Design, Synthesis, and Investigation of Novel Nitric Oxide (NO)-Releasing Aromatic Aldehydes as Drug Candidates for the Treatment of Sickle Cell Disease
Abstract
1. Introduction
2. Results
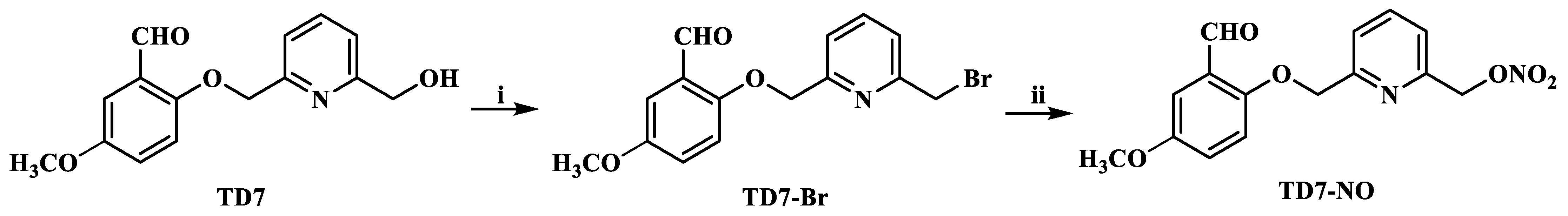
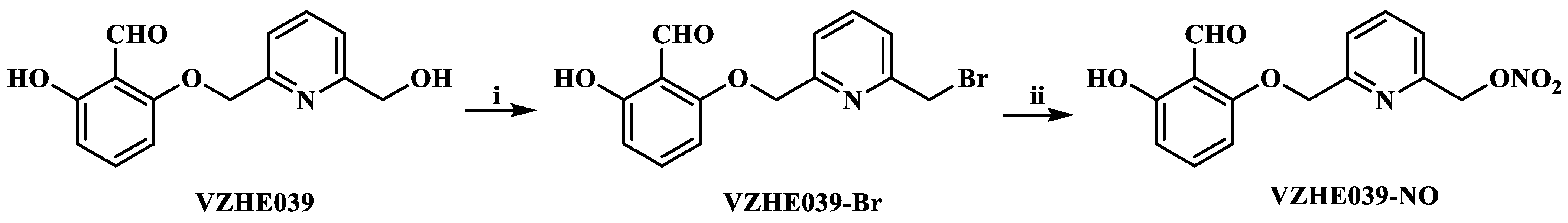
2.1. Chemistry
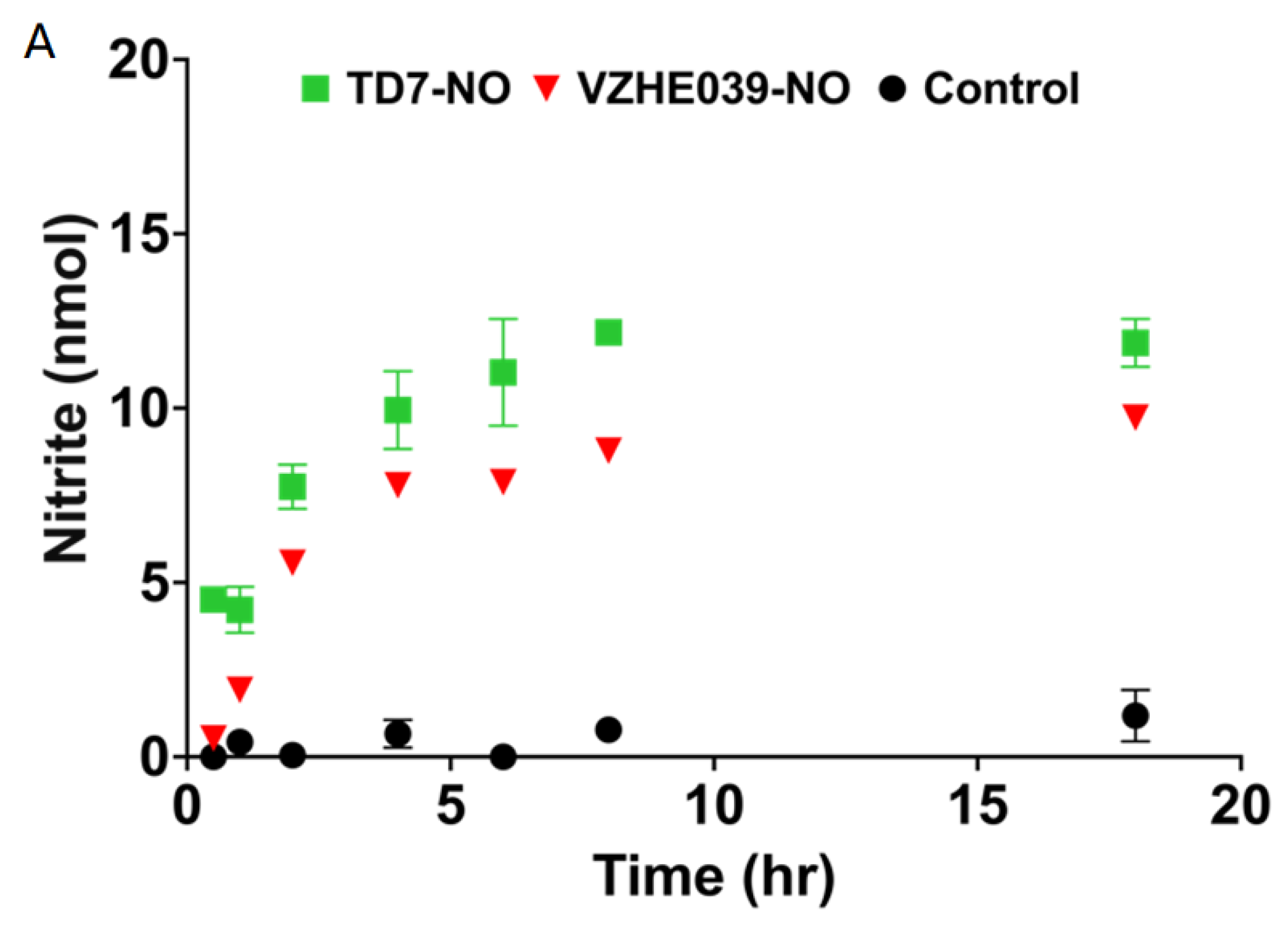
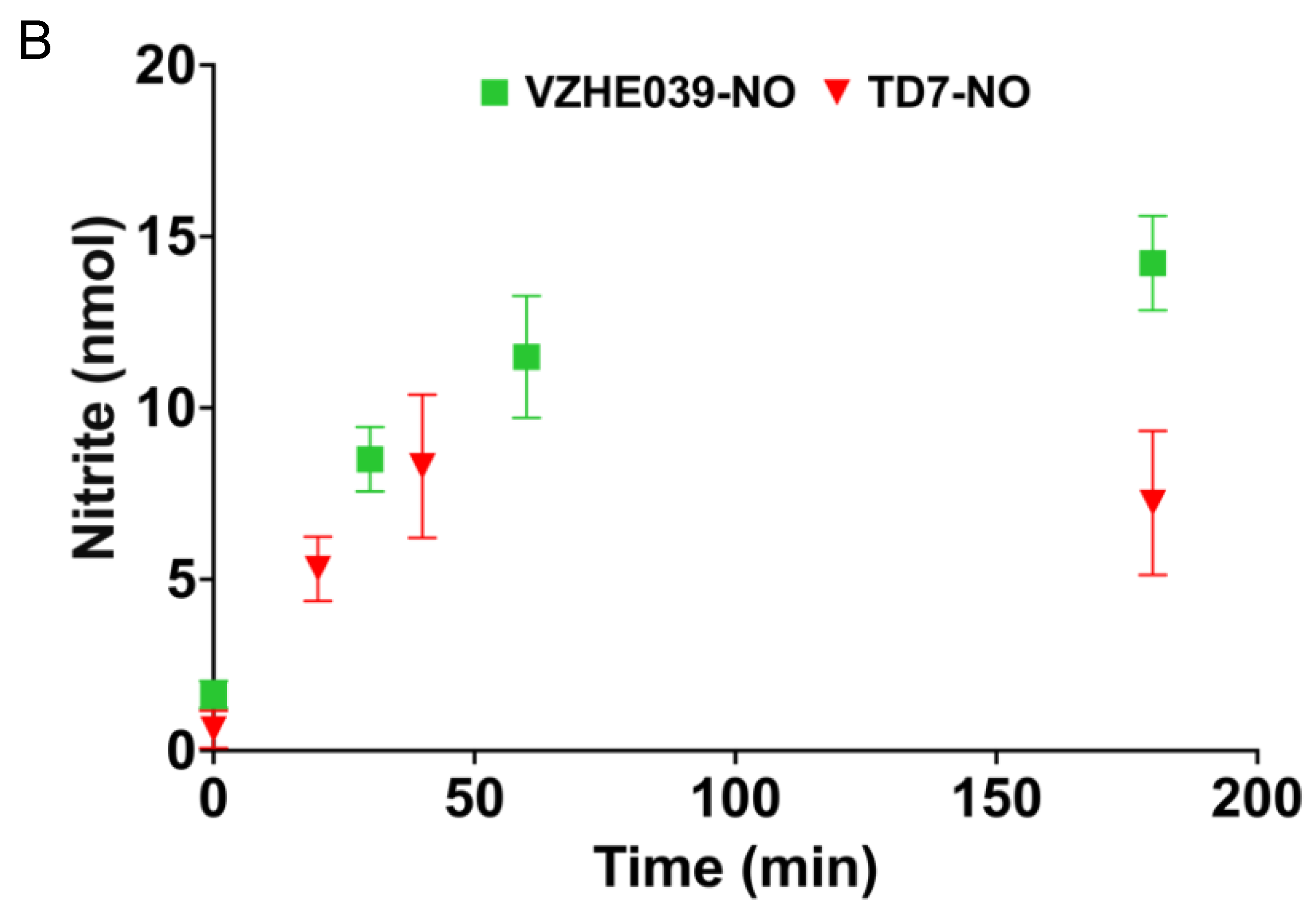
2.2. Nitrate Ester %Derivatives of TD7 (TD7-NO) and VZHE039 (VZHE039-NO) Are Capable of Releasing NO
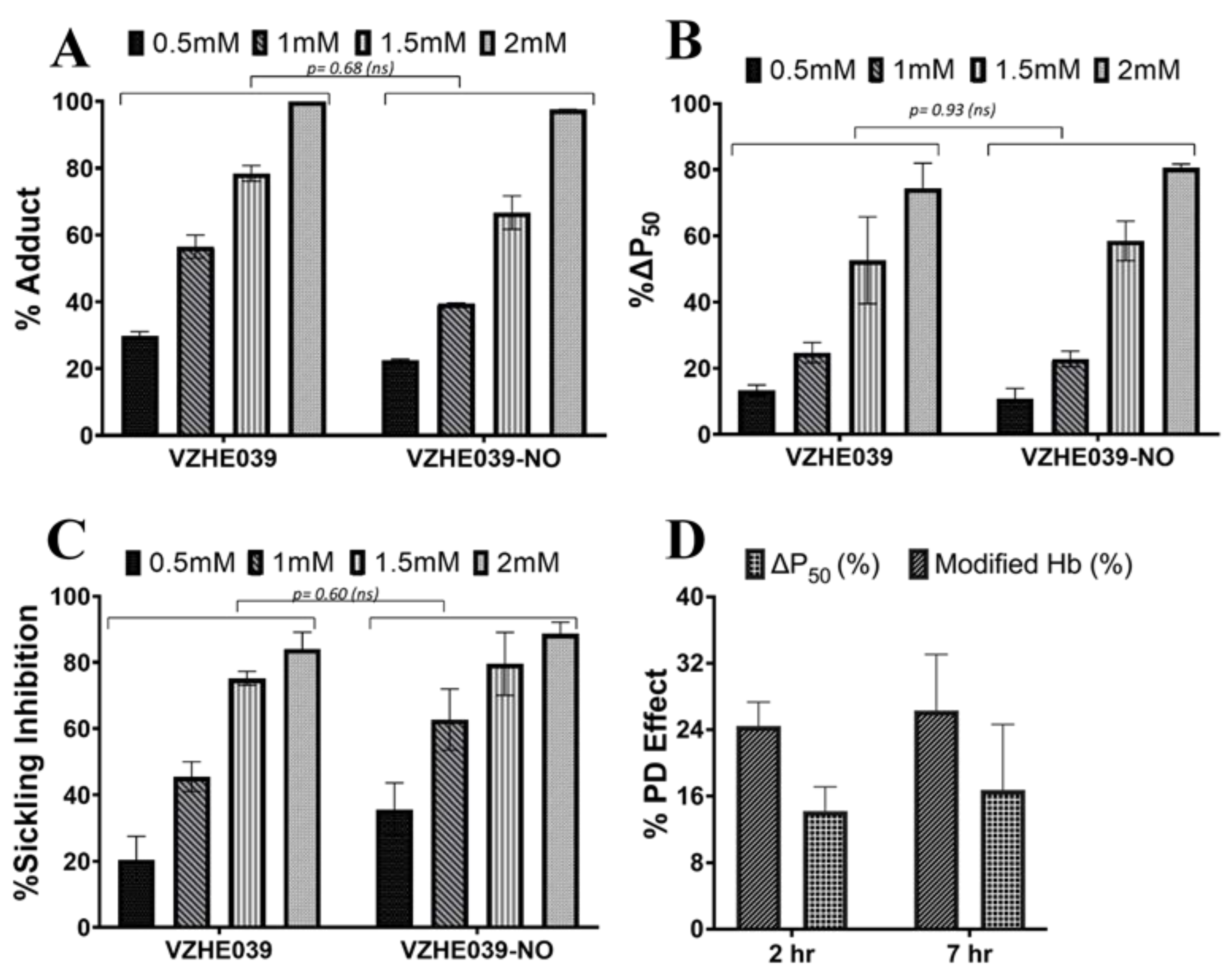
2.3. VZHE039-NO Exhibits Favorable Pharmacologic Effect In Vitro and In Vivo
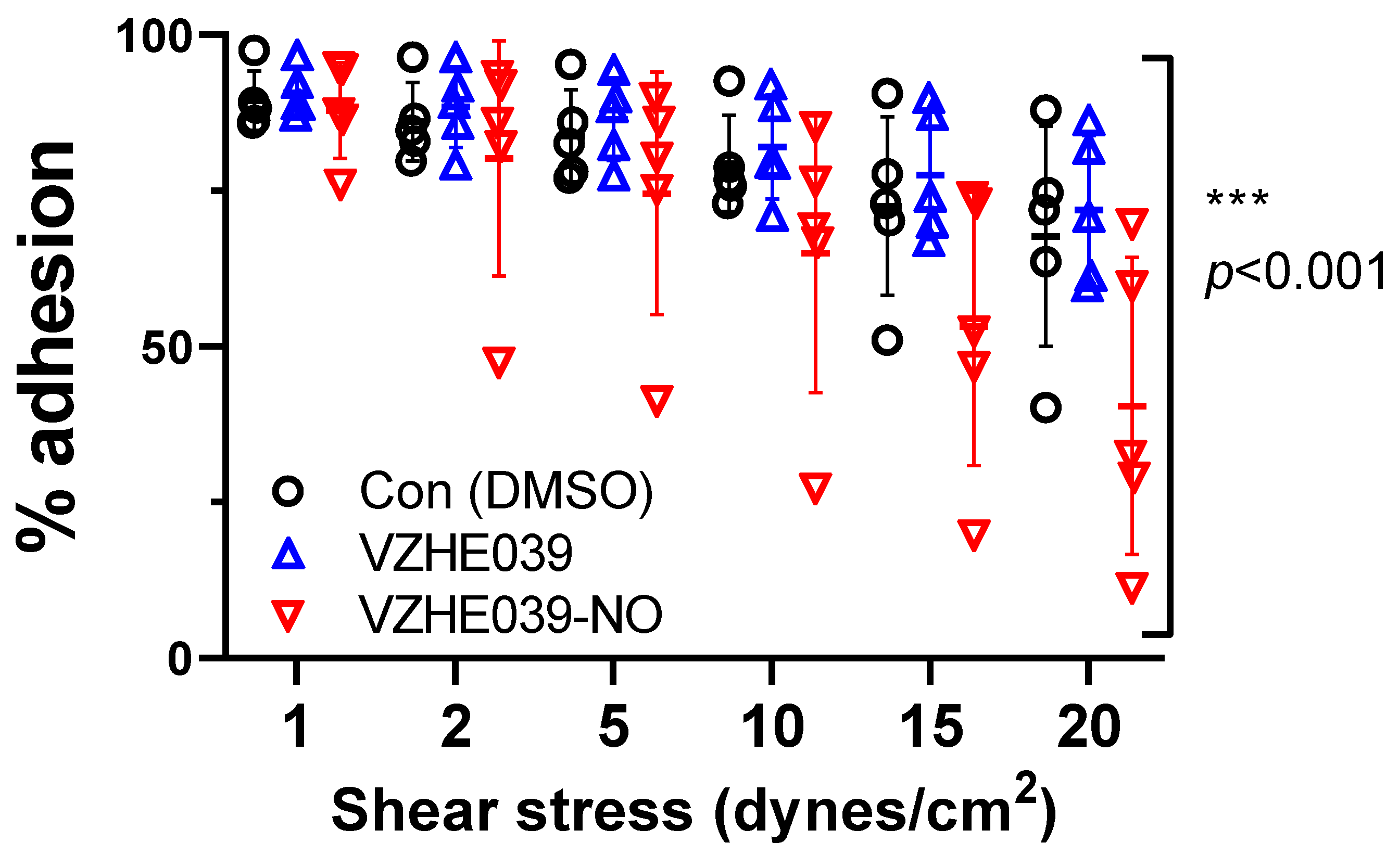
2.4. VZHE039-NO Showed Antiadhesive Effect
2.5. VZHE039-NO Binds Hb Similarly as VZHE039 to Stabilize Liganded Hb
3. Discussion
4. Experimental Section
4.1. Study Approvals
4.2. Chemistry
4.2.1. Materials and General Procedures
4.2.2. Synthesis of (6-((2-Formyl-4-methoxyphenoxy)methyl)pyridin-2-yl)methyl Nitrate (TD7-NO)
4.2.3. Synthesis of (6-((2-formyl-3-hydroxyphenoxy)methyl)pyridin-2-yl)methyl Nitrate (VZHE039-NO)
4.3. Study of NO Release from the Nitrate Esters in Whole Blood In Vitro
4.4. Study of NO Release from the Nitrate Esters in Whole Blood from Wild-Type Mice Dosed with the Compounds
4.5. In Vitro Hb Modification, Oxygen Equilibrium and Antisickling Studies Using Human Homozygous Sickle Cell (SS) Blood
4.6. In Vivo Pharmacologic Study of VZHE039-NO in Wild-Type Mice
4.7. Study of VZHE039 and VZHE039-NO on Adhesivity of SS RBCs
4.8. Crystallographic Study of Hb in Complex with VZHE039-NO
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Safo, M.K.; Kato, G.J. Therapeutic Strategies to Alter the Oxygen Affinity of Sickle Hemoglobin. Hematol. Oncol. Clin. N. Am. 2014, 28, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Safo, M.K.; Aljahdali, A.; Burnett, J.; Abraham, D.J.; Abdulmalik, O. Therapeutic Strategies for the Treatment of Sickle Cell Disease. In Burger’s Medicinal Chemistry and Drug Discovery; Wiley: Hoboken, NJ, USA, 2021; pp. 1–31. ISBN 978-0-470-27815-4. [Google Scholar]
- Pagare, P.P.; Rastegar, A.; Abdulmalik, O.; Omar, A.M.; Zhang, Y.; Fleischman, A.; Safo, M.K. Modulating Hemoglobin Allostery for Treatment of Sickle Cell Disease: Current Progress and Intellectual Property. Expert Opin. Ther. Pat. 2022, 32, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Eaton, W.A.; Bunn, H.F. Treating Sickle Cell Disease by Targeting HbS Polymerization. Blood 2017, 129, 2719–2726. [Google Scholar] [CrossRef]
- Ghatge, M.S.; Ahmed, M.H.; Omar, A.S.M.; Pagare, P.P.; Rosef, S.; Kellogg, G.E.; Abdulmalik, O.; Safo, M.K. Crystal Structure of Carbonmonoxy Sickle Hemoglobin in R-State Conformation. J. Struct. Biol. 2016, 194, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, F.; Corvo, I.; Bergalli, L.; Ilarraz, A.; Cabrera, M.; Gil, J.; Susuki, B.M.; Caffrey, C.R.; Timson, D.J.; Robert, X.; et al. Novel and Selective Inactivators of Triosephosphate Isomerase with Anti-Trematode Activity. Sci. Rep. 2020, 10, 2587. [Google Scholar] [CrossRef]
- Cretegny, I.; Edelstein, S.J. Double Strand Packing in Hemoglobin S Fibers. J. Mol. Biol. 1993, 230, 733–738. [Google Scholar] [CrossRef]
- Eaton, W.A.; Hofrichter, J. Sickle Cell Hemoglobin Polymerization. Adv. Protein Chem. 1990, 40, 63–279. [Google Scholar]
- Harrington, D.J.; Adachi, K.; Royer, W.E. The High Resolution Crystal Structure of Deoxyhemoglobin S. J. Mol. Biol. 1997, 272, 398–407. [Google Scholar] [CrossRef]
- Ferrone, F.A. Polymerization and Sickle Cell Disease: A Molecular View. Microcirculation 2004, 11, 115–128. [Google Scholar] [CrossRef]
- Rhoda, M.D.; Martin, J.; Blouquit, Y.; Garel, M.C.; Edelstein, S.J.; Rosa, J. Sickle Cell Hemoglobin Fiber Formation Strongly Inhibited by the Stanleyville II Mutation (Alpha 78 Asn Leads to Lys). Biochem. Biophys. Res. Commun. 1983, 111, 8–13. [Google Scholar] [CrossRef]
- Burchall, G.; Maxwell, E. Haemoglobin Stanleyville II Modifies Sickle Disease Phenotype. Pathology 2010, 42, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Benesch, R.E.; Kwong, S.; Edalji, R.; Benesch, R. Alpha Chain Mutations with Opposite Effects on the Gelation of Hemoglobin S. J. Biol. Chem. 1979, 254, 8169–8172. [Google Scholar] [CrossRef]
- Aliyu, Z.Y.; Gordeuk, V.; Sachdev, V.; Babadoko, A.; Mamman, A.I.; Akpanpe, P.; Attah, E.; Suleiman, Y.; Aliyu, N.; Yusuf, J.; et al. Prevalence and Risk Factors for Pulmonary Artery Systolic Hypertension among Sickle Cell Disease Patients in Nigeria. Am. J. Hematol. 2008, 83, 485–490. [Google Scholar] [CrossRef]
- Akinsheye, I.; Klings, E.S. Sickle Cell Anemia and Vascular Dysfunction: The Nitric Oxide Connection. J. Cell. Physiol. 2010, 224, 620–625. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L. Pathophisiology of Sickle Cell Disease and New Drugs for the Treatment. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009024. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.D.; Bryant, C.J.; Nguyen, J.; Bowlin, P.R.; Kielbik, M.C.; Bischof, J.C.; Hebbel, R.P.; Vercellotti, G.M. Transgenic Sickle Mice Have Vascular Inflammation. Blood 2003, 101, 3953–3959. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef]
- Cieri-Hutcherson, N.E.; Hutcherson, T.C.; Conway-Habes, E.E.; Burns, B.N.; White, N.A. Systematic Review of L-Glutamine for Prevention of Vaso-Occlusive Pain Crisis in Patients with Sickle Cell Disease. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 1095–1104. [Google Scholar] [CrossRef]
- Kaufman, M.B. Pharmaceutical Approval Update. Pharm. Ther. 2017, 42, 620–621. [Google Scholar]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; Gordeuk, V.R.; et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, B.; Chuang, C.; Dufu, K.; Patel, M.P.; Silva-Garcia, A.; Johnson, C.; Lu, Q.; Partridge, J.R.; Patskovska, L.; Patskovsky, Y.; et al. Discovery of GBT440, an Orally Bioavailable R-State Stabilizer of Sickle Cell Hemoglobin. ACS Med. Chem. Lett. 2017, 8, 321–326. [Google Scholar] [CrossRef]
- Oksenberg, D.; Dufu, K.; Patel, M.P.; Chuang, C.; Li, Z.; Xu, Q.; Silva-Garcia, A.; Zhou, C.; Hutchaleelaha, A.; Patskovska, L.; et al. GBT440 Increases Haemoglobin Oxygen Affinity, Reduces Sickling and Prolongs RBC Half-Life in a Murine Model of Sickle Cell Disease. Br. J. Haematol. 2016, 175, 141–153. [Google Scholar] [CrossRef]
- Vichinsky, E.; Hoppe, C.C.; Ataga, K.I.; Ware, R.E.; Nduba, V.; El-Beshlawy, A.; Hassab, H.; Achebe, M.M.; Alkindi, S.; Brown, R.C.; et al. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N. Engl. J. Med. 2019, 381, 509–519. [Google Scholar] [CrossRef]
- Abraham, D.J.; Mehanna, A.S.; Wireko, F.C.; Whitney, J.; Thomas, R.P.; Orringer, E.P. Vanillin, a Potential Agent for the Treatment of Sickle Cell Anemia. Blood 1991, 77, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Safo, M.K.; Abdulmalik, O.; Danso-Danquah, R.; Burnett, J.C.; Nokuri, S.; Joshi, G.S.; Musayev, F.N.; Asakura, T.; Abraham, D.J. Structural Basis for the Potent Antisickling Effect of a Novel Class of Five-Membered Heterocyclic Aldehydic Compounds. J. Med. Chem. 2004, 47, 4665–4676. [Google Scholar] [CrossRef]
- Abdulmalik, O.; Safo, M.K.; Chen, Q.; Yang, J.; Brugnara, C.; Ohene-Frempong, K.; Abraham, D.J.; Asakura, T. 5-Hydroxymethyl-2-Furfural Modifies Intracellular Sickle Haemoglobin and Inhibits Sickling of Red Blood Cells. Br. J. Haematol. 2005, 128, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Safo, M.K.; Bruno, S. Allosteric Effectors of Hemoglobin: Past, Present and Future. In Chemistry and Biochemistry of Oxygen Therapeutics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 285–300. ISBN 978-1-119-97542-7. [Google Scholar]
- Dufu, K.; Yalcin, O.; Ao-Ieong, E.S.Y.; Hutchaleelala, A.; Xu, Q.; Li, Z.; Vlahakis, N.; Oksenberg, D.; Lehrer-Graiwer, J.; Cabrales, P. GBT1118, a Potent Allosteric Modifier of Hemoglobin O2 Affinity, Increases Tolerance to Severe Hypoxia in Mice. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H381–H391. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, T.M.; Pagare, P.P.; Ghatge, M.S.; Chen, Q.; Musayev, F.N.; Venitz, J.; Zhang, Y.; Abdulmalik, O.; Safo, M.K. Rational Modification of Vanillin Derivatives to Stereospecifically Destabilize Sickle Hemoglobin Polymer Formation. Acta Crystallogr. D Struct. Biol. 2018, 74, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Pagare, P.P.; Ghatge, M.S.; Musayev, F.N.; Deshpande, T.M.; Chen, Q.; Braxton, C.; Kim, S.; Venitz, J.; Zhang, Y.; Abdulmalik, O.; et al. Rational Design of Pyridyl Derivatives of Vanillin for the Treatment of Sickle Cell Disease. Bioorg. Med. Chem. 2018, 26, 2530–2538. [Google Scholar] [CrossRef]
- Abdulmalik, O.; Pagare, P.P.; Huang, B.; Xu, G.G.; Ghatge, M.S.; Xu, X.; Chen, Q.; Anabaraonye, N.; Musayev, F.N.; Omar, A.M.; et al. VZHE-039, a Novel Antisickling Agent That Prevents Erythrocyte Sickling under Both Hypoxic and Anoxic Conditions. Sci. Rep. 2020, 10, 20277. [Google Scholar] [CrossRef]
- Pagare, P.P.; Ghatge, M.S.; Chen, Q.; Musayev, F.N.; Venitz, J.; Abdulmalik, O.; Zhang, Y.; Safo, M.K. Exploration of Structure-Activity Relationship of Aromatic Aldehydes Bearing Pyridinylmethoxy-Methyl Esters as Novel Antisickling Agents. J. Med. Chem. 2020, 63, 14724–14739. [Google Scholar] [CrossRef] [PubMed]
- Mvalo, T.; Topazian, H.; Kamthunzi, P.; Chen, J.; Kambalame, I.; Mafunga, P.; Mumba, N.; Chiume-Chiphaliwali, M.; Paseli, K.; Key, N.; et al. Increasing Hydroxyurea Use in Children with Sickle Cell Disease at Kamuzu Central Hospital, Malawi. Blood Adv. 2018, 2, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Brandow, A.M.; Panepinto, J.A. Hydroxyurea Use in Sickle Cell Disease: The Battle with Low Prescription Rates, Poor Patient Compliance and Fears of Toxicities. Expert Rev. Hematol. 2010, 3, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sinha, C.B.; Bakshi, N.; Ross, D.; Krishnamurti, L. From Trust to Skepticism: An in-Depth Analysis across Age Groups of Adults with Sickle Cell Disease on Their Perspectives Regarding Hydroxyurea. PLoS ONE 2018, 13, e0199375. [Google Scholar] [CrossRef]
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-Nitrosylation in Health and Disease: A Current Perspective. Trends Mol. Med. 2009, 15, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Dranka, B.P.; Bailey, S.M.; Lancaster, J.R.; Darley-Usmar, V.M. What Part of NO Don’t You Understand? Some Answers to the Cardinal Questions in Nitric Oxide Biology. J. Biol. Chem. 2010, 285, 19699–19704. [Google Scholar] [CrossRef]
- Mack, A.K.; Kato, G.J. Sickle Cell Disease and Nitric Oxide: A Paradigm Shift? Int. J. Biochem. Cell Biol. 2006, 38, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Kassim, A.A.; DeBaun, M.R. Sickle Cell Disease, Vasculopathy, and Therapeutics. Annu. Rev. Med. 2013, 64, 451–466. [Google Scholar] [CrossRef]
- Reiter, C.D.; Gladwin, M.T. An Emerging Role for Nitric Oxide in Sickle Cell Disease Vascular Homeostasis and Therapy. Curr. Opin. Hematol. 2003, 10, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Head, C.A.; Swerdlow, P.; McDade, W.A.; Joshi, R.M.; Ikuta, T.; Cooper, M.L.; Eckman, J.R. Beneficial Effects of Nitric Oxide Breathing in Adult Patients with Sickle Cell Crisis. Am. J. Hematol. 2010, 85, 800–802. [Google Scholar] [CrossRef]
- Sullivan, K.J.; Goodwin, S.R.; Evangelist, J.; Moore, R.D.; Mehta, P. Nitric Oxide Successfully Used to Treat Acute Chest Syndrome of Sickle Cell Disease in a Young Adolescent. Crit. Care Med. 1999, 27, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ruiz, R.; Montero-Huerta, P.; Homi, J.; Head, C.A. Inhaled Nitric Oxide Improves Survival Rates during Hypoxia in a Sickle Cell (SAD) Mouse Model. J. Am. Soc. Anesthesiol. 2001, 94, 1113–1118. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Kato, G.J.; Weiner, D.; Onyekwere, O.C.; Dampier, C.; Hsu, L.; Hagar, R.W.; Howard, T.; Nuss, R.; Okam, M.M.; et al. Nitric Oxide for Inhalation in the Acute Treatment of Sickle Cell Pain Crisis: A Randomized Controlled Trial. JAMA 2011, 305, 893–902. [Google Scholar] [CrossRef]
- Santos, R.M.; Lourenço, C.F.; Gerhardt, G.A.; Cadenas, E.; Laranjinha, J.; Barbosa, R.M. Evidence for a Pathway That Facilitates Nitric Oxide Diffusion in the Brain. Neurochem. Int. 2011, 59, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Lourenço, C.F.; Ledo, A.; Barbosa, R.M.; Laranjinha, J. Nitric Oxide Inactivation Mechanisms in the Brain: Role in Bioenergetics and Neurodegeneration. Int. J. Cell Biol. 2012, 2012, 391914. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-Arginine-Nitric Oxide Pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Nathan, C.; Xie, Q.W. Nitric Oxide Synthases: Roles, Tolls, and Controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Mondoro, T.H.; Ryan, B.B.; Hinczenko, B.W.; Schechter, A.N.; Vostal, J.G.; Alayash, A.I. Biological Action of Nitric Oxide Donor Compounds on Platelets from Patients with Sickle Cell Disease. Br. J. Haematol. 2001, 112, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Conran, N.; Torres, L. Minireview CGMP Modulation Therapeutics for Sickle Cell Disease. Exp. Biol. Med. 2019, 244, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Cokic, V.P.; Smith, R.D.; Beleslin-Cokic, B.B.; Njoroge, J.M.; Miller, J.L.; Gladwin, M.T.; Schechter, A.N. Hydroxyurea Induces Fetal Hemoglobin by the Nitric Oxide–Dependent Activation of Soluble Guanylyl Cyclase. J. Clin. Investig. 2003, 111, 231–239. [Google Scholar] [CrossRef]
- Morris, C.R.; Kuypers, F.A.; Lavrisha, L.; Ansari, M.; Sweeters, N.; Stewart, M.; Gildengorin, G.; Neumayr, L.; Vichinsky, E.P. A Randomized, Placebo-Controlled Trial of Arginine Therapy for the Treatment of Children with Sickle Cell Disease Hospitalized with Vaso-Occlusive Pain Episodes. Haematologica 2013, 98, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.; Laufs, U.; Huang, Z.; Nakamura, T.; Huang, P.; Moskowitz, M.A.; Liao, J.K. Stroke Protection by 3-Hydroxy-3-Methylglutaryl (HMG)-CoA Reductase Inhibitors Mediated by Endothelial Nitric Oxide Synthase. Proc. Natl. Acad. Sci. USA 1998, 95, 8880–8885. [Google Scholar] [CrossRef] [PubMed]
- Amin-Hanjani, S.; Stagliano, N.E.; Yamada, M.; Huang, P.L.; Liao, J.K.; Moskowitz, M.A. Mevastatin, an HMG-CoA Reductase Inhibitor, Reduces Stroke Damage and Upregulates Endothelial Nitric Oxide Synthase in Mice. Stroke 2001, 32, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Ataga, K.I. Novel Therapies in Sickle Cell Disease. Hematology 2009, 2009, 54–61. [Google Scholar] [CrossRef]
- Adam, S.S.; Hoppe, C. Potential Role for Statins in Sickle Cell Disease. Pediatric Blood Cancer 2013, 60, 550–557. [Google Scholar] [CrossRef]
- Tayeh, M.A.; Marletta, M.A. Macrophage Oxidation of L-Arginine to Nitric Oxide, Nitrite, and Nitrate. Tetrahydrobiopterin Is Required as a Cofactor. J. Biol. Chem. 1989, 264, 19654–19658. [Google Scholar] [CrossRef]
- Mayer, B.; John, M.; Böhme, E. Purification of a Ca2+/Calmodulin-Dependent Nitric Oxide Synthase from Porcine Cerebellum: Cofactor-Role of Tetrahydrobiopterin. FEBS Lett. 1990, 277, 215–219. [Google Scholar] [CrossRef]
- Katusic, Z.S.; d’Uscio, L.V.; Nath, K.A. Vascular Protection by Tetrahydrobiopterin: Progress and Therapeutic Prospects. Trends Pharmacol. Sci. 2009, 30, 48–54. [Google Scholar] [CrossRef]
- Mittleman, R.S.; Wilson, R.S.; Sykes, K.; Mihova, M.; Chickering, J.G.; Ruff, D.; Hall, M.; Milne, T.G.; Currie, M.G.; Chien, Y.T. Multiple-Ascending-Dose Study of the Soluble Guanylate Cyclase Stimulator, IW-1701, in Healthy Subjects. Blood 2017, 130, 3533. [Google Scholar]
- McMahon, T.J.; Shan, S.; Riccio, D.A.; Batchvarova, M.; Zhu, H.; Telen, M.J.; Zennadi, R. Nitric Oxide Loading Reduces Sickle Red Cell Adhesion and Vaso-Occlusion in Vivo. Blood Adv. 2019, 3, 2586–2597. [Google Scholar] [CrossRef]
- McMahon, T.J. Red Blood Cell Deformability, Vasoactive Mediators, and Adhesion. Front. Physiol. 2019, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.G.; Deshpande, T.M.; Ghatge, M.S.; Mehta, A.Y.; Omar, A.S.M.; Ahmed, M.H.; Venitz, J.; Abdulmalik, O.; Zhang, Y.; Safo, M.K. Design, Synthesis, and Investigation of Novel Nitric Oxide (NO)-Releasing Prodrugs as Drug Candidates for the Treatment of Ischemic Disorders: Insights into NO-Releasing Prodrug Biotransformation and Hemoglobin-NO Biochemistry. Biochemistry 2015, 54, 7178–7192. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, C.; Praveen Rao, P.N.; Knaus, E.E. Novel Nonsteroidal Antiinflammatory Drugs Possessing a Nitric Oxide Donor Diazen-1-Ium-1,2-Diolate Moiety: Design, Synthesis, Biological Evaluation, and Nitric Oxide Release Studies. J. Med. Chem. 2005, 48, 4061–4067. [Google Scholar] [CrossRef]
- Thatcher, G.R.J.; Nicolescu, A.C.; Bennett, B.M.; Toader, V. Nitrates and NO Release: Contemporary Aspects in Biological and Medicinal Chemistry. Free Radic Biol. Med. 2004, 37, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Alhashimi, R.T.; Ghatge, M.S.; Donkor, A.K.; Deshpande, T.M.; Anabaraonye, N.; Alramadhani, D.; Danso-Danquah, R.; Huang, B.; Zhang, Y.; Musayev, F.N.; et al. Design, Synthesis, and Antisickling Investigation of a Nitric Oxide-Releasing Prodrug of 5HMF for the Treatment of Sickle Cell Disease. Biomolecules 2022, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- Safo, M.K.; Abraham, D.J. X-Ray Crystallography of Hemoglobins. Methods Mol. Med. 2003, 82, 1–19. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP4 Suite and Current Developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Echols, N.; Headd, J.J.; Hung, L.-W.; Jain, S.; Kapral, G.J.; Grosse Kunstleve, R.W.; et al. The Phenix Software for Automated Determination of Macromolecular Structures. Methods 2011, 55, 94–106. [Google Scholar] [CrossRef]
- Echols, N.; Grosse-Kunstleve, R.W.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Headd, J.J.; McCoy, A.J.; Moriarty, N.W.; Read, R.J.; Richardson, D.C.; et al. Graphical Tools for Macromolecular Crystallography in PHENIX. J. Appl. Crystallogr. 2012, 45, 581–586. [Google Scholar] [CrossRef]
- Brünger, A.T.; Adams, P.D.; Clore, G.M.; DeLano, W.L.; Gros, P.; Grosse-Kunstleve, R.W.; Jiang, J.S.; Kuszewski, J.; Nilges, M.; Pannu, N.S.; et al. Crystallography & NMR System: A New Software Suite for Macromolecular Structure Determination. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 905–921. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Ghatge, M.S.; Donkor, A.K.; Musayev, F.N.; Deshpande, T.M.; Al-Awadh, M.; Alhashimi, R.T.; Zhu, H.; Omar, A.M.; Telen, M.J.; et al. Design, Synthesis, and Investigation of Novel Nitric Oxide (NO)-Releasing Aromatic Aldehydes as Drug Candidates for the Treatment of Sickle Cell Disease. Molecules 2022, 27, 6835. https://doi.org/10.3390/molecules27206835
Huang B, Ghatge MS, Donkor AK, Musayev FN, Deshpande TM, Al-Awadh M, Alhashimi RT, Zhu H, Omar AM, Telen MJ, et al. Design, Synthesis, and Investigation of Novel Nitric Oxide (NO)-Releasing Aromatic Aldehydes as Drug Candidates for the Treatment of Sickle Cell Disease. Molecules. 2022; 27(20):6835. https://doi.org/10.3390/molecules27206835
Chicago/Turabian StyleHuang, Boshi, Mohini S. Ghatge, Akua K. Donkor, Faik N. Musayev, Tanvi M. Deshpande, Mohammed Al-Awadh, Rana T. Alhashimi, Hongmei Zhu, Abdelsattar M. Omar, Marilyn J. Telen, and et al. 2022. "Design, Synthesis, and Investigation of Novel Nitric Oxide (NO)-Releasing Aromatic Aldehydes as Drug Candidates for the Treatment of Sickle Cell Disease" Molecules 27, no. 20: 6835. https://doi.org/10.3390/molecules27206835
APA StyleHuang, B., Ghatge, M. S., Donkor, A. K., Musayev, F. N., Deshpande, T. M., Al-Awadh, M., Alhashimi, R. T., Zhu, H., Omar, A. M., Telen, M. J., Zhang, Y., McMahon, T. J., Abdulmalik, O., & Safo, M. K. (2022). Design, Synthesis, and Investigation of Novel Nitric Oxide (NO)-Releasing Aromatic Aldehydes as Drug Candidates for the Treatment of Sickle Cell Disease. Molecules, 27(20), 6835. https://doi.org/10.3390/molecules27206835








