3.1. Crystal Structure
Based on their crystal structure, all the fluorocarbonates in this research were divided into three groups: group I—KCaCO3F, KSrCO3F, RbSrCO3F (space group P-6m2); group II—KZnCO3F, KCdCO3F, RbCdCO3F (P-6c2); group III—RbMgCO3F, RbCaCO3F, CsCaCO3F (P-62m). Each group clearly demonstrates the roles of its alkali metal, alkaline-earth metal, or the one that contained a completely full nd10 shell in addition to the full ns2 shell.
Atoms of crystal KSrCO
3F are layered as follows: strontium (Sr), carbon (C), and oxygen (O) are located in plane
z =
c/2, whereas potassium (K) and fluorine (F) atoms are in plane
z = 0. Here and below, coordinate
z is chosen along axes
c, x, and
y in plane
ab. Atomic position coordinates are as follows: K(0,0,0), Sr(2/3,1/3,1/2), C(1/3,2/3,1/2), F(2/3,1/3,0), and O(-2
xO,-
xO,1/2) in lattice vectors
a =
b,
c. Thus, the independent parameters of the lattice were determined by optimizing its geometry and involved three quantities:
a,
c, and
xO.
Table 1 shows the independent lattice parameters calculated using PBE + D3 and B3LYP, as well as those measured experimentally in [
2,
8,
9]. They make it easy to calculate interatomic distances.
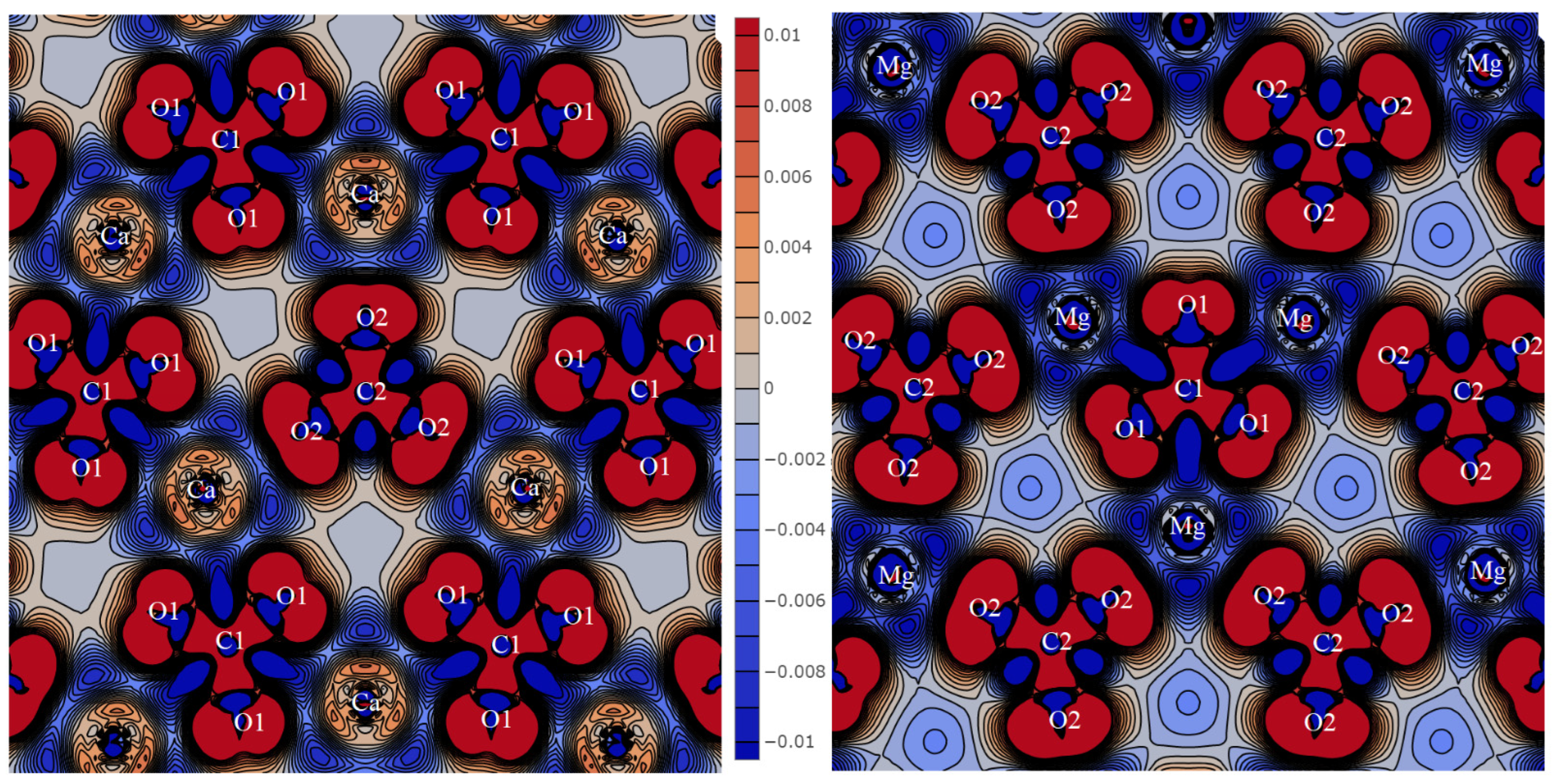
Figure 1 shows the deformation density distribution Δρ for KSrCO
3F obtained by subtracting the density of non-interacting atoms from the crystal density. It illustrates the redistribution of the electronic charge during the chemical bond formation. Positive Δρ areas are red, while the areas of negative values are blue. Density values here and below are given in atomic units.
Each Sr atom is surrounded by six oxygen atoms separated by 2.622 Å. All distances are given for functional PBE + D3, and
Table 1 provides them for B3LYP. In
Figure 1 (left), the electron charge flows out of the inner atomic regions and flows into the outer ones. In the case of Sr, the charge follows in such a way that the interaction results in a positively charged ion +1.82 |
e|, where
e is the electron charge. The ion charge was calculated according to the Mulliken scheme, which splits it between atoms, which is not entirely true. The charge flows out from the inner regions of the carbon and oxygen atoms. Then, it flows into the region between them and behind the oxygen nuclei symmetrically to bond line C–O (1.308 Å). As a result, carbon acquires a positive charge of +0.39 |
e|, while oxygen acquires a negative charge of −0.70 |
e|. The overlap population of electron clouds on bond line C-O (
PC-O) is 0.539
e, which indicates that they develop a strong covalent chemical bond and a stable carbonate ion. The excess charge behind the oxygen nuclei in plane
ab,
z = c/2 produces a stable SrCO
3 structure. All carbonate groups line up in parallel, which gives the maximal contribution to the macroscopic SHG effect [
2].
In plane
ab,
z = 0 (
Figure 1, right), each potassium atom is surrounded by three fluorine atoms at 3.018 Å. The electronic charge flows out of the potassium atom so that its ionic charge is +0.75 |
e|. It then flows onto fluorine with its ionic charge of −0.84 |
e|. Fluorine has a greater affinity for the electron and attracts electronic charges from both cations. Since carbonate ion has three oxygen atoms, it has a higher negative charge. The overlap population is
PK-F = 0.019
e, so bond K-F is an ionic chemical. The entire layer acquires an excess negative charge of −0.09 |
e|, which provides additional electrostatic adhesion of layers ···Sr-CO
3··· and ···K-F··· to each other.
Figure 2 shows the distribution of the deformation density in plane
xz (
y = a/2) in the primitive cell of KSrCO
3F. It has strontium and fluorine atoms, as well as closely spaced oxygen and potassium atoms, so that the layers of Sr, C, and O alternate with those of K and F. The fluorine atoms are located above and below the strontium atoms at 2.354 Å. The overlap population for Sr-F is 0.003
e, which is even less than Sr-O at 0.008
e with a larger distance. Thus, the resulting ionic chemical bond provides infinite ···F-Sr-F··· chains. Each potassium atom is surrounded by six oxygen atoms at 2.909 Å. As maxima Δρ are located behind the oxygen nuclei, the overlap population on line K-O is 0.018
e. Thus, the nearest environment of negatively charged ions is polyhedra KO
6F
3 for potassium and SrO
6F
2 for strontium. According to [
38], if the coordination number
NOF is nine, the radius of the potassium ion R
A equals 1.55 Å, and for a strontium ion with
NOF = 8,
RB equals 1.26 Å. As a result, the average radius of cation
RC = (
RA +
RB)/2 is 1.405 Å. This value allowed us to establish all ordinary patterns in this research.
When the alkali metal cation Rb replaces K in isostructural RbSrCO3F, the replacement triggers no fundamental change in the parameters of the crystal structure. Constants a and c increase, as do all interatomic distances, but the quantitative crystallographic environment remains the same. The cationic charge of strontium changes insignificantly and reaches +1.81 |e|. For the rubidium ion, the charge is +0.84 |e| larger than for potassium, and its cationic radius is 1.63 Å. In KCaCO3F, the calcium ion radius is 1.12 Å smaller than that of strontium. Therefore, lattice constants a and c and the interatomic distances are also smaller than in other crystals. The charge of the calcium ion is much less than that of strontium and equals +1.45 |e|, while the charge of potassium reaches +0.76 |e|. Due to the smaller calcium radius, the overlap populations of Ca-O and Ca-F increase by 0.049 and 0.036 e, respectively. Unlike KSrCO3F, the charge of the Ca-CO3 layer is negative and equals −0.024 |e|.
The average radius of cation RC in the series of KCaCO3F, KSrCO3F, and RbSrCO3F increases as 1.335, 1.405, and 1.445 Å, respectively. They have a stable linear dependency on the geometric parameters of the lattice. Lattice constant a increases as a(Å) = 2.815 + 1.713·RC, and so do the interatomic distances: RA-O(Å) = 0.405 + 1.778·RC, RB-O(Å) = 1.371 + 0.888·RC. The change in the distances triggers the change in the parameters of the chemical bond. As RC increases, the charges of CO3 anions (−1.48, −1.71, −1.78 |e|) and fluorine anions (−0.73, −0.84, −0.87 |e|) also increase. The overlap population of bond C–O also goes up (0.516, 0.539, 0.551 e), which means that the covalent chemical bond in the carbonate group also increases.
Zinc and cadmium atoms have completely full 3d10 and 4d10 electron shells, which are energetically close to the upper full 4s2 shell. Therefore, d-electrons participate in the hybridization of electron states. As a result, fluorocabonates with zinc and cadmium have different properties.
Lattice cell KCdCO
3F contains two formula units. Unlike group I, lattice constant
c is twice as large, and its alternating layers are more numerous. The coordinates of nonequivalent atoms in units
a and
c are as follows: K(2/3,1/3,1/2), Cd(0,0,1/4), C(1/3,2/3,1/4), F(0,0,1/2), and O(
xO,
yO,1/4), i.e., a total of four independent quantities (see
Table 1 for their values).
The cadmium atom is surrounded by three carbonate groups in plane
z =
c/4 (
Figure 3, left). Unlike KSrCO
3F, they are deployed in such a way that only three oxygen atoms are located at 2.238 Å, and three others are at a greater distance of 2.882 Å. This rotation leads to the fact that carbonate groups in the neighboring planes separated by
c/2 are parallel to each other and have the same direction. These planes are connected through infinite chains ···F-Cd-F···, the shortest distance being 2.219 Å. Thus, cadmium cation forms polyhedra CdO
6F2. For coordination number
NOF = 8, cadmium ion radius is 1.1 Å [
38]. The calculated cadmium ion charge is much lower than those calculated for Ca or Sr and equals +1.07 |
e|, which also leads to a lower charge of the carbonate group, which equals −1.23 |
e|. The small cadmium radius is accompanied by relatively small Cd-O and Cd-F distances. As a result, they have a larger electron shell overlap population of 0.089 and 0.073
e, respectively, than other cations. It is only 0.016
e for three oxygen atoms separated by large distances.
Each potassium atom in KCdCO3F is surrounded by six oxygen atoms at 2.804 Å and three fluorine atoms at 2.946 Å. Thus, the radius of its ion is 1.55 Å, and its charge is +0.78 |e|. The charge of fluorine atoms in the same plane is −0.62 |e| and the total charge is much greater than in group I.
When zinc replaces cadmium in KZnCO
3F, it decreases the lattice constants and all interatomic distances, including K-O (2.733 Å). Potassium atom charge falls down to +0.75 |
e|, while that of fluorine rises as high as −0.68 |
e|. The crystalline environment of the zinc atom differs from that of cadmium in KCdCO
3F. It is surrounded by three oxygen atoms at 2.002 Å, two fluorine atoms, and three carbons, while three more oxygen atoms come last at 3.028 Å. This pattern occurs because the carbonate groups change their direction axis relative to the cations (
Figure 3, right). Zinc ion charge is +1.24 |
e| and its radius for
NOF = 8 is 0.90 Å. Such a small radius has a considerable overlap population of 0.112 and 0.066
e for Zn-O and Zn-F, respectively. Thus, the proportion of the covalent component of the chemical bond in KZnCO
3F is much higher than in other fluorocarbonates.
When rubidium replaces potassium, the resulting RbCdCO3F experiences an increase in all interatomic distances: Rb-O rises to 2.898 Å and Rb-F increases to 3.009 Å. As a result, the rubidium ion radius charged +0.87 |e| is 1.66 Å. Fluorine shares the same plane with a charge of −0.64 |e| and the total positive charge is very significant. The environment of cadmium is the same as that of zinc. Each cadmium ion with a charge of +1.06 |e| is surrounded by the three nearest oxygen atoms at 2.221 Å followed by two fluorine atoms and carbon, while three oxygen atoms come last at 3.018 Å. The radius of the cadmium ion is small (1.1 Å), which provides a significant overlap of electron shells with oxygen at 0.089 e and fluorine at 0.073 e, as well as a high share of the covalent component in the chemical bond.
Group II demonstrates the linear dependency of the crystal parameters on the average cation radius, which increases as 1.225, 1325, and 1.365 Å in the series of KZnCO3F, KCdCO3F, and RbCdCO3F. For constant c(Å) = 1.918 + 5.234·RC and distances RC-O(Å) = 1.274 + 0.022·RC (0.99), the increasing bond length increases the population of overlap PC-O, which is 0.483, 0.493, and 0.506 e in this series.
In RbCaCO
3F, atoms occupy the following positions: Rb(0,-
xRb,0), Ca(-
xCa,-
xCa,1/2), carbon C1 (1/3,2/3,1/2), C2(0,0,1/2), C1(1/3,2/3,1/2), C2(0,0,1/2), F(-
xF,-
xF,0), O1(
xO1,-
yO1,1/2), and O2(-
xO2,-
xO2,1/2) (see
Table 1 for their values). This compound has three carbonate groups. Two C1O
3 groups occupy equivalent positions, their C1-O1 distances being 1.308 Å. In the third C2O
3 group, the distance of C2-O2 is 1.302 Å. The rubidium atom is surrounded by four O1 oxygen atoms at 3.015 Å, one fluorine atom at 3.117 Å, four more O2 oxygen atoms at 3.149 Å, and, finally, two more fluorine atoms at 3.155 Å. In polyhedron RbO
8F
3, the rubidium ion charge is +0.86 |
e| and its radius is 1.69 Å [
38]. The charge of the entire plane per one formula unit is +0.11 |
e|.
The calcium atom is surrounded by two fluorine atoms at 2.223 Å, one O2 oxygen atom at 2.275 Å, and two O1 at 2.458 Å and 2.488 Å from two C1O
3 (
Figure 4, left). In CaO
5F2, calcium ion charge is +1.45 |
e|, and its radius is 1.06 Å. The surrounding carbonate groups C1O
3 and C2O
3 with distances of 1.308 and 1.302 Å are charged as −1.54 |
e| and −1.59 |
e|, respectively. For the deformation density in plane
ab, carbonate groups C1O
3 point in the same direction (
Figure 4), while groups C2O
3 point in the opposite direction (see the center of
Figure 4). Group III fluorocarbonates differ from the others.
CsCaCO3F maintains the same cationic environment. The cesium ion radius is 1.85 Å in CsO8F3 and its charge is +0.66 |e|. Fluorine is charged at −0.71 |e|, which is also lower than in a rubidium crystal. Calcium also forms pentagonal bipyramids with an ion charge of +1.48 |e| and a radius of 1.06 Å. The alignment of four O1 and one O2 equatorial atoms leads to an asymmetric alignment of three carbonate groups: two groups C1O3 with a distance for C1-O1 of 1.307 Å and a charge of −1.44 |e| are parallel to each other and share the edges with CaO5F2, while the third C2O3 group has a distance of 1.304 Å and a charge of −1.54 |e| and is antiparallel to the rest.
The magnesium ion in RbMgCO
3F is the smallest of all the alkaline-earth metals in this research, so its environment differs from the other group III crystals. The rubidium atom is surrounded by ten oxygen atoms as follows: two O2 at 2.936 Å, four O1 at 3.086 Å, and four O1 at 3.162 Å. One fluorine atom is located at 3.060 and two are located at 3.106 Å. The rubidium ion in RbO
10F
3 has a charge of +0.85 |
e| and a radius of 1.83 Å. The fluorine charge of −0.72 |
e| is responsible for the excess charge of the entire plane. The magnesium atom also has a MgO
4F
2 environment, which is different from other alkaline-earth ions (
Figure 4, right): the magnesium atom is surrounded by two fluorine atoms at 1.966 Å, two oxygen atoms O1 at 1.989 Å, and two oxygen atoms O2 at 2.173 Å from two C2O
3. In this environment, the magnesium charge is +1.37 |
e| and its radius is 0.72 Å. This change is associated with the reversal of carbonate groups, which polarizes the small magnesium cation.
RC1-O1 = 1.298 Å in two ions C1O
3 is less than 1.303 Å in one C2O
3, and the charge is −1.51 |
e| greater than −1.47 |
e|. These peculiarities manifest in the elastic and vibrational properties of group III fluorocarbonates. Due to its small radius, the overlap population of Mg-O at 0.095
e and Mg-F at 0.081
e is significantly higher than for Ca-O at 0.04
e.
Like other groups, the average cation radius in the series of RbMgCO3F, RbCaCO3F, and CsCaCO3F is as high as 1.275, 1.375, and 1.455 Å, respectively. Crystallographic parameters a(Å) = 6.90 + 1.671·RC and RA-O(Å) = 1.880 + 0.828·RC demonstrate a linear dependence.
We compared the experimental crystallographic data for fluorocarbonates of group I [
2], group II [
8], RbMgCO
3F [
9], RbCaCO
3F, and CsCaCO
3F [
2]. The mean-square deviation for eight structural parameters
VR:
a,
c,
V,
RA-O,
RA-F,
RB-O,
RB-F, and
RC-O (mean for group III) is 0.42% for PBE + D3 functional, 1.53% for PBE, and 1.26% for B3LYP for nine fluorocarbonates. PBE + D3 gives a minimal deviation of 0.35% for lattice constant
a, 0.3% for constant
c, 0.58% for the shortest distances between cations A and oxygen, 0.36% for cations B and oxygen, and 0.79% for hybrid functional B3LYP for C-O. PBE + D3 decreases constant
a in KSrCO
3F (0.6%), RbSrCO
3F (0.25%), KZnCO
3F (0.3%), and KCdCO
3F (0.6%). It decreases constant
c in KZnCO
3F (0.4%), RbCdCO
3F (0.2%), RbMgCO
3F (0.2 %), and RbCaCO
3F (0.04%). Functional PBE in all fluorocarbonates increases both constant
a and constant
c. Functional B3LYP leads to a similar result, except for KZnCO
3F and RbCdCO
3F, where constant
c is lower by 0.1%. Thus, van der Waals interactions provide an agreement between the calculated and experimental parameters of the crystal structure in fluorocarbonates ABCO
3F.
All fluorocarbonates demonstrate a simple linear relationship between the structural parameters VR calculated by different functionals. For KCaCO3F, it is VRPBE = −0.052 + 1.025·VRPBE + D3. For volume V, the value calculated by functional PBE + D3 is 100.794 Å3, while the value obtained by the interpolation formula for PBE is 103.279 Å3, its precise value being 103.281 Å3. For functional B3LYP, the formula is VRB3LYP = −0.047 + 1.022·VRPBE + D3. On the contrary, gradient functional PBESOL and hybrid functional PBESOL0 lead to decreased lattice constants and actual interatomic distances: VRPBESOL = 0.038 + 0.982·VRPBE + D3, VRPBESOL0 = 0.057 + 0.972·VRPBE + D. Their mean-square deviations are 0.7 and 1.0%, which exceeds those for PBE + D3 by 0.45%.
All fluorocarbonates demonstrated a stable linear dependence. The lattice cell volume per formula unit depends on the average cation radius
V/
Z(Å
3) = −59.75 + 121.16·
RC (0.99), the average distance from cations A and B to the nearest oxygen (
RA–O +
RB-O)/2(Å) = 0.253 + 1.738·
RC (0.91), and the average distance between cations A and B and fluorine (
RA–F +
RB-F)/2(Å) = 1.13 + 1.1·
RC (0.98) and
RC-O(Å) = 1.264 + 0.03·
RC (0.86). These formulae can predict the corresponding values for other fluorocarbonates.
Figure 5 shows the cell volumes per formula unit calculated using functional PBE + D3 and those measured experimentally in [
2,
8,
9]. It also illustrates the average distances between cations and fluorine atoms, as well as the predicted and experimental values for KMgCO
3F (
Z = 3), RbZnCO
3F (
Z = 2), RbPbCO
3F, and CsPbCO
3F (
Z = 1), which were beyond the scope of this work.
Figure 5 clearly demonstrates a sufficient agreement between the calculated, predicted, and experimental values.
3.2. Electron Structure
The energy distribution of electrons in a crystal has a band structure that reflects the dependence of the electron energy on wave vector
E(
k). The relevant points of the Brillouin zone were Г(0,0,0), M(1/2,0,0), L(1/2,0,1/2), A(0,0,1/2), K(1/3,1/3,0), H(1/3,1/3,1/2).
Figure 6 visualizes calculations for the band structures of typical representatives KSrCO
3F, KZnCO
3F, and RbCaCO
3F.
The last full state is taken as the energy reference point. Therefore, the occupied (valence) states have negative energies, and the unoccupied (conduction bands) have positive ones. Here and below, we give PBE + D3 calculations by default. The valence bands are narrow in energy E and practically flat, depending on k. This pattern is typical of crystals with an ionic chemical bond. The conduction bands have a greater dispersion than the valence bands. The energy distance between the top of the valence band and the bottom of the conduction band determines an important parameter we defined as the band gap Eg. The zones are divided into direct and indirect. In direct zones, the electron transition occurs from an occupied to an unoccupied state, both belonging to the same wave vector k. In indirect zones, they belong to different ones.
In the fluorocarbonate group I, the top of the valence band and the bottom of the conduction band occur at point A. The band gaps in this series are 5.18, 5.12, and 5.32 eV. The uppermost valence bands are 0.78, 0.50, and 0.02 eV wide. Each is separated from the previous band by a band gap of 0.6–0.7 eV. This band is formed of 98% pxy-oxygen. The bottom unoccupied bands are also separated from the subsequent ones by a band gap of 0.08, 0.40, and 1.67 eV. They are formed of about 60% pz- carbon and of about 39% oxygen. Only the following conduction bands are metallic in nature for 50–70% of s-potassium, rubidium, calcium, and strontium. The share of CO3 is 15–30%.
In KZnCO
3F and RbCdCO
3F, the top of the valence band and the bottom of the conduction band are at point Г. In this series, its width is 5.18 eV (3.17 in [
8], 3.29 in [
15]) and 5.27 eV (5.05 in [
7], 5.35 in [
8]). In KCdCO
3F, the top of the valence band is on the line and the bottom of the conduction band is at point Г. The width of the indirect gap is 5.008 eV, while the straight one is 5.013 eV (5.11 in [
7], 5.30 in [
8]). The uppermost two valence bands are separated from the previous ones by a small band gap. Their widths are 0.58, 0.65, and 0.76 eV in KZnCO
3F, KCdCO
3F, and RbCdCO
3F, respectively. They are formed of 80–85% p
xy-oxygen, 12% of
d-zinc, 7% of
d-cadmium, and about 5% of
pz-fluorine. The lower two unoccupied bands are formed of ~50%
pz-carbon and ~35% of oxygen. They overlap with the subsequent ones, which are ~60% of
d-metallic nature of zinc or cadmium.
In RbMgCO3F, the top of the valence band and the bottom of the conduction band are at point A. The band gap is 5.07 eV. A band gap of 0.75 eV separates the uppermost two valence bands from the previous ones and their width is 0.5 eV. They are formed of 98% of the pxy-states of O1 oxygen and have an anti-bonding character. The lower three unoccupied bands are also separated from the subsequent ones by a band gap of 0.4 eV. The lowest one is 87% formed of pz- states of carbon and oxygen from C2O3, and the other two are 92% formed of C1O3. Only the next conduction band has a metallic character at 29% s-rubidium and 37% magnesium.
The band structure of RbCaCO3F and CsCaCO3F fundamentally differs from that of the rubidium–magnesium fluorocarbonate. The top of the valence band is at point Г, while the bottom of the conduction band is at point A and its indirect band gap is 4.97 and 5.16 eV. The top three valence bands are 1.1 and 1.0 eV wide. They are separated by a band gap of 0.35 eV from the preceding ones. The top one of these bands is 95% formed of pxy-oxygen from C2O3, whereas the other two are 92% formed of C1O3. The bottom unoccupied three bands are separated from the subsequent bands by band gaps 0.44 and 0.11 eV. The two bottom ones are formed of pz- carbon (68%) and oxygen from C1O3, while the third one is formed of C2O3 (88%). The following conduction bands are metallic: s- rubidium—39%, cesium—73%, and calcium—26.8%, respectively.
The total and partial density distribution can characterize the nature of the electronic states (
Figure 7). The energy position and width of bands
N(E) reflect the hybridization degree.
The states of alkali metals hardly affect the formation of the upper valence region between −5 and 0 eV. In KCaCO3F and KSrCO3F, the K3s- and K3p-states of potassium are located in the region of −27.8, −28.4 and −11.9, −12.2 eV, respectively. Their respective widths are about 0.01 and 0.16 eV. Therefore, they participate very little in the chemical bond formation, hence the insignificant overlap population of their electron shells with other atoms. In KZnCO3F and KCdCO3F, the energy positions of bands K3s (−27.1, −27.2 eV) and K3p (−11.0, −11.3 eV) shift towards higher values. The width of the former remains insignificant, while that of the latter one rises to 0.28 and 0.17 eV, respectively. The energies of 4s- and 4p-states of rubidium shift towards lower values as the cation B radius increases: from −25.6 and −9.3 eV with widths of 0.05 and 0.71 eV in RbMgCO3F and −26.1 and −9.8 eV with widths of 0.02 and 0.36 eV in RbCaCO3F to −28.4 and −12.3 eV with widths of 0.00 and 0.15 eV in RbSrCO3F. The energy positions of Cs5s and Cs5p are −20.2 and −6.8 eV, and their widths are the most significant so far, namely 0.37 and 1.42 eV. This means that as the cation A radius increases at a fixed B, its states shift towards higher energies and the bandwidths increase.
Mg
2s-, Mg
2p-, Ca
3s-, and Sr
4s-lie in the energy region of the core states and are not marked in
Figure 7. Ca
3p- maintains its energy position as −19.6 eV, while its width increases from 1.33 eV in KCaCO
3F to 1.57 eV in RbCaCO
3F. These energies coincide with the region of states C
2s-, O
2s-, and F
2s-. Therefore, their considerable width indicates mutual hybridization. The energies of Sr
4s- states in KSrCO
3F are at −15.3 eV with a width of 0.74 eV, while in RbSrCO
3F they are at −15.6 eV with a width of 0.62 eV, which is higher in energy than the bands of hybrid anion
s-states.
Zinc 3d states range from −5.5 to −4.0 eV with a maximal N(E) at −4.3 eV. They overlap with the region of C2p-O2p hybrid states from below and F2p-O2p states from above. Zinc states have a high degree of hybridization not only in this energy region but even in the upper valence states. For KCdCO3F, the band of cadmium 4d states ranges from –7.2 to –5.4 eV with maxima at −6.2 and −5.2 eV. The total share of cadmium for this energy range is 70%, oxygen—20%, and fluorine—5%. It is this bundle of energy bands that is responsible for the overlap population of Cd-F electron shells at 0.046 e and Cd-O at 0.057 e, which is approximately 70% of their total population. In RbCdCO3F, cadmium d-bands are located in the same energy region with a maximum of 6.3 eV. Anion–cation hybridization p-d is responsible for the overlap population of the Cd-O bond as 0.057 e and Cd-F as 0.037 e. The bottom hybrid zones with maxima at −6.7 and −7.5 eV provides 0.03 e of Cd-O overlap population. However, the upper bands with maxima at −5.1 eV are anti-bonding for Cd-O and bonding for C-O with 0.115 e of overlap population.
Structure N(E) of RbMgCO3F reflects the anion states since magnesium has almost no effect on its formation. The lowest bands with maxima at −22.1 and −21.4 eV are 0.74 eV wide. They are formed by hybridized s-states of carbon and oxygen, which are responsible for the C1-O1 overlap population of 0.106 e and C2-O2 of 0.101 e. Three bands at −20.8 eV are 0.12 eV wide. This group is formed by 95% of the fluorine s-states. The bands with N(E) maxima at −18.9 and −18.3 eV are 0.69 eV wide. They are formed by two-thirds by C1O3 s-states and by one-third by C2O3. They provide an overlap population of 0.178 e for C1-O1 and 0.16e for C2-O2. The energies of the p-states of oxygen and carbon (60% C1O3, 30% C2O3) range from −8.3 to −5.4 eV with N(E) maxima at −8.0, −6.6, −6.2, and −5.5 eV. They provide an overlap of 0.071 e for Mg-O1, 0.014 e for Mg-O2, 0.131 e for C1-O1, and 0.178 e for C-O2. The bands in the energy range from –4.2 to –3.5 eV are formed of the pz-states of fluorine (73%) and magnesium (6%). They contribute 0.037 e to overlap population Mg-F. These crystalline orbitals provide π-conjugation of the layers in the crystal by developing ···F-Mg-F···chains.
3.3. Effect of Pressure on Crystal Structure
Pressure provides a good opportunity to study the interactions inside a crystal of structural elements. External voltage makes atoms leave their equilibrium positions. As a result, the bond lengths change in one way or another, depending on the chemical bonding strength. We used hydrostatic compression when the cell volume was reduced by a given value. After that, the structure parameters underwent complete optimization by calculating the total energy minimum. The resulting dependence
E(V) was approximated by the third-order Birch–Murnaghan analytical equation of state. The distances–pressure dependence
R(P) are described using compressibility modulus
KR =
R0/(
dR/
dP), where the derivative is calculated from a quadratic dependence.
Figure 8 exemplifies dependencies
E(
V),
P(
V),
a(
P)/
a0, and
c(
P)/
c0. The equilibrium values of the crystal parameters (
Table 1) are marked as zero.
Table 2 shows the numerical values of EoS parameters and moduli obtained by functional PBE + D3.
For each group of fluorocarbonates, the cell volume per formula unit increases as
V0/
Z(Å
3) = −59.27 + 120.83·
RC (0.99) following the increase in the average cation radius. The volume modulus decreases, which provides a reliable correlation for the entire
K0(GPa) = 168.37 − 78.24·
RC (0.81). Fluorocarbonates have similar values of moduli
K0. They are lower than those of calcite or dolomite and approach 65.24 GPa for aragonite [
39]. RbMgCO
3F and KZnCO
3F with the smallest grade B cations have the maximal
K0 values (minimal compressibility). KCdCO
3F and RbMgCO
3F have the maximal SGH coefficients. Their maximal volume modulus increase rate with
K1 pressure is maximal. Their linear correlation coefficient SGH = −16.8 + 4.11·
K1 is 0.70. The ion exceeds
D for the dispersive energy per one formula unit
Edisp/
Z, which is also at its maximum in these carbonates: −22.96 and −23.04 kJ/mol.
For each group of crystals, compressibility moduli Ka along axis a and Kc along axis c decrease as the average cation radius increases. However, group II is an exception: this rule does not work for module Kc in RbCdCO3F. As for groups I and III Ka > Kc, this ratio increases in the former following the growing average cation radius and decreases in the latter. In group II, Ka < Kc and their ratio also decrease following the growing cation radius. In KZnCO3F, both directions along axes a and c also change with pressure.
Compressibility modulus
KA-O for the distance between metal A and oxygen O decreases in each group with an increase in the average cation radius, while the distances
RA-O grow. The linear dependency between them is stable, e.g., in group I:
KA-O(GPa) = 425.8–97.92·
RA-O. This pattern also exists for
RB-O distances in groups I and III. However, group II has a much higher compressibility modulus
KB-O than other fluorocarbonates with alkaline-earth metals. As mentioned above, Zn-O and Cd-O have high overlap populations. The fact that the region between the nuclei contains electrons prevents the distance from decreasing. This tendency is especially obvious in bond C-O, where compressibility modulus varies from 1000 to 1500 GPa, i.e., it is practically incompressible. In
Table 2, group III has the average
KC-O value between two non-equivalent CO
3 groups to illustrate the comparison. The compressibility modulus
KC1-O1 for C1O
3 in RbMgCO
3F is 922 GPa. For
KC2-O2, it is as high as 2042 GPa, which is almost 2.5 times bigger. On the contrary, in RbCaCO
3F and CsCaCO
3F, compressibility modulus
KC1-O1 is 1753 (1572) GPa for C1O
3 group and ≤871 (830) GPa for
KC2-O2. This fact can be explained by the different values of distances C–O, which have already manifested themselves in the band structure.
The shortest distances between alkali metal atom A and fluorine atom
RA-F occur in plane
ab (see
Figure 1), and those between cations B and fluorine
RB-F occur along axis
c (
Figure 2). Under pressure, they change in proportion to the change in lattice constants
a and
c, respectively. For example, in KCaCO
3F, it is
RA-F(Å) ≈ 0.577·
a and
RR-F(Å) ≈ 0.577·
c. Linear moduli have a similar relationship, which can be used to calculate them:
KA-F(GPa) = −44.89 + 1.22·
Ka (0.97),
KB-F(GPa) = −9.95 + 1.05·
Kc. 3.4. Elastic Properties
Elastic properties determine how a certain material deforms or changes shape under applied forces. In microscopic theory, the elastic behavior of a crystal is related to the second derivative of the free energy with respect to reversible physical deformation. Thus, crystal elasticity is a very sensitive tool that can detect interactions and forces that occur between atoms and determine the structure and stability of crystals.
The elastic constant matrix
Cij (
i, j = 1, 2, 3) for hexagonal crystals contains five independent constants and
C66 = (
C11-
C12)/2 (see
Table 3 for PBE + D3 calculations).
Elastic constants determine the properties of mechanical stability, which look as follows in hexagonal crystals [
40]
C44 > 0,
C11 > |
C12| and
.
Table 3 demonstrates that these conditions are satisfied for all crystals. Diagonal constants
C11 and
C33 indicate the response of the crystal to external stress along one axis, while
C44 and
C66 do the same for the plane shift. For groups I and II,
C33 is greater than
C11, which means that direction
c is harder than
a(b). As
C12 >
C13, it means that if the same normal stress acts in direction
x, the crystals in direction
z shrink more than in direction
y. Small
C44 values indicate that shift deformation occurs more easily if stresses are applied to horizontal planes
ab. Since
C66 >
C44, a shift in plane
ab causes less response than in the perpendicular direction. C
44 value can serve as an indicator to determine the cracking of a hexagonal crystal along axis
c.
In fluorocarbonates, C11 decreases as the average cation radius increases. Group I has the following dependence: C11(GPa) = 306.0 − 137.8·RC. This downward trend in C11 indicates that the bond between and cations becomes more ionic.
No experimental data for elastic constants are available; therefore, we have to compare their values obtained with different functionals. Constants C11 and C33 always satisfy the following condition: C11,PBE + D3 > C11,B3LYP > C11,PBE, which means that the van der Waals interaction enhances the bond between BCO3 molecules and between layers. The shift constant, on the contrary, always satisfies C44,B3LYP > C44,PBE > C44,PBE + D3. Elastic constants Cij calculated by different functionals have a linear dependency. For example, for KSrCO3F, it looks like CPBE(GPa) = 1.38 + 0.90·CPBE + D3, CB3LYP(GPa) = 1.01 + 0.94·CPBE + D3; for KCdCO3F, it is CPBE(GPa) = 1.20 + 0.91·CPBE + D3, CB3LYP(GPa) = 0.97 + 0.92·CPBE + D3; and for RbMgCO3F, it is CPBE(GPa) = 1.23 + 0.92·CPBE + D3, CB3LYP(GPa) = 1.13 + 0.97·CPBE + D3.
Natural minerals are polycrystalline aggregates and represent a set of randomly oriented single crystals. The study of their mechanical properties is possible in two extreme cases: (1) any uniform deformation in a polycrystalline aggregate is equated to the value of external deformation; (2) uniform stress is equated to external stress. These two assumptions of uniform local strain and uniform local stress are known as the Voigt (
KV,
GV) [
41] and Reuss (
KR,
GR) [
42] approximations. These values can be obtained from the elastic constants using known formulae [
43]. The Hill averaging method provides the best results [
44]: volume and shift moduli can be determined as
KH = (
KV +
KR)/2,
GH = (
GV +
GR)/2. These values [
45] make it possible to calculate the Young modulus
EH = 9
KHGH/(3
KH +
GH) and the Poisson ratio µ = (3
KH − 2
GH)/(2(3
KH +
GH)) (
Table 4).
A crystal cannot physically exist outside
KV/
KR < 1 and
GV/
GR < 1. For all fluorocarbonates, these ratios are ≥1, but by a very small amount. The
KV/
KR ratio is minimal for KZnCO
3F (1.000) and KCaCO
3F (1.0003); it is maximal for RbMgCO
3F (1.034) and RbCdCO
3F (1.0205). Other crystals have intermediate values. These ratios are included in the so-called universal anisotropy index [
46]
AU = 5·
GV/
GR +
KV/
KR-6, which takes into account elastic anisotropy and is equally valid for all types of crystals (symmetries). The orthorhombic phase of carbon has the highest anisotropy, where
AU is 397.3. Trigonal carbon has 284.0 [
47]. Only RbCaCO
3F (1.6) and KSrCO
3F has
AU ≥ 1 (1.18). It is at its lowest in CsCaCO
3F (0.27) and RbCdCO
3F (0.41).
Anisotropy is an important property of any polycrystalline material. The anisotropy coefficient for each modulus can be calculated through its maximal and minimal values for each direction [
48]. For linear compressibility (β = 1/
KH), the direction
a(b) has a minimal value of β
min, and axis
c has a maximal value of β
max (
Figure 9). The maximal anisotropy coefficients for β belong to RbCdCO
3F (1.75) and RbMgCO
3F (1.71), while the minimal value belongs to KZnCO
3F (1.00). The minimal value of shift modulus
Gmin in all fluorocarbonates is in the direction of axis
c and the maximal value is in various combinations of
a(b)c. The maximal anisotropy coefficient for the shift modulus belongs to RbCaCO
3F (3.33): it is ≥ 2 everywhere except RbCdCO
3F (1.82) and CsCaCO
3F (1.67). The maximal Young modulus
Emax is along axis
c. Its maximal anisotropy coefficient belongs to RbCaCO
3F (2.58) and RbSrCO
3F (2.45), whereas the lowest value is in CsCaCO
3F (1.44). The Poisson ratio also has anisotropy but it is impossible to indicate any single selected direction for all materials.
For each group of crystals, elastic moduli KH, GH, and EH decrease as the average cation radius increases. For each modulus, its value is maximal in group III and minimal in group I. Fluorocarbonates with a small grade B cation radius have the maximal KH. The modulus has a reliable linear dependence on average cation radius KH(GPa) = 171.1–79.9·RC (0.81).
Shift modulus
G describes plastic deformation resistance and volume modulus
K reflects fracture resistance. Therefore, the brittle or plastic behavior of solids can be predicted based on the simple empirical relationship that occurs between them. The critical value that separates them is 1.75. If
K/
G > 1.75, the material is plastic; otherwise, it is brittle.
Table 4 demonstrates that all the fluorocarbonates in this research are plastic materials.
The Poisson ratio provides information about the characteristics of the bond strengths. For central forces in solids (ionic crystals), value μ = 0.25 (G/K = 0.6) is the bottom limit, while 0.5 (G/K = 0) is the top limit. At μ ≤ 0.25 (G/K > 0.6), the interatomic forces are non-central, which indicates that the bond has a covalent component. In fluorocarbonates, μ ≥ 0.28 indicates central forces and ionic bonding. The Poisson’s ratio and ratio (G/K) = 1.238–2.588 µ have a reliable linear dependency: group II fluorocarbonates with Zn and Cd and µ > 0.34 have chemical bonding with a greater covalent character than other fluorocarbonates. The same crystals also have greater plasticity, since for them K/G > 2.8.
The resulting set of elastic moduli can be used for semi-empirical estimates of some other physical properties of polycrystalline materials. For instance, acoustic velocities in a solid can be obtained from the volume modulus, shift modulus, and density ρ. In an anisotropic material, wave energy propagates in two modes, i.e., longitudinal and transverse. As a rule, the longitudinal mode is faster: particle oscillations move parallel to wave energy propagation. The transverse mode is slower: particle oscillations are perpendicular to wave energy propagation. Acoustic velocities of longitudinal (
vp) and transverse (
vs) waves can be theoretically defined as [
49]:
vP = ((
KH + 4
/3
GH)/ρ)
1/2,
vS = (
GH/ρ)
1/2. The highest velocities of the longitudinal and transverse waves belong to KCaCO
3F and RbMgCO
3F. In each group, they decrease following the increase in the average cation radius. For example, it is
vs(km/s) = 11.74 − 6.22·
RC,
vP(km/s) = 22.10 − 11.77·
RC in group I.
3.5. Vibration Spectrum
The spectra of infrared light absorption (IRS) and Raman light scattering (RS) of fluorocarbonates are divided into two regions: (1) lattice vibrations that involve metal atoms, fluorine, translational, and rotational displacements of whole carbonate groups; (2) intramolecular vibrations of atoms of carbonate groups. Free
ion (symmetry D
3h) has four main IR-active vibrational modes [
50]: symmetric stretching
ν1 (1100 cm
−1), out-of-plane bending
ν2 (800 cm
−1), singular asymmetric stretching
ν3 (1400 cm
−1), and singular plane deformation mode ν4 (700 cm−1). The same types of vibrations are active in RS.
Table 5 illustrates a classification of optical modes according to irreducible representations of the symmetry groups of fluorocarbonates, the nature of vibrations as external (lattice) or internal, and their activity in IRS and RS.
Table 5 contains no data for symmetries
A1u and
A2g, which are inactive for the indicated spectra.
Table 6 gives wave numbers of vibrational modes.
For group I fluorocarbonates of symmetry P-6m2, only one mode where polarization vectors transform according to the irreducible representation is active in RS. Its αxx, αyy, and αzz tensor components are nonzero. This vibration belongs to the intramolecular v1 type. As the number of carbonate groups increases, the total number of these vibrations for group II with symmetry P-6c2 and group III with symmetry P-62m is 2 and 6, respectively. Doubly degenerate modes of symmetry are active both in IRS with the polarization of the electric field vector E||z and in RS with nonzero components αxx, αxy, and αyy. Group I fluorocarbonates have only five such modes: two internal modes v4 and v3 and three lattice modes. Crystals of symmetry P-62m and Z = 3 have only fourteen: six internal modes, three v4 modes, three v3 modes, and eight lattice modes.
Figure 10 shows the normal long-wave oscillations calculated using functional B3LYP, as well as the IRS absorption and RS of group I fluorocarbonates obtained by their Gaussian broadening. The intensities are calculated as a percentage for ease of comparison. In the IRS spectrum of each crystal, the most intense intramolecular vibration
v3 is taken as 100%. Its intensity decreases in the series as 2207, 2161, and 1964 km/mol with a decrease in the average Born charge of
ZC cation. For all the carbonates in this study, the maximal intensity averaged over number
Z of modes
v3 equals
I(km/mol) = −2337 + 2727·
ZC (0.91). The dynamic charge of alkali cations
ZA correlates with their electronic charge
ZA(|
e|) = 1.85 − 0.83·
QA (0.93), and this correlation is the same for
ZB(|
e|) = 2.83 − 0.445·
QB (0.96). The intensity of line
v1 of the symmetry
was taken as 100% in RS.
In IRS, KCaCO3F, modes v4 of and modes v2 of symmetry have an intensity of ~1.5% and are almost invisible in the spectrum. RS is dominated by mode v1 with wave numbers that decrease in series I as 1110, 1096, and 1096 cm−1, as well as by modes v4 (~35% intensity) and modes v3 (2, 32, and 19% intensity), which makes it possible to identify fluorocarbonates.
Not only wave number and intensity can characterize lattice modes. In the case of carbonate ions, the squared oscillation amplitude (percentage) of an atom or group of atoms is another option. In IRS group I, lattice modes of symmetry with wave numbers 130, 91, and 78 cm−1 are formed by translational vibrations of cations A with CO3 anions and cations B with fluorine anions in opposite directions of plane xy. Alkali metal atoms provide the maximal contribution of 40–50% to the displacement vector amplitudes, while fluorine atoms are responsible for 50–40%. Modes with wave numbers 164, 138, and 148 cm−1 appear when A and F atoms (50%) shift in one direction, while B and CO3 (40%) atoms shift in the other direction. These are translational vibrations of two atomic planes relative to each other. The most intense modes at 210, 208, and 200 cm−1 are formed by translational shifts of cations A and B (46–10%) and anions CO3 (50–90%) and F in opposite directions. Symmetry vibrations move cations and anions in opposite directions on axis z. Modes with low intensity and wave numbers 306, 313, and 245 cm−1 are formed by vibrations of atoms A, CO3 (65%) and B, F. Modes of high intensity at 471, 456, and 395 cm−1 are formed by vibrations of atoms B (27–10%) and fluorine (69–90%). In RS, librational oscillations of CO3 correspond to symmetry modes .
For intramolecular vibrations of KCdCO
3F in IRS (
Figure 11), mode
v3 of symmetry
has a wave number of 1499 cm
−1, its experimental value being1432 cm
−1 [
7]. In RbCdCO
3F, it is 1505 cm
−1 (experimental 1442 cm
−1). Modes
v4 with wave numbers ~720 cm
−1 (730–680 cm
−1) have a low intensity that starts at ~1%. Modes
v2 of symmetry
with wave numbers 856 cm
−1 (853 cm
−1) and 847 cm
−1 (843 cm
−1) also have low intensity. In RS of cadmium fluorocarbonates, symmetry
with wave numbers 1098 and 1100 cm
−1 demonstrate the most intense vibration, while modes
v4 and
v3 have an intensity of ~7–10%.
For IRS of lattice vibrations in KCdCO3F, symmetry modes with wave numbers 231 cm−1 and 117 cm−1 prove to be the most intense ones (13%). In the first vibration, cadmium (3%) and CO3 (94%) atoms are displaced in opposite directions of plane xy. In the second vibration, Cd and CO3 atoms shift in the same direction, while potassium (40%) and fluorine (56%) atoms shift in the other direction (in-plane shifts). Symmetry mode with wave number 429 cm−1 appears as potassium and fluorine atoms (~90%) shift in one direction z, while Cd and CO3 (~10%) shift in another direction. Symmetry mode with wave number 217 cm−1 results from translational vibrations of cadmium atoms (15%) and UCO3 anions (80%).
As for group II fluorocarbonates, the most intense (~15–20%) lattice vibrations in RS are symmetry modes with wave numbers 340, 300, and 310 cm−1, as well as symmetry modes . This symmetry corresponds to immobile Zn (Cd) and C atoms, as well as potassium (rubidium) and fluorine atoms that vibrate in plane xy (~33%) and CO3 atoms that rotate in the direction of axis z (~65%).
In IRS of RbMgCO
3F (
Figure 12), mode
v3 with wave number 1539 cm
−1 is the most intense one (5852 km/mol). It is followed by a mode with 13% intensity and a wave number of 1591 cm
−1. The mode with 0.5% intensity at 1624 cm
−1 comes last (experimental wave number 1495 and 1650 cm
−1) [
9]. Wave number gaps of 50 and 30 cm
−1 prove that molecules interact. The contribution of the polarization vectors to mode I is 82% of C1O
3 and 17% of C2O
3. For mode II, it is 67% and 32%, respectively. For mode III, it is 50% of each. Three modes
v4 of symmetry
have wave numbers of 705, 709, and 732 cm
−1 (680 cm
−1). Their intensity is ≤0.5%. The C2O
3 group is responsible for 99% of the last mode. Two modes of type
v2 with intensities of ~2% have wave numbers of 842 and 846 cm
−1 (890 cm
−1). The third mode of this type with wave number 843 cm
−1 belongs to symmetry
and is outside of the IRS scope. In BRS, modes v1 of symmetry
with wave numbers 1184 cm
−1 (100%) and 1107 cm
−1 (52%) prove to be the most intense ones. They develop by 99.6% due to vibrations of oxygen atoms O1 and by 99.9% due to vibrations of oxygen atoms O2 along the C–O bond line.
The other two group III fluorocarbonates show changes in RS. RbMgCO3F has the second v1 maximum located to the left of the main one. However, in RbCaCO3F and CsCaCO3F it is on the right. The fact is that in fluorocarbonate with magnesium, bond length RC1-O1 is shorter than RC2-O2, whereas, in fluorocarbonate calcium, RC1-O1 is longer than RC2-O2. These structural features also lead to other changes in the spectrum. The positions of the wave numbers and mode v3 intensities swap places. As a result, the central maximum changes at 1536 cm−1 in CsCaCO3F. The position of the maxima in band v4 also changes, while maintaining its width.
In lattice vibrations of RbMgCO
3F, the IRS demonstrates an intense (37%) symmetry mode
with wave number 354 cm
−1. It depends on 60% of magnesium atom vibration, 23% on C1O
3, and 16% on C2O
3. Symmetry mode
with wave number 497 cm
−1 (experimental 540 cm
−1 [
9]) and an intensity of 5% depends on 27% magnesium atom vibration and 71% on fluorine atoms. In RS, the most intense modes (10 and 6%) belong to symmetry
with wave numbers 159 cm
−1 and 219 cm
−1. Both are librational. The first is formed by vibrations of O1 oxygen atoms. The second involves magnesium and fluorine atoms in addition to O1 (36%) and O2 (30%).
The energy of zero-point vibrations is an important energy characteristic of the phonon spectra of crystals. No accurate thermodynamic analysis of fluorocarbonate development is possible without it. The zero-point energy in terms of one formula unit has a correlation coefficient of 0.7. It decreases following the increase in the average ionic radius EZP(kJ/mol) = 83.89 − 22.34·RC. The wave numbers of intramolecular vibrations also depend on the average radius of the cation. For mode v4, they increase in group I while decreasing (mean value) in groups II and III. As a result, their linear dependence has a correlation coefficient of at least 0.75 of v4(cm−1) = 883 − 123·RC. Out-of-plane vibration mode v2 (cm−1) = 690 − 123·RC behaves approximately the same way. For the fully symmetric mode that dominates RS, it is v1(cm−1) = 1315 − 151·RC. These formulas can predict the IRS and RS of fluorocarbonates.
Tran et al. measured wave numbers of some intramolecular modes for RbMgCO
3F in [
9]. Their mean-square deviation for B3LYP is 4.7%, for PBE—5.9%, and for PBE + D3—4.6%. In the IRS of RbMgCO
3F, functional PBE calculations of wave numbers for twenty active vibrations are connected by a linear dependency with the functional B3LYP as
vPBE(cm
−1) = −2.47 + 0.97·
vB3LYP. For PBE + D3, it is
vPBE + D3(cm
−1) = 4.74 + 0.97·
vB3LYP. For B3LYP, some frequency values are higher and some are lower than for PBE + D3, but they are always greater than PBE. For example, the lattice mode Mg-F of symmetry
for the functionals, B3LYP and PBE have a wave number of 497 cm
−1, and for PBE + D3 its value is 537 cm
−1. Therefore, intermolecular interaction agrees with the experimental value for this lattice mode. The situation is different for intramolecular modes, e.g., for KCaCO
3F, it is as follows:
vPBE(cm
1) = −18.2 + 0.98·
vB3LYP,
vPBE + D3(cm
−1) = −22.9 + 0.99·v
B3LYP. Thus, the functionals PBE and PBE + D3 provide lower wave numbers than B3LYP. The ratio of zero-point energy obtained by functional B3LYP is 1.001 ÷ 1.036 times greater than by functional PBE and 1.00 ÷ 1.027 times greater than the functional PBE + D3.