One-Pot Synthesis of N-Rich Porous Carbon for Efficient CO2 Adsorption Performance
Abstract
:1. Introduction
2. Results and Discussion
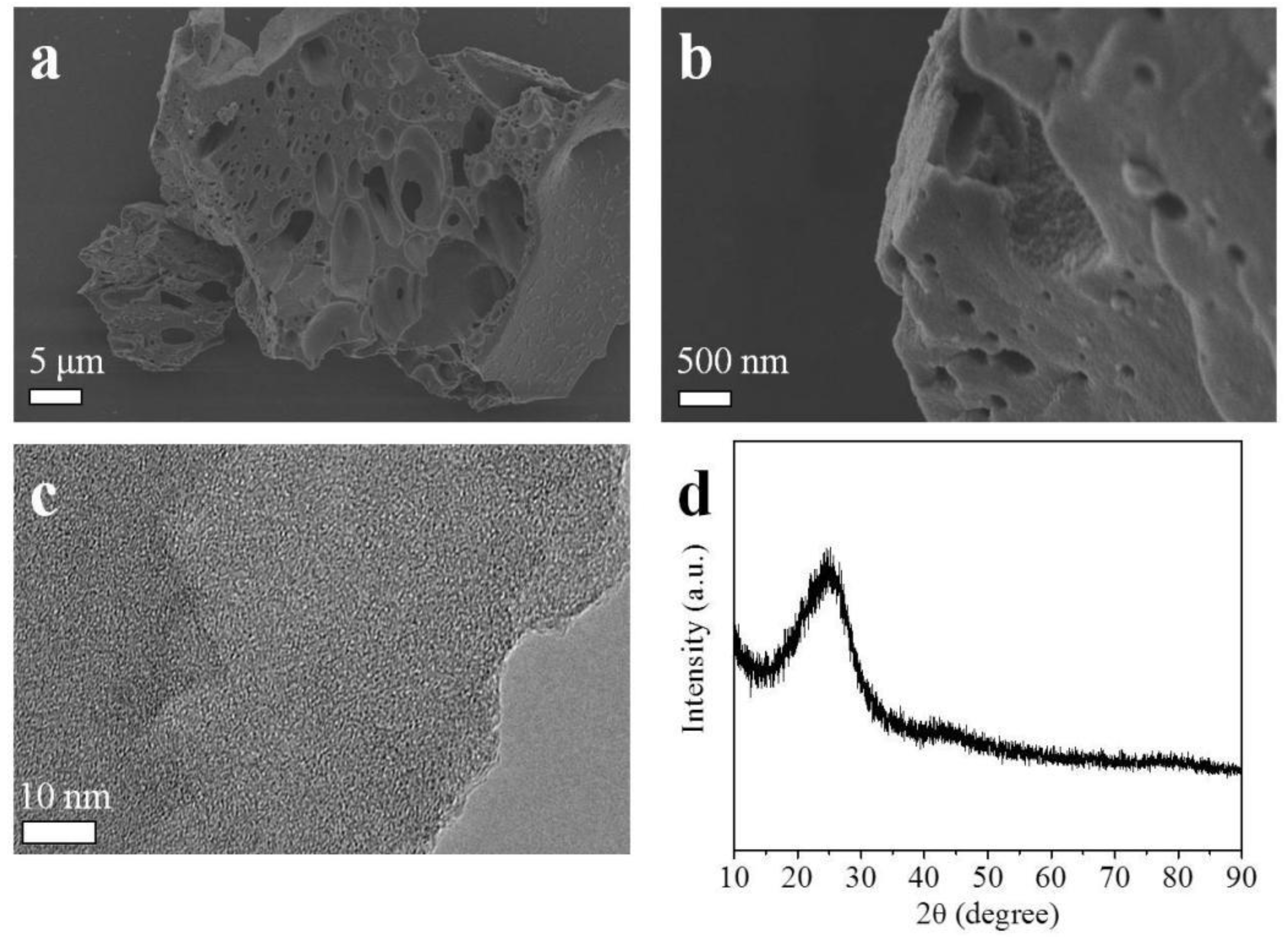
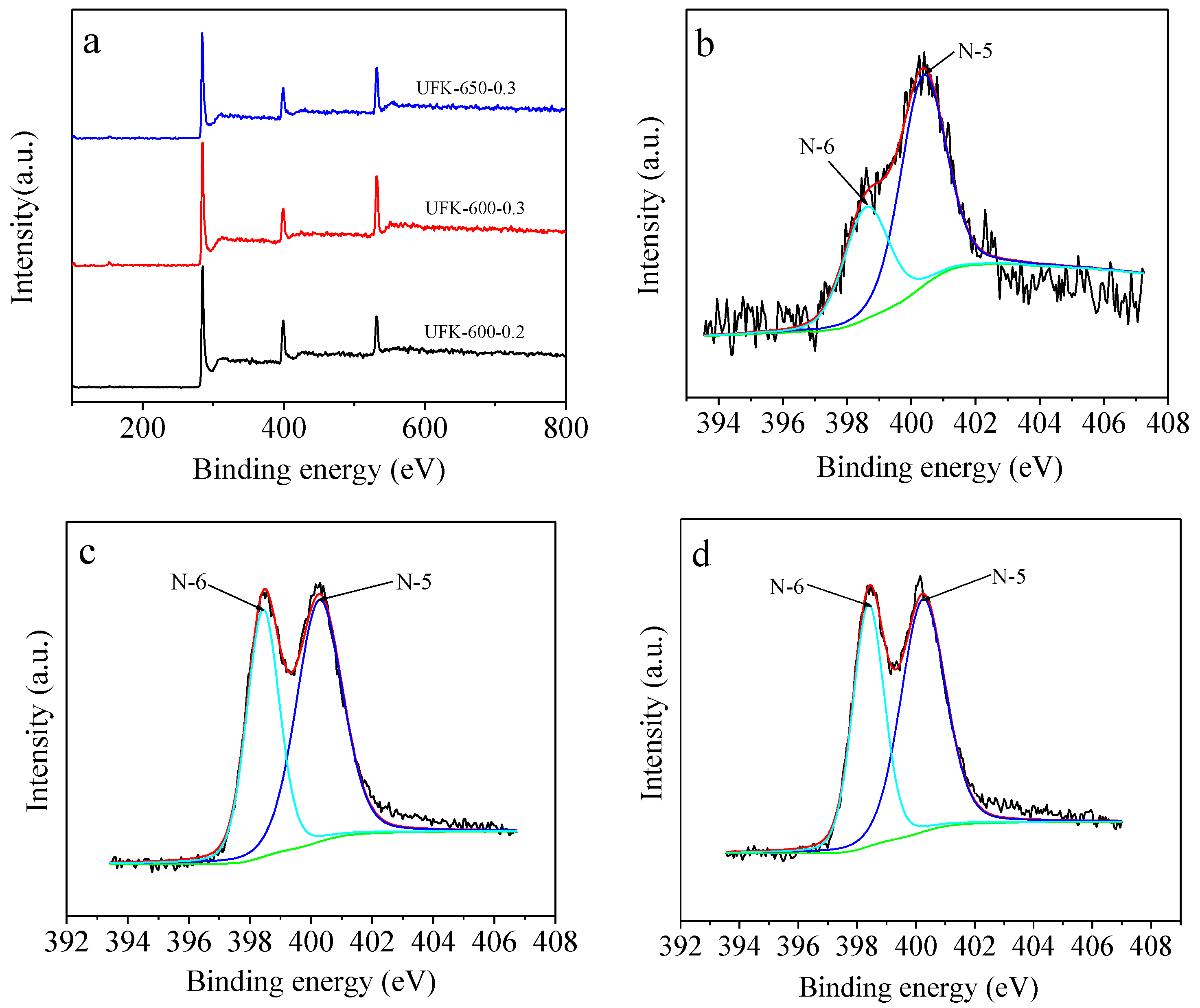
2.1. Morphological, Phase Structural, and Surface Chemical Properties
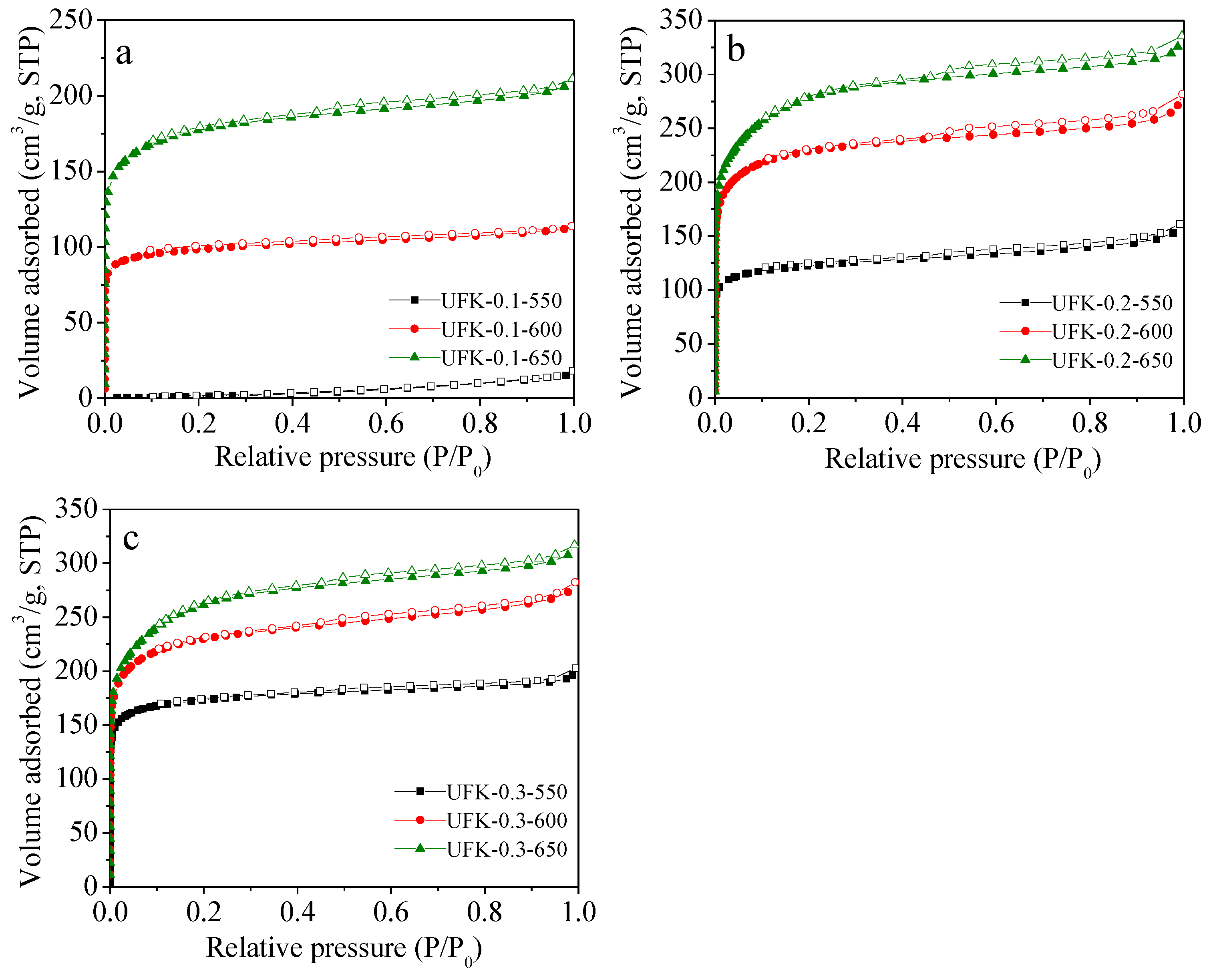
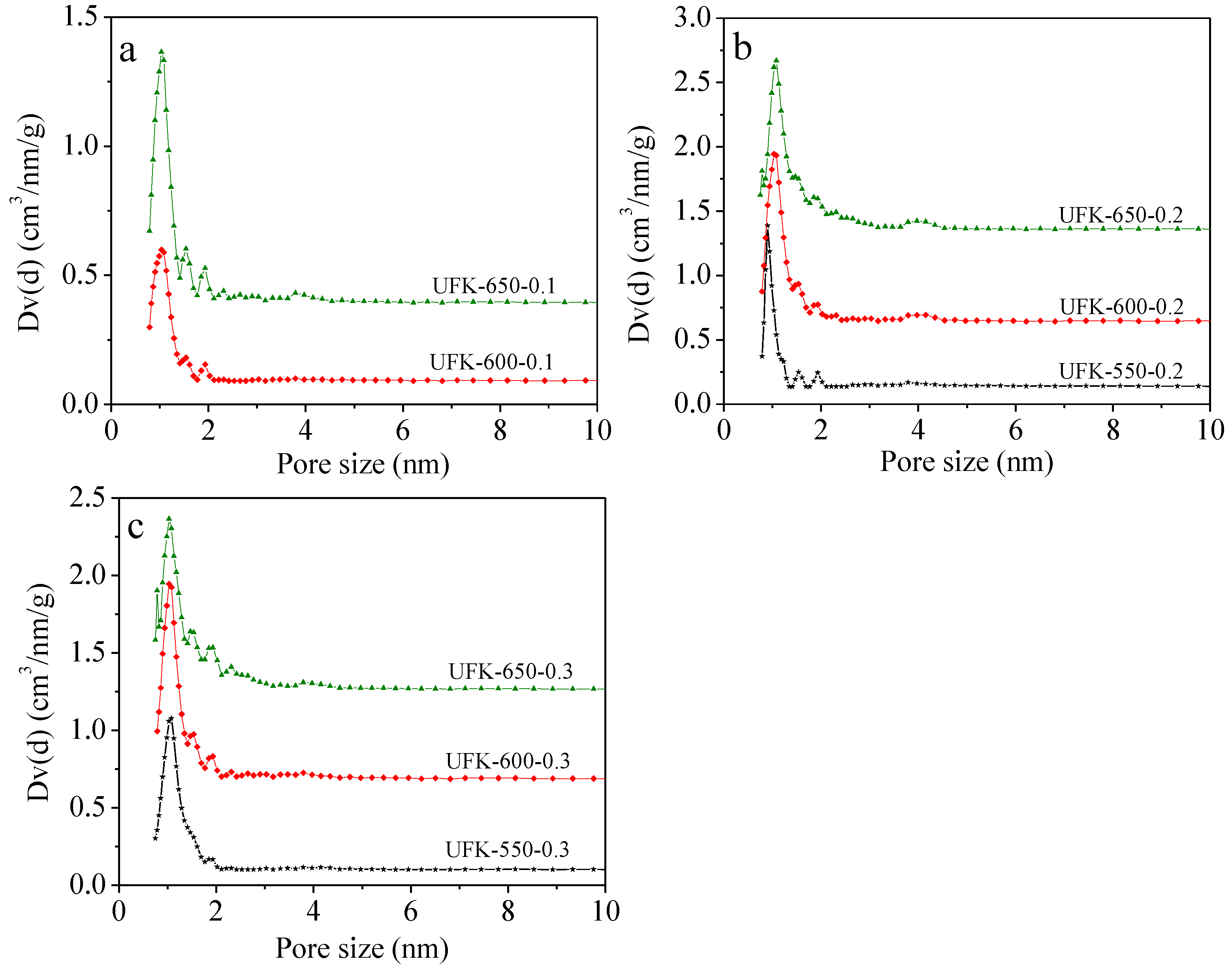
2.2. Porous Textual Properties
2.3. CO2 Adsorption Analysis for the Porous Carbons
3. Synthesis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol. Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef] [Green Version]
- Feron, P.H.; Cousins, A.; Jiang, K.; Zhai, R.; Garcia, M. An update of the benchmark post-combustion CO2-capture technology. Fuel 2020, 273, 117776. [Google Scholar] [CrossRef]
- Yan, H.Y.; Zhang, G.J.; Xu, Y.; Zhang, Q.Q.; Liu, J.; Li, G.Q.; Zhao, Y.Q.; Wang, Y.; Zhang, Y.F. High CO2 adsorption on amine-functionalized improved macro-/mesoporous multimodal pore silica. Fuel 2022, 315, 123195. [Google Scholar] [CrossRef]
- Shen, S.; Shi, X.; Li, C.; Guo, H.; Long, Q.; Wang, S.; Yin, X. Nonaqueous (amine + glycol ether) solvents for energy-efficient CO2 capture: New insights into phase change behaviors and assessment of capture performance. Sep. Purif. Technol. 2022, 300, 121908. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Chen, F.-F.; Huang, K.; Zhou, Y.; Tian, Z.-Q.; Zhu, X.; Tao, D.-J.; Jiang, D.; Dai, S. Multi-Molar Absorption of CO2 by the Activation of Carboxylate Groups in Amino Acid Ionic Liquids. Angew. Chem. 2016, 55, 7166–7170. [Google Scholar] [CrossRef]
- Yuan, X.; Xiao, J.; Yilmaz, M.; Zhang, T.C.; Yuan, S. N, P Co-doped porous biochar derived from cornstalk for high performance CO2 adsorption and electrochemical energy storage. Sep. Purif. Technol. 2022, 299, 121719. [Google Scholar] [CrossRef]
- Xiao, J.; Yuan, X.; Zhang, T.C.; Ouyang, L.; Yuan, S. Nitrogen-doped porous carbon for excellent CO2 capture: A novel method for preparation and performance evaluation. Sep. Purif. Technol. 2022, 298, 121602. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wu, J.J.; Wang, X.; Liu, M.Y.; Liu, Y.M. Synthesis, Characterization and Application of Amine-Functionalized Hierarchically Micro-Mesoporous Silicon Composites for CO2 Capture in Flue Gas. Molecules 2022, 27, 3429. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-L.; Zhang, J.-B.; Zhang, J.-Y.; Zhong, F.-Y.; Wu, P.-K.; Huang, K.; Fan, J.-P.; Liu, F. Chitosan-derived mesoporous carbon with ultrahigh pore volume for amine impregnation and highly efficient CO2 capture. Chem. Eng. J. 2019, 359, 1159–1165. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef]
- Guo, Y.F.; Tan, C.; Sun, J.; Li, W.L.; Zhang, J.B.; Zhao, C.W. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.X.; Liu, L.; Zhang, Y.; Yang, J.F.; Zeng, Z.L.; Deng, S.G. Controllable synthesis of bifunctional porous carbon for efficient gas-mixture separation and high-performance supercapacitor. Chem. Eng. J. 2018, 348, 57–66. [Google Scholar] [CrossRef]
- Ma, C.; Lu, T.; Shao, J.; Huang, J.; Hu, X.; Wang, L. Biomass derived nitrogen and sulfur co-doped porous carbons for efficient CO2 adsorption. Sep. Purif. Technol. 2022, 281, 119899. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Demir, M.; Hu, X.; Liu, S.; Wang, L. Water chestnut shell-derived N/S-doped porous carbons and their applications in CO2 adsorption and supercapacitor. Fuel 2022, 326, 125119. [Google Scholar] [CrossRef]
- Shao, L.S.; Li, Y.; Huang, J.H.; Liu, Y.N. Synthesis of Triazine-Based Porous Organic Polymers Derived N-Enriched Porous Carbons for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 2856–2865. [Google Scholar] [CrossRef]
- Shi, J.; Cui, H.; Xu, J.; Yan, N.; Liu, Y. Design and fabrication of hierarchically porous carbon frameworks with Fe2O3 cubes as hard template for CO2 adsorption. Chem. Eng. J. 2020, 389, 124459. [Google Scholar] [CrossRef]
- Shi, S.; Liu, Y. Nitrogen-doped activated carbons derived from microalgae pyrolysis by-products by microwave/KOH activation for CO2 adsorption. Fuel 2021, 306, 121762. [Google Scholar] [CrossRef]
- Huang, J.; Bai, J.; Demir, M.; Hu, X.; Jiang, Z.; Wang, L. Efficient N-Doped Porous Carbonaceous CO2 Adsorbents Derived from Commercial Urea-Formaldehyde Resin. Energy Fuels 2022, 36, 5825–5832. [Google Scholar] [CrossRef]
- Comroe, M.L.; Kolasinski, K.W.; Saha, D. Direct Ink 3D Printing of Porous Carbon Monoliths for Gas Separations. Molecules 2022, 27, 5653. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, P.; Sun, M.; Liu, W.; Li, D.; Wu, W.; Yan, W.; Shang, J.; Yu, J. Fabricating Mechanically Robust Binder-Free Structured Zeolites by 3D Printing Coupled with Zeolite Soldering: A Superior Configuration for CO2 Capture. Adv. Sci. 2019, 6, 1901317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Policicchio, A.; Conte, G.; Agostino, R.G.; Caputo, P.; Rossi, C.O.; Godbert, N.; Nicotera, I.; Simari, C. Hexagonal Mesoporous Silica for carbon capture: Unrevealing CO2 microscopic dynamics by Nuclear Magnetic Resonance. J. CO2 Util. 2022, 55, 101809. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Yu, J.; Xie, L.-H.; Li, J.-R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 Capture and Separations Using MOFs: Computational and Experimental Studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Liu, P.; Wang, Y.; Yang, J.; Li, J.; Li, L. CO2 Capture from High-Humidity Flue Gas Using a Stable Metal-Organic Framework. Molecules 2022, 27, 5608. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-B.; Kang, Y.-H.; Shi, Y.-Q.; Jiang, Y.; Liu, X.-Q. Highly Selective Capture of the Greenhouse Gas CO2 in Polymers. ACS Sustain. Chem. Eng. 2015, 3, 3077–3085. [Google Scholar] [CrossRef]
- Yuan, R.R.; Sun, H.; He, H.M. Rational Construction of a Responsive Azo-Functionalized Porous Organic Framework for CO2 Sorption. Molecules 2021, 26, 4993. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, Y.; Wang, L.; Yan, W.; Chen, T.; Huang, J.; Liu, Y.-N. N-rich porous organic polymers based on Schiff base reaction for CO2 capture and mercury(II) adsorption. J. Colloid Interface Sci. 2021, 587, 121–130. [Google Scholar] [CrossRef]
- Nobandegani, M.S.; Yu, L.; Hedlund, J. Zeolite membrane process for industrial CO2/CH4 separation. Chem. Eng. J. 2022, 446, 137223. [Google Scholar] [CrossRef]
- Lu, T.; Bai, J.; Demir, M.; Hu, X.; Huang, J.; Wang, L. Synthesis of potassium Bitartrate-derived porous carbon via a facile and Self-Activating strategy for CO2 adsorption application. Sep. Purif. Technol. 2022, 296, 121368. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Hu, X.; Jiang, Z.; Wang, L. Nitrogen-doped porous carbons from polyacrylonitrile fiber as effective CO2 adsorbents. J. Environ. Sci. 2023, 125, 533–543. [Google Scholar] [CrossRef]
- Shao, J.; Ma, C.; Zhao, J.; Wang, L.; Hu, X. Effective nitrogen and sulfur co-doped porous carbonaceous CO2 adsorbents derived from amino acid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127750. [Google Scholar] [CrossRef]
- Aydin, H.; Kurtan, U.; Demir, M.; Karakus, S. Synthesis and Application of a Self-Standing Zirconia-Based Carbon Nanofiber in a Supercapacitor. Energy Fuels 2022, 36, 2212–2219. [Google Scholar] [CrossRef]
- Demir, M.; Tessema, T.-D.; Farghaly, A.A.; Nyankson, E.; Saraswat, S.K.; Aksoy, B.; Islamoglu, T.; Collinson, M.M.; El-Kaderi, H.M.; Gupta, R.B. Lignin-derived heteroatom-doped porous carbons for supercapacitor and CO2 capture applications. Inter. J. Energy Res. 2018, 42, 2686–2700. [Google Scholar] [CrossRef]
- Jia, L.; Yu, Y.; Li, Z.P.; Qin, S.N.; Guo, J.R.; Zhang, Y.Q.; Wang, J.C.; Zhang, J.C.; Fan, B.G.; Jin, Y. Study on the Hg0 removal characteristics and synergistic mechanism of iron-based modified biochar doped with multiple metals. Bioresour. Technol. 2021, 332, 125086. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef]
- Altinci, O.C.; Demir, M. Beyond Conventional Activating Methods, a Green Approach for the Synthesis of Biocarbon and Its Supercapacitor Electrode Performance. Energy Fuels 2020, 34, 7658–7665. [Google Scholar] [CrossRef]
- Altay, B.N.; Aksoy, B.; Banerjee, D.; Maddipatla, D.; Fleming, P.D.; Bolduc, M.; Cloutier, S.G.; Atashbar, M.Z.; Gupta, R.B.; Demir, M. Lignin-derived carbon-coated functional paper for printed electronics. ACS Appl. Electron. Mater. 2021, 3, 3904–3914. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Tuning ratios of KOH and NaOH on acetic acid-mediated chitosan-based porous carbons for improving their textural features and CO2 uptakes. J. CO2 Util. 2020, 40, 101212. [Google Scholar] [CrossRef]
- Rehman, A.; Heo, Y.-J.; Nazir, G.; Park, S.-J. Solvent-free, one-pot synthesis of nitrogen-tailored alkali-activated microporous carbons with an efficient CO2 adsorption. Carbon 2021, 172, 71–82. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S.-J. From chitosan to urea-modified carbons: Tailoring the ultra-microporosity for enhanced CO2 adsorption. Carbon 2020, 159, 625–637. [Google Scholar] [CrossRef]
- Thakkar, H.; Lawson, S.; Rownaghi, A.A.; Rezaei, F. Development of 3D-printed polymer-zeolite composite monoliths for gas separation. Chem. Eng. J. 2018, 348, 109–116. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.-H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef] [Green Version]
- Li, H.M.; Li, J.H.; Thomas, A.; Liao, Y.Z. Ultra-High Surface Area Nitrogen-Doped Carbon Aerogels Derived From a Schiff-Base Porous Organic Polymer Aerogel for CO2 Storage and Supercapacitors. Adv. Funct. Mater. 2019, 29, 1904785. [Google Scholar] [CrossRef]
- Yancy-Caballero, D.; Leperi, K.T.; Bucior, B.J.; Richardson, R.K.; Islamoglu, T.; Farha, O.K.; You, F.; Snurr, R.Q. Process-level modelling and optimization to evaluate metal–organic frameworks for post-combustion capture of CO2. Mol. Syst. Des. Eng. 2020, 5, 1205–1218. [Google Scholar] [CrossRef]
- Liu, S.; Rao, L.; Yang, P.; Wang, X.; Wang, L.; Ma, R.; Yue, L.; Hu, X. Superior CO2 uptake on nitrogen doped carbonaceous adsorbents from commercial phenolic resin. J. Environ. Sci. 2020, 93, 109–116. [Google Scholar] [CrossRef]
- Liu, Z.; Du, Z.; Song, H.; Wang, C.; Subhan, F.; Xing, W.; Yan, Z. The fabrication of porous N-doped carbon from widely available urea formaldehyde resin for carbon dioxide adsorption. J. Colloid Interface Sci. 2014, 416, 124–132. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, H.; Li, C.C.; Wang, S.H.; Li, L.; Song, C.W.; Wang, T.H. Hierarchical flaky porous carbon derived from waste polyimide film for high-performance aqueous supercapacitor electrodes. Int. J. Energy Res. 2021, 46, 370–382. [Google Scholar] [CrossRef]
- Demir, M.; Taymaz, B.H.; Sarıbel, M.; Kamış, H. Photocatalytic Degradation of Organic Dyes with Magnetically Separable PANI/Fe3O4 Composite under Both UV and Visible-light Irradiation. Chemistryselect 2022, 7, e202103787. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, Á.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Influence of Porous Texture and Surface Chemistry on the CO2 Adsorption Capacity of Porous Carbons: Acidic and Basic Site Interactions. ACS Appl. Mater. Interfaces 2014, 6, 21237–21247. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Cao, M.; Hu, C. A novel activating strategy to achieve highly porous carbon monoliths for CO2 capture. J. Mater. Chem. A 2014, 2, 4819–4826. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Xu, J.-Y.; Liu, L.-C.; Zhou, J.-C.; Cai, J.-J. High-surface-area porous carbons produced by the mild KOH activation of a chitosan hydrochar and their CO2 capture. N. Carbon Mater. 2021, 36, 1081–1090. [Google Scholar] [CrossRef]
- Li, J.H.; Shi, C.; Bao, A.; Jia, J.C. Development of Boron-Doped Mesoporous Carbon Materials for Use in CO2 Capture and Electrochemical Generation of H2O2. ACS Omega 2021, 6, 8438–8446. [Google Scholar] [CrossRef]
- Youk, S.; Hofmann, J.P.; Badamdorj, B.; Volkel, A.; Antonietti, M.; Oschatz, M. Controlling pore size and pore functionality in sp(2)-conjugated microporous materials by precursor chemistry and salt templating. J. Mater. Chem. A 2020, 8, 21680–21689. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.X.; Dutta, S.; Ok, Y.S.; Wu, K.C.W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, G.Y.; Gao, M.M.; Huang, L.; Li, L.; Liu, S.W.; Xie, C.X.; Zhang, Y.H.; Yu, S.T. N-doped porous carbon from different nitrogen sources for high-performance supercapacitors and CO2 adsorption. J. Alloys Compd. 2019, 786, 826–838. [Google Scholar] [CrossRef]
- Hu, Y.J.; Tong, X.; Zhuo, H.; Zhong, L.X.; Peng, X.W.; Wang, S.; Sun, R.C. 3D hierarchical porous N-doped carbon aerogel from renewable cellulose: An attractive carbon for high-performance supercapacitor electrodes and CO2 adsorption. RSC Adv. 2016, 6, 15788–15795. [Google Scholar] [CrossRef]
- Balahmar, N.; Mitchell, A.C.; Mokaya, R. Generalized Mechanochemical Synthesis of Biomass-Derived Sustainable Carbons for High Performance CO2 Storage. Adv. Energy Mater. 2015, 5, 1500867. [Google Scholar] [CrossRef]
- Sui, Z.-Y.; Cui, Y.; Zhu, J.-H.; Han, B.-H. Preparation of Three-Dimensional Graphene Oxide–Polyethylenimine Porous Materials as Dye and Gas Adsorbents. ACS Appl. Mater. Interfaces 2013, 5, 9172–9179. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Ben, T.; Li, Y.; Zhu, L.; Zhang, D.; Cao, D.; Xiang, Z.; Yao, X.; Qiu, S. Selective adsorption of carbon dioxide by carbonized porous aromatic framework (PAF). Energy Environ. Sci. 2012, 5, 8370–8376. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Vafaeinia, M.; Khosrowshahi, M.S.; Mashhadimoslem, H.; Emrooz, H.B.M.; Ghaemi, A. Oxygen and nitrogen enriched pectin-derived micro-meso porous carbon for CO2 uptake. RSC Adv. 2021, 12, 546–560. [Google Scholar] [CrossRef]
- Kaye, S.S.; Long, J.R. Hydrogen Storage in the Dehydrated Prussian Blue Analogues M3[Co(CN)6]2 (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 2005, 127, 6506–6507. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Wang, Y.; Wang, B. Transformation of waste cornstalk into versatile porous carbon adsorbent for selective CO2 capture and efficient methanol adsorption. J. Environ. Chem. Eng. 2021, 9, 106149. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Park, S.-J. Effect of heat treatment on CO2 adsorption of KOH-activated graphite nanofibers. J. Colloid Interface Sci. 2010, 352, 498–503. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.-F.; Zhong, J.-J.; Qian, X.; Song, S.-L.; Zhang, Y.-G.; Li, D.-H. Nitrogen-Enriched Porous Polyacrylonitrile-Based Carbon Fibers for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 11608–11616. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Q.; Cheng, Q.; Zhao, Y.; Cui, Y.; Wang, T.; Han, B. Graphene-manganese oxide hybrid porous material and its application in carbon dioxide adsorption. Chin. Sci. Bull. 2012, 57, 3059–3064. [Google Scholar] [CrossRef]
- Zhou, D.; Cheng, Q.Y.; Cui, Y.; Wang, T.; Li, X.; Han, B.H. Graphene-terpyridine complex hybrid porous material for carbon dioxide adsorption. Carbon 2014, 66, 592–598. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, S.; Su, H.; Wang, M.; Qiao, W.; Ling, L.; Long, D. Facile preparation and ultra-microporous structure of melamine-resorcinol-formaldehyde polymeric microspheres. Chem. Commun. 2013, 49, 3763–3765. [Google Scholar] [CrossRef]
- Zheng, L.; Li, W.B.; Chen, J.L. Nitrogen doped hierarchical activated carbons derived from polyacrylonitrile fibers for CO2 adsorption and supercapacitor electrodes. RSC Adv. 2018, 8, 29767–29774. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Huang, J.; Zhang, X.; Shao, G.; He, Q.; Cao, S.; Yuan, S. Ultra-microporous N-doped carbon from polycondensed framework precursor for CO2 adsorption. Microporous Mesoporous Mater. 2018, 257, 19–26. [Google Scholar] [CrossRef]
- Madhu, J.; Madurai Ramakrishnan, V.; Santhanam, A.; Natarajan, M.; Palanisamy, B.; Velauthapillai, D.; Lan Chi, N.T.; Pugazhendhi, A. Comparison of three different structures of zeolites prepared by template-free hydrothermal method and its CO2 adsorption properties. Environ. Res. 2022, 214, 113949. [Google Scholar] [CrossRef]
- Hsieh, S.-L.; Li, F.-Y.; Lin, P.-Y.; Beck, D.E.; Kirankumar, R.; Wang, G.-J.; Hsieh, S. CaO recovered from eggshell waste as a potential adsorbent for greenhouse gas CO2. J. Environ. Manage. 2021, 297, 113430. [Google Scholar] [CrossRef]
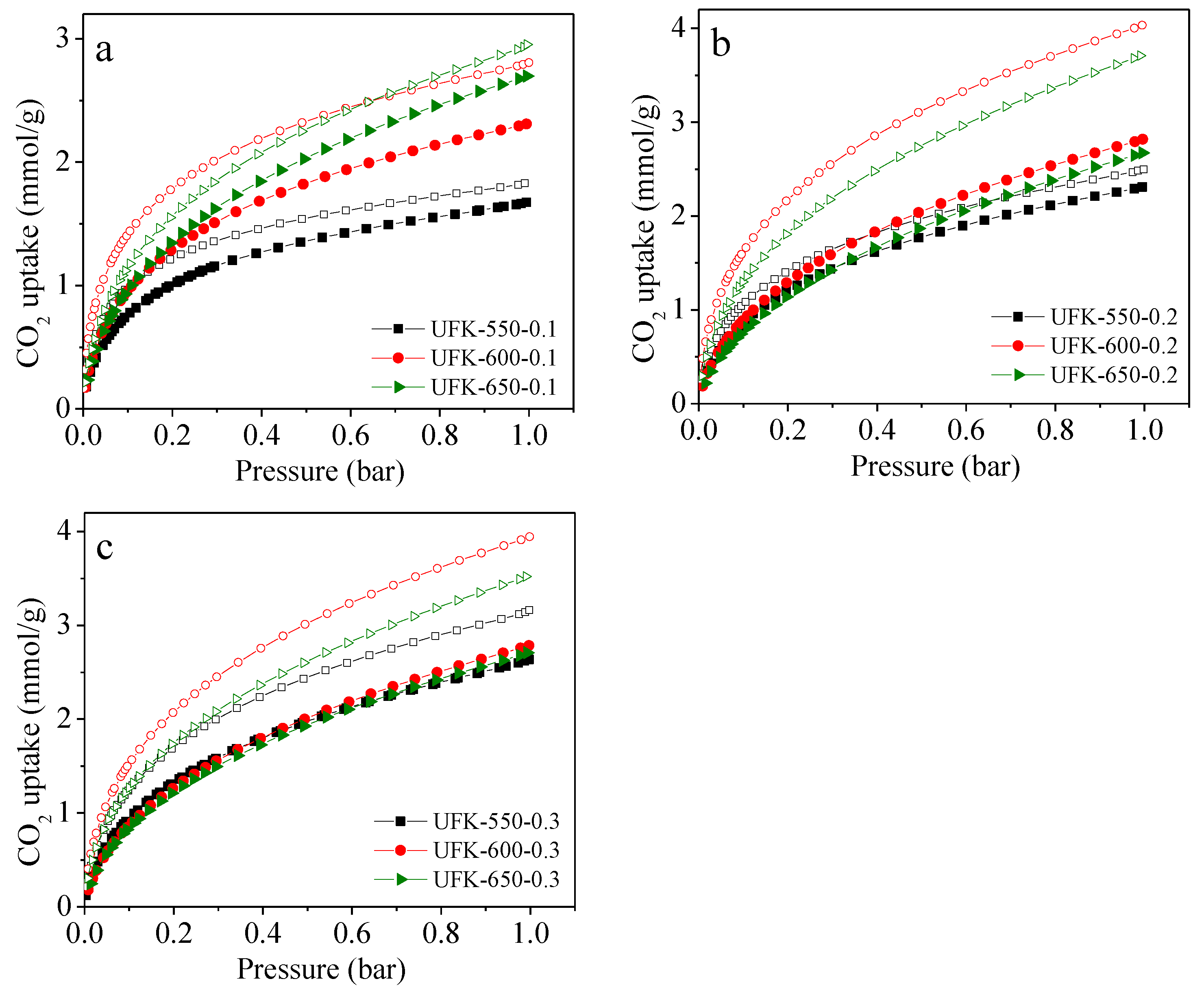

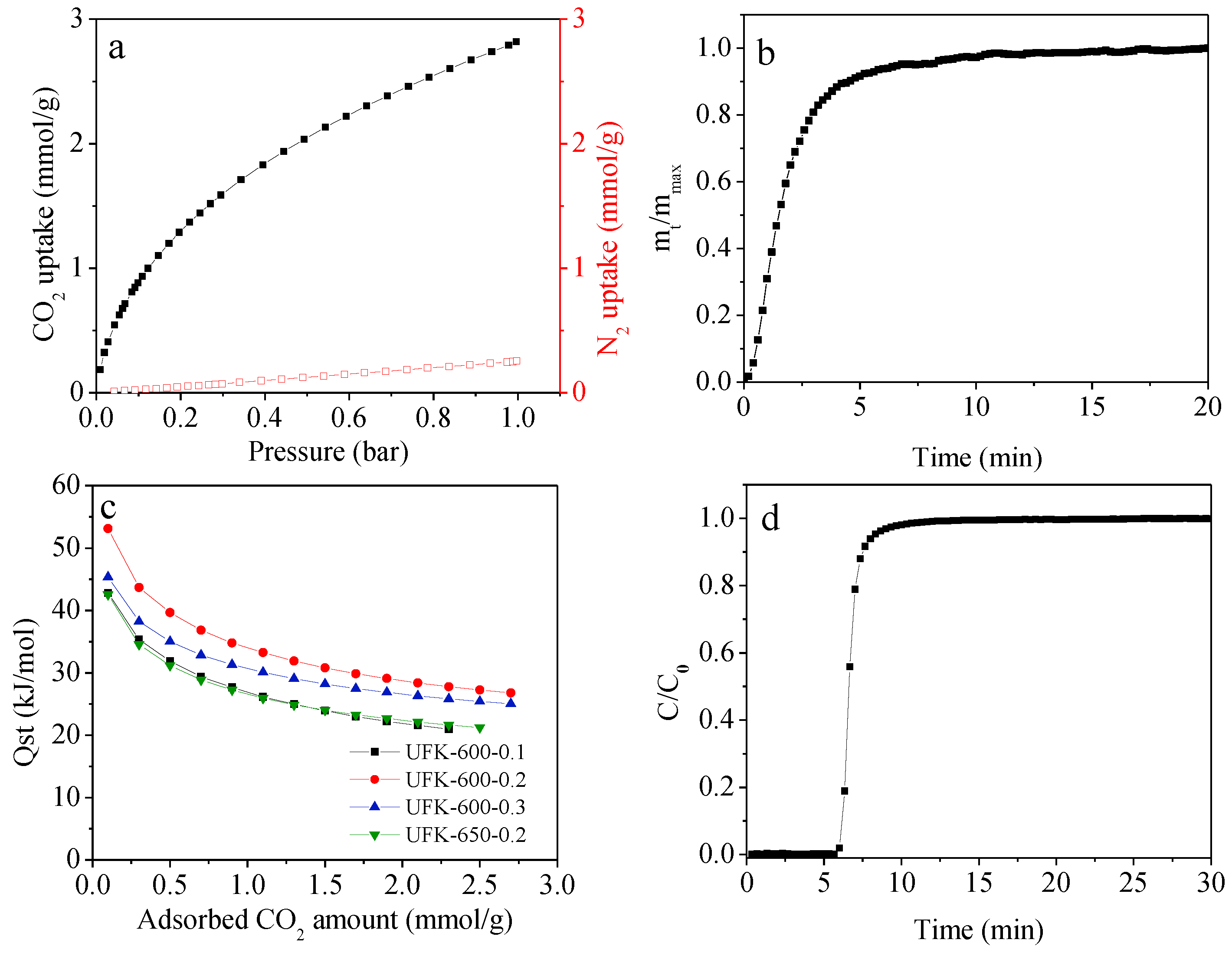
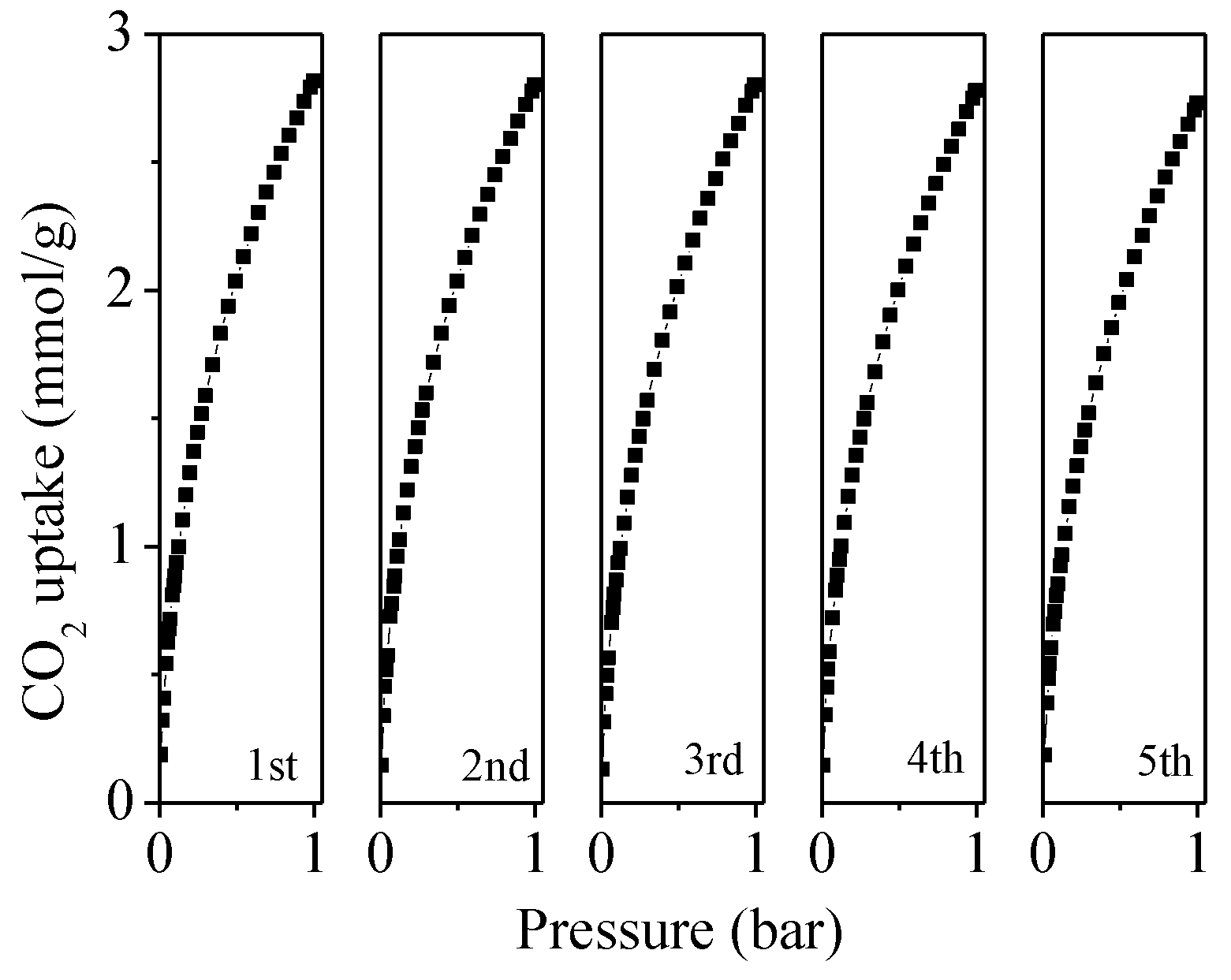
| Sample | SBET a (m2/g) | V0 b (cm3/g) | Vt c (cm3/g) | N (wt%) | C (wt%) | H (wt%) | CO2 Uptake (mmol/g) | |
|---|---|---|---|---|---|---|---|---|
| 25 °C | 0 °C | |||||||
| UFK-550-0.1 | 8 | 0.03 | - | 23.51 | 57.43 | 2.72 | 1.67 | 1.83 |
| UFK-550-0.2 | 474 | 0.25 | 0.17 | 22.42 | 58.09 | 3.41 | 2.31 | 2.50 |
| UFK-550-0.3 | 688 | 0.31 | 0.26 | 20.34 | 56.03 | 3.25 | 2.64 | 3.16 |
| UFK-600-0.1 | 388 | 0.18 | 0.14 | 21.32 | 58.23 | 3.01 | 2.31 | 2.81 |
| UFK-600-0.2 | 865 | 0.44 | 0.33 | 19.69 | 59.62 | 3.90 | 2.82 | 4.03 |
| UFK-600-0.3 | 895 | 0.44 | 0.32 | 18.32 | 55.13 | 3.56 | 2.78 | 3.95 |
| UFK-650-0.1 | 662 | 0.33 | 0.25 | 19.34 | 57.82 | 3.29 | 2.70 | 2.95 |
| UFK-650-0.2 | 980 | 0.52 | 0.41 | 18.20 | 59.63 | 3.71 | 2.67 | 3.71 |
| UFK-650-0.3 | 950 | 0.49 | 0.37 | 16.85 | 58.67 | 3.52 | 2.71 | 3.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Bai, J.; Huang, J.; Demir, M.; Altay, B.N.; Hu, X.; Wang, L. One-Pot Synthesis of N-Rich Porous Carbon for Efficient CO2 Adsorption Performance. Molecules 2022, 27, 6816. https://doi.org/10.3390/molecules27206816
Yu Q, Bai J, Huang J, Demir M, Altay BN, Hu X, Wang L. One-Pot Synthesis of N-Rich Porous Carbon for Efficient CO2 Adsorption Performance. Molecules. 2022; 27(20):6816. https://doi.org/10.3390/molecules27206816
Chicago/Turabian StyleYu, Qiyun, Jiali Bai, Jiamei Huang, Muslum Demir, Bilge Nazli Altay, Xin Hu, and Linlin Wang. 2022. "One-Pot Synthesis of N-Rich Porous Carbon for Efficient CO2 Adsorption Performance" Molecules 27, no. 20: 6816. https://doi.org/10.3390/molecules27206816





