Treatment of Liquid Digestate by Green Algal Isolates from Artificial Eutrophic Pond
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of the Liquid Fraction of Digestate Used for the Tests
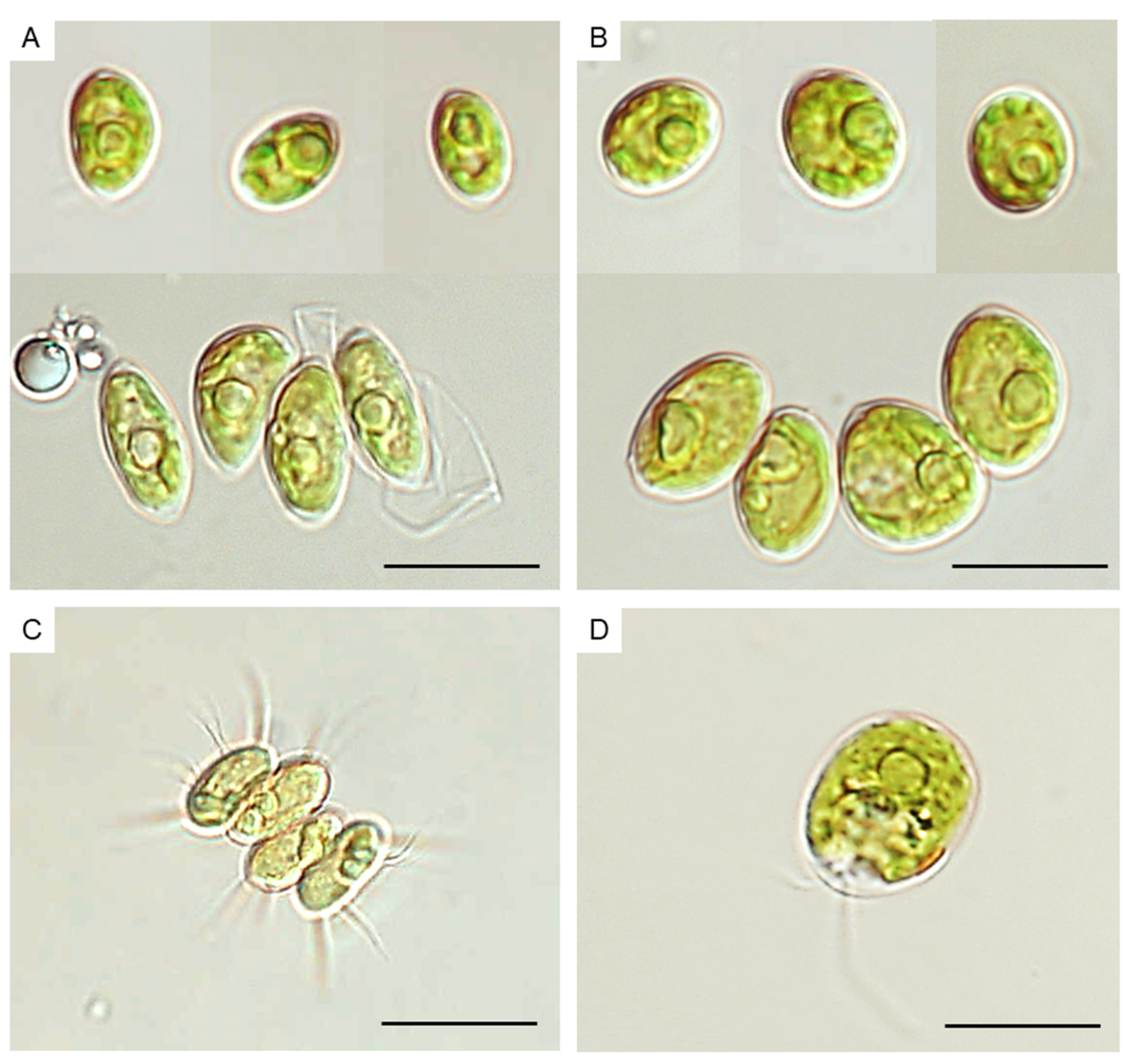
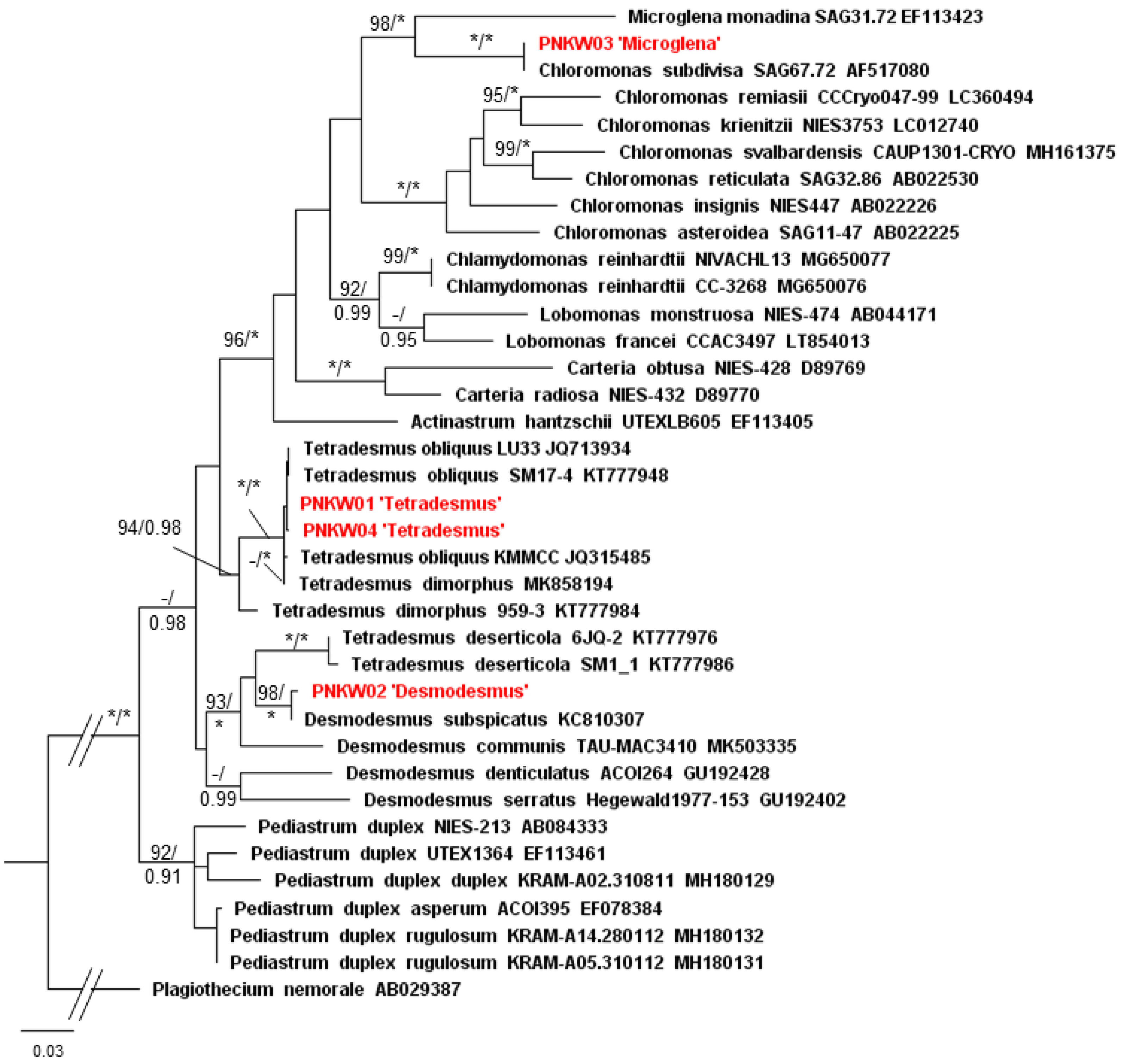
2.2. Isolation and Taxonomic Identification of Microalgae
2.3. Calibration of Maximum Absorbance for Optical Density
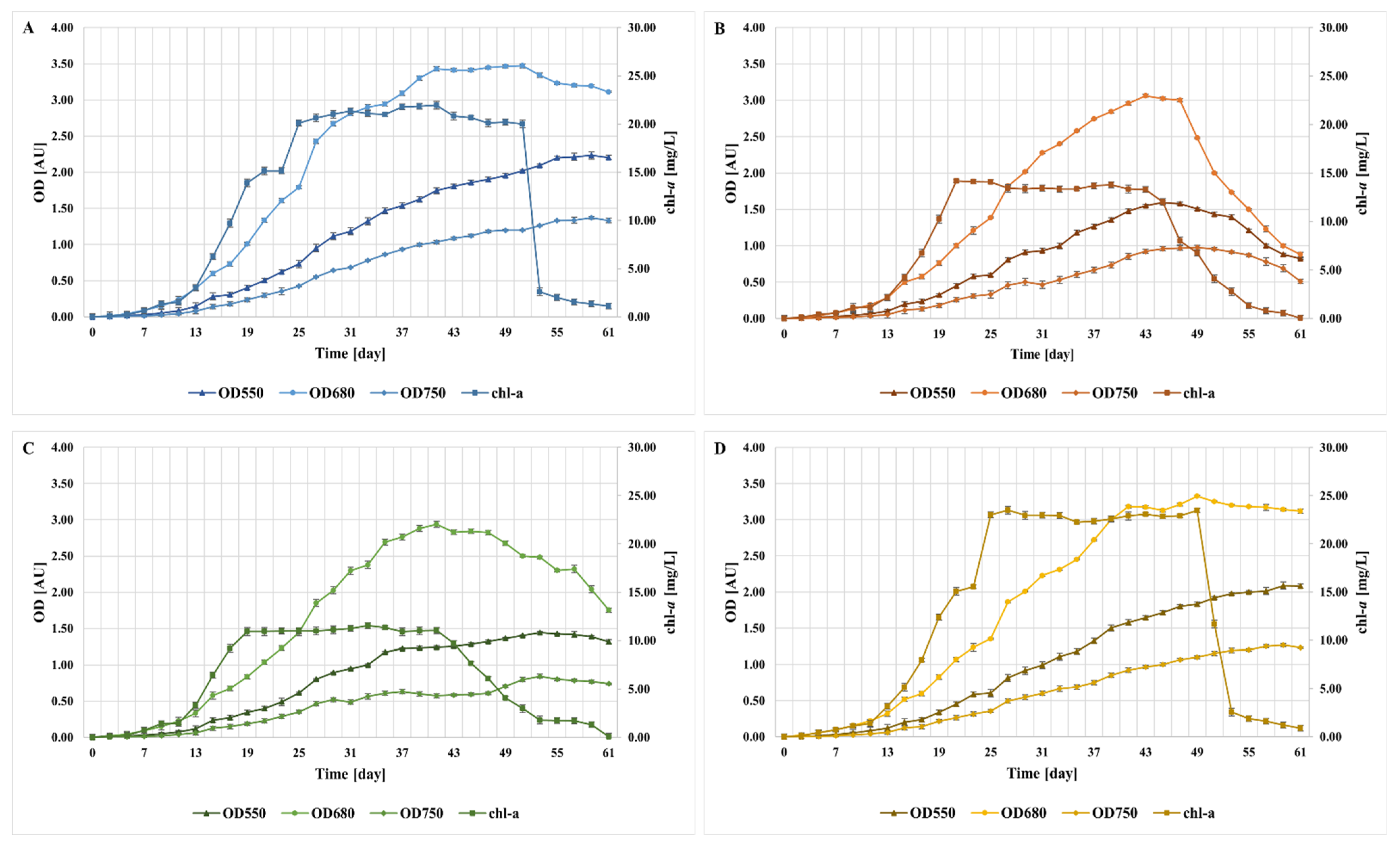
2.4. The Growth Curves for Isolated Microalgae
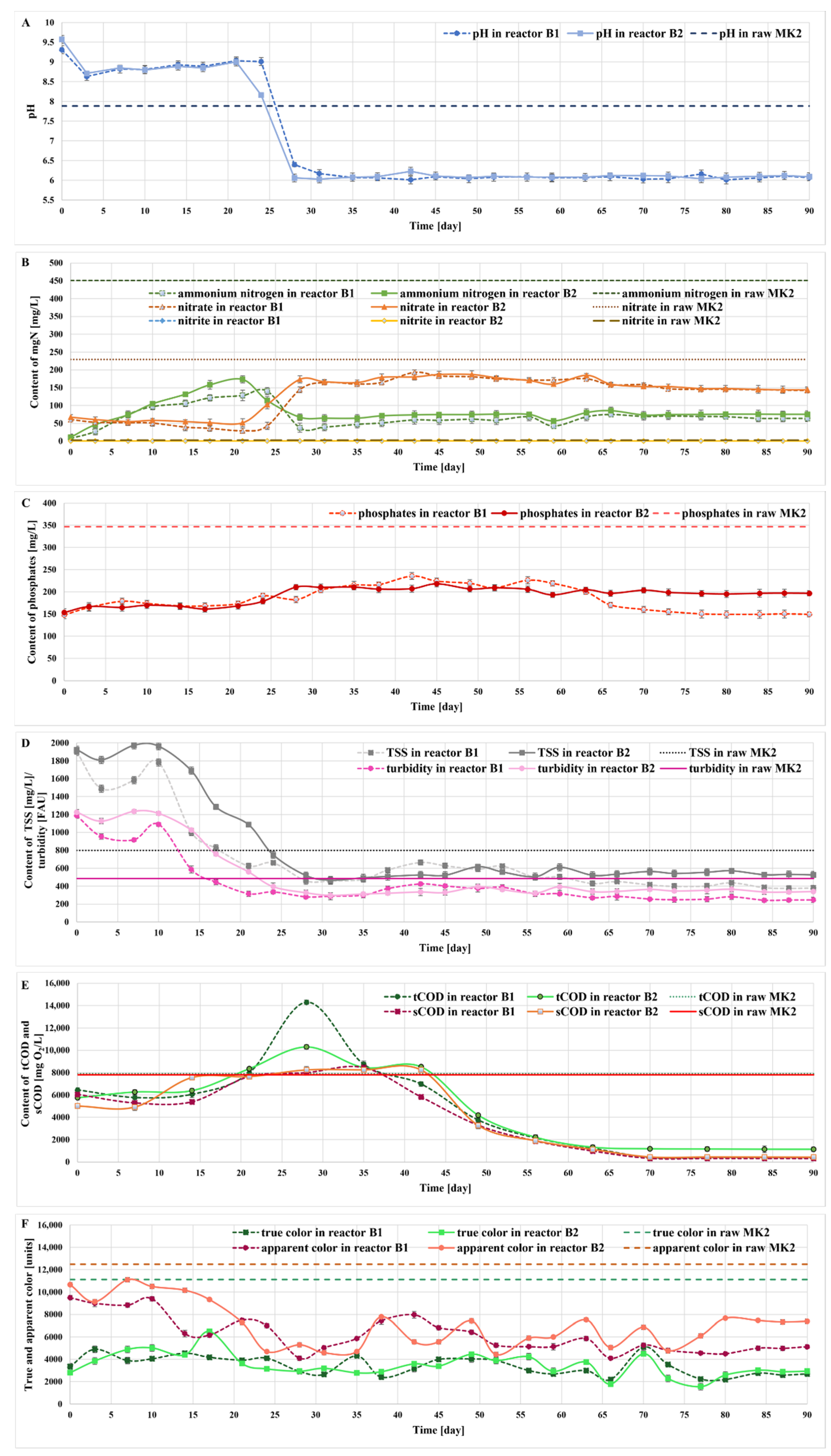
2.5. Treatment of the Liquid Digestate in Photobioreactors
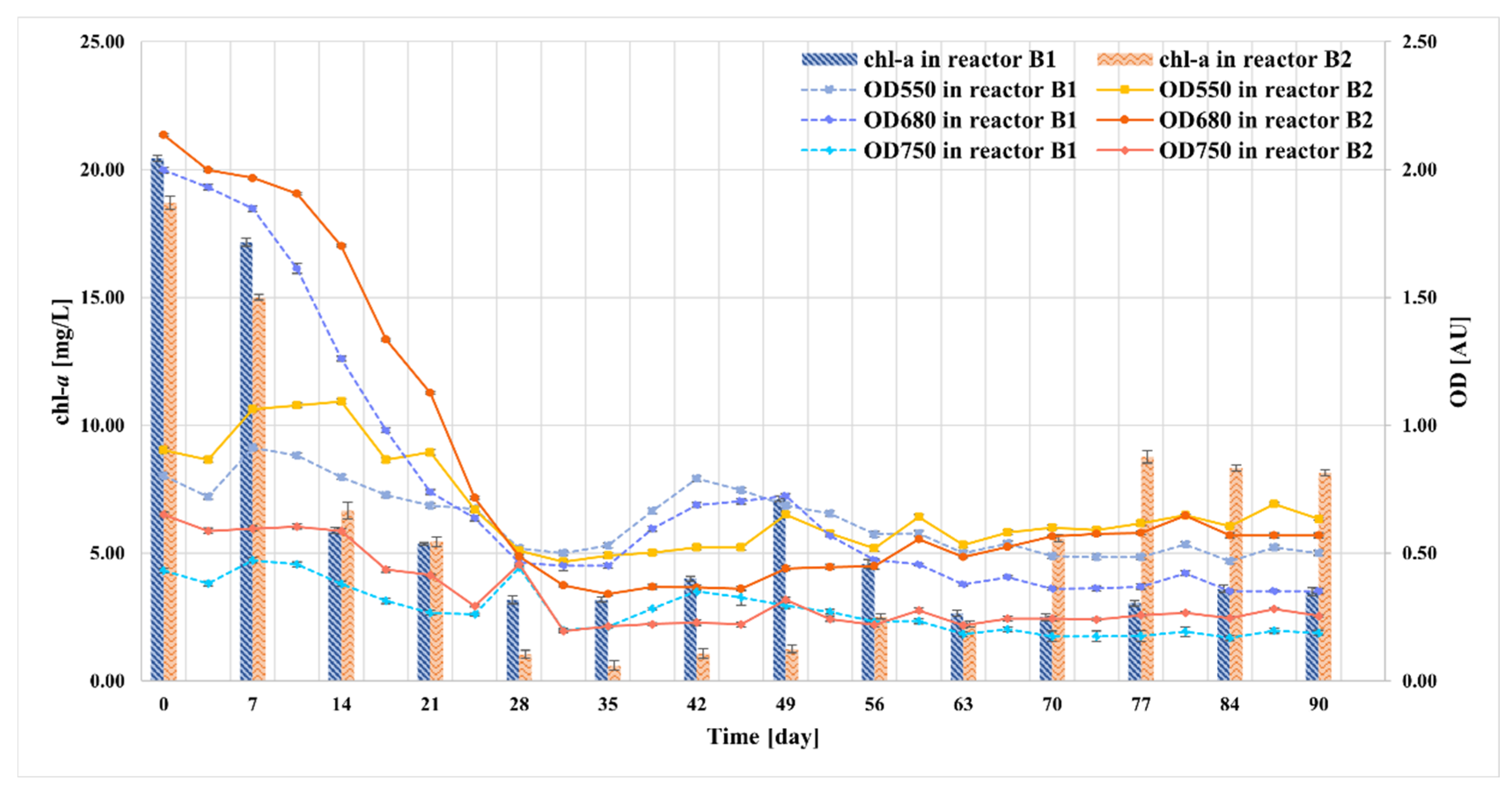
2.6. Monitoring the Growth of Microalgae in Bioreactors
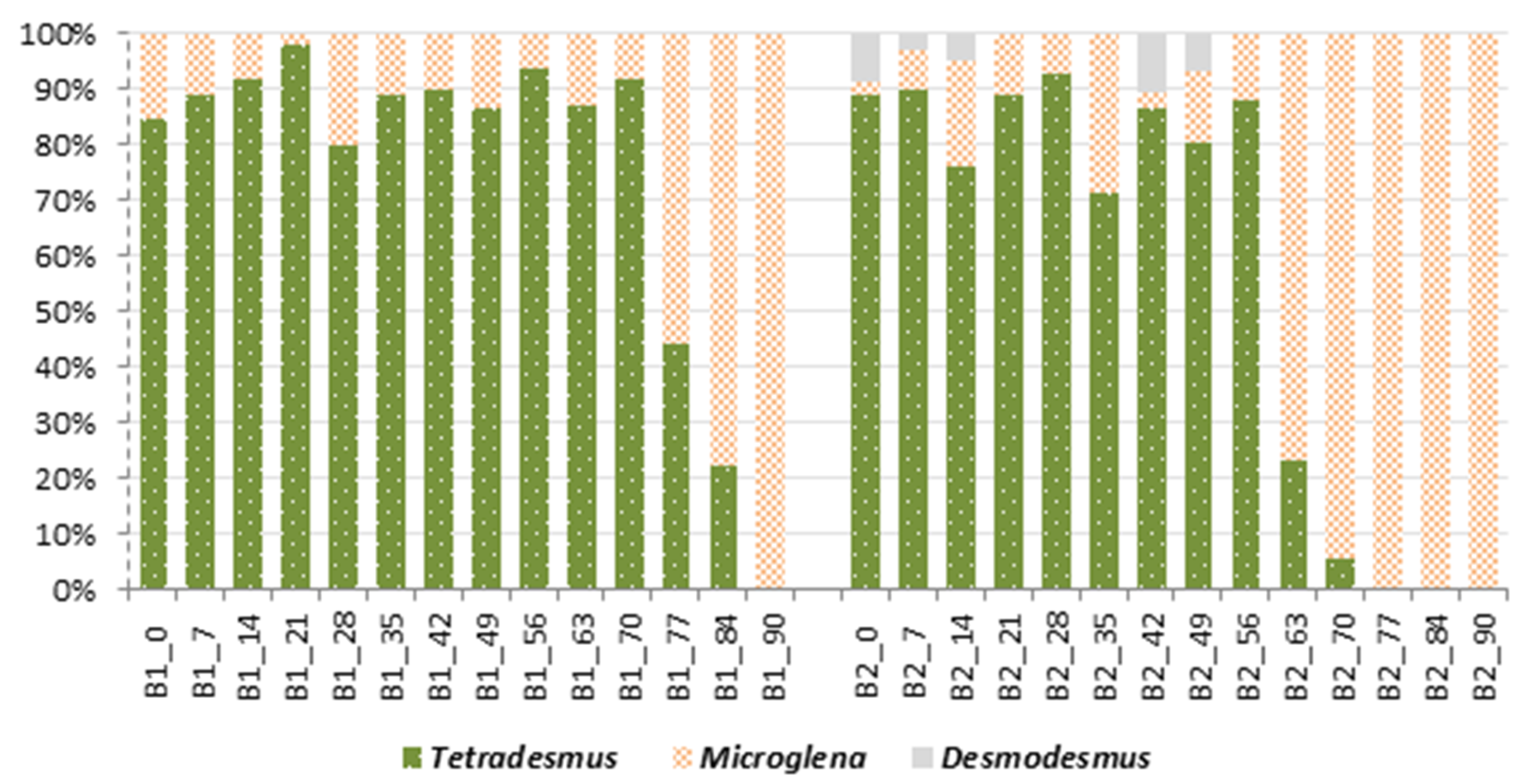
2.7. Qualitative and Quantitative Structure of Microalgal Culture in Bioreactors
3. Materials and Methods
3.1. Environmental Sampling and Media
3.2. Experiments
3.2.1. Isolation of Microalgae from the Environment
3.2.2. Determination of the Growth Curves for Isolated Microalgae
3.2.3. Liquid Digestate Treatment by Mixed Microalgae in Bioreactors
3.3. Analyses
3.3.1. Physical and Chemical Analyses of Liquid Digestate
3.3.2. Calibration of Maximum Absorbance for Optical Density Measurement
3.3.3. Optical Density Measurement
3.3.4. Measurement of the Chlorophyll a Concentration
3.3.5. Taxonomic Analyses of Microalgae from Cultures
3.3.6. The Structure of Microalgae Culture in Bioreactors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Romero-güiza, M.S.; Mata-alvarez, J.; María, J.; Rivera, C. Nutrient recovery technologies for anaerobic digestion systems: An overview Tecnologías de recuperación de nutrientes para los sistemas de digestión anaeróbica: Revisión Tecnologias de recuperação de nutrientes para os sistemas de digestão anaeróbia: R. Bucaramanga 2015, 29, 7–26. [Google Scholar]
- Wang, W.; Lee, D.-J. Valorization of anaerobic digestion digestate: A prospect review. Bioresour. Technol. 2021, 323, 124626. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Z.; Isaguirre, C.; Liu, Y.; Liao, W. Fungal fermentation on anaerobic digestate for lipid-based biofuel production. Biotechnol. Biofuels 2016, 9, 253. [Google Scholar] [CrossRef]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Albini, E.; Pecorini, I.; Ferrara, G. Improvement of Digestate Stability Using Dark Fermentation and Anaerobic Digestion Processes. Energies 2019, 12, 3552. [Google Scholar] [CrossRef]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-bacterial aggregates: Applications and perspectives for wastewater treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Szwarc, K.; Szwarc, D.; Zieliński, M. Purification of Post-Fermentation Effluent Using Chlorella vulgaris Microalgae. Multidiscip. Digit. Publ. Inst. Proc. 2018, 2, 1285. [Google Scholar] [CrossRef]
- Wang, H.; Qi, B.; Jiang, X.; Jiang, Y.; Yang, H.; Xiao, Y.; Jiang, N.; Deng, L.; Wang, W. Microalgal interstrains differences in algal-bacterial biofloc formation during liquid digestate treatment. Bioresour. Technol. 2019, 289, 121741. [Google Scholar] [CrossRef] [PubMed]
- Uggetti, E.; Sialve, B.; Latrille, E.; Steyer, J.P. Anaerobic digestate as substrate for microalgae culture: The role of ammonium concentration on the microalgae productivity. Bioresour. Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Wu, D.; Wang, L.; Yang, Z.; Yan, W.; Jin, Y.; Chen, F.; Song, Y.; Cheng, X. Biochemical wastewater from landfill leachate pretreated by microalgae achieving algae’s self-reliant cultivation in full wastewater-recycling chain with desirable lipid productivity. Bioresour. Technol. 2021, 340, 125640. [Google Scholar] [CrossRef]
- Paskuliakova, A.; Tonry, S.; Touzet, N. Phycoremediation of landfill leachate with chlorophytes: Phosphate a limiting factor on ammonia nitrogen removal. Water Res. 2016, 99, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Zhao, G.; Zhang, H. Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl. Microbiol. Biotechnol. 2015, 99, 6493–6501. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Quattrocelli, P.; Bordiean, A. Effects of Liquid Digestate Treatment on Sustainable Microalgae Biomass Production. BioEnergy Res. 2022, 15, 357–370. [Google Scholar] [CrossRef]
- Massa, M.; Buono, S.; Langellotti, A.L.; Castaldo, L.; Martello, A.; Paduano, A.; Sacchi, R.; Fogliano, V. Evaluation of anaerobic digestates from different feedstocks as growth media for Tetradesmus obliquus, Botryococcus braunii, Phaeodactylum tricornutum and Arthrospira maxima. New Biotechnol. 2017, 36, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Lakaniemi, A.M.; Rintala, J.A. Cultivation of Scenedesmus acuminatus in different liquid digestates from anaerobic digestion of pulp and paper industry biosludge. Bioresour. Technol. 2017, 245, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org/about/ (accessed on 27 April 2022).
- Nielsen, S.L.; Hansen, B.W. Evaluation of the robustness of optical density as a tool for estimation of biomass in microalgal cultivation: The effects of growth conditions and physiological state. Aquac. Res. 2019, 50, 2698–2706. [Google Scholar] [CrossRef]
- Santos-Ballardo, D.U.; Rossi, S.; Hernández, V.; Gómez, R.V.; del Carmen Rendón-Unceta, M.; Caro-Corrales, J.; Valdez-Ortiz, A. A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquaculture 2015, 448, 87–92. [Google Scholar] [CrossRef]
- Yatirajula, S.K.; Shrivastava, A.; Saxena, V.K.; Kodavaty, J. Flow behavior analysis of Chlorella Vulgaris microalgal biomass. Heliyon 2019, 5, e01845. [Google Scholar] [CrossRef]
- Iasimone, F.; Zuccaro, G.; D’Oriano, V.; Franci, G.; Galdiero, M.; Pirozzi, D.; De Felice, V.; Pirozzi, F. Combined yeast and microalgal cultivation in a pilot-scale raceway pond for urban wastewater treatment and potential biodiesel production. Water Sci. Technol. 2018, 77, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Steele, D.J. Cellular Viability and the Occurrence and Significance of Chlorophyll Allomers during Phytoplankton Turnover. Ph.D. Thesis, Bournemouth University, Poole, UK, 2014. [Google Scholar]
- Sarkis, S.; Lovatelli, A. Installation and Operation of a Modular Bivalve Hatchery; FAO: Rome, Italy, 2007; Volume 492, ISBN 9788578110796. [Google Scholar]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Franchino, M.; Comino, E.; Bona, F.; Riggio, V.A. Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate. Chemosphere 2013, 92, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhou, Y.; Pang, A.; Ning, L.; Rodgers, K.; Liu, Y.; Dong, R. Fed-batch cultivation of Desmodesmus sp. in anaerobic digestion wastewater for improved nutrient removal and biodiesel production. Bioresour. Technol. 2015, 184, 116–122. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res. 2011, 45, 3351–3358. [Google Scholar] [CrossRef]
- Marténez, M.E.; Sánchez, S.; Jiménez, J.M.; El Yousfi, F.; Muñoz, L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Larsdotter, K.; la Cour Jansen, J.; Dalhammar, G. Phosphorus removal from wastewater by microalgae in Sweden—A year-round perspective. Environ. Technol. 2010, 31, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Kumar, P.; Malik, A.; Vijay, V.K. Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: A closed loop bioenergy generation process. Bioresour. Technol. 2014, 158, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hodaifa, G.; Martínez, M.E.; Sánchez, S. Influence of pH on the culture of Scenedesmus obliquus in olive-mill wastewater. Biotechnol. Bioprocess Eng. 2009, 14, 854–860. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Science: Amsterdam, The Netherlands, 2005; ISBN 9780120884261. [Google Scholar]
- El-Sheekh, M.M.; El-Gamal, A.; Bastawess, A.E.; El-Bokhomy, A. Production and characterization of biodiesel from the unicellular green alga Scenedesmus obliquus. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 783–793. [Google Scholar] [CrossRef]
- Verma, S.; Bagul, S.Y.; Choudhary, P.; Chakdar, H.; Das, S.; Siddiqui, N.; Saxena, A.K. Microscope Assisted Uni-algal isolation through Dilution (MAU-D): A simple modified technique for tapping diverse cyanobacteria. 3 Biotech 2021, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.D.; Clesceri, L.S.; Franson, M.A.H.; Association, A.P.H.; Rice, E.W.; Greenberg, A.E.; Association, A.W.W.; Federation, W.E. Standard Methods for the Examination of Water & Wastewater; Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005; ISBN 9780875530475. [Google Scholar]
- Lúcia, H.R.R.; Alexandre, A.; Maria, T.R.-R.; Nelson, F.F. Algal density assessed by spectrophotometry: A calibration curve for the unicellular algae Pseudokirchneriella subcapitata. J. Environ. Chem. Ecotoxicol. 2011, 3, 225–228. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994; Volume 10, ISBN 0521350204. [Google Scholar]
- Lee, Y.-K.; Chen, W.; Shen, H.; Han, D.; Li, Y.; Jones, H.D.T.; Timlin, J.A.; Hu, Q. Basic Culturing and Analytical Measurement Techniques. In Handbook of Microalgal Culture; Wiley Online Books; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 37–68. ISBN 9781118567166. [Google Scholar]
- Ettl, H.; Gärtner, G. Chlorophyta II. Tetrasporales, Chlorococcales, Gloeodendrales. Süswasserflora Von Mitteleur. 1988, 10, 1–436. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. Partitionfinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Dahlkild, Å.; Källersjö, M.; Lohtander, K.; Tehler, A. Photobiont Diversity in the Physciaceae (Lecanorales). Bryologist 2001, 104, 527–536. [Google Scholar] [CrossRef]
- Piercey-Normore, M.D.; DePriest, P.T. Algal Switching among Lichen Symbioses. Am. J. Bot. 2001, 88, 1490–1498. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In PCR—Protocols and Applications—A Laboratory Manual; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1990; pp. 315–322. ISBN 0-12-372180-6. [Google Scholar]
- Hoshina, R.; Kato, Y.; Kamako, S.; Imamura, N. Genetic Evidence of “American” and “European” Type Symbiotic Algae of Paramecium bursaria Ehrenberg. Plant Biol. 2005, 7, 526–532. [Google Scholar] [CrossRef]
- Nozaki, H.; Itoh, M.; Sano, R.; Uchida, H.; Watanabe, M.M.; Kuroiwa, T. Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from rbcL gene sequence data. J. Phycol. 1995, 31, 970–979. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]

| Indicator | Type of Liquid Fraction of Digestate | ||
|---|---|---|---|
| MK1 | MK2 | GS | |
| pH | 7.55 ± 0.01 | 7.88 ± 0.25 | 4.43 ± 0.01 |
| Total Solids, TS [g/kg] | 2.09 ± 0.01 | 3.23 ± 0.17 | 2.84 ± 0.01 |
| Volatile Solids, VS [g/kg] | 0.54 ± 0.01 | 1.73 ± 0.19 | 1.35 ± 0.02 |
| Volatile Solids, VS [% TS] | 25.91 ± 0.38 | 53.38 ± 3.19 | 47.58 ±0.37 |
| Total Suspended Solids, TSS [mg/L] | 185.00 ± 2.00 | 798.33 ± 158.62 | 7.33 ± 0.58 |
| Turbidity [FAU] | 129.00 ± 3.00 | 485.00 ± 111.00 | 111.00 ± 3.00 |
| Apparent Color [units] | 1631.00 ± 17.00 | 12,483.00 ± 2586.00 | 410.00 ± 1.00 |
| True Color [units] | 563.00 ± 1.00 | 11,117.00 ± 2835.00 | 369.00 ± 10.00 |
| Total Chemical Oxygen Demand, tCOD [mg O2/L] | 7743.00 ± 168.00 | 7900.00 ± 754.00 | 7413.00 ± 90.00 |
| Soluble Chemical Oxygen Demand, sCOD [mg O2/L] | 6810.00 ± 287.00 | 7800.00 ± 540.00 | 6417.00 ± 57.00 |
| Total Volatile Fatty Acids, TVFA [mg/L] | 3076.00 ± 70.00 | 3110.00 ± 1128.00 | 3097.00 ± 86.00 |
| Ammonium Nitrogen, N-NH4 [mg/L] | 131.33 ± 1.53 | 580.00 ± 95.50 | 128.67 ± 1.53 |
| Nitrates (V), N-NO3 [mg/L] | 766.67 ± 57.74 | 1016.67 ± 116.90 | 1013.33 ± 57.74 |
| Nitrates (III), N-NO2 [mg/L] | 5.67 ± 0.58 | 9.33 ± 3.01 | 6.00 ± 1.00 |
| Phosphates, P-PO4 [mg/L] | 360.00 ± 69.28 | 346.67 ± 26.58 | 810.00 ± 40.00 |
| Sulfates [mg/L] | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sulfides [μg/L] | 0.00 ± 0.00 | 1550.00 ± 615.63 | 0.00 ± 0.00 |
| Chlorides [mg/L] | 330.00 ± 10.00 | 216.67 ± 36.70 | 283.33 ± 11.55 |
| Iron [mg/L] | 21.00 ± 4.00 | 4.00 ± 2.28 | 9.33 ± 0.58 |
| Copper [mg/L] | 5.67 ± 0.58 | 5.67 ± 0.52 | 7.00 ± 1.00 |
| Zinc [mg/L] | 10.33 ± 1.53 | 15.17 ± 1.72 | 14.00 ± 1.00 |
| Aluminum [mg/L] | 9.83 ± 0.40 | 10.30 ± 8.81 | 0.60 ± 0.20 |
| Marker | Primer | F/R | Reference | Reaction Conditions |
|---|---|---|---|---|
| ITS | ITS1AKL | F | [44] | 95 °C (3 min) 35 × [94 °C (1 min)/52 °C (1 min)/72 °C (2 min)] 72 °C (7 min) |
| Nr-LSU-0012-3′ | R | [45] | ||
| ITS3 | F | [46] | ||
| HLR3R | R | [47] | ||
| rbcL | rbcL1 | F | [48] | 95 °C (2 min) 35 × [94 °C (1 min)/50 °C (1 min)/72 °C (3 min)] 72 °C (8 min) |
| rbcL1421 | R |
| ITS1 | 5.8S gDNA | ITS2 | rbcL Codon | |
|---|---|---|---|---|
| ML | TrN + I + G | TIM + I + G | JC | GTR + I |
| BI | F81 | HKY | JC | GTR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolewska, E.; Borowski, S.; Nowicka-Krawczyk, P.; Banach, K. Treatment of Liquid Digestate by Green Algal Isolates from Artificial Eutrophic Pond. Molecules 2022, 27, 6856. https://doi.org/10.3390/molecules27206856
Sobolewska E, Borowski S, Nowicka-Krawczyk P, Banach K. Treatment of Liquid Digestate by Green Algal Isolates from Artificial Eutrophic Pond. Molecules. 2022; 27(20):6856. https://doi.org/10.3390/molecules27206856
Chicago/Turabian StyleSobolewska, Ewelina, Sebastian Borowski, Paulina Nowicka-Krawczyk, and Katarzyna Banach. 2022. "Treatment of Liquid Digestate by Green Algal Isolates from Artificial Eutrophic Pond" Molecules 27, no. 20: 6856. https://doi.org/10.3390/molecules27206856
APA StyleSobolewska, E., Borowski, S., Nowicka-Krawczyk, P., & Banach, K. (2022). Treatment of Liquid Digestate by Green Algal Isolates from Artificial Eutrophic Pond. Molecules, 27(20), 6856. https://doi.org/10.3390/molecules27206856









