Abstract
A series of novel indole Schiff base derivatives (2a–2t) containing a 1,3,4-thiadiazole scaffold modified with a thioether group were synthesized, and their structures were confirmed using FT-IR, 1H NMR, 13C NMR, and HR-MS. In addition, the antifungal activity of synthesized indole derivatives was investigated against Fusarium graminearum (F. graminearum), Fusarium oxysporum (F. oxysporum), Fusarium moniliforme (F. moniliforme), Curvularia lunata (C. lunata), and Phytophthora parasitica var. nicotiana (P. p. var. nicotianae) using the mycelium growth rate method. Among the synthesized indole derivatives, compound 2j showed the highest inhibition rates of 100%, 95.7%, 89%, and 76.5% at a concentration of 500 μg/mL against F. graminearum, F. oxysporum, F. moniliforme, and P. p. var. nicotianae, respectively. Similarly, compounds 2j and 2q exhibited higher inhibition rates of 81.9% and 83.7% at a concentration of 500 μg/mL against C. lunata. In addition, compound 2j has been recognized as a potential compound for further investigation in the field of fungicides.
1. Introduction
Food crop diseases caused by fungi have become one of the concerns in the global agricultural sector [1]. Fungal diseases directly cause a reduction in crop yield and quality, which results in a huge economic loss for farmers worldwide [1,2]. Furthermore, some pathogenic fungi can secrete toxins and metabolites that are harmful to humans and livestock [3,4,5,6]. For example, F. oxysporum is a soil-borne fungal pathogen widely distributed throughout the world that can infect more than 100 valuable crops by causing blight and root rot, seriously affecting plant growth, yield, and quality [7,8,9,10,11,12,13]. Similarly, F. graminearum is responsible for fusarium head blight (FHB) disease in barley, rice, and oat, and stem rot and spike rot in maize, which severely affects the production of these crops on a global scale [14,15,16,17,18]. Meanwhile, mycotoxins such as trichothecenes and zearalenone produced by F. graminis are harmful to humans and livestock [19]. The use of fungicides is the most common and well-known method for controlling these fungal diseases. However, the excessive or improper use of antifungal agents leads to an increase in the resistance of fungi to fungicides. Thus, the discovery of new antifungal compounds with a new mechanism of action is of great significance for future development in agriculture.
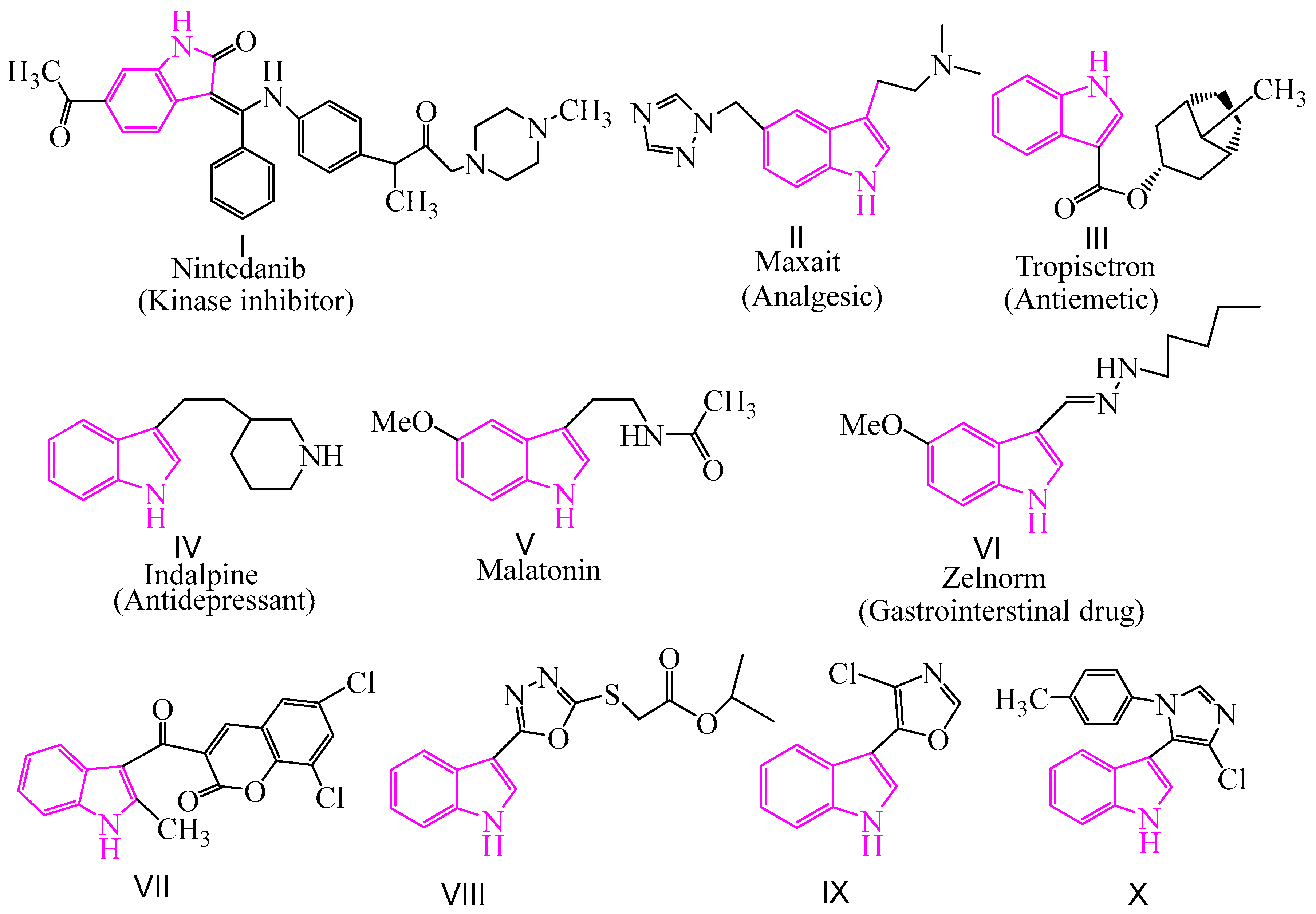
In recent years, heterocyclic pesticides have become the mainstream of pesticide research because of their flexible structure, low toxicity, and high activity. Indole is an important nitrogen-containing heterocyclic compound. Indole and indole derivatives have a broad spectrum of biological activities such as antifungal [20,21,22,23,24,25,26], antibacterial [27,28,29], antimycobacterial [30], antitubercular [31,32,33], antioxidant [34], antimalarial [35,36,37], antiviral [38,39,40,41], anti-leishmanial [42,43], anti-inflammatory [44], and anti-tumor [45,46,47] activities. The design and synthesis of new indole derivatives with excellent biological activity is one of the emerging fields in pharmaceutical chemistry. There are various indole-based drugs available for the treatment of human fatal diseases (I–VI in Figure 1). Furthermore, some indole derivatives containing coumarin [48], thiofuran [49], oxazole [50], and imidazole [51] at the 3-position of the indole ring were found to exhibit obvious fungicidal activity (VII–X in Figure 1). However, the usage of commercial indole-based pesticides for the treatment of plant fungal diseases has not yet been explored.
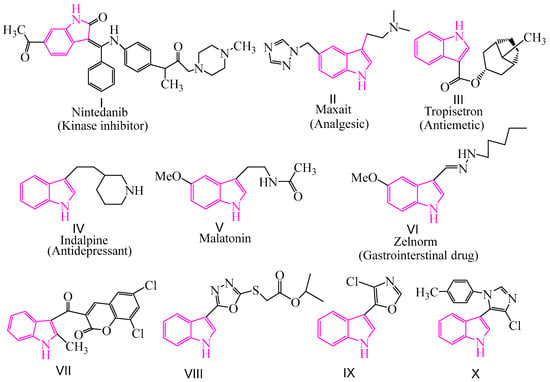
Figure 1.
The structures of commercial drugs and antifungal active compounds containing indole.
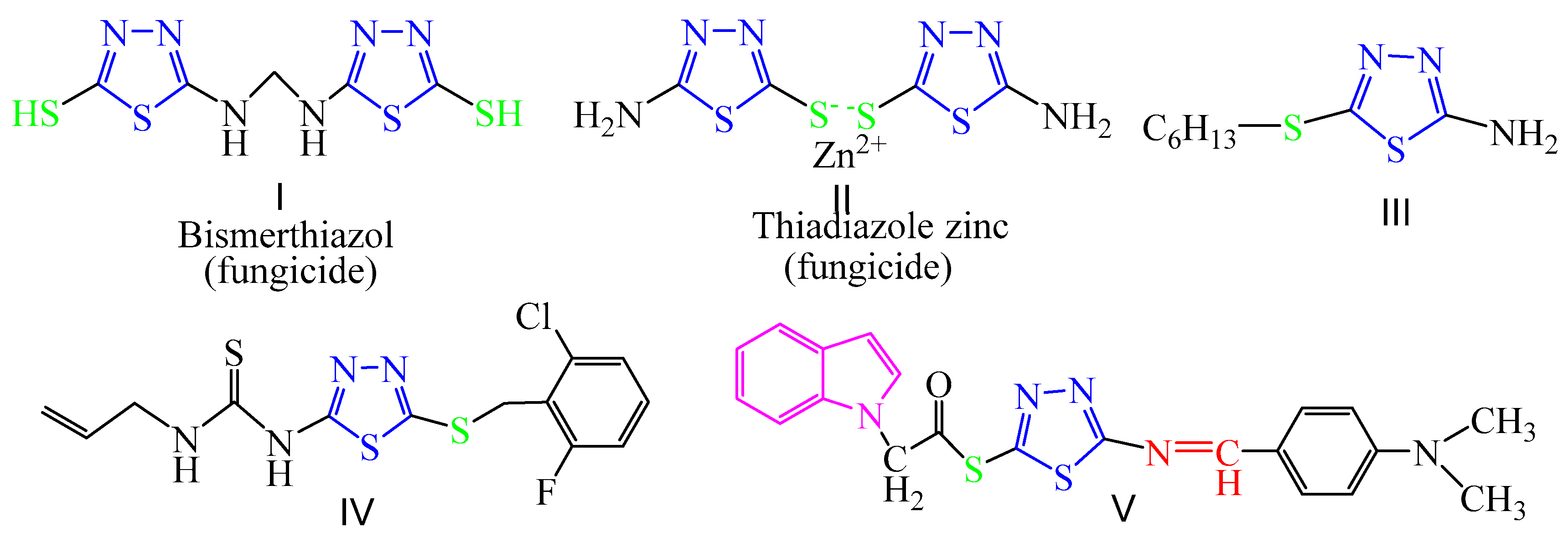
1,3,4-thiadiazole derivatives were widely used as pesticides in agrochemical chemistry and studied for years due to their excellent biological activities, including antifungal [52,53], insecticidal [54], acaricidal [54], antibacterial [55], and herbicidal activities [56]. Heterocyclic thioether compounds also possess high antifungal activities [57], and the thioether-bound 1,3,4-thiadiazole scaffold is an important pharmacophore [58]. Some thioether-bound 1,3,4-thiadiazole derivatives such as bismerthiazol and 2,5-dimercapto-1,3,4-thiadiazole zinc salts (I, II in Figure 2) have been used as commercial fungicides for plant fungal diseases. Schiff base, a class of compounds with imine groups (-CH=N-), is a common pharmacological group in many compounds, which has a wide range of biological activities [59,60]. The introduction of Schiff bases into 1,3,4-thiadiazoleis is interesting to study, and the 1,3,4-thiadiazole Schiff base derivatives also have biological activities [61,62]. For example, compound V in Figure 2 were found to exhibit obvious fungicidal activity [63].
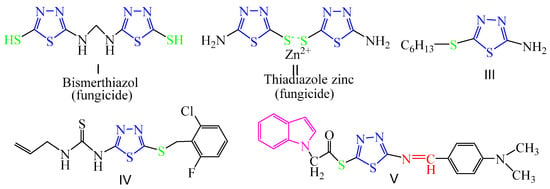
Figure 2.
The structures of fungicides and antifungal activity compounds containing 1,3,4-thiadiazole.
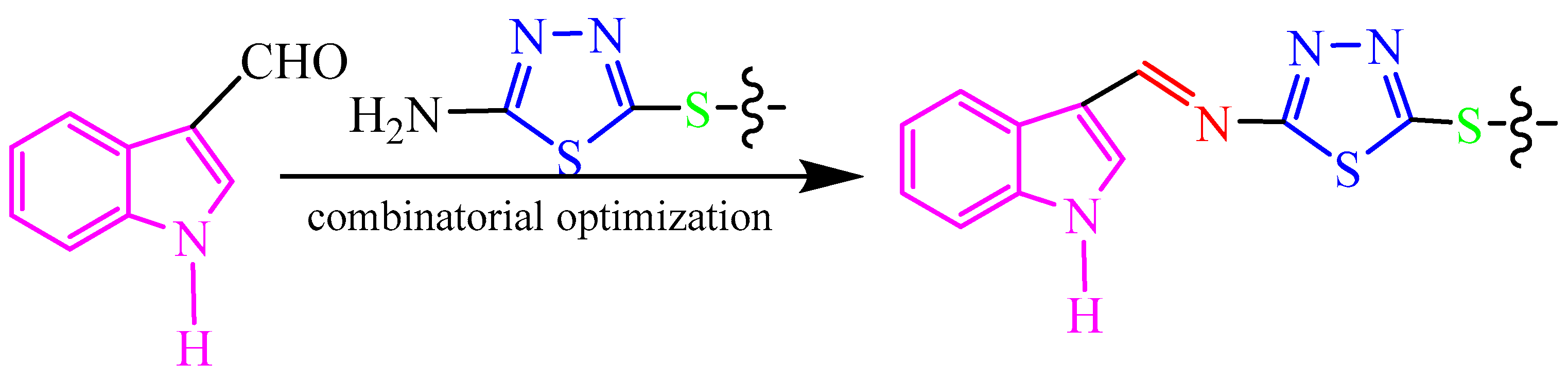
In this research, our aim is to find new antifungal compounds to control fungal diseases from farmland. Based on the different advantages of indoles, thiadiazoles, thioethers and Schiff bases, and in continuation of our long-term research on the heterocyclic derivatives such as 1,3,4-thiadiazole [64], coumarin [65,66,67,68,69,70,71], indole [72], and chitosan [73] as agricultural antifungal agents, herein, we designed and synthesized a series of target compounds 2a–2t containing indole, thioether-modified 1,3,4-thiadiazole, and imine. These compounds have obvious inhibitory activities against plant pathogenic fungi, which have not been reported in the literature at home and abroad. The structure–activity relationship of the new derivatives against fungi was determined. This structure–activity relationship lays a foundation for the research and development of drugs to control plant fungal diseases in the future. According to the preliminary inhibition experiments results, compound 2j had been recognized as a potential compound for further investigation in the field of fungicides. The design of target compounds is shown in Scheme 1.
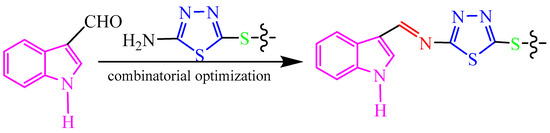
Scheme 1.
Design of target compounds.
2. Results and Discussion
2.1. Synthesis
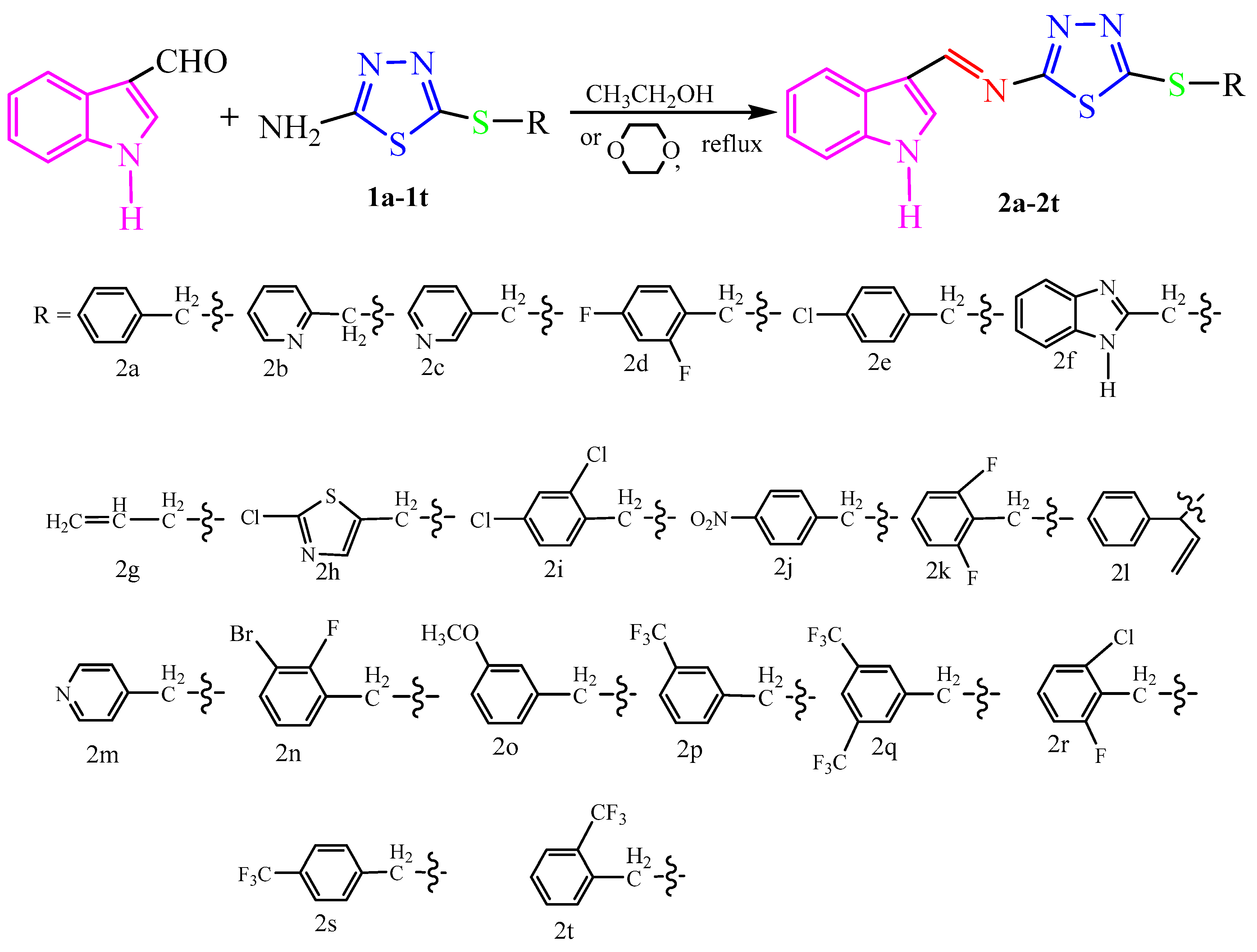
The synthetic pathway used in the preparation of novel indole derivatives 2a–2t containing thioether-modified 1,3,4-thiadiazole is shown in Scheme 2. The (1H)-indole-3-formaldehyde was condensed with 2-amino-5-alkylthio-1,3,4-thiadiazole in ethanol or 1,4-dioxane solvent in the presence of a CH3COOH catalyst to obtain the target compounds. The progress of the reaction was monitored using HPLC and TLC. The reaction progress monitoring revealed that it took approximately 4–6 h to completely consume the 2-amino-5-alkylthio-1,3,4-thiadiazole, and the target compounds 2a–2i can be obtained with a yield range of 62–94% after refluxing at 80 °C with ethanol as the solvent. However, compounds 2k–2t showed low yields or no product under the same conditions. After refluxing at 100 °C with 1,4-Dioxane instead of ethanol as solvent, the result was higher yields of compounds 2k–2t. Conclusively, the formation of compounds 2k–2t required higher temperatures compared to the formation of compounds 2a–2j.
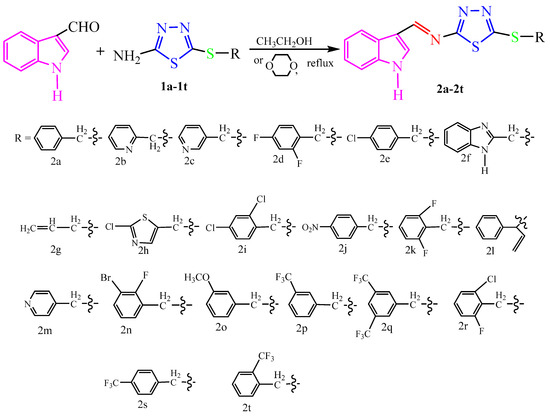
Scheme 2.
Synthesis route of the target compounds 2a–2t.
The structures of the synthesized compounds 2a–2t were confirmed using different spectroscopic techniques, such as FT-IR, 1H NMR, 13C NMR, and HR-MS analyses. The FT-IR spectra of the synthesized compounds 2a–2t showed one or two separate absorption bands in the 3267–3506 cm−1 region, which corresponds to the N–H stretching of the indole ring. The peaks corresponding to aromatic =C–H and C=N stretching bands were identified in the 3040–3097 cm−1 and 1605–1698 cm−1 regions, respectively. The peak was observed at 1035–1087 cm−1 and corresponds to the thioether bond C–S–C stretching. The 1H NMR spectra of compounds 2a–2t showed the pyrrole N–H protons of the indole moiety as one singlet in the δ 11.18–12.34 ppm region and the C–H protons of the imine group as one singlet in the δ 8.90–10.68 ppm region. The thioether (SCH2) C–H proton signals of compounds 2j and 2k were observed at δ 3.75 and 4.37 ppm, respectively, as a doublet due to the ortho coupling with the ethylene C–H. In the other compounds, the thioether (SCH2) C–H protons were found as one singlet in the δ 4.29–4.83 ppm region. The 13C NMR spectrum showed the resonances of C=N, S–C, 1,3,4-thiadiazole C2, and 1,3,4-thiadiazole C5 through the signals at δ 152.80–165.56, 19.02–38.09, 170.49–181.65, and 161.88–170.08 ppm, respectively. The HR-MS of compounds 2a–2t was conducted using the electrospray ionization method (ESI). In the HR-MS spectra of compounds 2a–2t, [M + H+], [M + Na+] or [M–H+] peaks were observed, which confirmed their precise molecular weights.
The synthesized compounds 2a–2t had moderate solubility in ethanol and methanol, and good solubility in DMF, DMSO, acetone, and chloroform. The synthetic molecules are stable in any of the above solvents.
2.2. In Vitro Antifungal Activity
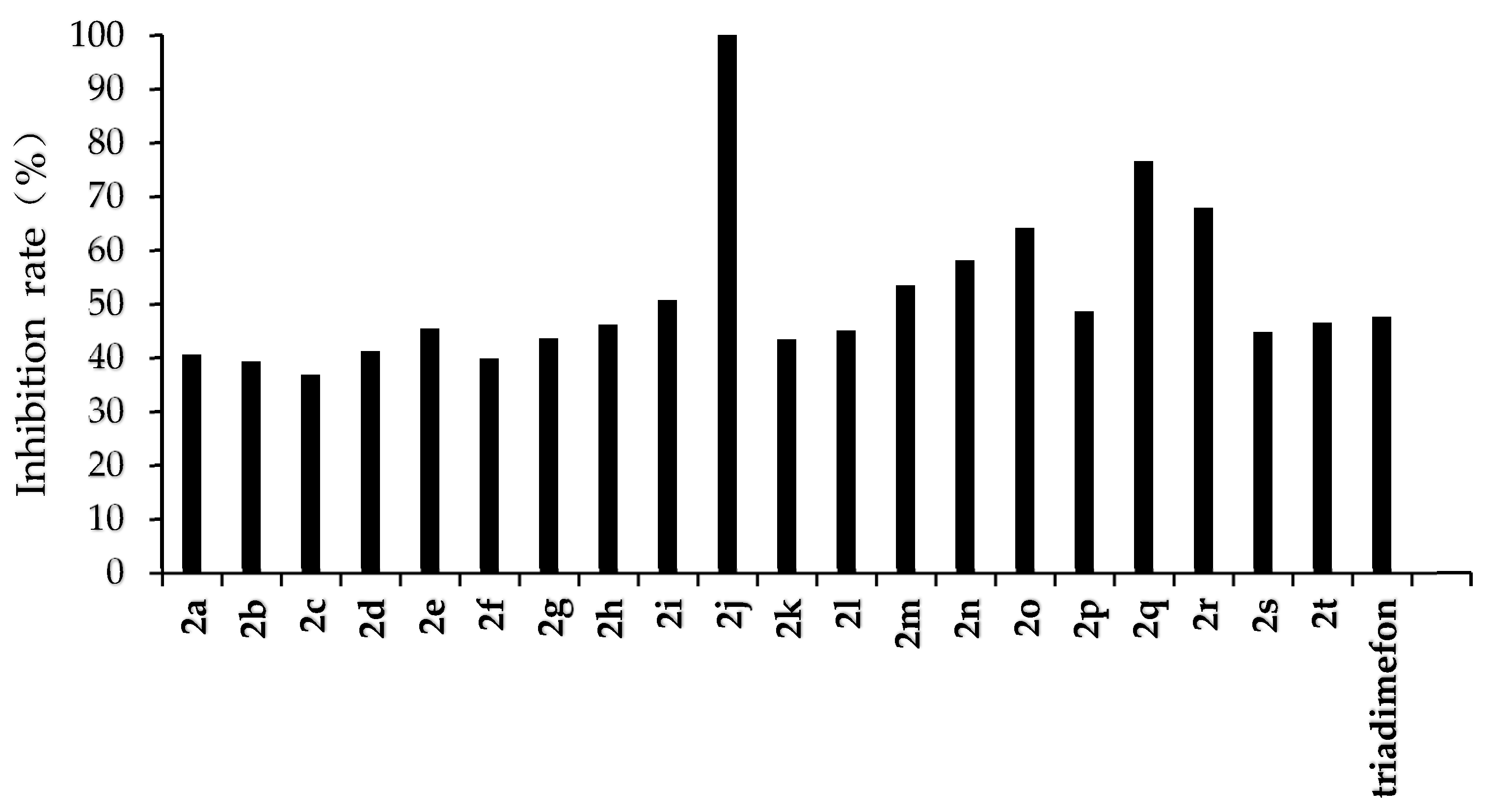
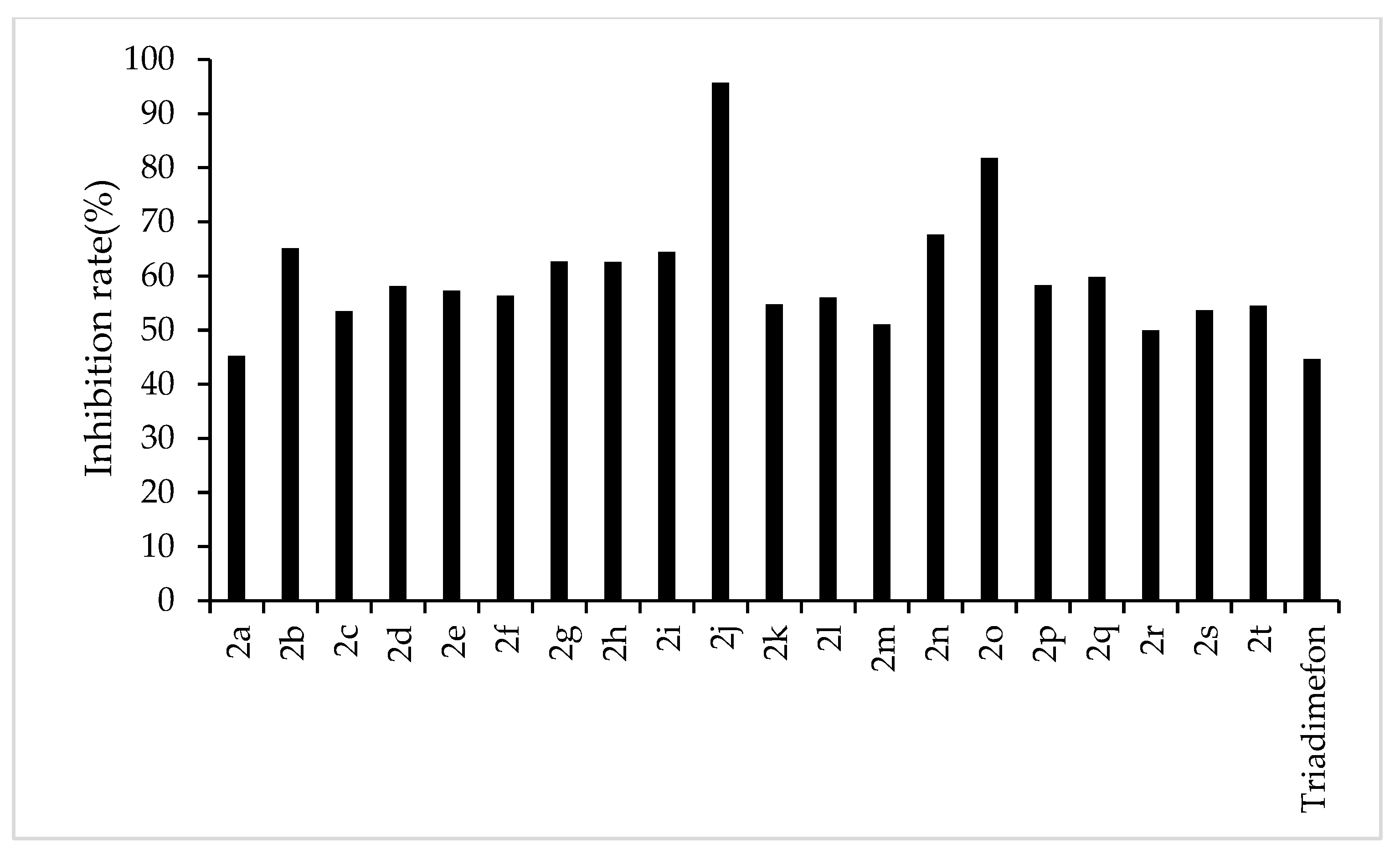
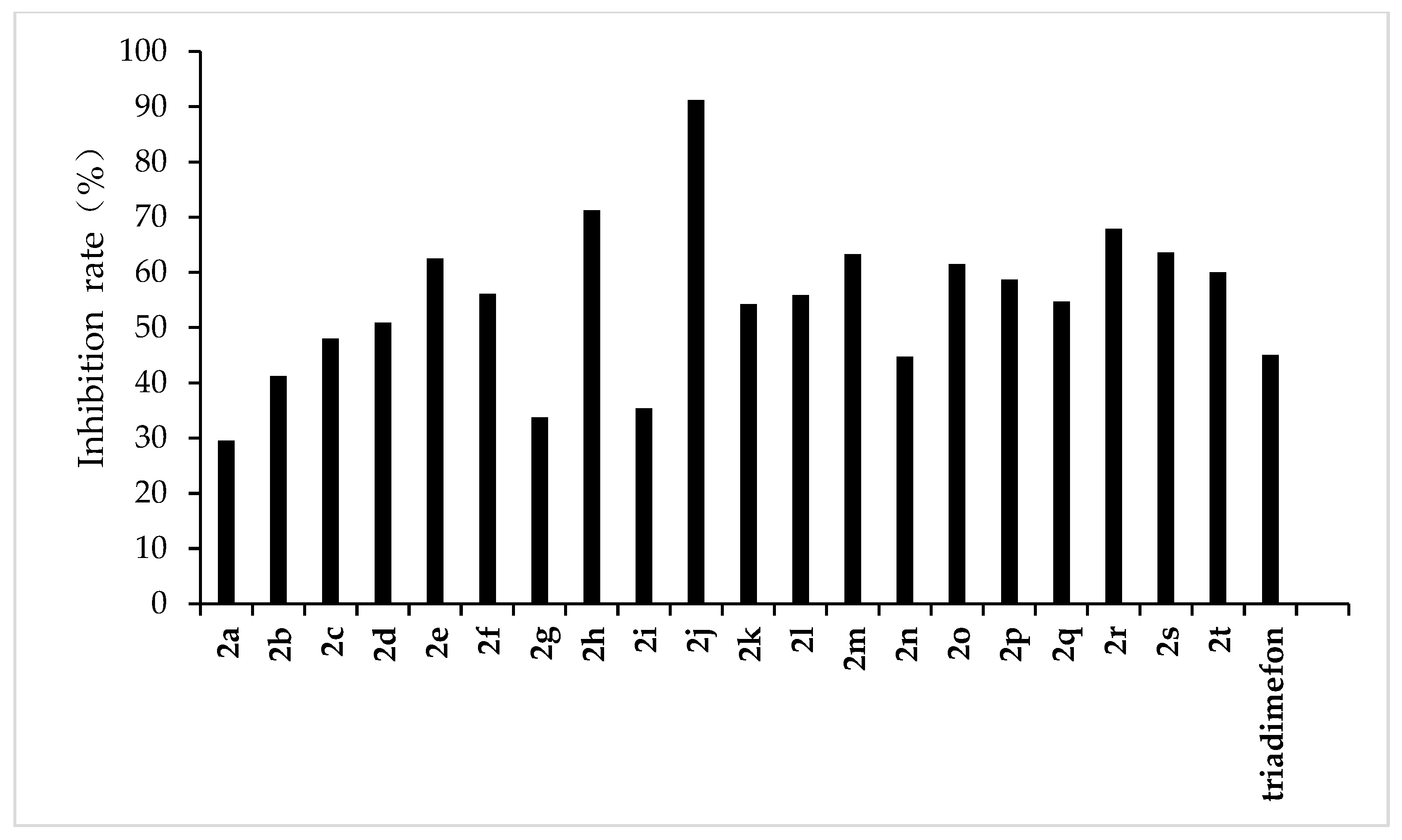
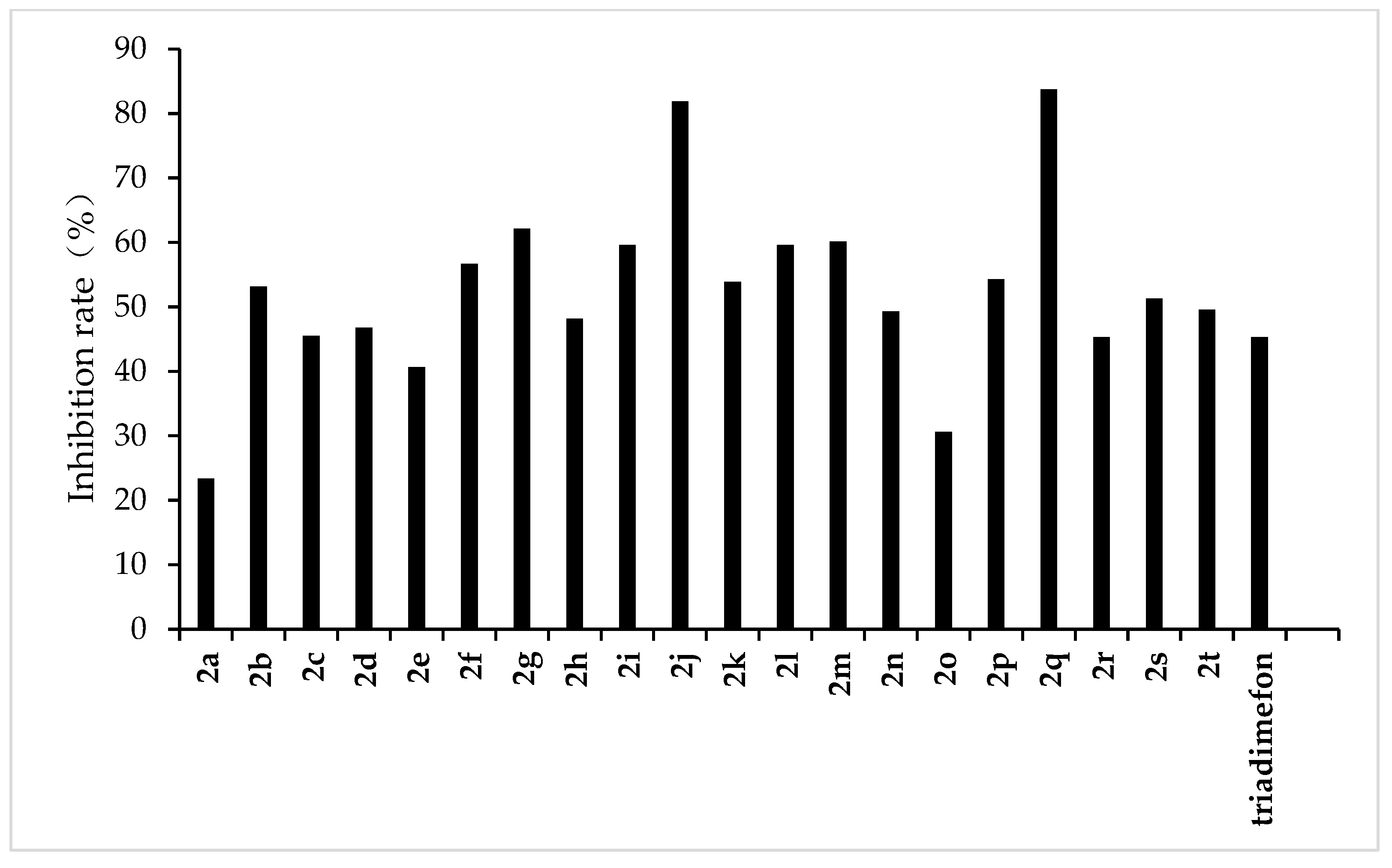
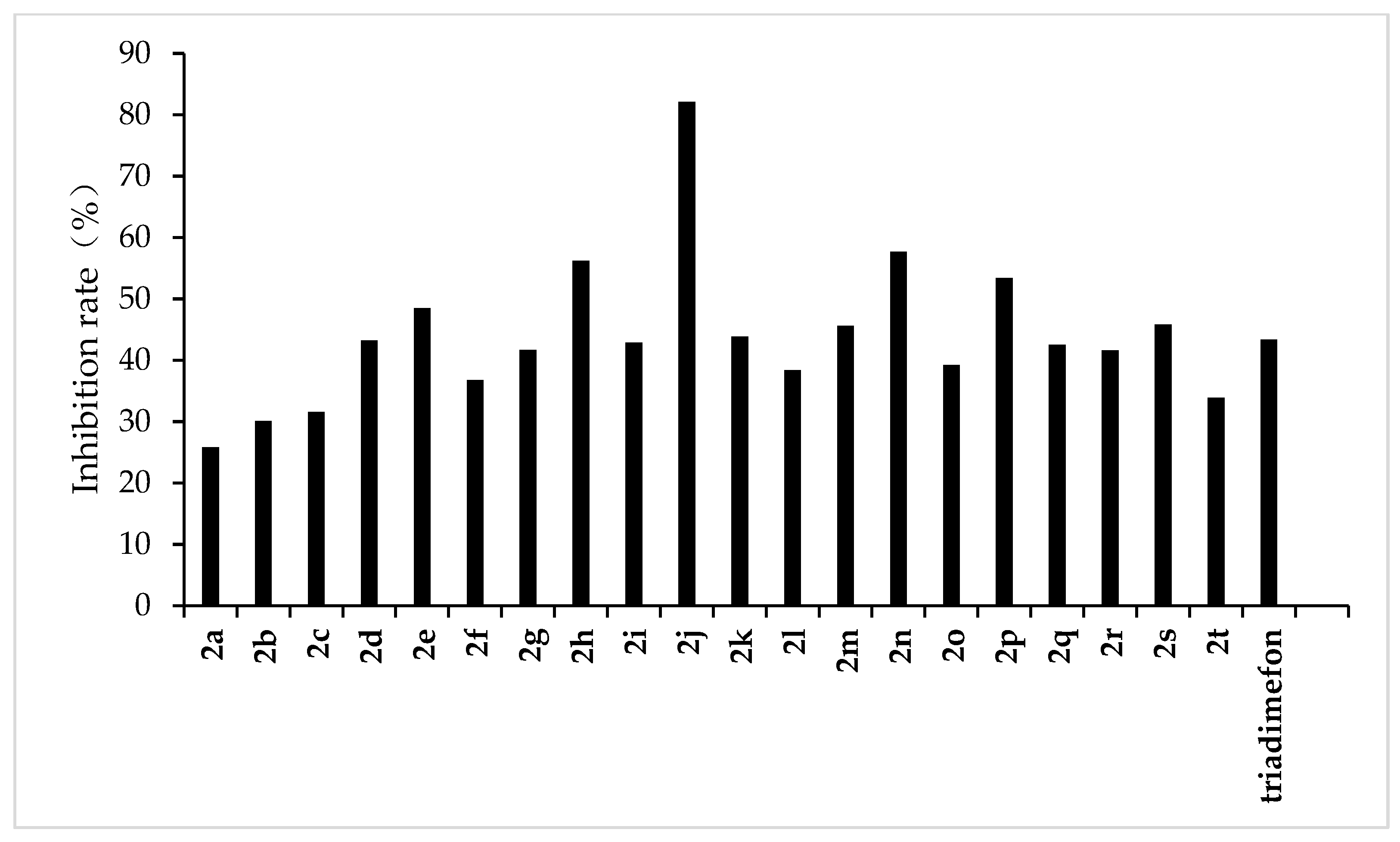
The results of the preliminary inhibition experiments of the target compounds 2a–2t against F. graminearum, F. oxysporum, F. moniliforme, C. lunata, and P. p. var. nicotianae are shown in Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7, respectively. Photos of some of the compounds in the fungal inhibition experiment are in the Supplemental Materials.
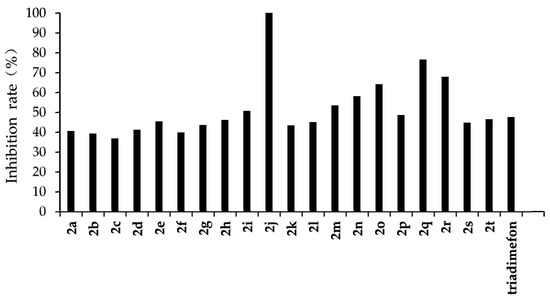
Figure 3.
Antifungal activity of the target compounds (2a–2t) against F. graminearum.
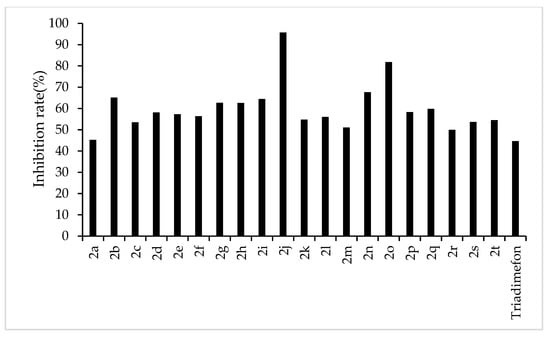
Figure 4.
Antifungal activity of the target compounds (2a–2t) against F. oxysporum.
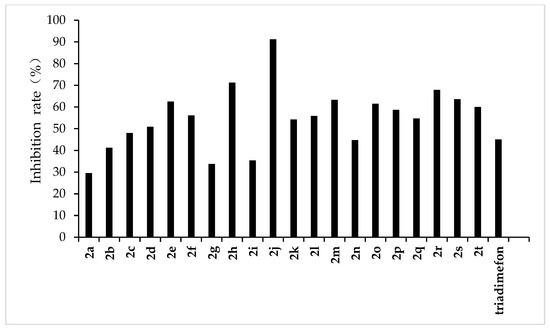
Figure 5.
Antifungal activity of the synthesized compounds (2a–2t) against F. moniliforme.
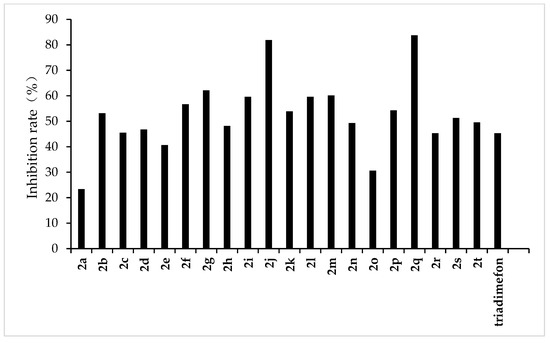
Figure 6.
Antifungal activity of the target compounds (2a–2t) against C. lunata.
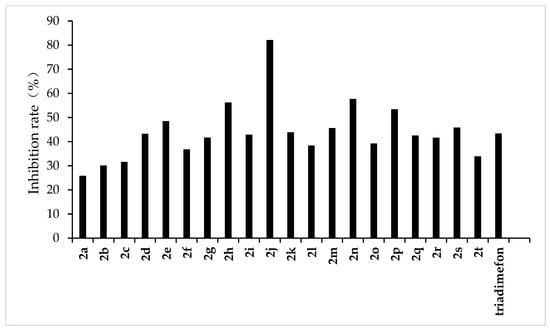
Figure 7.
Antifungal activity of the synthesized compounds (2a–2t) against P. p. var. nicotianae.
From the experimental results, we found that the target compounds 2a‒2t has different inhibitory activities against the experimental fungi. For example, at the concentration of 500 µg/mL, the inhibitory rate of the target compound 2a–2t against F. graminearum was within the range of 36.8–100% (in Figure 3). Among the tested compounds, compounds 2i, 2j, 2m, 2n, 2o, 2p, 2q, and 2r exhibited higher inhibition rates than the control reagent triadimefon (inhibition index: 47.6%). At the same concentration, the inhibitory rate of compounds 2a–2t against F. oxysporum was in the range of 45.2–95.7% (in Figure 4), which was higher than that of the control drug triadimefon (the inhibitory rate of 45.2%). Some compounds, such as compound 2j and compound 2q, showed a broad spectrum of good antifungal activity. The inhibition rates of compound 2j against F. Graminearum, F. oxysporum, F. Moniliforme, C. lunata, and P. p. var. nicotianae were 100%, 95.7%, 91.2%, 81.9%, and 82.1%, respectively. Compound 2q showed better inhibitory activity against F. graminearum and C. lunata with inhibition rates of 76.5% and 83.7%, respectively.
The structure–activity relationship indicated that different substituents attached to the benzene ring of the target compounds would have obvious effects on the inhibitory activity of the experimental fungi. The introduction of electron-withdrawing groups such as –NO2, –CF3, –F, –Cl on the benzene ring resulted in an increase in the antifungal activity of compounds such as 2j, 2i, 2k, 2e, 2p, 2q, 2r, 2s, and 2t, compared to compound 2a. The higher antifungal activity of those compounds may be due to the electron-withdrawing group on the benzene ring, which decreases the electron cloud density and results in an increase in the accessibility of the target molecules toward the fungicide cell. In addition, different positions of the same substituents have different effects on the inhibitory activities of different fungi. For example, when –CF3 is in different positions (ortho:2t, meta:2p and para:2s), it has little effect on the inhibition rate of compound against F. Graminearum, F. Oxysporum, F. Moniliforme and C. Lunata, but has a great effect on the inhibition rate of compound against P. p. var. nicotianae. The inhibition rates of meta compound (2p) and ortho compound (2t) against P. p. var. nicotianae were 53.4% and 33.9%, respectively. The inhibition rates of 3, 5-di-substituted –CF3 compound (2q) against F. Graminearum and C. lunata were 76.5% and 83.7%, respectively, which were higher than that of mono-substituted –CF3 compounds (2p, 2s and 2t). However, the inhibition rates of 3, 5-di-substituted –CF3 compound (2q) against the other three fungi were almost the same as that of mono-substituted –CF3 compounds (2p, 2s, 2t). The compounds with different substituted pyridine positions had different inhibitory activities against fungi. The inhibition rates of 4-position pyridine compound (2m) against F. Graminearum, F. Moniliforme, C. Lunata, and P. p. var. nicotianae were higher than that of 2-position and 3-position pyridine compounds (2b and 2c). However, the inhibition rate of 2-position pyridine compound (2b) against F. oxysporum was higher than that of 3-position and 4-position pyridine compounds (2c and 2m).
3. Materials and Methods
3.1. Chemicals and Instruments
All reagents and chemicals were procured from a commercial supplier (Shanghai Aladdin Reagent Co., Ltd., Shanghai, China) and used as received. The method described in the literature was used to synthesize the intermediate 1 (1a–1t, 2-ammino-5-alkylthio-1,3,4-thiadiazoles) [64]. Five crop-threatening pathogenic fungi (F. graminearum, F. oxysporum, F. moniliforme, C. lunata, and P. p. var. nicotianae) were obtained from the College of Plant Protection of Henan Agricultural University.
The Fourier transformed infrared (FT-IR) spectra were recorded using a Thermo Scientific Nicolet IS10 FT-IR spectrometer (Nicolet Technologies Co., Madison, America) and the frequencies were given in cm–1. The proton nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR) spectra were obtained using a Bruker DPX-400 spectrometer (Brucker Technologies Co., Karlsruhe, German) in acetone or dimethyl sulfoxide (DMSO) solvent with tetramethylsilane (TMS) as an internal standard. A thin-layer chromatography (TLC) was performed on silica gel 60 F254 (Shanxi ersai biotechnology Co., Ltd., Xian, China). A high-performance liquid chromatography (HPLC) from Thermo Fisher Science and Technology Ltd. with C18 chromatographic column was used in the process of the reaction. The high resolution-mass spectroscopy (HR-MS) was performed using an Ultimate 3000RE-Q-ExactiveTM Orbitrap, Thermo Fisher-ESI instrument (Thermo Fisher Technologies Co., Waltham, America). Melting points were determined using a Taike X-4 melting point apparatus. The reaction yields, except for compound 2a, were not optimized.
3.2. General Procedure for the Preparation of Compounds 2a–2t
A total of 3.6 mmol of 3-indoxformaldehyde and 3 mmol of the intermediate 1a (2-amino-5-S-benzyl-1,3,4-thiadiazole) were taken in the round bottom flask and dissolved in ethanol, and then a few drops of acetic acid were added as a catalyst. The resulting mixture was refluxed for 5 h at 80 °C. Once the reaction was completed according to thin layer chromatography (TLC) or high-performance liquid chromatography (HPLC), the reaction solution was cooled and then filtered using vacuum filtration to obtain the crude product. The crude product was then purified using ethanol recrystallization to obtain the desired product 2a. The preparation method for compounds 2b–2t was the same as for compound 2a.
3.3. Spectral Data
(E)-N-(5-(benzylthio)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl) methanimine (2a)
Orange yellow crystal; M. p. 200.5–201.4 °C; yield 72%; IR (ν, cm–1 KBr): 3506 (N–H), 3069 (Ar–H), 1630 (C=N), 1524, 1513, 1402, 1336, 1204 (thiadiazole ring), 1042 (C–S–C); 1H NMR (400 MHz, DMSO, d6, δ, ppm): 12.31 (s, 1H, N–H), 8.92 (s, 1H, HC=N), 8.30 (d, J = 8.0 Hz, 2H, Ar–H), 7.55 (d, J = 8.0 Hz, 1H, Ar–H), 7.46 (d, J = 8.0 Hz, 2H, Ar–H), 7.35 (t, J = 8.0 Hz, 2H, Ar–H), 7.29 (t, J = 8.0 Hz, 3H, Ar–H), 4.56 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 176.51, 163.41 (thiadiazole ring), 160.51 (C=N), 139.14, 138.02, 137.05, 129.56, 129.04, 128.10, 124.93, 124.23, 122.67, 122.38, 114.76, 113.09, 37.83 (SCH2); HR-MS (ESI): calcd. for C18H14N4S2: [M + Na+] 373.0558; found: 373.0559.
(E)-N-(5-((pyridin-2-ylmethyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine (2b)
Yellow needle-shaped crystal; M. p. 209.1–210.5 °C; yield 81%; IR (ν, cm–1 KBr): 3442 (N–H), 3091(Ar–H), 1619 (C=N), 1596, 1573, 1478, 1429, 1374, 1245 (thiadiazole ring), 1046 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.29 (s, 1H, N–H), 8.93 (s, 1H, HC=N), 8.55 (d, J = 4.0 Hz, 1H, thiadiazole-H), 8.32 (s, 1H, Ar–H), 8.29 (d, J = 4.0 Hz, 1H, Ar–H), 7.78–7.83 (m, 1H, Ar–H), 7.55 (d, J = 8.0 Hz, 2H, Ar–H), 7.34–7.26 (m, 3H, Ar–H), 4.67 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 176.55, 163.50 (thiadiazole ring-C), 160.70 (C=N), 156.48, 149.78, 139.19, 138.01, 137.49, 124.91, 124.24, 123.76, 123.21, 122.68, 122.69, 122.37, 114.74, 113.11, 36.26 (SCH2); HR-MS (ESI): calcd. for C17H13N5S2: [M + Na+] 374.0510; found: 374.0509.
(E)-N-(5-((pyridin-3-ylmethyl)thio)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine (2c)
Yellow-green needle-shaped crystal; M. p. 207.5–208.4 °C; yield 92%; IR (ν, cm–1 KBr): 3436 (N–H), 3055 (Ar–H), 1605 (C=N), 1580, 1479, 1431, 1294,1241 (thiadiazole ring), 1059 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.32 (s, 1H, N–H), 8.92 (s, 1H, HC=N), 8.66 (s, 1H, Ar–H), 8.49 (d, J = 4.0 Hz, 1H, thiadiazole-H), 8.31 (s, 1H, Ar–H), 8.29 (d, J = 4.0 Hz, 1H, Ar–H), 7.88 (d, J = 8.0 Hz, 1H, Ar–H), 7.55 (d, J = 8.0 Hz, 1H, Ar–H), 7.37–7.40 (m, 1H, Ar–H), 7.29 (m, 2H, Ar–H), 4.59 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 176.75, 163.59 (thiadiazole ring-C), 159.83 (C=N), 150.48, 149.15, 139.27, 138.02, 137.11, 133.44, 124.92, 124.26, 124.09, 122.71, 122.37, 114.75, 113.12, 34.82 (SCH2); HR-MS (ESI): calcd. for C17H13N5S2: [M + Na+] 374.0510; found: 374.051.
(E)-N-(5-((2,4,5-trifluorobenzyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)metha- nimine (2d)
Bright yellow needle-shaped crystal; M.p. 206.2–207.5 °C; yield 73%; IR (ν, cm–1 KBr): 3277 (N–H), 3091(Ar–H), 1620 (C=N),1519, 1423, 1401, 1320, 1239 (thiadiazole ring), 1065 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.32 (s, 1H, N–H), 8.94 (s, 1H, HC=N), 8.32 (s, 1H, Ar–H), 8.28 (d, J = 8.0 Hz, 1H, Ar–H), 7.60–7.68 (m, 2H, Ar–H), 7.54 (d, J = 4.0 Hz, 1H, Ar–H), 7.26–7.32 (m, 2H, Ar–H), 4.54 (s, 2H,-SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 177.12, 163.70 (thiadiazole ring-C), 159.12 (C=N), 153.98, 139.39, 138.04, 136.79, 128.86, 127.49, 124.92, 124.27, 122.72, 122.37, 119.75, 119.70, 119.55, 114.75, 113.14, 30.81 (SCH2); HR-MS (ESI): calcd. for C18H11F3N4S2: [M + Na+] 427.0275; found: 427.0276.
(E)-N-(5-((4-chlorobenzyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine (2e)
Beige needle-shaped crystal; M. p. 201.8–202.6 °C; yield 65%; IR (ν, cm–1 KBr): 3332 (N–H), 3085 (Ar–H), 1616 (C=N), 1513, 1453, 1428, 1373, 1292 (thiadiazole ring), 1035 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.14 (s, 1H, N–H), 9.94 (s, 1H, HC=N), 8.29 (s, 1H, thiadiazole-H), 8.10 (d, J = 8.0 Hz, 1H, Ar–H), 7.52 (d, J = 8.0 Hz, 1H, Ar–H), 7.38 (d, J = 4.0 Hz, 3H, Ar–H), 7.31 (s, 1H, Ar–H), 7.22–7.27 (m, 2H, Ar–H), 4.29 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 170.49, 163.55 (thiadiazole ring-C), 160.17 (C=N), 149.51, 138.00, 136.88, 136.40, 132.50, 128.89, 124.90, 124.28, 122.72, 122.37, 114.74, 113.11, 38.09 (SCH2); HR-MS (ESI): calcd. for C18H13ClN4S2: [M + Na+] 407.0168; found: 407.0167.
(E)-N-(5-(((1H-benzo[d]imidazol-2-yl)methyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine (2f)
Brown-red needle-shaped crystals; M. p. 245.8–246.5 °C; yield 72%; IR (ν, cm–1 KBr): 3307 (N–H), 3073 (Ar–H), 1634 (C=N), 1585, 1504, 1454, 1315, 1298 (thiadiazole ring), 1036 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.12 (s, 1H, N–H), 10.68 (s, 1H, N–H), 8.39 (s, 1H, HC=N), 8.28 (d, J = 8.0 Hz, 1H, Ar–H), 8.17 (s, 1H, Ar–H), 7.98 (d, J = 8.0 Hz, 1H, Ar–H), 7.80 (s, 1H, Ar–H), 7.75 (d, J = 8.0 Hz, 1H, Ar–H), 7.53 (d, J = 8.0 Hz, 1H, Ar–H), 7.38–7.45 (m, 2H, Ar–H), 7.20–7.27 (m, 2H, Ar–H), 4.36 (s, 1H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 176.03, 170.08 (thiadiazole ring-C), 152.80 (C=N), 149.29, 136.58, 130.17, 129.65, 127.70, 126.98, 123.48, 123.09, 121.30, 118.99, 118.74, 118.61, 114.71, 112.78, 112.03, 111.63, 19.02 (SCH2); HR-MS (ESI): calcd. for C19H14N6S2: [M–H+]: 389.0683; found: 389.070.
(E)-N-(5-((2,6-difluorobenzyl)thio)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine (2g)
Light yellow solid powder; M. p. 177.0–177.7 °C; yield 72%; IR (ν, cm–1 KBr): 3287 (N–H), 3065 (Ar–H), 1622 (C=N), 1580, 1496, 1409, 1384, 1246 (thiadiazole ring), 1045 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 11.35 (s, 1H, N–H), 9.08 (s, 1H, HC=N), 8.48 (s, 1H, Ar–H), 8.28 (d, J = 4.0 Hz, 1H, Ar–H), 7.58–7.61 (m, 1H, Ar–H), 7.43–7.48 (m, 1H, Ar–H), 7.30–7.34 (m, 2H, Ar–H), 7.09 (t, J = 8.0 Hz, 2H, Ar–H), 4.63 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 176.99, 162.17 (thiadiazole ring-C), 158.82 (C=N), 137.89, 137.57, 130.42, 124.99, 123.96, 122.43, 122.27, 121.33, 115.24, 112.31, 111.70, 111.45, 25.48 (SCH2); HR-MS (ESI): calcd. for C18H12F2N4S2: [M + Na+] 409.0369; found: 409.0369.
(E)-N-(5-(((2-chlorothiazol-5-yl)methyl)thio)-1,3,4-thiadiazol-2-yl)-1-(1H-in-dol-3-yl)me- thanimine (2h)
Yellow solid powder; M. p.167.6–169.2 °C; yield 65%; IR (ν, cm–1 KBr): 3267 (N–H), 3084 (Ar–H), 1636 (C=N), 1577, 1504, 1462, 1325, 1297 (thiadiazole ring), 1045 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.31 (s, 1H, N–H), 8.95 (s, 1H, HC=N), 8.33 (s, 1H, thiadiazole–H), 8.29 (d, J = 4.0 Hz, 1H, Ar–H), 7.66 (s, 1H, Ar–H), 7.55 (d, J = 8.0 Hz, 1H, Ar–H), 7.30 (t, J = 4.0 Hz, J = 8.0 Hz, 2H, thiadiazole–H), 4.81 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 177.12, 163.74 (thiadiazole ring-C), 162.28 (C=N), 159.30, 150.89, 141.44, 139.37, 138.69, 138.02, 124.92, 124.28,122.74, 122.38, 114.76, 113.13, 29.51 (SCH2); HR-MS (ESI): calcd. for C15H10ClN5S3: [M + Na+] 413.9685; found: 413.96824.
(E)-N-(5-((2,4-dichlorobenzyl)thio)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine (2i)
Yellow solid powder; M. p. 182.9–183.8 °C; yield 68%; IR (ν, cm–1 KBr): 3273 (N–H), 3093 (Ar–H), 1633 (C=N), 1572, 1504, 1426, 1325, 1238 (thiadiazole ring), 1045 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.31 (s, 1H, N–H), 8.93 (s, 1H, HC=N), 8.32 (s, 1H, Ar–H), 8.29 (d, J = 8.0 Hz, 1H, Ar–H), 7.68 (d, J = 4.0 Hz, 1H, Ar–H), 7.61 (d, J = 8.0 Hz, 1H, Ar–H), 7.54 (d, J = 4.0 Hz, 1H, Ar–H), 7.45–7.42 (m, 1H, Ar–H), 7.31–7.261 (m, 2H, Ar–H), 4.62 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 177.03, 163.68 (thiadiazole ring-C), 159.29 (C=N), 139.33, 138.02, 134.82, 133.88, 133.82, 133.23, 129.38, 128.05, 124.91, 124.27, 122.72, 122.37, 114.75, 113.12, 35.32 (SCH2); HR-MS (ESI): calcd. for C18H12Cl2N4S2: [M–H+] 416.98022; found: 416.9815
(E)-N-(5-((4-nitrobenzyl)thio)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine (2j)
Brown solid powder; M. p. 194.8–195.5 °C; yield 75%; IR (ν, cm–1 KBr): 3433 (N–H), 3042 (Ar–H), 1698 (C=N), 1580, 1518, 1443, 1345,1244 (thiadiazole ring), 1087 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.29 (s, 1H, N–H), 8.91 (s, 1H, HC=N), 8.28 (t, J = 12.0 Hz, J = 8.0 Hz, 2H, Ar–H), 8.22 (d, J = 8.0 Hz, 2H, Ar–H), 7.75 (d, J = 8.0 Hz, 2H, Ar–H), 7.54 (d, J = 8.0 Hz, 1H, Ar–H), 7.30–7.27 (m, 2H, Ar–H), 4.70 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 176.80, 163.55 (thiadiazole ring-C), 159.61(C=N), 147.26, 145.66, 139.26, 138.02, 132.52, 130.80, 124.91, 124.24, 124.11, 122.69, 122.26, 114.74, 113.09, 36.72 (SCH2); HR-MS (ESI): calcd. for C18H13N5O2S2: [M–H+] 394.0472; found: 394.0478.
(E)-N-(5-(allylthio)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl) methanimine (2k)
Reddish-brown powder; M. p. 190.3–190.7 °C; yield 72%; IR (ν, cm–1 KBr): 3313 (N–H), 3097 (Ar–H), 1639 (C=N), 1574, 1521, 1445, 1392, 1297 (thiadiazole ring), 1047 (C–S–C); 1H NMR (400 MHz, Acetone d6, δ, ppm): 11.20 (s, 1H, N–H), 10.05 (s, 1H, HC=N), 8.23 (t, J = 8.0 Hz, 2H, Ar–H), 7.56 (d, J = 4.0 Hz, 1H, Ar–H), 7.25–7.29 (m, 1H, Ar–H), 6.64 (s, 1H, =CH), 5.91–6.01 (m, 1H, =CH), 5.25 (d, J = 12.0 Hz, 1H, =CH), 5.12 (d, J = 8.0 Hz, 1H, =CH), 3.75 (d, J = 8.0 Hz, 2H, SCH2); 13C NMR (101 MHz, Acetone d6, δ, ppm): 181.65, 170.07 (thiadiazole ring-C), 162.28 (C=N), 150.68, 137.51, 133.41, 124.66, 123.58, 122.15, 121.31, 119.06, 118.63, 118.06, 112.31, 37.48 (SCH2); HR-MS (ESI): calcd. for C14H12N4S2: [M + Na+] 323.0401; found: 323.0401.
(E)-N-(5-((1-phenylallyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine (2l)
Yellow solid powder; M. p. 217.0–218.3 °C; yield 69%; IR (ν, cm–1 KBr): 3470 (N–H), 3040 (Ar–H), 1642 (C=N), 1574, 1510, 1457, 1373, 1241 (thiadiazole ring), 1064 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.29 (s, 1H, N–H), 8.92 (s, 1H, HC=N), 8.31 (s, 1H, Ar–H), 8.29 (d, J = 8.0 Hz, 1H, Ar–H), 7.54 (d, J = 8.0 Hz, 1H, Ar–H), 7.44 (s, 3H, Ar–H), 7.29 (d, J = 4.0 Hz, 2H, Ar–H), 6.72 (q, J = 12.0 Hz, J = 8.0 Hz, J = 12.0 Hz, 1H, Ar–H), 5.83 (d, J = 20.0 Hz, 1H, Ar–H), 5.26 (d, J = 12.0 Hz, 1H, =CH), 4.55 (s, 2H, =CH2), 4.37 (t, J = 4.0 Hz, 1H, SCH); 13C NMR (101 MHz, DMSO d6, δ, ppm): 176.53, 163.46 (thiadiazole ring-C), 160.42 (C=N), 139.19, 138.01, 136.95, 136.77, 136.64, 129.84, 126.79, 124.92, 122.69, 122.37, 115.02, 114.74, 113.10, 37.62 (SCH2); HR-MS (ESI): calcd. for C20H16N4S2: [M + Na+] 399.0714; found: 399.0714.
(E)-N-(5-((pyridin-4-ylmethyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine (2m)
Orange solid powder; M. p. 183.2–184.5 °C; yield 65%; IR (ν, cm–1 KBr): 3442 (N–H), 3043 (Ar–H), 1633 (C=N), 1577, 1521, 1445, 1396, 1244 (thiadiazole ring), 1087 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 12.32 (s, 1H, N–H), 8.92 (s, 1H, HC=N), 8.66 (s, 1H, Ar–H), 8.49 (d, J = 8.0 Hz, 1H, Ar–H), 8.31 (s, 1H, Ar–H), 8.28 (d, J = 4.0 Hz, 1H, Ar–H), 7.88 (d, J = 8.0 Hz, 1H, Ar–H), 7.55 (d, J = 8.0 Hz, 1H, Ar–H), 7.38 (q, J = 4.0 Hz, 1H, Ar–H), 7.26–7.32 (m, 2H, Ar–H), 4.59 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 172.6, 169.31 (thiadiazole ring-C), 165.56 (C=N), 159.22, 156.40, 155.03, 137.40, 137.16, 124.63, 123.61, 122.12, 121.33, 119.21, 112.09, 35.79 (SCH2); HR-MS (ESI): calcd. for C17H13N5S2: [M + H+] 352.0691; found: 352.0691
(E)-N-(5-((3-bromo-2-fluorobenzyl)thio)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)meth- animine (2n)
Yellow solid powder; M. p. 198.0–199.3 °C; yield 76%; IR (ν, cm–1 KBr): 3439 (N–H), 3053 (Ar–H), 1605 (C=N), 1577, 1482, 1459, 1392, 1241 (thiadiazole ring), 1053 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 11.36 (s, 1H, N–H), 9.05 (s, 1H, HC=N), 8.46 (d, J = 8.0 Hz, 1H, Ar–H), 8.27(s, 1H, Ar–H), 7.55–7.60 (m, 2H, Ar–H), 7.42–7.47 (m, 2H, Ar–H), 7.31–7.34 (m, 2H, Ar–H), 4.60 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO d6, δ, ppm): 177.03, 163.68 (thiadiazole ring-C), 159.29 (C=N), 138.02, 134.82, 133.88, 133.82, 133.23, 129.58, 128.05, 124.91, 124.27, 122.72, 122.37, 114.75, 113.12, 35.32 (SCH2); HR-MS (ESI): calcd. for C18H12BrFN4S2: [M + Na+] 468.9569; found: 468.9575.
(E)-N-(5-((3-methoxybenzyl)thio)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)methanimine(2o)
Yellow solid powder; M. p. 194.2–195.4 °C; yield 73%; IR (ν, cm–1 KBr): 3464 (N–H), 3064 (Ar–H), 1636 (C=N), 1577, 1462, 1440, 1389, 1244 (thiadiazole ring), 1050 (C–S–C); 1H NMR (400 MHz, DMSO d6, δ, ppm): 11.18 (s, 1H, N–H), 8.9 (s, 1H, HC=N), 8.31 (d, J = 8.0 Hz, 1H, Ar–H), 8.06–8.12 (m, 2H, Ar–H), 7.44 (d, 1H, J = 8.0 Hz, Ar–H), 7.12–7.19 (m, 3H, Ar–H), 6.94 (t, J = 8.0 Hz, 2H, Ar–H), 4.42 (s, 2H, SCH2), 3.66 (s, 3H, OCH3); 13C NMR (101 MHz, DMSO d6, δ, ppm): 176.07, 161.88 (thiadiazole ring-C), 159.98 (C=N), 138.28, 137.86, 137.32, 129.62, 129.50, 123.92, 123.61, 122.42, 122.22, 122.11, 121.31, 115.24, 114.71, 113.20, 112.28, 54.63 (OCH3), 37.52 (SCH2); HR-MS (ESI): calcd. for C19H16N4OS2: [M + Na+] 403.0663; found: 403.0667.
(E)-N-(5-((3-(trifluoromethyl)benzyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)meth- animine (2p)
Yellow solid powder; M. p. 201.3–202.2 °C; yield 68%; IR (ν, cm–1 KBr): 3419 (N–H), 3069 (Ar–H), 1670 (C=N), 1577, 1462, 1426, 1328, 1246 (thiadiazole ring), 1064 (C–S–C); 1H NMR (400 MHz, Acetone d6, δ, ppm): 11.34 (s, 1H, N–H), 9.06 (d, J = 20.0 Hz, 1H, HC=N), 8.45 (d, J = 20.0 Hz, 1H, Ar–H), 8.27 (d, J = 8.0 Hz, 1H, Ar–H), 7.4 (q, J = 8.0 Hz, J = 12.0 Hz, 2H, Ar–H), 7.59 (d, J = 8.0 Hz, 1H, Ar–H), 7.20–7.46 (m, 4H, Ar–H), 4.70 (d, J = 12.0 Hz, 2H, SCH2); 13C NMR (101 MHz, Acetone d6, δ, ppm): 176.31, 162.05 (thiadiazole ring-C), 159.67 (C=N), 142.10, 137.87, 137.44, 129.91, 125.39, 123.95, 122.41, 122.25, 115.23, 112.31, 36.54 (SCH2); HR-MS (ESI): calcd. for C19H13F3N4S2: [M + Na+] 441.0431found: 441.0431.
(E)-N-(5-((3,5-bis(trifluoromethyl)benzyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)me -thanimine (2q)
Yellow solid powder; M. p. 201.3–202.2 °C; yield 72%; IR (ν, cm–1 KBr): 3456 (N–H), 3066 (Ar–H), 1650 (C=N), 1577, 1496, 1437, 1375, 1243 (thiadiazole ring), 1050 (C–S–C); 1H NMR (400 MHz, Acetone d6, δ, ppm): 11.35 (s, 1H, N–H), 9.03 (s, 1H, HC=N), 8.45 (d, J = 8.0 Hz, 1H, Ar–H), 8.26 (d, J = 4.0 Hz, 1H, Ar–H), 8.24 (s, 2H, Ar–H), 7.99 (s, 1H, Ar–H), 7.59 (t, J = 4.0 Hz, 1H, Ar–H), 7.29–7.35 (m, 2H, Ar–H), 4.83 (s, 2H, SCH2); 13C NMR (101 MHz, Acetone d6, δ, ppm): 176.58, 162.18 (thiadiazole ring-C), 159.13 (C=N), 141.31, 137.88, 137.56, 131.36, 131.04, 129.99, 124.97, 123.96, 122.41, 122.28, 121.23, 115.21, 112.32, 35.79 (SCH2); HR-MS (ESI): calcd. for C20H12F6N4S2: [M–H+] 487.0486; found: 487.0486.
(E)-N-(5-((2-chloro-6-fluorobenzyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)meth- animine (2r)
Yellow solid powder; M. p. 194.6–195.5 °C; yield 63%; IR(ν, cm–1 KBr): 3489 (N–H), 3063 (Ar–H), 1622 (C=N), 1577, 1493, 1431, 1381, 1243 (thiadiazole ring), 1061 (C–S–C); 1H NMR (400 MHz, Acetone d6, δ, ppm): 11.34 (s, 1H, N–H), 9.09 (s, 1H, HC=N), 8.47 (d, J = 8.0 Hz, 1H, Ar–H), 8.28 (d, J = 4.0 Hz, 1H, Ar–H), 7.60 (t, J = 4.0 Hz, 1H, Ar–H), 7.39–7.45 (m, 2H, Ar–H), 7.35–7.32 (m, 2H, Ar–H), 7.22 (t, J = 8.0 Hz, 1H, Ar–H), 4.73 (s, 2H, SCH2); 13C NMR (101 MHz, Acetone d6, δ, ppm): 177.02, 162.14 (thiadiazole ring-C), 160.16 (C=N), 158.79, 137.88, 137.54, 130.56, 130.46, 125.75, 123.96, 122.43, 122.27, 117.01, 115.26, 114.63, 114.41, 112.30, 37.18 (SCH2); HR-MS (ESI): calcd. for C18H12ClFN4S2: [M + Na+] 425.0074; found: 425.0071.
(E)-N-(5-((4-(trifluoromethyl)benzyl)thiol)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)meth- animine (2s)
Yellow solid powder; M. p. 198.1–199.0 °C; yield 65%; IR(ν, cm–1 KBr): 3442 (N–H), 3043 (Ar–H), 1653 (C=N), 1574, 1490, 1442, 1389, 1243 (thiadiazole ring), 1081 (C–S–C); 1H NMR (400 MHz, Acetone d6, δ, ppm): 12.29 (s, 1H, N–H), 8.91 (s, 1H, HC=N), 8.28 (t, J = 12.0 Hz, J = 8.0 Hz, 2H, Ar–H), 8.22 (d, J = 8.0 Hz, 2H, Ar–H), 7.75 (d, J = 8.0 Hz, 2H, Ar–H), 7.54 (d, J = 8.0 Hz, 1H, Ar–H), 7.28 (s, 2H, Ar–H), 4.70 (s, 2H, SCH2); 13C NMR (101 MHz, Acetone d6, δ, ppm): 176.31, 162.05 (thiadiazole ring-C), 159.67 (C=N), 142.10, 137.87, 137.44, 129.91, 128.91, 125.42, 124.99, 123.95, 122.41, 122.25, 115.23, 112.31, 36.54 (SCH2); HR-MS (ESI): calcd. for C19H13F3N4S2: [M–H+] 417.0456; found: 417.0496.
(E)-N-(5-((2-(trifluoromethyl)benzyl)thio)-1,3,4-thiadiazol-2-yl)-1-(1H-indol-3-yl)meth- animine (2t)
Yellow solid powder; M. p. 194.7–195.6 °C; yield 63%; IR (ν, cm–1KBr): 3444 (N–H), 3043 (Ar–H), 1636 (C=N), 1561, 1496, 1448, 1336, 1246 (thiadiazole ring), 1055 (C–S–C); 1H NMR (400 MHz, Acetone d6, δ, ppm): 12.34 (s, 1H, N–H), 9.03 (d, J = 20.0 Hz, 1H, HC=N), 8.46 (t, J = 8.0 Hz, 1H, Ar–H), 8.27 (d, J = 8.0 Hz, 1H, Ar–H), 7.58–7.78 (m, 4H, Ar–H), 7.23–7.39 (m, 3H, Ar–H), 4.71 (d, J = 8.0 Hz, 2H, SCH2); 13C NMR (101 MHz, Acetone d6, δ, ppm) δ: 176.31, 162.05 (thiadiazole ring-C), 159.67 (C=N), 142.10, 142.10, 137.87, 137.44, 129.91, 128.94, 125.76, 125.42, 124.99, 123.95, 123.06, 122.41, 122.25, 115.23, 112.31, 36.54 (SCH2); HR-MS (ESI): calcd. for C19H13F3N4S2: [M–H+] 417.0456; found: 417.0496.
Spectra for structural information about the compounds are provided in the Supplemental Materials.
3.4. In Vitro Antifungal Assay
The antifungal activities of the novel compounds 2a–2t were tested based on the reported method [74]. The synthesized compounds were dissolved in a 20% acetone water solution. The solution of each compound was added to sterilized potato dextrose agar to obtain a final concentration of 500 μg/mL. After the mixture was cooled, the mycelium of the fungi was transferred to the test plate and incubated at 25 °C for 4–7 days. When the mycelium reached the edges of the control plate (without the added samples), the inhibitory index was calculated using the following formula:
where Da is the diameter of the growth zone in the test plate, and Db is the diameter of the growth zone in the control plate. Each experiment was performed three times and the data points were averaged. The commercial fungicide triadimefon (100 μg/mL) was used as a control and tested in the same manner.
Inhibitory index (%) = (1 − Da/Db)
4. Conclusions
In the present study, a series of novel indole derivatives containing 1,3,4-thiadiazole scaffolds modified with thioether groups were efficiently designed and synthesized. In addition, their antifungal activities were investigated against F. graminearum, F. oxysporum, F. moniliforme, C. lunata, and P. p. var. nicotianae. The antifungal activity test results show that some of the indole analogs exhibited better antifungal activity than the control reagent triadimefon. Compound 2j was identified as the most active against F. graminearum, F. oxysporum, F. moniliforme, and P. p. var. nicotianae with the inhibition rates of 100%, 95.7%, 89%, and 76.5%, respectively. Compounds 2j and 2q exhibited better antifungal activity against C. lunata with inhibition rates of 81.9% and 83.7%, respectively. Compound 2j, as the representative compound, was used for further mechanistic studies. The indole derivatives containing modified 1,3,4-thiadiazole with the electron-withdrawing –NO2 group on the benzene ring showed better antifungal activity. Conclusively, the structural optimization of indole derivatives containing modified 1,3,4-thiadiazole with the electron-withdrawing groups on the benzene ring is a potential strategy to prepare analogs with improved antifungal activity.
5. Patents
There is a patent resulting from the work reported in this manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27206858/s1, Ⅰ: The IR, 1H NMR, 13C NMR, and HRMS of the target compounds (2a–2t) Figures S1–S80; page: 12–41; II: The physical photos of the inhibitory activity of the target compounds against test fungi, Figures S81–S85; page: 42–46.
Author Contributions
Conceptualization, C.W., C.X. and G.Y.; methodology, L.F. and Z.P.; synthesis of target compounds, C.W., S.F., X.L. and J.Z.; validation, C.W., L.S., L.W. and S.F.; spectrum analysis, C.W., C.X., G.Y. and Z.P.; preliminary test of inhibiting fungal activity, C.W., G.Y., C.X., J.Z. and X.L.; data curation, C.W., L.F. and Z.P.; writing—original draft preparation, C.W., G.Y. and C.X.; writing—review and editing, C.W., G.Y. and C.X.; supervision, C.W. and C.X.; project administration, C.W., C.X., L.F.,and G.Y.; funding acquisition, C.W., C.X., L.F. and G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Program for Tackling Key Problems in Science and Technology of Henan Province (222102310243), the Natural Science Foundation of Henan Province (222300420456, 222300420459, 222300420188), the Key Scientific Research Projects in Henan Colleges and Universities (21A150021) and the special fund for topnotch talents in Henan Agricultural University (30500925).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Wang C.X. thanks her family for their support and encouragement throughout all her research career. The authors thank Huang X.S. and Huang R.D. for their unvaluable support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gur, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Pennisi, E. Armed and dangerous. Science 2010, 27, 804–805. [Google Scholar]
- Helena, P.V.; Josue, J.S.; Larissa, S.F. The first report of A. novo parasiticus, A. arachidicola and A. pseudocaelatus in Brazilian corn kernels. Int. J. Food Microbiol. 2017, 243, 46–51. [Google Scholar]
- Kos, J.; Halnal, E.J.; Malachov, A. Mycotoxins in maize harvested in Republic of Serbia in the period 2012–2015. Part 1: Regulated mycotoxins and its derivatives. Food Chem. 2020, 312, 126034. [Google Scholar] [CrossRef]
- Ayodel, A.O.; Tumisi, M.; Rhulani, M. A review on novel non—Thermal food processing techniques for mycotoxin reduction. Int. J. Food Sci. Technol. 2020, 56, 13–27. [Google Scholar]
- Eskola, M.; Skola, M.; Kos, G.; Elliott, C.T. Worldwide contamination of food—Crops with mycotoxins: Validity of the widely cited FAO estimate of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Der Does, H.C.; Borkovich, K.A. Comparative genomics reveals mobile pathogeni- city chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Molina, M. Arabidopsis defense response against Fusarium oxysporum. Trends Plant Sci. 2008, 13, 145–150. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current status of Fusarium oxysporum formae specials and races. Phytopathology 2018, 109, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Lopez-Berges, M.S.; Hera, C.; Sulyok, M. The velvet complex governs mycotoxin production and virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Microbiol. 2013, 87, 49–65. [Google Scholar] [CrossRef]
- Thatcher, L.F.; Gao, L.L.; Singh, K.B. Jasmonate signalling and defence responses in the model legume Medicago trncatula—A focus on responses to Fusarium wilt disease. Plants 2016, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Gosw, A.S.; Trail, F.; Xu, J.R. Fungal genes expressed during plant disease development in Fusarium/wheat interaction. Fungal Genet Newsl. 2003, 50, 292–602. [Google Scholar]
- Emerson, M.D.; Ponte, J.G.; Eliana, B.F. Deoxynivalenol and nivalenol in commercial wheat grain related to Fusarium head blight epidemics in southern Brazil. Food Chem. 2012, 132, 1087–1091. [Google Scholar]
- Stack, R.W. Return of an Old Problem: Fusarium Head Blight of Small Grains; APS Press: St. Paul, MN, USA, 1999. [Google Scholar]
- Stack, R.W. History of Fusarium head blight with emphasis on North America. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 1–34. [Google Scholar]
- Nganje, W.E.; Bangsund, D.A.; Leistriiz, F.L. Estimating the economic impact of a crop disease: The case of Fusarium head blight in US wheat and barley. In National Fusarium Head Blight Forum Proceeding; East Lansing Michigan State University: Michigan, MI, USA, 2002; pp. 75–281. [Google Scholar]
- Mcmulle, N.M.; Jones, R.; Gallenber, G.D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 13401348. [Google Scholar]
- Han, X.Y.; Zhong, Y.F.; Li, S.B.; Liang, G.C.; Zhou, G.; Wang, X.K.; Chen, B.H.; Song, Y.L. Synthesis, characterization and antifungal evaluation of novel derivatives containing indole skeleton. Chem. Pharm. Bull. 2016, 64, 1411–1416. [Google Scholar] [CrossRef][Green Version]
- Xu, G.; Zhao, J.; Jiang, Y.; Zhang, P.; Li, W. Design, Synthesis and Antifungal Activity of Novel Indole Derivatives Linked with the 1,2,3-Triazole Moiety via the CuAAC Click Reaction. J. Chem. Res. 2016, 40, 269–272. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Jia, C.-Y.; Gu, Y.-C.; Mulholland, N.; Turner, S.; Beattie, D.; Zhang, W.-H.; Yang, G.-F.; Clough, J. Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem. 2017, 126, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, T.G.; Yilmaz, N.; Ece, A.; Altanlar, N.; Olgen, S. Invitro antibacterial and antifungal activity and computational evaluation of novel indole derivatives containing 4-substituted piperazine moieties. Lett. Drug Des. Dis. 2018, 15, 1079–1086. [Google Scholar] [CrossRef]
- Sumiya, T.; Ishigaki, M.; Oh, K. Synthesis of Imidazole and Indole Hybrid Molecules and Antifungal Activity against Rice Blast. Int. J. Chem. Eng. Appl. 2017, 8, 233–236. [Google Scholar] [CrossRef]
- Pagniez, F.; Lebouvier, N.; Na, Y.M.; Ourliac-Garnier, I.; Picot, C.; Le Borgne, M.; Le Pape, P. Biological exploration of a novel 1,2,4-triazole-indole hybrid molecule as antifungal agent. J. Enzym. Inhib. Med. Chem. 2020, 35, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabli, R.I.; Alsulami, M.A.; Bukhari, S.I.; Moubayed, N.M.S.; Al-Mutairi, M.S.; Attia, M.I. Design, synthesis, and antimicrobial activity of certain new indole-1,2,4 triazole conjugates. Molecules 2021, 26, 2292. [Google Scholar] [CrossRef]
- Mruthyunjayaswamy, B.H.M.; Basavarajaiah, S.M. Synthesis and antimicrobial activity of some 5-chloro-3-phenyl-1H-indole-2-carbonyl azide derivatives. Indian J. Chem. 2018, 57, 390–399. [Google Scholar]
- Kong, Q.; Pan, W.; Xu, H.; Xue, Y.; Guo, B.; Meng, X.; Luo, C.; Wang, T.; Zhang, S.; Yang, Y. Design, synthesis, and biological evaluation of novel pyrimido [4,5-b] indole derivatives against gram-negative multidrug-resistant pathogens. J. Med. Chem. 2021, 64, 8644–8665. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.-L.; Liu, J.; Fang, W.-Y.; Ravindar, L.; Rakesh, K. Indole-based derivatives as potential antibacterial activity against methicillin-resistance Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 194, 112245. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, K.H.M.E.; Mashayekhi, V.; Azerang, P.; Sardari, S.; Kobarfard, F.; Rostamizadeh, K. Synthesis and Antimycobacterial Activity of Novel Thiadiazolylhydrazones of 1-Substituted Indole-3-carboxaldehydes. Chem. Biol. Drug Des. 2014, 83, 224–236. [Google Scholar] [CrossRef]
- Khan, G.A.; War, J.A.; Kumar, A.; Sheikh, I.A.; Saxena, A.; Das, R. A facile synthesis of novel indole derivatives as potential antitubercular agents. J. Taibah Univ. Sci. 2017, 11, 910–921. [Google Scholar] [CrossRef]
- Champciaux, B.; Raynaud, C.; Viljoen, A.; Chene, L.; Thibonnet, J.; Vincent, S.P.; Kremer, L.; Thiery, E. Synthesis and biological evaluation of 3,4-dihydro-1H-[1,4] oxazepino [6,5,4-hi] indol-1-ones and 4,6-dihydrooxepino [5,4,3-cd] indol-1(3H)-ones as Mycobacterium tuberculosis inhibitors. Bioorganic Med. Chem. 2021, 43, 116248. [Google Scholar] [CrossRef]
- Cihan-Üstündag, G.; Naesens, L.; Şatana, D.; Erköse-Genç, G.; Mataracı-Kara, E.; Çapan, G. Design, synthesis, antitubercular and antiviral properties of new spirocyclic indole derivatives. Mon. Chem. Chem. Mon. 2019, 150, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, M.; Baldisserotto, A.; Lampronti, I.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Indole derivatives as multifunctional drugs: Synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity of indole hydrazones. Bioorganic Chem. 2019, 85, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Elshemy, H.A.; Zaki, M.A.; Mohamed, E.I.; Khan, S.I.; Lamie, P.F. A multicomponent reaction to design antimalarial pyridyl-indole derivatives: Synthesis, biological activities and molecular docking. Bioorg. Chem. 2020, 97, 103673. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, S.N.; Meissner, K.A.; Ferraz, W.R.; Trossini, G.H.; Wrenger, C.; A Stefani, H. Indole-3-glyoxyl tyrosine: Synthesis and antimalarial activity against Plasmodium falciparum. Future Med. Chem. 2019, 11, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Saxena, A.; Saha, B. An insight in anti-malarial potential of indole scaffold: A review. Eur. J. Med. Chem. 2021, 218, 113400. [Google Scholar] [CrossRef]
- Che, Z.; Tian, Y.; Liu, S.; Hu, M.; Chen, G. Synthesis and in vitro anti-HIV-1 evaluation of some N-arylsulfonyl-3-formylindoles. Braz. J. Pharm. Sci. 2018, 54, 3–9. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Song, H.; Liu, Y.; Wang, L.; Wang, Q. Expanding indole diversity: Direct 1-step synthesis of 1,2-fused indoles and spiroindolines from 2-halo anilines for fast SAR antiviral elucidation against tobacco mosaic virus (TMV). Mol. Divers. 2017, 21, 61–68. [Google Scholar] [CrossRef]
- Sevinçli, Z.; Duran, G.N.; Özbil, M.; Karalı, N. Synthesis, molecular modeling and antiviral activity of novel 5-fluoro-1H-indole-2,3-dione 3-thiosemicarbazones. Bioorganic Chem. 2020, 104, 104202. [Google Scholar] [CrossRef]
- Wei, C.; Zhao, L.; Sun, Z.; Hu, D.; Song, B. Discovery of novel indole derivatives containing dithioacetal as potential antiviral agents for plants. Pestic. Biochem. Physiol. 2020, 166, 104568. [Google Scholar] [CrossRef]
- Tiwari, S.; Kirar, S.; Banerjee, U.C.; Neerupudi, K.B.; Singh, S.; Wani, A.A.; Bharatam, P.V.; Singh, I.P. Synthesis of N-substituted indole derivatives as potential antimicrobial and antileishmanial agents. Bioorganic Chem. 2020, 99, 103787. [Google Scholar] [CrossRef]
- Ashok, P.; Chander, S.; Smith, T.K.; Prakash Singh, R.; Jha, P.N.; Sankaranarayanan, M. Biological evaluation and structure activity relationship of 9-methyl-1-phenyl-9H-pyrido[3,4-b] indole derivatives as anti-leishmanial agents. Bioorg. Chem. 2019, 84, 98–105. [Google Scholar] [CrossRef]
- Alka, A.S.; Maheshkumar, P.P.; Kang, M.J.; Irvine, N.; Kim, G.D. Biomedical application of Indole-3-carbinol: A mini-review. Phytochem. Lett. 2021, 41, 49–54. [Google Scholar]
- Ma, J.L.; Li, J.; Guo, P.H.; Liao, X.C.; Cheng, H.C. Synthesis and antitumor activity of novel indole derivatives containinα-aminophosphonate moieties. Arab. J. Chem. 2021, 14, 103256. [Google Scholar] [CrossRef]
- Pecnard, S.N.; Hamze, A.L.; Bignon, J.M.; Prost, B.T.; Deroussent, A.; Laura, G.Y.; Aez, R.P.; Ji, Y.P.; Marc, D.; Mouad, A.; et al. Anticancer properties of indole derivatives as Iso Combretastatin A-4 analogues. Eur. J. Med. Chem. 2021, 223, 113656. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Barbarossa, A.; Carocci, A.; Fazio, A.; La Torre, C.; Caruso, A.; Ponassi, M.; Rosano, C.; et al. Synthesis, anticancer and antioxidant properties of new indole and pyranoindole derivatives. Bioorganic Chem. 2020, 105, 104440. [Google Scholar] [CrossRef] [PubMed]
- Umar Basha, K.N.; Gnanamani, S.; Shanmugam, P.; Venugopal, S.; Murthy, S.; Ramasamy, B. Synthesis, antioxidant, and antimicrobial activity of 3-(1 H -indole-3-carbonyl)- 2 H -chromen-2-ones. J. Heterocycl. Chem. 2021, 58, 2000–2008. [Google Scholar] [CrossRef]
- Song, Z.-L.; Zhu, Y.; Liu, J.-R.; Guo, S.-K.; Gu, Y.-C.; Han, X.; Dong, H.-Q.; Sun, Q.; Zhang, W.-H.; Zhang, M.-Z. Diversity-oriented synthesis and antifungal activities of novel pimprinine derivative bearing a 1,3,4-oxadiazole-5-thioether moiety. Mol. Divers. 2021, 25, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Z.; Chen, Q.; Mulholland, N.; Beattie, D.; Irwin, D.; Gu, Y.-C.; Yang, G.-F.; Clough, J. Synthesis and fungicidal activity of novel pimprinine analogues. Eur. J. Med. Chem. 2012, 53, 283–291. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, D.-C.; Liu, C.; Song, Z.-L.; Liu, J.-R.; Guo, S.-K.; Tan, J.-Y.; Qiu, R.-L.; Jin, B.; Zhang, H.; et al. Streptochlorin analogues as potential antifungal agents: Design, synthesis, antifungal activity and molecular docking study. Bioorg. Med. Chem. 2021, 35, 116073. [Google Scholar] [CrossRef]
- Muğlu, H.; Yakan, H.; Shouaib, H.A. New 1,3,4-thiadiazoles based on thiophene-2-carboxylic acid: Synthesis, characterization, and antimicrobial activities. J. Mol. Struct. 2020, 1203, 127470. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Liu, H.; Chen, D.; Li, H.; Chen, Z.; Xu, W. Synthesis and antimicrobial bioassays of 1,3,4-thiadiazole sulfone derivatives containing amide moiety: A study based on molecular dynamics (MD) simulations, MM/GBSA, and molecular docking. J. Saudi Chem. Soc. 2022, 26, 101415. [Google Scholar] [CrossRef]
- Lv, M.; Liu, G.C.; Jia, M.H.; Xu, H. Synthesis of matrinic amide derivatives containing 1,3,4-thiadiazole scaffffold as insecticidal/acaricidal agents. Bioorg. Chem. 2018, 81, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cai, H.; Yuan, T.; Li, S.; Gan, X.; Song, B. Novel vanillin derivatives containing a 1,3,4-thiadiazole moiety as potential antibacterial agents. Bioorganic Med. Chem. Lett. 2020, 30, 127113. [Google Scholar] [CrossRef] [PubMed]
- He, L.E.; Wu, Y.Y.; Shi, D.Q. Design, synthesis, and herbicidal evaluation of novel uracil derivatives containing 1,3,4-thiadiazolyl moiety. J. Heterocyclic Chem. 2015, 52, 1308–1313. [Google Scholar] [CrossRef]
- Knerr, P.J.; Tzekou, A.; Ricklin, D.; Qu, H.C.; Chen, H.; Donk, W.A.; Lambris, J.D. Synthesis and activity of thioether-containing analogues of the complement inhibitor compstatin. ACS Chem. Biol. 2011, 15, 753–759. [Google Scholar] [CrossRef]
- Xu, W.M.; Li, S.Z.; He, M.; Yang, S.; Li, X.Y.; Li, P. Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1, 2, 3-thiadiazole and 1,3, 4-oxadiazole/thiadiazole moiety. Bioorg. Med. Chem. Lett. 2013, 23, 5821–5829. [Google Scholar] [CrossRef]
- Elangovan, N.; Thomas, R.; Sowrirajan, S. Synthesis of Schiff base (E)-4-((2-hydroxy-3,5- diiodobenzylidene) amino)-N-thiazole-2-yl)benzenesulfon amide with antimicrobial potential, structural features, experimental biological screening and quantum mechanical studies. J. Mol. Struct. 2022, 1250, 131762. [Google Scholar] [CrossRef]
- Manivel, S.; Gangadharappa, B.S.; Elangovan, N.; Thomas, R.; Abu Ali Ola, A.; Saleh Dalia, I. Schiff base (Z)-4-((furan-2-ylmethylene) amino) benzene sulfonamide: Synthesis, solvent interactions through hydrogen bond, structural and spectral properties, quantum chemical modeling and biological studies. J. Mol. Liq. 2022, 350, 118531. [Google Scholar] [CrossRef]
- Gür, M.; Yerlikaya, S.; Şener, N.; ÖzkInalı, S.; Baloglu, M.; Gökçe, H.; Altunoglu, Y.C.; Demir, S.; Şener, İ. Antiproliferative-antimicrobial properties and structural analysis of newly synthesized Schiff bases derived from some 1,3,4-thiadiazole compounds. J. Mol. Struct. 2020, 1219, 128570. [Google Scholar] [CrossRef]
- Lou, J.Y.; Wang, H.S.; Wang, S.Y.; Han, J.J.; Wang, M.Y. Synthesis, antimicrobial activity and 3D-QSAR study of novel 5-substituted-1,3,4-thiadiazole Schiff base derivatives. J. Mol. Struct. 2022, 1267, 133629. [Google Scholar] [CrossRef]
- Singh, G.; Kalra, P.; Singh, A.; Sharma, G.; Pawan, S.; Cristóbal Espinosa-Ruíz, M.; Esteban, M.A. A quick microwave preparation of isatin hydrazone schiff base conjugated organosilicon compounds: Exploration of their antibacterial, antifungal, and antioxidative potentials. J. Organomet. Chem. 2021, 953, 122051. [Google Scholar] [CrossRef]
- Wang, C.X.; Song, H.L.; Liu, W.Q.; Xu, C.L. Design, synthesis and antifungal activity of novel thioureas containing 1,3,4-thiadiazole and thioether skeleton. Chem. Res. Chin. Univ. 2016, 32, 615–620. [Google Scholar] [CrossRef]
- Yang, G.Y.; Shi, L.J.; Pan, Z.L.; Wu, L.L.; Fan, L.X.; Wang, C.X.; Xu, C.L.; Liang, J. The synthesis of coumarin thiazoles containing a trifluoromethyl group and their antifungal activities. Arab. J. Chem. 2021, 14, 102880–102888. [Google Scholar] [CrossRef]
- Yang, G.Y.; Wang, C.X.; Fan, S.F.; Xie, P.H.; Jin, Q.; Xu, C.L. Microwave assisted solvent-free synthesis of 3-(trifluoroacetyl)coumarins. Chin. J. Org. Chem. 2015, 35, 1173–1178. [Google Scholar] [CrossRef]
- Shi, L.J.; Liu, Y.; Wang, C.X.; Yuan, X.X.; Liu, X.B.; Wu, L.L.; Pan, Z.L.; Yu, Q.C.; Xu, C.L.; Yang, G.Y. Synthesis of 1-(b-coumarinyl)-1-(b-indolyl) trifluoroethanols through regioselective Friedel-Crafts alkylation of indoles with b-(trifluoroacetyl) coumarins catalyzed by Sc (OTf) 3. RSC Adv. 2020, 10, 13929–13935. [Google Scholar] [CrossRef]
- Bao, J.P.; Xu, C.L.; Yang, G.Y.; Wang, C.X.; Zheng, X.; Yuan, X.X. Novel 6a,12b-dihydro-6H,7H-chromeno[3,4-c] chromen-6-ones: Synthesis, structure and antifungal activity. Molecules 2019, 24, 1745. [Google Scholar] [CrossRef]
- Yang, G.Y.; Jin, Q.; Xu, C.L.; Fan, S.F.; Wang, C.X.; Xie, P.H. Synthesis, characterization and antifungal activity of coumarin-functionalized chitosan derivatives. Int. J. Biol. Macromol. 2018, 106, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zhang, M.Y.; Lu, M.X.; He, Y.H.; Fan, L.X.; Zhang, X.L.; Wu, J.K.; Yang, Y.X. Synthesis of spiropyrans via the Rh (III)-catalyzed annulation of 3-aryl-2H-benzo[b][1,4] oxazines with diazoetoesters. Chem. Commun. 2022, 58, 5144–5147. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Yang, J.T.; Wang, C.X.; Fang, S.F.; Xie, P.H.; Xu, C.L. Microwave-assisted TsOH/SiO2-catalyzed one-pot synthesis of novel fluoro-substituted coumarin hydrazones under solvent-free conditions. J. Fluorine Chem. 2014, 168, 1–8. [Google Scholar] [CrossRef]
- Yuan, X.X.; Wu, L.L.; Xu, C.L.; Pan, Z.L.; Shi, L.J.; Yang, G.Y.; Wang, C.X.; Fan, S.F. A consecutive one-pot two-step approach to novel trifluoromethyl-substituted bis(indolyl)methane derivatives promoted by Sc (OTf)3 and p-TSA. Tetrahedron Lett. 2019, 60, 151329–151343. [Google Scholar] [CrossRef]
- Wang, Z.M.; Xu, C.L.; Zhao, M.Q.; Zhao, C.Y. One-pot synthesis of narrowly distributed silver nanoparticles using phenolichydroxyl modified chitosan and their antimicrobial activities. RSC Adv. 2014, 4, 47021–47030. [Google Scholar] [CrossRef]
- Huang, Z.X. Guidance of the Phytochemical Protection Experiments; China Agricultural Press: Beijing, China, 1993; pp. 56–59. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).