kept around 90:10 er, yet for the minor diastereoisomer, the enantiomeric excess remained at an average level.
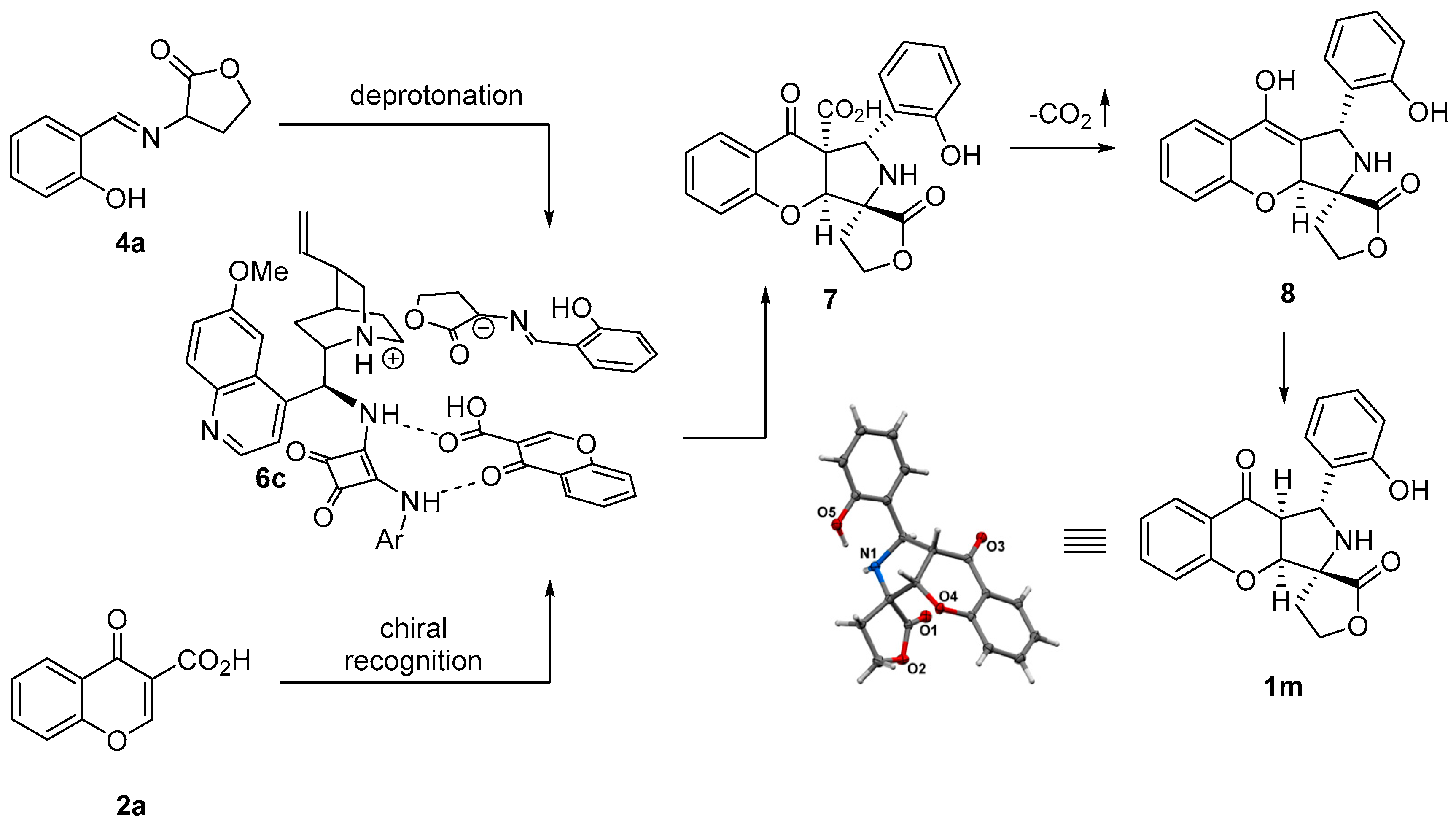
The absolute configurations of the major stereoisomer of chromone 1m were unambiguously assigned by single-crystal X-ray analysis (Scheme 7) [45]. The absolute configurations of the remaining polycyclic products 1a-x were assigned by analogy. Given these configurational assignments, the reaction mechanism explaining the observed stereochemistry of the products was proposed (Scheme 7). The reaction was initiated through the deprotonation of 4a by the Brønsted base catalyst 6c to give the corresponding azomethine ylide that participated in a (3+2)-cycloaddition. Importantly, it was postulated that, in this reaction, 6c acted as a bifunctional catalyst. Firstly, a Brønsted base moiety in 6c deprotonated the starting imine 4a to form the corresponding ion pair. Secondly, the H-bonding unit of 6c recognized the chromone-3-carboxylic acid 2a. The subsequent cycloaddition of ylide with chromone-3-carboxylic acid yielded 1m. The decarboxylation of 7 is the key step of the reaction, allowing for the removal of the activating group. The protonation of the enolate 8 thus obtained yielded the desired chromanones 1m.
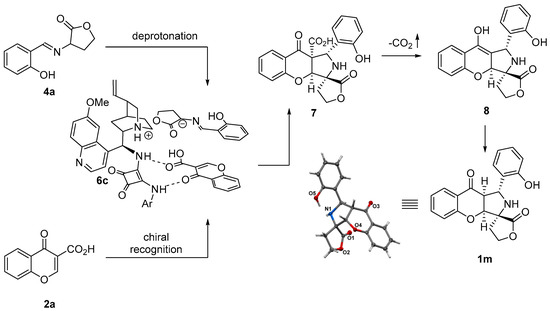
Scheme 7.
Mechanism of decarboxylative (3+2)-cycloaddition.
To demonstrate the usefulness of cycloadducts 1 the annulation of 1k with formaldehyde was attempted (Scheme 8). It was found that under acidic conditions in the presence of an aq. solution of formaldehyde, product 9a bearing an oxazine moiety was obtained in 95% yield. Notably, the reaction proceeded with full preservation of the stereochemical information introduced at the decarboxylative cycloaddition step, as 9a was obtained as a single diastereoisomer.
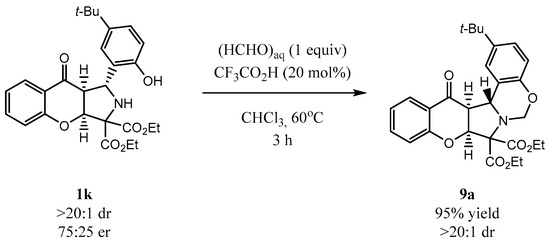
Scheme 8.
Synthesis of oxazine 9a from 1k.
3. Materials and Methods
3.1. General Methods
NMR spectra were acquired on a Bruker Ultra Shield 700 instrument (Bruker Corporation, Billerica, MA, USA), running at 700 MHz for 1H and 176 MHz for 13C, respectively. Chemical shifts (δ) were reported in ppm relative to residual solvent signals (CDCl3: 7.26 ppm for 1H NMR and 77.16 ppm for 13C NMR). Mass spectra were recorded on a Bruker Maxis Impact spectrometer using electrospray (ES+) ionization (referenced to the mass of the charged species). Analytical thin layer chromatography (TLC) was performed using pre-coated aluminium-backed plates (Merck Kieselgel 60 F254) and visualized by ultraviolet irradiation. Unless otherwise noted, analytical-grade solvents and commercially available reagents were used without further purification. For flash chromatography (FC), silica gel (Silica gel 60, 230–400 mesh, Merck, Darmstadt, Germany) was used. The enantiomeric ratio (er) of the products were determined by chiral stationary phase HPLC by Ultra Performance Convergence Chromatography (UPCC), using Daicel Chiralpak IA, IB, IC, and IG columns as chiral stationary phases. Diethyl iminomalonates 3 and iminodihydrofuran-2-one 4 were prepared from the corresponding 2-hydroxyaldehyde following the literature procedure [46]. Chromone-3-carboxylic acids 2 were prepared from the corresponding 2-hydroxyacetophenones following the literature procedure [47].
3.2. General Procedure
3.2.1. General Procedure for the Synthesis of Substituted (1R,3aS,9aS)-Diethyl 1-(2-Hydroxyphenyl)-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1a-l
An ordinary screw-cap vial was charged with a magnetic stirring bar, the corresponding chromone-3-carboxylic acid 2 (0.1 mmol, 1 equiv), CHCl3 (0.2 mL), catalyst 6c (0.005 mmol, 0.05 equiv), and the corresponding 2-hydroxyarylideneaminomalonates 3 (0.1 mmol, 1 equiv). The reaction mixture was stirred at 0 °C and monitored by 1H NMR spectroscopy. After the complete consumption of the chromone-3-carboxylic acid 2, the mixture was directly subjected to FC on silica gel (hexane:ethyl acetate 5:1) to provide the desired products 1.
(1R,3aS,9aS)-Diethyl 1-(2-hydroxyphenyl)-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1a
Pure product was isolated via flash chromatography on silica gel (hexane/ethyl acetate 5:1) as yellow oil in 93% yield, 5:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.20 (s, 1H), 7.91 (ddd, J = 7.9, 1.8, 0.4 Hz, 1H), 7.55–7.50 (m, 1H), 7.17 (ddd, J = 8.1, 7.3, 1.8 Hz, 1H), 7.10 (ddd, J = 7.9, 7.3, 1.0 Hz, 1H), 6.93 (ddd, J = 8.4, 1.0, 0.5 Hz, 1H), 6.85 (dd, J = 8.2, 1.2 Hz, 1H), 6.74 (td, J = 7.4, 1.2 Hz, 1H), 6.59 (ddt, J = 7.6, 1.8, 0.5 Hz, 1H), 5.67 (dd, J = 3.5, 0.8 Hz, 1H), 4.91 (d, J = 11.3 Hz, 1H), 4.50 (dqd, J = 10.7, 7.1, 1.4 Hz, 1H), 4.43–4.38 (m, 1H), 4.33 (qd, J = 7.1, 3.0 Hz, 2H), 3.34 (dd, J = 11.3, 3.5 Hz, 1H), 1.42 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 188.04, 167.90, 166.75, 158.68, 157.29, 136.73, 129.72, 129.09, 127.31, 122.78, 120.44, 119.71, 119.37, 118.09, 117.84, 83.75, 76.16, 64.23, 63.42, 63.17, 55.29, 14.34, 14.07. HRMS calculated for [C23H24NO7+]: 426.1553, found: 426.1550. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 3.13 min, τminor = 2.96 min, (90:10 er). 〖[α]〗_D^20 = −44.9 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 1-(2-hydroxyphenyl)-7-methyl-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1b
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as yellow oil solid in 85% yield, 6:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.23 (s, 1H), 7.69 (dd, J = 2.3, 1.1 Hz, 1H), 7.33 (ddd, J = 8.4, 2.3, 0.8 Hz, 1H), 7.17 (ddd, J = 8.1, 7.4, 1.7 Hz, 1H), 6.84 (dd, J = 8.1, 1.2 Hz, 1H), 6.82 (d, J = 8.4 Hz, 1H), 6.73 (td, J = 7.4, 1.2 Hz, 1H), 6.59 (dd, J = 7.6, 1.6 Hz, 1H), 5.63 (d, J = 3.5 Hz, 1H), 4.89 (d, J = 11.3 Hz, 1H), 4.49 (dq, J = 10.7, 7.1 Hz, 1H), 4.44–4.37 (m, 1H), 4.33 (qd, J = 7.1, 2.7 Hz, 6H), 4.29 (s, 1H), 3.31 (dd, J = 11.3, 3.5 Hz, 1H), 2.33 (s, 3H), 1.42 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 188.27, 167.94, 166.80, 157.30, 156.71, 137.76, 132.35, 129.68, 129.11, 126.86, 120.47, 119.33, 119.30, 117.87, 117.82, 83.69, 76.14, 64.25, 63.39, 63.13, 55.29, 20.58, 14.34, 14.06. HRMS calculated for [C24H26NO7+]: 440.1709, found: 440.1711. The er was determined by UPC2 using a chiral Chiralpack IA column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 3.54 min, τminor = 3.65 min, (87:13 er). 〖[α]〗_D^20 = −49.9 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 1-(2-hydroxyphenyl)-6-methyl-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1c
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as yellow solid m.p.: 201–203 °C in 90% yield, 9:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.21 (s, 1H), 7.79 (d, J = 8.0 Hz, 1H), 7.17 (ddd, J = 8.4, 7.5, 1.7 Hz, 1H), 6.93–6.88 (m, 1H), 6.84 (dd, J = 8.4, 1.1 Hz, 1H), 6.76–6.70 (m, 2H), 6.59 (dd, J = 7.6, 1.7 Hz, 1H), 5.63 (d, J = 3.5 Hz, 1H), 4.88 (dd, J = 11.2, 6.6 Hz, 1H), 4.49 (dq, J = 10.6, 7.1 Hz, 1H), 4.42 (dq, J = 10.6, 7.1 Hz, 1H), 4.33 (qd, J = 7.1, 2.1 Hz, 2H), 4.27 (d, J = 6.9 Hz, 1H), 3.31 (dd, J = 11.2, 3.5 Hz, 1H), 2.38 (s, 3H), 1.43 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 187.71, 167.93, 166.78, 158.69, 157.27, 148.46, 129.65, 129.07, 127.18, 124.09, 120.54, 119.34, 118.06, 117.80, 117.41, 83.71, 76.13, 64.30, 63.38, 63.12, 55.21, 22.10, 14.35, 14.06. HRMS calculated for [C24H26NO7+]: 440.1709, found: 440.1710. The er was determined by UPC2 using a chiral Chiralpack IA column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 3.54 min, τminor = 3.41 min, (86:14 er). 〖[α]〗_D^20 = −38.6 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 7-fluoro-1-(2-hydroxyphenyl)-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1d
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as yellow solid m.p.: 84–86 °C in 91% yield, 13:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.11 (s, 1H), 7.55 (dd, J = 7.9, 3.2 Hz, 1H), 7.26–7.22 (m, 1H), 7.18 (td, J = 7.7, 1.6 Hz, 1H), 6.92 (dd, J = 9.0, 4.0 Hz, 1H), 6.84 (dd, J = 8.2, 1.1 Hz, 1H), 6.74 (td, J = 7.5, 1.1 Hz, 1H), 6.58 (dd, J = 7.5, 1.6 Hz, 1H), 5.65 (d, J = 3.5 Hz, 1H), 4.88 (dd, J = 11.3, 5.1 Hz, 1H), 4.52–4.44 (m, 1H), 4.44–4.36 (m, 1H), 4.32 (dtt, J = 9.6, 5.7, 3.0 Hz, 3H), 3.34 (dd, J = 11.3, 3.5 Hz, 1H), 1.41 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 187.33, 167.22 (d, J= 211.77 Hz), 158.70, 157.29, 157.26, 154.81, 129.82, 129.06, 124.24 (d, J = 24.4 Hz), 120.31 (d, J = 6.6 Hz), 120.21, 119.89 (d, J = 7.4 Hz), 119.42, 117.88, 112.40 (d, J = 23.7 Hz), 84.00, 76.06, 64.08, 63.46, 63.22, 54.97, 14.33, 14.05. HRMS calculated for [C23H23FNO7+]: 444.1459, found: 444.1458. The er was determined by UPC2 using a chiral Chiralpack IA column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 3.21 min, τminor = 3.34 min, (89:11 er). 〖[α]〗_D^20 = −46.0 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 7-bromo-1-(2-hydroxyphenyl)-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1e
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as pale yellow solid m.p.: 98–101 °C in 97% yield, 20:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.06 (s, 1H), 8.01 (d, J = 2.5 Hz, 1H), 7.61 (dd, J = 8.8, 2.5 Hz, 1H), 7.18 (ddd, J = 8.3, 7.5, 1.6 Hz, 1H), 6.86–6.81 (m, 2H), 6.74 (td, J = 7.5, 1.2 Hz, 1H), 6.58 (dd, J = 7.5, 1.6 Hz, 1H), 5.66 (d, J = 3.5 Hz, 1H), 4.86 (d, J = 11.3 Hz, 1H), 4.52–4.45 (m, 1H), 4.44–4.38 (m, 1H), 4.33 (qd, J = 7.1, 3.4 Hz, 2H), 4.30 (s, 1H), 3.35 (dd, J = 11.3, 3.5 Hz, 1H), 1.41 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 186.86, 167.78, 166.57, 157.52, 157.23, 139.36, 129.87, 129.73, 129.05, 120.88, 120.16, 120.12, 119.46, 117.91, 115.54, 83.93, 76.10, 64.19, 63.51, 63.27, 54.95, 14.34, 14.06. HRMS calculated for [C23H23BrNO7+]: 504.0658, found: 504.0661. The er was determined by UPC2 using a chiral Chiralpack IA column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 3.76 min, τminor = 3.92 min, (87:13 er). 〖[α]〗_D^20 = −40.7 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 7-chloro-1-(2-hydroxyphenyl)-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1f
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as pale yellow solid m.p.: 118–121 °C in 89% yield 9:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.07 (s, 1H), 7.86 (d, J = 2.7 Hz, 1H), 7.47 (dd, J = 8.8, 2.7 Hz, 1H), 7.21–7.16 (m, 1H), 6.90 (d, J = 8.8 Hz, 1H), 6.85 (d, J = 8.1 Hz, 1H), 6.74 (td, J = 7.6, 1.2 Hz, 1H), 6.58 (dd, J = 7.6, 1.6 Hz, 1H), 5.66 (d, J = 3.5 Hz, 1H), 4.87 (d, J = 10.5 Hz, 1H), 4.48 (dd, J = 10.7, 7.1 Hz, 1H), 4.41 (dd, J = 10.7, 7.1 Hz, 1H), 4.33 (qd, J = 7.1, 3.4 Hz, 2H), 4.29 (s, 1H), 3.35 (dd, J = 11.3, 3.5 Hz, 1H), 1.41 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 186.99, 167.79, 166.59, 157.24, 157.05, 136.57, 129.88, 129.05, 128.43, 126.64, 120.45, 120.12, 119.85, 119.46, 117.91, 83.97, 76.10, 64.19, 63.51, 63.27, 54.99, 14.35, 14.07. HRMS calculated for [C23H23ClNO7+]: 474.1163, found: 474.1164. The er was determined by UPC2 using a chiral Chiralpack IA column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 3.41 min, τminor = 3.58 min, (88:12 er). 〖[α]〗_D^20 = −54.0 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 7-chloro-1-(2-hydroxyphenyl)-6-methyl-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1g
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as pale yellow oil in 88% yield, 8:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.11 (s, 1H), 7.85 (s, 1H), 7.18 (ddd, J = 8.2, 7.3, 1.7 Hz, 1H), 6.84 (dd, J = 8.2, 1.2 Hz, 1H), 6.74 (td, J = 7.4, 1.2 Hz, 1H), 6.57 (dd, J = 7.7, 1.7 Hz, 1H), 5.63 (d, J = 3.5 Hz, 1H), 4.85 (d, J = 11.3 Hz, 1H), 4.48 (dq, J = 10.7, 7.1 Hz, 1H), 4.42 (dq, J = 10.7, 7.1 Hz, 1H), 4.33 (qd, J = 7.1, 2.4 Hz, 2H), 4.29 (s, 1H), 3.31 (dd, J = 11.3, 3.5 Hz, 1H), 2.40 (s, 3H), 1.42 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 186.82, 167.83, 166.63, 157.23, 156.85, 146.03, 129.79, 129.05, 129.02, 126.94, 120.25, 120.15, 119.41, 118.60, 117.86, 83.90, 76.09, 64.26, 63.46, 63.21, 54.96, 21.01, 14.35, 14.05. HRMS calculated for [C24H25ClNO7+]: 474.1320, found: 474.1319. The er was determined by UPC2 using a chiral Chiralpack IG column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 4.94 min, τminor = 4.54 min, (88:12 er). 〖[α]〗_D^20 = −37.0 (c = 1.0, CH2Cl2).
(7aS,10R,10aS)-Diethyl 10-(2-hydroxyphenyl)-11-oxo-9,10,10a,11-tetrahydrobenzo[5,6]chromeno[2,3-c]pyrrole-8,8(7aH)-dicarboxylate 1h
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as pale yellow oil in 90% yield 5:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 9.35 (ddd, J = 8.6, 1.2, 0.6 Hz, 1H), 7.98 (dt, J = 9.0, 0.6 Hz, 1H), 7.78 (ddt, J = 8.1, 1.3, 0.6 Hz, 1H), 7.64 (ddd, J = 8.6, 6.9, 1.4 Hz, 1H), 7.47 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H), 7.18 (ddd, J = 8.2, 7.4, 1.7 Hz, 1H), 7.03 (d, J = 8.9 Hz, 1H), 6.87 (dd, J = 8.2, 1.2 Hz, 1H), 6.72 (td, J = 7.4, 1.2 Hz, 1H), 6.60–6.58 (m, 1H), 5.78 (dd, J = 3.9, 0.8 Hz, 1H), 4.97 (d, J = 11.1 Hz, 1H), 4.52 (dq, J = 10.6, 7.1 Hz, 1H), 4.44 (dq, J = 10.6, 7.1 Hz, 1H), 4.35 (qq, J = 7.1, 3.6 Hz, 2H), 3.43 (dd, J = 11.1, 3.6 Hz, 1H), 1.45 (t, J = 7.1 Hz, 3H), 1.36 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 189.78, 167.97, 165.87, 161.85, 157.22, 137.74, 130.44, 129.43, 129.30, 129.22, 128.96, 128.14, 125.53, 124.90, 119.20, 118.91, 118.39, 117.27, 111.79, 81.50, 78.05, 63.37, 63.30, 63.03, 53.40, 14.37, 14.21. HRMS calculated for [C27H26NO7+]: 475.1631, found: 475.1634. The er was determined by UPC2 using a chiral Chiralpack IA column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 4.61 min, τminor = 4.44 min, (92:8 er). 〖[α]〗_D^20 = −48.6 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 1-(2-hydroxy-5-methylphenyl)-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1i
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as pale yellow solid m.p.: 144–146 °C in 73% yield, 6:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 9.89 (s, 1H), 7.92 (dd, J = 7.8, 1.8 Hz, 1H), 7.54 (ddd, J = 8.5, 7.2, 1.8 Hz, 1H), 7.13–7.09 (m, 1H), 6.97 (dd, J = 8.2, 2.1 Hz, 1H), 6.93 (dd, J = 8.5, 0.9 Hz, 1H), 6.75 (d, J = 8.2 Hz, 1H), 6.39 (d, J = 2.1 Hz, 1H), 5.66 (d, J = 3.5 Hz, 1H), 4.85 (d, J = 10.5 Hz, 1H), 4.52–4.47 (m, 1H), 4.43–4.37 (m, 1H), 4.33 (q, J = 7.1 Hz, 2H), 4.24 (s, 1H), 3.35 (dd, J = 10.5, 3.5 Hz, 1H), 2.17 (s, 3H), 1.42 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 188.05, 167.97, 166.84, 158.71, 154.88, 136.68, 130.32, 129.49, 128.43, 127.33, 122.75, 120.18, 119.76, 118.09, 117.64, 83.84, 76.09, 64.20, 63.39, 63.14, 55.22, 20.59, 14.35, 14.07. HRMS calculated for [C24H26NO7+]: 440.1709, found: 440.1712. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 2.96 min, τminor = 2.70 min, (82:18 er). 〖[α]〗_D^20 = −44.4 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 1-(5-chloro-2-hydroxyphenyl)-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1j
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as white solid m.p.: 167–169 °C in 87% yield, 7:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.19 (s, 1H), 7.92 (dd, J = 7.8, 1.6 Hz, 1H), 7.56–7.51 (m, 1H), 7.14–7.09 (m, 2H), 6.92 (d, J = 8.3 Hz, 1H), 6.77 (d, J = 8.9 Hz, 1H), 6.60 (d, J = 2.5 Hz, 1H), 5.65 (d, J = 3.6 Hz, 1H), 4.84 (dd, J = 11.3, 4.7 Hz, 1H), 4.48 (dq, J = 10.4, 7.1 Hz, 1H), 4.39 (dq, J = 10.4 7.1 Hz, 1H), 4.32 (qd, J = 7.1, 3.9 Hz, 2H), 4.29 (s, 1H), 3.31 (dd, J = 11.3, 3.6 Hz, 1H), 1.41 (t, J = 7.1 Hz, 3H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 187.77, 167.75, 166.58, 158.66, 155.91, 136.88, 129.53, 128.62, 127.43, 123.99, 122.92, 122.19, 119.60, 119.17, 118.08, 83.62, 76.15, 63.51, 63.48, 63.24, 55.11, 14.32, 14.04. HRMS calculated for [C23H23ClNO7+]: 460.1163, found: 460.1162. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 2.94 min, τminor = 2.74 min, (88:12 er). 〖[α]〗_D^20 = −59.6 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 1-(5-(tert-butyl)-2-hydroxyphenyl)-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1k
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as pale yellow oil in 75% yield, 20:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.04 (s, 1H), 7.92 (dd, J = 7.8, 1.7 Hz, 1H), 7.54 (ddd, J = 8.7, 7.2, 1.7 Hz, 1H), 7.18 (dd, J = 8.5, 2.5 Hz, 1H), 7.14–7.08 (m, 1H), 6.93 (dd, J = 8.3, 0.9 Hz, 1H), 6.77 (d, J = 8.5 Hz, 1H), 6.54 (d, J = 2.5 Hz, 1H), 5.67 (d, J = 3.6 Hz, 1H), 4.92 (d, J = 11.3 Hz, 1H), 4.50 (dq, J = 10.6, 7.1 Hz, 1H), 4.41 (dd, J = 10.6, 7.1 Hz, 1H), 4.32 (q, J = 7.1 Hz, 2H), 4.28 (s, 1H), 3.32 (dd, J = 11.3, 3.6 Hz, 1H), 1.42 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H), 1.21 (s, 9H). 13C NMR (176 MHz, Chloroform-d) δ 187.92, 167.93, 166.79, 158.64, 154.75, 141.73, 136.60, 127.09, 126.32, 126.07, 122.78, 119.79, 119.58, 118.10, 117.20, 83.81, 76.30, 64.68, 63.39, 63.12, 55.53, 33.98, 31.50, 14.34, 14.07. HRMS calculated for [C27H32NO7+]: 482.2179, found: 482.2181. The er was determined by UPC2 using a chiral Chiralpack IG column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 3.94 min, τminor = 4.14 min, (75:25 er). [α]20 = −43.4 (c = 1.0, CH2Cl2).
(1R,3aS,9aS)-Diethyl 1-(2-hydroxy-5-nitrophenyl)-9-oxo-1,2,9,9a-tetrahydrochromeno[2,3-c]pyrrole-3,3(3aH)-dicarboxylate 1l
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 5:1) as pale yellow solid m.p.: 152–154 °C in 68% yield, 7:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 11.44 (s, 1H), 8.09 (dd, J = 9.0, 2.7 Hz, 1H), 7.93 (dd, J = 7.8, 1.7 Hz, 1H), 7.60 (d, J = 2.7 Hz, 1H), 7.57 (ddd, J = 8.6, 7.2, 1.7 Hz, 1H), 7.17–7.14 (m, 1H), 6.95 (dd, J = 8.6, 0.9 Hz, 1H), 6.90 (d, J = 9.0 Hz, 1H), 5.67 (d, J = 3.6 Hz, 1H), 4.98 (dd, J = 11.2, 6.1 Hz, 1H), 4.51 (dq, J = 10.6, 7.1 Hz, 1H), 4.46–4.39 (m, 2H), 4.38–4.30 (m, 2H), 3.29 (dd, J = 11.2, 3.6 Hz, 1H), 1.43 (t, J = 7.1 Hz, 3H), 1.35 (t, J = 7.1 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 187.50, 167.48, 166.23, 163.51, 158.65, 140.32, 137.18, 127.59, 125.84, 125.36, 123.24, 120.94, 119.51, 118.28, 118.12, 83.41, 76.30, 63.75, 63.47, 63.26, 55.25, 14.32, 14.03. HRMS calculated for [C23H23N2O9+]: 471.1402, found: 471.1403. The er was determined by UPC2 using a chiral Chiralpack IA column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 3.53 min, τminor = 3.64 min, (70:30 er). 〖[α]〗_D^20 = −68.2 (c = 1.0, CH2Cl2).
3.2.2. General Procedure for the Synthesis of Substituted 1-(2-Hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1m-x
An ordinary screw-cap vial was charged with a magnetic stirring bar, the corresponding chromone-3-carboxylic acid 2 (0.1 mmol, 1 equiv), CH2Cl2 (0.4 mL), catalyst 6c (0.02 mmol, 0.02 equiv), and the corresponding 2-hydroxyarylideneaminolactones 4 (0.1 mmol, 1 equiv). The reaction mixture was stirred at 0 °C and monitored by 1H NMR spectroscopy. After the complete consumption of the chromone-3-carboxylic acid 2, the mixture was directly subjected to FC on silica gel (CH2Cl2:acetone 100:1) to provide the desired products 1m-x.
(1R,3R,3aS,9aS)-1-(2-Hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1m
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as white solid m.p.: 201–203 °C in 58% yield, 2:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 7.89 (dd, J = 7.8, 1.7 Hz, 1H), 7.54 (ddd, J = 8.4, 7.2, 1.7 Hz, 1H), 7.21 (ddd, J = 8.1, 7.4, 1.7 Hz, 1H), 7.09 (ddd, J = 8.1, 7.2, 1.0 Hz, 1H), 7.01 (dd, J = 8.4, 1.0 Hz, 1H), 6.87 (ddd, J = 8.1, 4.3, 1.5 Hz, 2H), 6.81 (td, J = 7.4, 1.2 Hz, 1H), 5.15 (d, J = 9.8 Hz, 1H), 5.10 (dd, J = 5.7, 0.7 Hz, 1H), 4.53 (ddd, J = 9.6, 8.5, 1.3 Hz, 1H), 4.44 (ddd, J = 11.1, 9.6, 5.7 Hz, 1H), 3.34 (dd, J = 9.8, 5.6 Hz, 1H), 2.60 (ddd, J = 13.0, 11.1, 8.5 Hz, 1H), 2.53 (ddd, J = 13.0, 5.7, 1.3 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 188.37, 174.69, 158.58, 157.05, 137.05, 129.80, 128.98, 127.15, 122.80, 121.80, 119.83, 119.40, 118.24, 117.77, 83.64, 69.40, 65.19, 63.91, 53.48, 36.37. HRMS calculated for [C20H18NO5+]: 352.1185, found: 352.1185. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 3.20 min, τminor = 3.05 min, (91.5:8.5 er).
(1R,3S,3aS,9aS)-1-(2-Hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1m′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as white solid m.p.: 188–190 °C in 29% yield, 2:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 7.90 (ddd, J = 7.8, 1.8, 0.4 Hz, 1H), 7.57 (ddd, J = 8.4, 7.2, 1.8 Hz, 1H), 7.20 (ddd, J = 8.2, 7.2, 1.8 Hz, 1H), 7.12 (ddd, J = 7.8, 7.2, 1.0 Hz, 1H), 7.05 (ddd, J = 8.2, 1.0, 0.4 Hz, 1H), 6.91 (dd, J = 8.1, 1.2 Hz, 1H), 6.80–6.78 (m, 1H), 6.77–6.74 (m, 1H), 5.04 (dd, J = 4.6, 0.7 Hz, 1H), 4.88 (d, J = 10.8 Hz, 1H), 4.53–4.49 (m, 1H), 4.43 (td, J = 9.4, 6.4 Hz, 1H), 3.64 (dd, J = 10.8, 4.6 Hz, 1H), 3.21 (ddd, J = 13.3, 6.4, 3.1 Hz, 1H), 2.43–2.37 (m, 1H). 13C NMR (176 MHz, Chloroform-d) δ 188.34, 177.30, 158.46, 156.92, 136.88, 129.97, 129.28, 127.52, 122.98, 121.02, 119.91, 119.47, 117.96, 117.95, 81.65, 69.06, 65.57, 63.70, 53.48, 30.57. HRMS calculated for [C20H18NO5+]: 352.1185, found: 352.1184. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 4.91 min, τminor = 5.19 min, (84:16 er).
(1R,3R,3aS,9aS)-1-(2-Hydroxyphenyl)-7-methyl-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1n
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow oil in 47% yield, 2:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.27 (s, 1H), 7.67 (d, J = 1.2 Hz, 1H), 7.35 (ddd, J = 8.4, 2.4, 0.7 Hz, 1H), 7.20 (ddd, J = 8.0, 7.4, 1.7 Hz, 1H), 6.91 (d, J = 8.4 Hz, 1H), 6.87 (dt, J = 7.7, 1.7 Hz, 2H), 6.80 (td, J = 7.4, 1.2 Hz, 1H), 5.13 (d, J = 9.9 Hz, 1H), 5.06 (dd, J = 5.4, 0.7 Hz, 1H), 4.52 (ddd, J = 9.6, 8.6, 1.2 Hz, 1H), 4.43 (ddd, J = 11.2, 9.6, 5.6 Hz, 1H), 3.45 (s, 1H), 3.31 (dd, J = 9.9, 5.4 Hz, 1H), 2.59 (ddd, J = 13.1, 11.2, 8.6 Hz, 1H), 2.52 (ddd, J = 13.1, 5.6, 1.2 Hz, 1H), 2.32 (s, 3H). 13C NMR (176 MHz, Chloroform-d) δ 188.60, 174.78, 157.05, 156.63, 138.11, 132.36, 129.74, 128.99, 126.66, 121.86, 119.77, 119.00, 118.02, 117.72, 83.62, 69.36, 65.19, 63.91, 53.51, 36.40, 20.57. HRMS calculated for [C21H20NO5+]: 366.1297, found: 366.1298. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 4.69 min, τminor = 4.55 min, (89:11 er).
(1R,3S,3aS,9aS)-1-(2-Hydroxyphenyl)-7-methyl-3a,4′,5′,9a-tetrah-ydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-di-one 1n′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow oil in 23% yield, 2:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 7.67 (d, J = 2.3 Hz, 1H), 7.37 (dd, J = 8.5, 2.3 Hz, 1H), 7.19 (ddd, J = 8.5, 7.1, 2.0 Hz, 1H), 6.94 (d, J = 8.4 Hz, 1H), 6.92–6.89 (m, 1H), 6.75 (dtd, J = 11.2, 7.6, 1.6 Hz, 2H), 4.99 (d, J = 4.5 Hz, 1H), 4.85 (d, J = 10.8 Hz, 1H), 4.50 (td, J = 8.8, 3.0 Hz, 1H), 4.41 (td, J = 9.3, 6.4 Hz, 1H), 3.59 (dd, J = 10.8, 4.5 Hz, 1H), 3.20 (ddd, J = 13.2, 6.4, 3.0 Hz, 1H), 2.38 (dt, J = 13.2, 8.9 Hz, 1H), 2.33 (d, J = 2.6 Hz, 3H). 13C NMR (176 MHz, Chloroform-d) δ 188.56, 177.40, 156.94, 156.49, 137.90, 132.58, 129.91, 129.31, 127.08, 121.07, 119.52, 119.41, 117.93, 117.73, 81.57, 69.03, 65.56, 63.73, 53.51, 30.52, 20.59. HRMS calculated for [C21H20NO5+]: 366.1297, found: 366.1294. The er was determined by UPC2 using a chiral Chiralpack IC column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 6.81 min, τminor = 6.16 min, (78:22 er).
(1R,3R,3aS,9aS)-1-(2-Hydroxyphenyl)-6-methyl-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1o
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow solid m.p.: 180–182 °C in 41% yield, 2:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.26 (s, 1H), 7.78 (d, J = 8.0 Hz, 1H), 7.20 (ddd, J = 8.7, 7.4, 1.7 Hz, 1H), 6.90 (d, J = 8.0 Hz, 1H), 6.87 (m, 2H), 6.83 (s, 1H), 6.80 (td, J = 7.4, 1.2 Hz, 1H), 5.13 (d, J = 9.8 Hz, 1H), 5.06 (d, J = 5.5 Hz, 1H), 4.53 (t, J = 9.0 Hz, 1H), 4.43 (ddd, J = 11.1, 9.6, 5.5 Hz, 1H), 3.43 (s, 1H), 3.31 (dd, J = 9.8, 5.5 Hz, 1H), 2.59 (ddd, J = 13.0, 10.9, 8.3 Hz, 1H), 2.52 (dd, J = 13.0, 5.5 Hz, 1H), 2.37 (s, 3H). 13C NMR (176 MHz, Chloroform-d) δ 188.04, 174.78, 158.58, 157.02, 148.88, 129.72, 128.94, 127.00, 124.12, 121.95, 119.78, 118.19, 117.71, 117.08, 83.65, 69.37, 65.19, 63.93, 53.38, 36.35, 22.11. HRMS calculated for [C21H20NO5+]: 366.1297, found: 366.1295. The er was determined by UPC2 using a chiral Chiralpack IC column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.87 min, τminor = 4.93 min, (88:12 er).
(1R,3S,3aS,9aS)-1-(2-Hydroxyphenyl)-6-methyl-3a,4′,5′,9a-tetrah-ydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1o′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow solid m.p.: 135–137 °C in 21% yield, 2:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 9.46 (s, 1H), 7.78 (dd, J = 8.1, 3.2 Hz, 1H), 7.19 (ddd, J = 8.8, 7.4, 1.8 Hz, 1H), 6.91 (ddd, J = 13.1, 8.1, 1.4 Hz, 2H), 6.86 (s, 1H), 6.80 (dd, J = 7.5, 1.8 Hz, 1H), 6.75 (td, J = 7.4, 1.4 Hz, 1H), 5.01 (dd, J = 4.7, 0.7 Hz, 1H), 4.85 (d, J = 10.7 Hz, 1H), 4.50 (ddd, J = 9.3, 8.3, 3.1 Hz, 1H), 4.41 (td, J = 9.3, 6.4 Hz, 1H), 3.59 (dd, J = 10.7, 4.7 Hz, 1H), 3.17 (ddd, J = 13.2, 6.4, 3.1 Hz, 1H), 2.40 (d, J = 0.7 Hz, 3H), 2.38–2.35 (m, 1H). 13C NMR (176 MHz, Chloroform-d) δ 188.54, 177.53, 158.65, 156.64, 148.40, 129.45, 129.34, 127.00, 123.82, 122.11, 119.27, 117.81, 117.39, 117.27, 81.84, 68.91, 65.76, 63.37, 53.18, 30.18, 21.97. HRMS calculated for [C21H20NO5+]: 366.1297, found: 366.1298. The er was determined by UPC2 using a chiral Chiralpack IC column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 6.18 min, τminor = 5.06 min, (82.5:17.5 er).
(1R,3R,3aS,9aS)-7-Chloro-1-(2-hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1p
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as yellow solid m.p.: 164–166 °C in 36% yield, 1.5:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 7.84 (dd, J = 2.7, 1.6 Hz, 1H), 7.48 (ddd, J = 8.9, 2.7, 0.6 Hz, 1H), 7.21 (ddd, J = 8.0, 7.3, 1.6 Hz, 1H), 6.98 (d, J = 8.9 Hz, 1H), 6.87 (d, J = 1.0 Hz, 1H), 6.86 (d, J = 1.2 Hz, 1H), 6.81 (td, J = 7.3, 1.2 Hz, 1H), 5.12 (d, J = 9.7 Hz, 1H), 5.10 (d, J = 5.7 Hz, 1H), 4.53 (ddd, J = 9.6, 8.4, 1.6 Hz, 1H), 4.43 (ddd, J = 10.9, 9.6, 5.8 Hz, 1H), 3.34 (dd, J = 9.7, 5.7 Hz, 1H), 2.62–2.56 (m, 1H), 2.54 (ddd, J = 13.1, 5.8, 1.6 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 187.38, 174.60, 156.98, 156.96, 136.85, 129.93, 128.98, 128.39, 126.43, 121.60, 120.11, 119.96, 119.92, 117.80, 83.73, 69.35, 65.22, 63.87, 53.06, 36.22. HRMS calculated for [C20H17ClNO5+]: 387.0688, found: 387.0686. The er was determined by UPC2 using a chiral Chiralpack IC column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.26 min, τminor = 4.64 min, (87:13 er).
(1R,3S,3aS,9aS)-7-Chloro-1-(2-hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1p′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as yellow solid m.p.: 155–157 °C in 24% yield, 1.5:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 7.86 (dd, J = 2.7, 0.4 Hz, 1H), 7.51 (dd, J = 8.8, 2.7 Hz, 1H), 7.23–7.19 (m, 1H), 7.02 (dd, J = 8.8, 0.4 Hz, 1H), 6.91 (dd, J = 7.8, 0.9 Hz, 1H), 6.77 (dd, J = 1.5, 0.9 Hz, 1H), 6.76 (dd, J = 2.1, 0.8 Hz, 1H), 5.03 (d, J = 4.4 Hz, 1H), 4.85 (dd, J = 10.9, 2.5 Hz, 1H), 4.52 (ddd, J = 9.3, 8.3, 3.2 Hz, 1H), 4.43 (td, J = 9.3, 6.5 Hz, 1H), 3.66 (dd, J = 10.9, 4.4 Hz, 1H), 3.19 (ddd, J = 13.3, 6.5, 3.2 Hz, 1H), 2.41 (ddd, J = 13.3, 9.1, 8.3 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 187.25, 177.10, 156.84, 156.82, 136.71, 130.15, 129.29, 128.66, 126.86, 120.68, 120.64, 119.71, 119.58, 118.02, 81.95, 69.06, 65.55, 63.66, 53.25, 31.07. HRMS calculated for [C20H17ClNO5+]: 387.0688, found: 387.0691. The er was determined by UPC2 using a chiral Chiralpack IA column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.39 min, τminor = 5.72 min, (71:29 er).
(1R,3R,3aS,9aS)-6-Fluoro-1-(2-hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1r
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow oil in 34% yield, 1.5:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.12 (s, 1H), 7.92 (dd, J = 8.8, 6.5 Hz, 1H), 7.21 (ddd, J = 8.1, 7.3, 1.7 Hz, 1H), 6.88 (ddd, J = 17.1, 7.9, 1.4 Hz, 2H), 6.84–6.78 (m, 1H), 6.71 (dd, J = 9.5, 2.3 Hz, 1H), 5.14 (d, J = 9.6 Hz, 1H), 5.12 (d, J = 5.8 Hz, 1H), 4.53 (ddd, J = 9.8, 8.4, 1.6 Hz, 1H), 4.44 (ddd, J = 10.8, 9.6, 5.8 Hz, 1H), 3.40 (s, 1H), 3.34 (dd, J = 9.6, 5.9 Hz, 1H), 2.60 (ddd, J = 13.1, 10.8, 8.4 Hz, 1H), 2.55 (ddd, J = 13.1, 5.9, 1.6 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 187.03, 174.60, 168.13, (d, J = 258.03 Hz), 160.24 (d, J = 13.9 Hz), 156.96, 129.89, 129.82 (d, J = 11.4 Hz), 128.90, 121.85, 119.90, 117.82, 116.27 (d, J = 2.78 Hz), 111.28 (d, J = 22.6 Hz), 105.20 (d, J = 24.9 Hz), 84.09, 69.32, 65.21, 63.81, 53.01, 36.20. HRMS calculated for [C20H17FNO5+]: 370.1091, found: 370.1088. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 4.59 min, τminor = 4.44 min, (86:14 er).
(1R,3S,3aS,9aS)-6-Fluoro-1-(2-hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1r′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow oil in 22% yield, 1.5:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 7.54 (ddd, J = 8.0, 3.2, 1.4 Hz, 1H), 7.29 (ddd, J = 9.0, 7.5, 3.2 Hz, 1H), 7.21–7.17 (m, 1H), 7.05 (dd, J = 9.0, 4.1 Hz, 1H), 6.89 (d, J = 8.0 Hz, 1H), 6.76–6.74 (m, 2H), 5.00 (d, J = 4.3 Hz, 1H), 4.84 (dd, J = 11.0, 3.0 Hz, 1H), 4.53–4.48 (m, 1H), 4.42 (td, J = 9.3, 6.4 Hz, 1H), 3.64 (dd, J = 11.0, 4.3 Hz, 1H), 3.19 (ddd, J = 13.3, 6.5, 3.0 Hz, 1H), 2.40 (dt, J = 13.3, 8.8 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 187.57, 177.27, 158.10 (d, J = 247.4 Hz), 156.89, 154.56, 130.06, 129.33, 124.42, 124.28, 120.7, 119.77 (d, J = 7.5 Hz), 119.50, 117.95, 112.64 (d, J = 23.6 Hz), 81.93, 69.01, 65.58, 63.60, 53.28, 30.48. HRMS calculated for [C20H17FNO5+]: 370.1091, found: 370.1090. The er was determined by UPC2 using a chiral Chiralpack IC column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 6.30 min, τminor = 5.48 min, (72:28 er).
(1R,3R,3aS,9aS)-7-Chloro-1-(2-hydroxyphenyl)-6-methyl-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1s
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow oil in 43% yield, 2:1 dr. 1H NMR (700 MHz, DMSO-d6) δ 9.90 (s, 1H), 7.76 (s, 1H), 7.45 (dd, J = 7.6, 1.7 Hz, 1H), 7.24 (td, J = 7.7, 1.7 Hz, 1H), 7.22 (d, J = 0.9 Hz, 1H), 6.93 (td, J = 7.4, 1.2 Hz, 1H), 6.89 (dd, J = 8.0, 1.2 Hz, 1H), 5.50 (d, J = 6.9 Hz, 1H), 5.16 (dd, J = 8.9, 5.3 Hz, 1H), 4.60–4.45 (m, 2H), 3.46 (s, 1H), 3.44 (dd, J = 8.9, 6.9 Hz, 1H), 2.74 (ddd, J = 12.9, 5.9, 2.5 Hz, 2H), 2.65 (m, 3H), 2.59–2.53 (m, 1H). 13C NMR (176 MHz, DMSO) δ 188.35, 175.49, 157.12, 155.51, 144.52, 128.37, 128.25, 126.27, 126.18, 125.21, 120.08, 118.83, 118.30, 115.40, 83.79, 69.22, 65.07, 59.17, 52.51, 34.84, 20.00. HRMS calculated for [C21H19ClNO5+]: 401.0844, found: 401.0842. The er was determined by UPC2 using a chiral Chiralpack IC column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.78 min, τminor = 4.92 min, (87:13 er).
(1R,3S,3aS,9aS)-7-Chloro-1-(2-hydroxyphenyl)-6-methyl-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1s′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow oil in 21% yield, 2:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 8.08 (d, J = 2.6 Hz, 1H), 7.84 (s, 1H), 7.20 (ddd, J = 8.2, 6.6, 2.3 Hz, 1H), 6.96 (s, 1H), 6.90 (dd, J = 8.2, 1.1 Hz, 1H), 6.78–6.74 (m, 1H), 5.01 (dd, J = 4.6, 0.7 Hz, 1H), 4.83 (d, J = 10.8 Hz, 1H), 4.67 (s, 1H), 4.50 (ddd, J = 9.3, 8.3, 3.2 Hz, 1H), 4.41 (td, J = 9.3, 6.5 Hz, 1H), 3.61 (dd, J = 10.8, 4.6 Hz, 1H), 3.16 (ddd, J = 13.2, 6.5, 3.2 Hz, 1H), 2.42 (s, 3H), 2.40–2.37 (m, 1H). 13C NMR (176 MHz, Chloroform-d) δ 187.12, 177.17, 156.84, 156.66, 146.20, 130.05, 129.26, 129.22, 127.15, 120.85, 120.04, 119.53, 118.81, 117.97, 81.86, 68.99, 65.54, 63.71, 53.18, 30.53, 29.85. HRMS calculated for [C21H19ClNO5+]: 401.0844, found: 401.0845. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.14 min, τminor = 5.52 min, (77:23 er).
(1R,3R,3aS,9aS)-1-(2-Hydroxy-5-methylphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1t
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as yellow oil in 43% yield, 1.5:1 dr. A few drops of DMSO-d6 were added to increase the solubility. 1H NMR (700 MHz, Chloroform-d) δ 9.80 (s, 1H), 7.71 (dd, J = 7.8, 1.8 Hz, 1H), 7.38 (ddd, J = 8.5, 7.2, 1.8 Hz, 1H), 6.92 (td, J = 7.5, 1.0 Hz, 1H), 6.84 (dd, J = 8.5, 1.0 Hz, 1H), 6.82 (dd, J = 8.2, 2.2 Hz, 1H), 6.72 (d, J = 2.1 Hz, 1H), 6.59 (d, J = 8.2 Hz, 1H), 5.04 (d, J = 6.9 Hz, 1H), 4.98 (d, J = 8.8 Hz, 1H), 4.35 (ddd, J = 9.2, 8.2, 2.6 Hz, 1H), 4.31 (td, J = 9.6, 6.2 Hz, 1H), 3.59 (s, 1H), 3.22 (dd, J = 8.8, 6.9 Hz, 1H), 2.44–2.41 (m, 1H), 2.09 (s, 3H). 13C NMR (176 MHz, Chloroform-d) δ 188.89, 175.05, 158.45, 154.07, 136.35, 129.56, 129.09, 128.34, 126.53, 121.97, 119.20, 117.70, 116.77, 83.15, 69.02, 65.08, 62.65, 52.47, 35.26, 29.45, 20.33. HRMS calculated for [C21H20NO5+]: 366.1341, found: 366.1333. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.18 min, τminor = 4.87 min, (86:14 er).
(1R,3S,3aS,9aS)-1-(2-Hydroxy-5-methylphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1t′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as yellow oil in 29% yield, 1.5:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 7.90 (dd, J = 7.8, 1.8 Hz, 1H), 7.57 (ddd, J = 8.7, 7.2, 1.8 Hz, 1H), 7.14–7.11 (m, 1H), 7.04 (d, J = 7.8 Hz, 1H), 6.99 (dd, J = 8.3, 2.2 Hz, 1H), 6.81 (dd, J = 8.2, 2.2 Hz, 1H), 6.61 (d, J = 2.2 Hz, 1H), 5.05 (d, J = 4.8 Hz, 1H), 4.82 (dd, J = 10.6, 7.1 Hz, 1H), 4.50 (td, J = 8.8, 3.2 Hz, 1H), 4.42 (td, J = 9.3, 6.5 Hz, 1H), 3.63 (dd, J = 10.6, 4.8 Hz, 1H), 3.17 (ddd, J = 13.2, 6.5, 3.2 Hz, 1H), 2.37 (dt, J = 13.2, 8.8 Hz, 1H), 2.18 (s, 3H). 13C NMR (176 MHz, Chloroform-d) δ 188.49, 177.36, 158.55, 154.45, 136.85, 130.53, 129.63, 128.59, 127.51, 122.90, 120.97, 117.92, 117.74, 81.79, 68.98, 65.59, 63.56, 53.34, 30.61, 29.85, 20.59. HRMS calculated for [C21H20NO5+]: 366.1341, found: 366.1338.
(1R,3R,3aS,9aS)-1-(5-Bromo-2-hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1u
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as white powder in 59% yield, 1.5:1 dr. A few drops of DMSO-d6 were added to increase the solubility. 1H NMR (700 MHz, Chloroform-d) δ 9.73 (s, 1H), 7.44 (dd, J = 7.9, 1.7 Hz, 1H), 7.15 (ddd, J = 8.4, 7.1, 1.7 Hz, 1H), 7.04 (dd, J = 2.5, 0.6 Hz, 1H), 6.84 (dd, J = 8.6, 2.5 Hz, 1H), 6.68 (ddd, J = 7.9, 7.1, 1.0 Hz, 1H), 6.59 (dd, J = 8.4, 1.0 Hz, 1H), 6.35 (d, J = 8.6 Hz, 1H), 4.85 (d, J = 7.3 Hz, 1H), 4.79 (d, J = 8.4 Hz, 1H), 4.14–4.05 (m, 2H), 3.52 (s, 1H), 2.93 (dd, J = 8.3, 7.5 Hz, 1H), 2.27 (ddd, J = 13.0, 6.0, 3.0 Hz, 1H), 2.23–2.15 (m, 2H). 13C NMR (176 MHz, Chloroform-d) δ 188.83, 174.83, 158.09, 154.65, 135.70, 130.67, 130.29, 127.06, 125.78, 121.14, 118.60, 117.72, 117.09, 110.28, 82.46, 68.52, 64.62, 59.65, 51.82, 34.32. HRMS calculated for [C20H17BrNO5+]: 430.0290, found: 430.0285.
(1R,3S,3aS,9aS)-1-(5-Bromo-2-hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1u′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as white powder in 39% yield, 1.5:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 9.53 (s, 1H), 7.91 (dt, J = 7.8, 1.9 Hz, 1H), 7.58 (ddd, J = 8.7, 7.2, 1.9 Hz, 1H), 7.27 (t, J = 2.8 Hz, 1H), 7.15–7.12 (m, 1H), 7.05 (dd, J = 8.3, 0.9 Hz, 1H), 6.92–6.89 (m, 1H), 6.78 (dd, J = 8.7, 5.9 Hz, 1H), 5.02 (dd, J = 4.6, 2.5 Hz, 1H), 4.80 (dd, J = 10.7, 2.5 Hz, 1H), 4.51 (td, J = 8.9, 2.9 Hz, 1H), 4.42 (td, J = 9.4, 6.4 Hz, 1H), 3.60 (ddd, J = 10.7, 4.6, 1.9 Hz, 1H), 3.20 (ddd, J = 13.3, 6.4, 2.9 Hz, 1H), 2.69 (s, 1H), 2.39 (dt, J = 13.3, 8.9 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 188.01, 177.36, 158.45, 156.08, 137.04, 132.69, 131.79, 127.62, 123.32, 123.14, 119.84, 117.95, 111.21, 81.47, 69.11, 65.62, 63.03, 53.26, 30.39, 29.85. HRMS calculated for [C20H17BrNO5+]: 430.0290, found: 430.0283. The er was determined by UPC2 using a chiral Chiralpack IC column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.67 min, τminor = 4.98 min, (70.5:29.5 er).
(1R,3R,3aS,9aS)-1-(5-Chloro-2-hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1w
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as white powder in 63% yield, 2:1 dr. A few drops of DMSO-d6 were added to increase the solubility. 1H NMR (700 MHz, Chloroform-d) δ 10.17 (s, 1H), 7.89 (dd, J = 7.9, 1.8 Hz, 1H), 7.53 (ddd, J = 8.4, 7.2, 1.8 Hz, 1H), 7.14 (dd, J = 8.6, 2.6 Hz, 1H), 7.09 (ddd, J = 7.9, 7.2, 1.0 Hz, 1H), 7.02–6.96 (m, 2H), 6.80 (d, J = 8.6 Hz, 1H), 5.13 (d, J = 8.8 Hz, 1H), 5.10 (d, J = 6.6 Hz, 1H), 4.52–4.49 (m, 1H), 4.47–4.43 (m, 1H), 3.30 (dd, J = 8.8, 6.6 Hz, 1H), 3.28 (s, 1H), 2.60–2.54 (m, 2H). 13C NMR (176 MHz, Chloroform-d) δ 188.13, 174.17, 157.49, 153.34, 134.85, 126.73, 126.69, 126.65, 124.95, 121.84, 120.26, 117.97, 116.38, 116.12, 82.04, 67.92, 63.87, 58.14, 51.43, 33.79. HRMS calculated for [C20H17ClNO5+]: 387.0688, found: 387.0694. The er was determined by UPC2 using a chiral Chiralpack IC column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 4.78 min, τminor = 4.50 min, (99:1 er).
(1R,3S,3aS,9aS)-1-(5-Chloro-2-hydroxyphenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1w′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as white powder in 32% yield, 2:1 dr. A few drops of DMSO-d6 were added to increase the solubility. 1H NMR (700 MHz, Chloroform-d) δ 9.44 (s, 1H), 7.92 (dd, J = 7.8, 1.7 Hz, 1H), 7.58 (ddd, J = 8.7, 7.2, 1.7 Hz, 1H), 7.16–7.13 (m, 2H), 7.05 (dd, J = 8.4, 0.9 Hz, 1H), 6.85 (dd, J = 8.7, 1.9 Hz, 1H), 6.79 (d, J = 2.5 Hz, 1H), 5.04 (d, J = 4.6 Hz, 1H), 4.82 (d, J = 10.7 Hz, 1H), 4.54–4.50 (m, 1H), 4.43 (td, J = 9.4, 6.4 Hz, 1H), 3.63 (dd, J = 10.7, 4.6 Hz, 1H), 3.21 (ddd, J = 13.3, 6.4, 2.9 Hz, 1H), 2.65 (s, 1H), 2.39 (dt, J = 13.3, 8.9 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 188.71, 177.29, 158.59, 154.99, 136.49, 128.77, 128.70, 126.85, 124.70, 123.52, 122.23, 119.43, 118.01, 117.72, 81.98, 68.80, 65.69, 61.67, 53.35, 30.00, 29.46. HRMS calculated for [C20H17ClNO5+]: 387.0688, found: 387.0686. The er was determined by UPC2 using a chiral Chiralpack IC column gradient from 100% CO2 up to 40%; MeCN, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.39 min, τminor = 4.81 min, (75:25 er).
(1R,3R,3aS,9aS)-1-(2-Hydroxy-5-nitrophenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1x
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow oil in 65% yield, 2:1 dr. A few drops of DMSO-d6 were added to increase the solubility. 1H NMR (700 MHz, Chloroform-d) δ 11.30 (s, 1H), 8.13 (dd, J = 9.0, 2.8 Hz, 1H), 8.06 (dd, J = 2.8, 0.7 Hz, 1H), 7.93 (ddd, J = 8.0, 1.8, 0.5 Hz, 1H), 7.58 (ddd, J = 8.4, 7.2, 1.8 Hz, 1H), 7.13 (ddd, J = 8.0, 7.2, 1.0 Hz, 1H), 7.01 (ddd, J = 8.4, 1.0, 0.5 Hz, 1H), 6.96 (d, J = 9.0 Hz, 1H), 5.29 (d, J = 9.2 Hz, 1H), 5.12 (dd, J = 6.8, 0.6 Hz, 1H), 4.58–4.52 (m, 1H), 4.52–4.46 (m, 1H), 3.29 (dd, J = 8.5, 6.8 Hz, 1H), 3.28 (s, 1H), 2.68–2.59 (m, 2H). 13C NMR (176 MHz, Chloroform-d) δ 188.70, 174.77, 161.62, 158.14, 139.50, 135.84, 126.04, 125.79, 124.20, 123.91, 121.24, 118.54, 117.20, 115.81, 82.73, 77.16, 68.63, 64.60, 58.64, 52.30, 34.72. HRMS calculated for [C20H17N2O7+]: 397.0991, found: 397.0994. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.20 min, τminor = 5.38 min, (89:11 er).
(1R,3S,3aS,9aS)-1-(2-Hydroxy-5-nitrophenyl)-3a,4′,5′,9a-tetrahydro-1H,2′H-spiro[chromeno[2,3-c]pyrrole-3,3′-furan]-2′,9(2H)-dione 1x′
Pure product was isolated by flash chromatography on silica gel (CH2Cl2/acetone 100:1) as pale yellow oil in 33% yield, 2:1 dr. 1H NMR (700 MHz, Chloroform-d) δ 10.70 (s, 1H), 8.09 (ddd, J = 9.0, 2.7, 1.7 Hz, 1H), 7.91 (dd, J = 7.8, 1.7 Hz, 1H), 7.69 (dd, J = 2.7, 0.6 Hz, 1H), 7.61 (ddd, J = 8.4, 7.2, 1.7 Hz, 1H), 7.18–7.15 (m, 1H), 7.07 (dd, J = 8.4, 1.0 Hz, 1H), 6.94 (dd, J = 9.0, 1.5 Hz, 1H), 5.02 (d, J = 4.1 Hz, 1H), 4.94 (d, J = 11.1 Hz, 1H), 4.56 (ddd, J = 9.4, 8.5, 2.5 Hz, 1H), 4.46 (td, J = 9.7, 6.3 Hz, 1H), 3.59 (ddd, J = 11.1, 4.1, 0.5 Hz, 1H), 3.31–3.27 (m, 1H), 2.46 (ddd, J = 13.3, 9.7, 8.5 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 187.58, 177.39, 163.16, 158.34, 140.30, 137.29, 127.73, 126.08, 125.80, 123.47, 121.32, 119.79, 118.52, 118.04, 81.11, 69.46, 65.71, 63.20, 53.46, 30.12. HRMS calculated for [C20H17N2O7+]: 397.0991, found: 397.0993. The er was determined by UPC2 using a chiral Chiralpack IB column gradient from 100% CO2 up to 40%; MeOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 5.20 min, τminor = 5.38 min, (74:26 er).
3.2.3. General Procedure for the synthesis of the Tetrahydro-spiro[benzo[e]chromeno[3′,2′:3,4]pyrrolo[1,2-c][1,3]oxazine 9a
An ordinary screw-cap vial was charged with a magnetic stirring bar, the corresponding pyrrolidine 1k (0.1 mmol, 1 equiv), 37% aq solution of formaldehyde (0.1 mmol, 1 equiv), trifluoroacetic acid (0.2 equiv), and CHCl3 (0.5 mL). The resulting mixture was stirred at 60 °C for 3 h. Subsequently, sat. aq NaHCO3 (1 mL) was added, phases were separated, and the aqueous layer was extracted with CH2Cl2 (2 × 2 mL). The combined organic layers were dried (anhyd. MgSO4). After filtration, volatiles were removed under reduced pressure, and the residue was directly subjected to FC on silica gel (eluent: hexane/EtOAc) to afford the desired product 9a.
(8aS,14aS,14bR)-Diethyl 2-(tert-butyl)-14-oxo-8a,14,14a,14b-tetrahydro-benzo[e]chromeno[3′,2′:3,4]pyrrolo[1,2-c][1,3]oxazine-8,8(6H)-dicarboxylate 9a
Pure product was isolated by flash chromatography on silica gel (hexane/ethyl acetate 15:1) as pale yellow oil in 95% yield. 1H NMR (700 MHz, Chloroform-d) δ 7.97 (ddd, J = 7.9, 1.8, 0.5 Hz, 1H), 7.60 (dd, J = 2.4, 0.9 Hz, 1H), 7.50 (ddd, J = 8.2, 7.2, 1.8 Hz, 1H), 7.13 (ddd, J = 8.5, 2.4, 0.7 Hz, 1H), 7.08 (ddd, J = 8.2, 7.2, 1.0 Hz, 1H), 6.91 (ddd, J = 8.5, 1.1, 0.5 Hz, 1H), 6.66 (d, J = 8.5 Hz, 1H), 5.38 (dt, J = 5.1, 0.6 Hz, 1H), 5.31 (dd, J = 11.4, 0.5 Hz, 1H), 5.14 (d, J = 7.4 Hz, 1H), 5.06 (dd, J = 11.4, 0.7 Hz, 1H), 4.49 (dq, J = 10.7, 7.1 Hz, 1H), 4.37 (dq, J = 10.7, 7.1 Hz, 1H), 4.15 (qd, J = 7.1, 2.2 Hz, 2H), 3.75 (dd, J = 7.4, 5.1 Hz, 1H). 13C NMR (176 MHz, Chloroform-d) δ 190.34, 168.58, 167.02, 160.20, 151.77, 144.93, 136.71, 127.41, 124.94, 124.61, 124.39, 122.51, 119.82, 118.37, 116.45, 83.94, 76.47, 62.94, 62.19, 59.95, 55.24, 34.56, 31.67 (3xC), 14.35, 13.59. HRMS calculated for [C28H32NO7+]: 494.2134, found: 494.2136. The er was determined by UPC2 using a chiral Chiralpack IA column gradient from 100% CO2 up to 40%; i-PrOH, 2.5 mL/min; detection wavelength = 245 nm; τmajor = 2.93 min, τminor = 3.30 min, (75:25 er).
4. Conclusions
In conclusion, a new decarboxylative (3+2)-cycloaddition of azomethine ylides 3 or 4 with chromone-3-carboxylic acids 2 was developed. The scope studies confirmed the high efficiency of the transformation with regard to both chromone-3-carboxylic acids and diethyl iminomalonates 3 or iminodihydrofuran-2-one 4, providing access to a wide variety of interesting hybrid molecules bearing two important heterocyclic scaffolds: chromanone–pyrolidine ring systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27206809/s1, Section 1: General methods; Section 2: Crystal and X-ray data; Section 3: NMR data; Section 4: HPLC data.
Author Contributions
Conceptualization, A.A.; methodology, E.K.; formal analysis, E.K. and A.A.; investigation, E.K. and A.A.; writing—original draft preparation, E.K.; writing—review and editing, A.A.; supervision, A.A.; project administration, A.A.; funding acquisition, A.A. and L.S.—X-ray analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Science Centre, Poland within the “Sonata” programme realized in the period 2017–2020, project number: UMO-2016/21/D/ST5/01668. This contribution has been completed while the first author (E.K.) was a Doctoral Candidate in the Interdisciplinary Doctoral School of Lodz University of Technology, Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andersen, Ø.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Saengchantara, S.T.; Wallace, T.W. Chromanols, chromanones, and chromones. Nat. Prod. Rep. 1986, 3, 465–475. [Google Scholar] [CrossRef]
- Masters, K.-S.; Bräse, S. Xanthones from fungi, Lichens, and bacteria: The natural products and their synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef]
- Picker, K.; Ritchie, E.; Taylor, W.C. The chemical constituents of Australian Flindersia species. XXI. An examination of the bark and the leaves of F. laevicarpa. Aust. J. Chem. 1976, 29, 2023–2036. [Google Scholar] [CrossRef]
- Zhao, D.-L.; Shao, C.-L.; Gan, L.-S.; Wang, M.; Wang, C.-Y. Chromone derivatives from a sponge-derived strain of the Fungus Corynespora cassiicola. J. Nat. Prod. 2015, 78, 286–293. [Google Scholar] [CrossRef]
- Nibbs, A.E.; Scheidt, K.A. Asymmetric methods for the synthesis of flavanones, chromanones, and azaflavanones. Eur. J. Org. Chem. 2012, 2012, 449–462. [Google Scholar] [CrossRef]
- McDonald, B.R.; Scheidt, K.A. Pyranone natural products as inspirations for catalytic reaction discovery and development. Acc. Chem. Res. 2015, 48, 1172–1183. [Google Scholar] [CrossRef]
- O’Hagan, D. Pyrrole, pyrrolidine, pyridine, piperidine, azepine and tropane alkaloids. Nat. Prod. Rep. 1997, 14, 637–651. [Google Scholar] [CrossRef]
- O’Hagan, D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat. Prod. Rep. 2000, 17, 435–446. [Google Scholar] [CrossRef]
- Li, X.; Li, J. Recent advances in the development of MMPIs and APNIs based on the pyrrolidine platforms. Mini-Rev. Med. Chem. 2010, 10, 794–805. [Google Scholar] [CrossRef]
- Haider, S.; Saify, Z.S.; Begum, N.; Ashrafl, S.; Zarreen, T.; Saeed, S.M.G. Emerging pharmaceutical applications of piperidine, pyrrolidine and its derivatives. World J. Pharm. Res. 2014, 3, 2277–7105. [Google Scholar]
- Bhakuni, D.S.; Rawat, D.S. Bioactive Marine Natural Products; Springer: New York, NY, USA, 1999. [Google Scholar]
- Pinder, A.R. The Alkaloids; Grundon, M.F., Ed.; Chemical Society: London, UK, 1982; Volume 12. [Google Scholar]
- McGeer, E.G.; Olney, J.W.; McGcer, P.L. Kainic Acid as a Tool in Neurobiology; Raven Press: New York, NY, USA, 1983. [Google Scholar]
- Murukami, S.; Takemoto, T.; Shimizu, Z. Studies on the effective principles of Digenea simplex Aq. I. J. Pharm. Soc. Jpn. 1953, 73, 1026–1028. [Google Scholar] [CrossRef]
- Witherup, K.M.; Ransom, R.W.; Graham, A.C.; Bernard, A.M.; Salvatore, M.J.; Lumma, W.C.; Anderson, P.S.; Pitzenberger, S.M.; Varga, S.L. Martinelline and Martinellic acid, novel G-protein linked receptor antagonists from the tropical plant Martinella iquitosensis (Bignoniaceae). J. Am. Chem. Soc. 1995, 117, 6682–6685. [Google Scholar] [CrossRef]
- Gandhi, P.T.; Narayanappa Athmarama, T.; Arunkumar, G.R. Novel nicotine analogues with potential anti-mycobacterial activity. Bioorg. Med. Chem. 2016, 24, 1637–1647. [Google Scholar] [CrossRef]
- Sweet, J.A.; Cavallari, J.M.; Price, W.A.; Ziller, J.W.; McGrath, D.V. Enantioselective addition of diethylzinc to aldehydes using 2-azanorbornylmethanols and 2-azanorbornylmethanethiol as a catalyst. Tetrahedron Asymmetry 1997, 8, 207. [Google Scholar] [CrossRef]
- Fache, F.; Schulz, E.; Tommasino, M.L.; Lemaire, M. Nitrogen-containing ligands for asymmetric homogeneous and heterogeneous catalysis. Chem. Rev. 2000, 100, 2159–2232. [Google Scholar] [CrossRef]
- Seayad, J.; List, B. Asymmetric organocatalysis. Org. Biomol. Chem. 2005, 3, 719. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef]
- Dalko, P.I.; Moisan, L. Enantioselective organocatalysis. Angew. Chem. Int. Ed. 2001, 40, 3726. [Google Scholar] [CrossRef]
- Carroll, F.I. Epibatidine analogs synthesized for characterization of nicotinic pharmacophores—A review. Heterocycles 2009, 79, 99–120. [Google Scholar] [CrossRef]
- Yu, J.; Shi, F.; Gong, L.-Z. Brønsted-Acid-Catalyzed Asymmetric Multicomponent Reactions for the Facile Synthesis of Highly Enantioenriched Structurally Diverse Nitrogenous Heterocycles. Acc. Chem. Res. 2011, 44, 1156–1171. [Google Scholar] [CrossRef]
- Han, M.-Y.; Jia, J.-Y.; Wang, W. Recent advances in organocatalytic asymmetric synthesis of polysubstituted pyrrolidines. Tetrahedron Lett. 2014, 55, 784–794. [Google Scholar] [CrossRef]
- Li, H.; Zu, L.; Xie, H.; Wang, J.; Wang, W. Highly enantio- and diastereoselective organocatalytic cascade aza-Michael–Michael reactions: A direct method for the synthesis of trisubstituted chiral pyrrolidines. Chem. Commun. 2008, 43, 5636–5638. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Goddertz, D.P.; Beceno, C.; Raabe, G. Asymmetric synthesis of polyfunctionalized pyrrolidines via a thiourea catalyzed domino Mannich/aza-Michael reaction. Adv. Synth. Catal. 2010, 352, 2863–2868. [Google Scholar] [CrossRef]
- Jui, N.T.; Garber, J.A.O.; Finelli, F.G.; MacMillan, D.W.C. Enantioselective organo-SOMO cycloadditions: A catalytic approach to complex pyrrolidines from olefins and aldehydes. J. Am. Chem. Soc. 2012, 134, 11400–11403. [Google Scholar] [CrossRef] [PubMed]
- Dalko, P.I. Formation of 3-, 4- and 5-Membered Cycles by Intermolecular Reactions in Comprehensive Enantioselective Organocatalysis; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gothelf, K.V.; Jørgensen, K.A. Asymmetric 1,3-dipolar cycloaddition reactions. Chem. Rev. 1998, 98, 863–909. [Google Scholar] [CrossRef]
- Moyano, A.; Rios, R. Asymmetric Organocatalytic Cyclization and Cycloaddition Reactions. Chem. Rev. 2011, 111, 4703–4832. [Google Scholar] [CrossRef]
- Przydacz, A.; Bojanowski, J.; Albrecht, A.; Albrecht, Ł. Hydroxyl-group-activated azomethine ylides in organocatalytic H-bond-assisted 1,3-dipolar cycloadditions and beyond. Org. Biomol. Chem. 2021, 19, 3075–3086. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-C.; Liu, J.-Y.; Liu, Z.; Hu, X.-Q.; Xu, P.-F. Quaternary carbon center forming [3+2] cyclization reaction by adjusting the substituents of substrates. J. Org. Chem. 2019, 84, 13871–13880. [Google Scholar] [CrossRef]
- Mantelingu, K.; Lin, Y.; Seidel, D. Intramolecular [3+2]-cycloadditions of azomethine ylides derived from secondary amines via redox-neutral C–H functionalization. Org. Lett. 2014, 16, 5910–5913. [Google Scholar] [CrossRef]
- Dai, Z.; Zhu, J.; Su, W.; Zeng, W.; Liu, Z.; Chen, M.; Zhou, Q. Phosphine-catalyzed stereoselective tandem annulation reaction for the synthesis of chromeno[4,3-b]pyrroles. Org. Lett. 2020, 22, 7008–7012. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y. Phosphine-catalyzed sequential (2+3)/(2+4) annulation of γ-vinyl allenoates: Access to the synthesis of chromeno[4,3-b]pyrroles. Chem. Commun. 2021, 57, 9934–9937. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-K.; Chien, H.-W.; Lin, Y.-J.; Karanam, P.; Chen, Y.-H.; Lin, W. Diversity-oriented synthesis of chromenopyrrolidines from azomethine ylides and 2-hydroxybenzylidene indandiones via base-controlled regiodivergent (3+2) cycloaddition. Chem. Commun. 2018, 54, 9921–9924. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Albrecht, Ł. Organocatalytic doubly annulative approach to 3,4-dihydrocoumarins bearing a fused pyrrolidine scaffold. J. Org. Chem. 2016, 81, 6800–6807. [Google Scholar] [CrossRef]
- Pan, Y.; Tan, C.-H. Catalytic Decarboxylative Reactions: Biomimetic Approaches Inspired by Polyketide Biosynthesis. Synthesis 2011, 13, 2044–2053. [Google Scholar] [CrossRef]
- Wang, Z.-L. Recent advances in catalytic asymmetric decarboxylative addition reactions. Adv. Synth. Catal. 2013, 355, 2745–2755. [Google Scholar] [CrossRef]
- Nakamura, S. Catalytic enantioselective decarboxylative reactions using organocatalysts. Org. Biomol. Chem. 2014, 12, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Bojanowski, J.; Albrecht, A. Carboxylic-acid-activated olefins in decarboxylative reactions. Asian J. Org. Chem. 2019, 8, 746–754. [Google Scholar] [CrossRef]
- Guo, D.-G.; Li, Z.; Han, X.-X.; Zhang, L.; Zhang, M.; Liu, X.-L. Decarboxylative, Diastereoselective and exo-Selective 1,3-Dipolar Cycloaddition for Diversity-Oriented Construction of Structural Spiro[Butyrolactone–Pyrrolidine–Chromanone] Hybrids. Synlett 2021, 32, 1447–1452. [Google Scholar] [CrossRef]
- Liu, X.-W.; Yue, J.; Li, Z.; Wu, D.; Tian, M.-Y.; Wang, Q.-L.; Zhou, Y. DMAP-catalyzed decarboxylative [3þ2] cycloadditions: A strategy for diastereoselective synthesis of trifluoromethylated chromanone-fused pyrrolidinyl spirooxindoles. Tetrahedron 2020, 76, 131678. [Google Scholar] [CrossRef]
- CCDC 2167375 and CCDC 2167382 Contain the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available online: https://www.ccdc.cam.ac.uk/structures (accessed on 11 September 2022).
- Tian, L.; Xu, G.-Q.; Li, Y.-H.; Liang, Y.-M.; Xu, P.-F. An efficient strategy for the synthesis of polysubstituted chromeno[4,3-b]pyrrolidine derivatives. Chem. Commun. 2014, 50, 2428–2430. [Google Scholar] [CrossRef]
- Ishizuka, N.; Matsunori, K.; Sakai, K.; Fujimoto, M.; Mihara, S.; Yamamori, T. Structure-activity relationships of a novel class of Endothelin-A receptor antagonists and discovery of potent and selective receptor antagonist, 2-(Benzo[1,3]dioxol-5-yl)-6-isopropyloxy-4-(4-methoxyphenyl)-2H-chromene-3-carboxylic acid (S-1255). 1. Study on structure-activity relationships and basic structure crucial for ETA antagonism. J. Med. Chem. 2002, 45, 2041–2055. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).