Response Surface Methodology Optimization in High-Performance Solid-State Supercapattery Cells Using NiCo2S4–Graphene Hybrids
Abstract
:1. Introduction
2. Results and Discussion
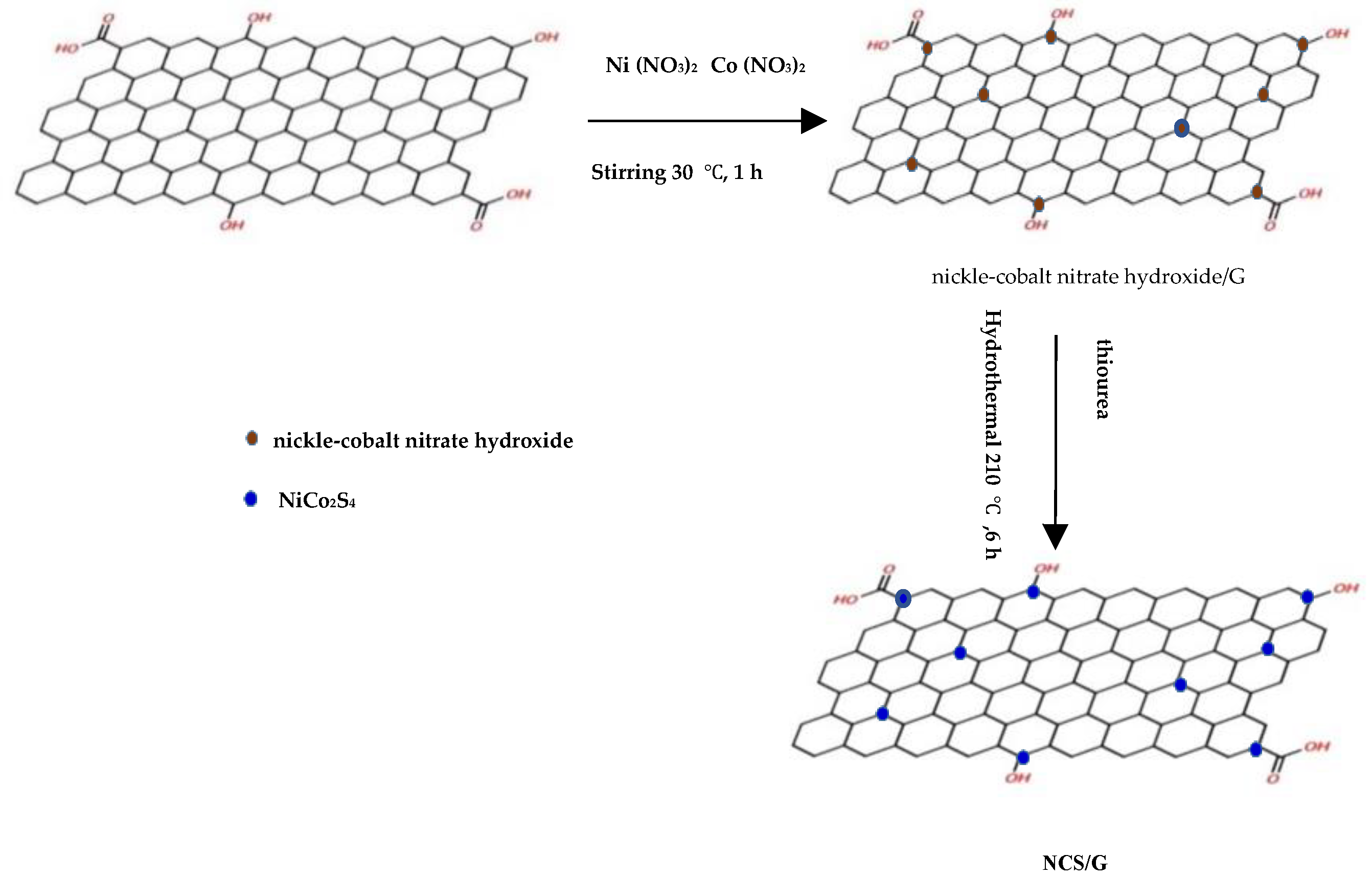
2.1. The Hydrothermal Synthesis of NCS@G Hybrids Using the Traditional Method
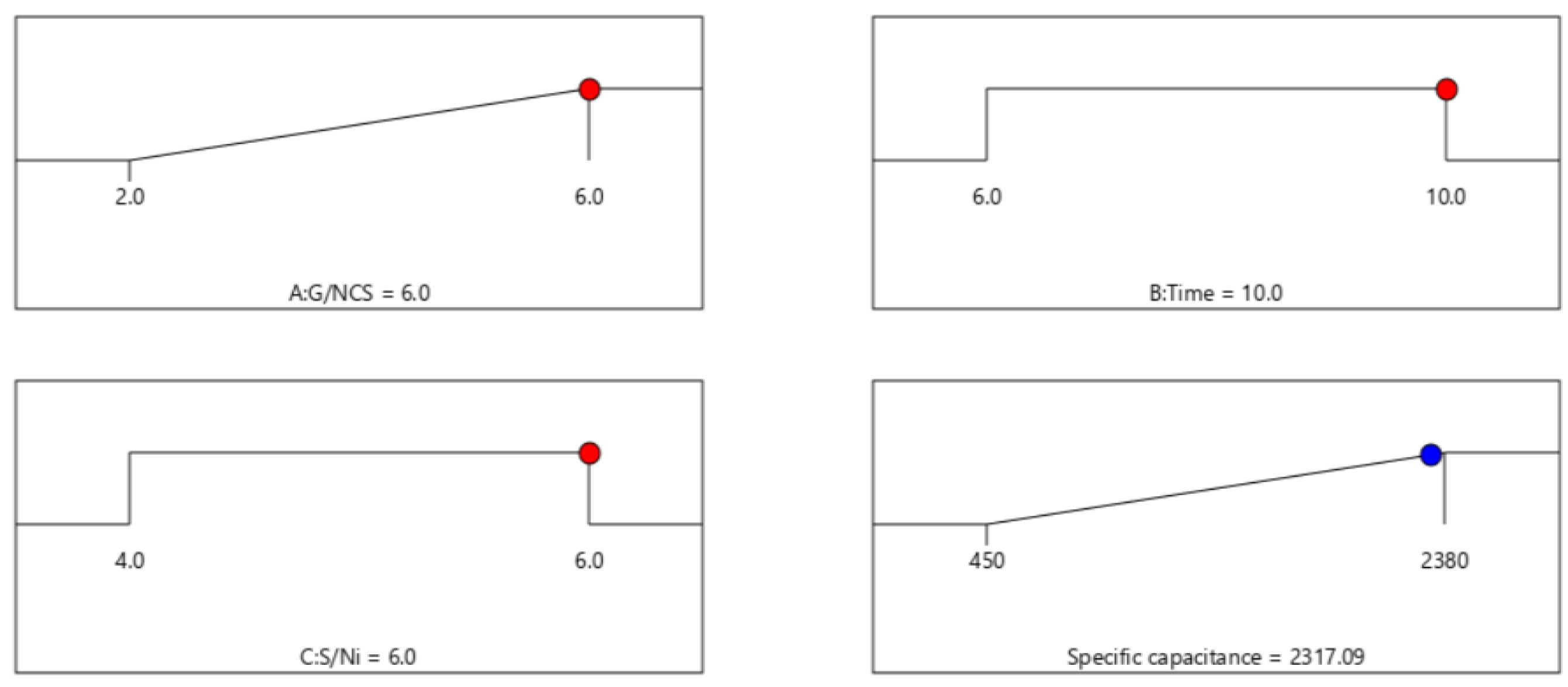
2.2. The Improvement of Specific Capacitance of NCS@G/Ni Composite Electrodes with CCD
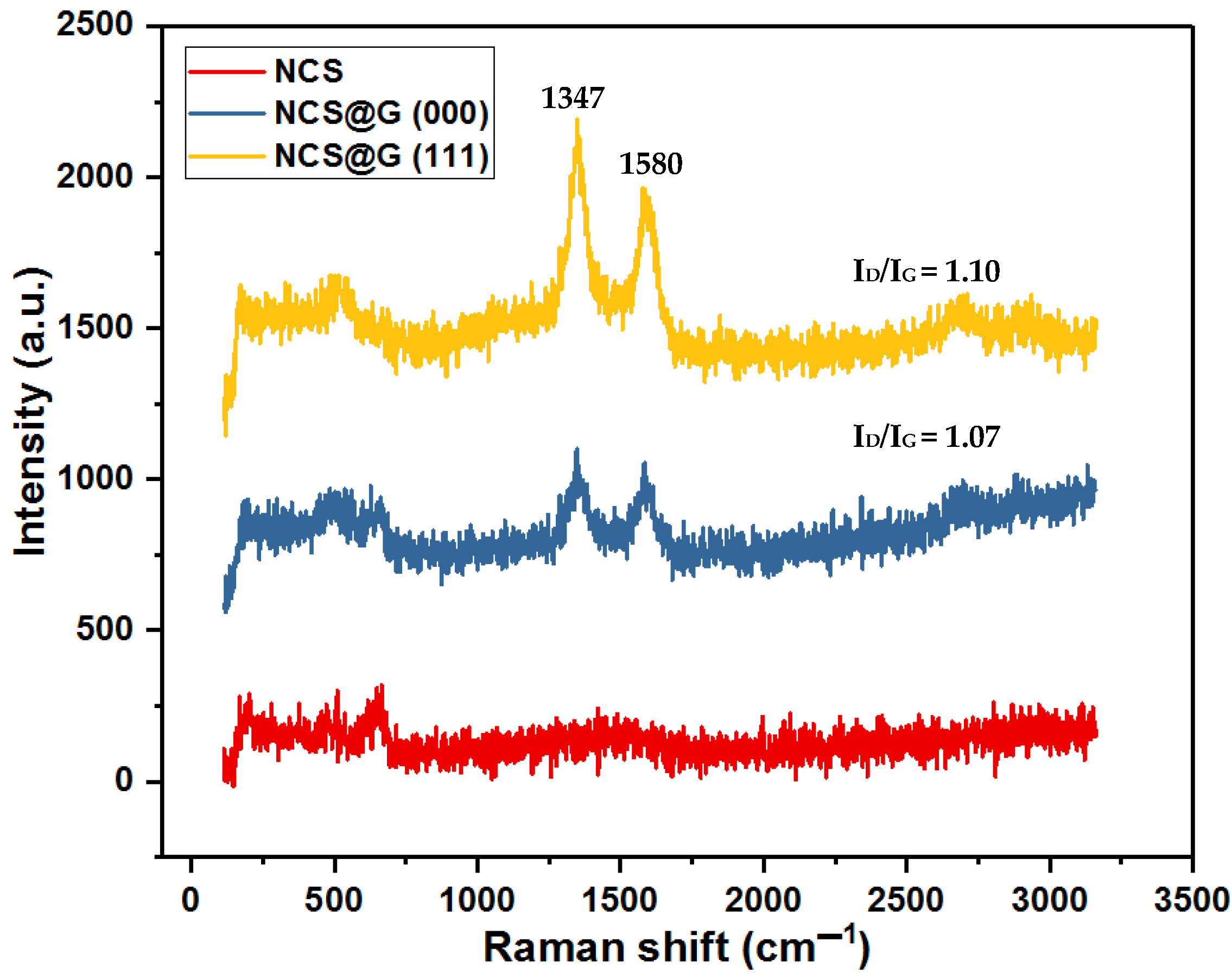
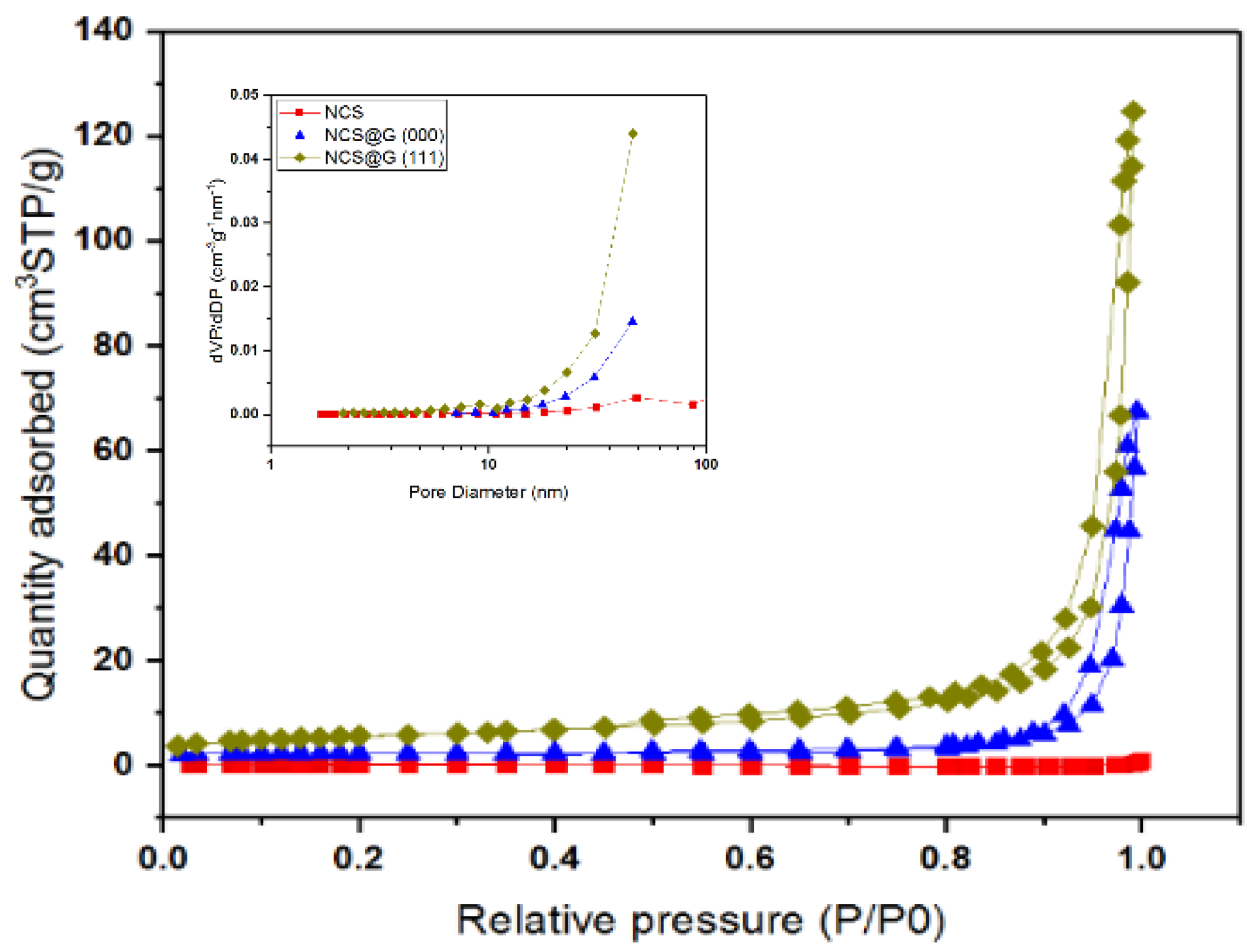
2.3. Structural, Morphological, and Textural Analysis of the NCS@G Hybrids
2.4. Electrochemical Performance of the NCS, NCS@G (000), and NCS@G (111) Electrodes
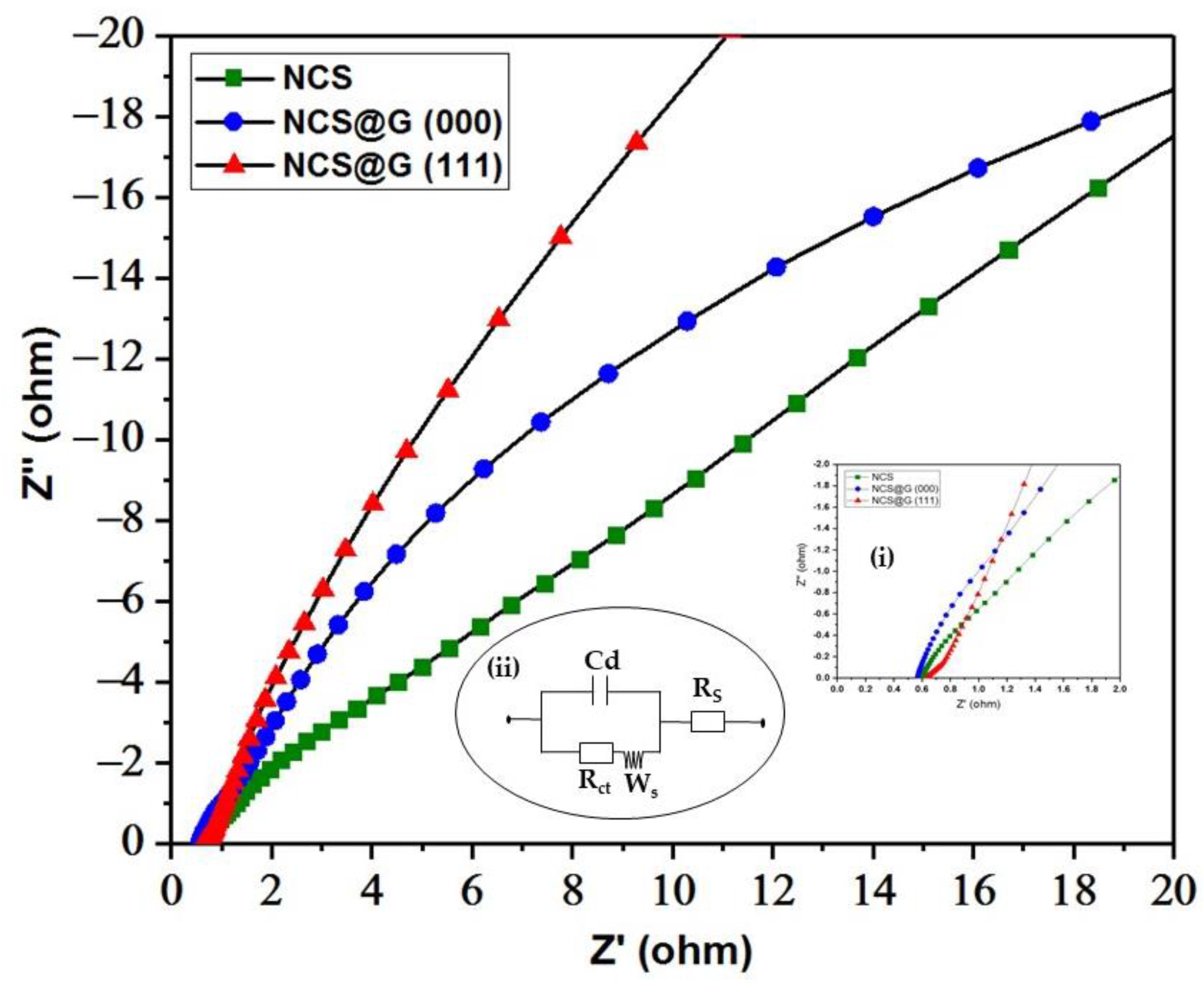
2.5. Electrochemical Impedance of the Electrode
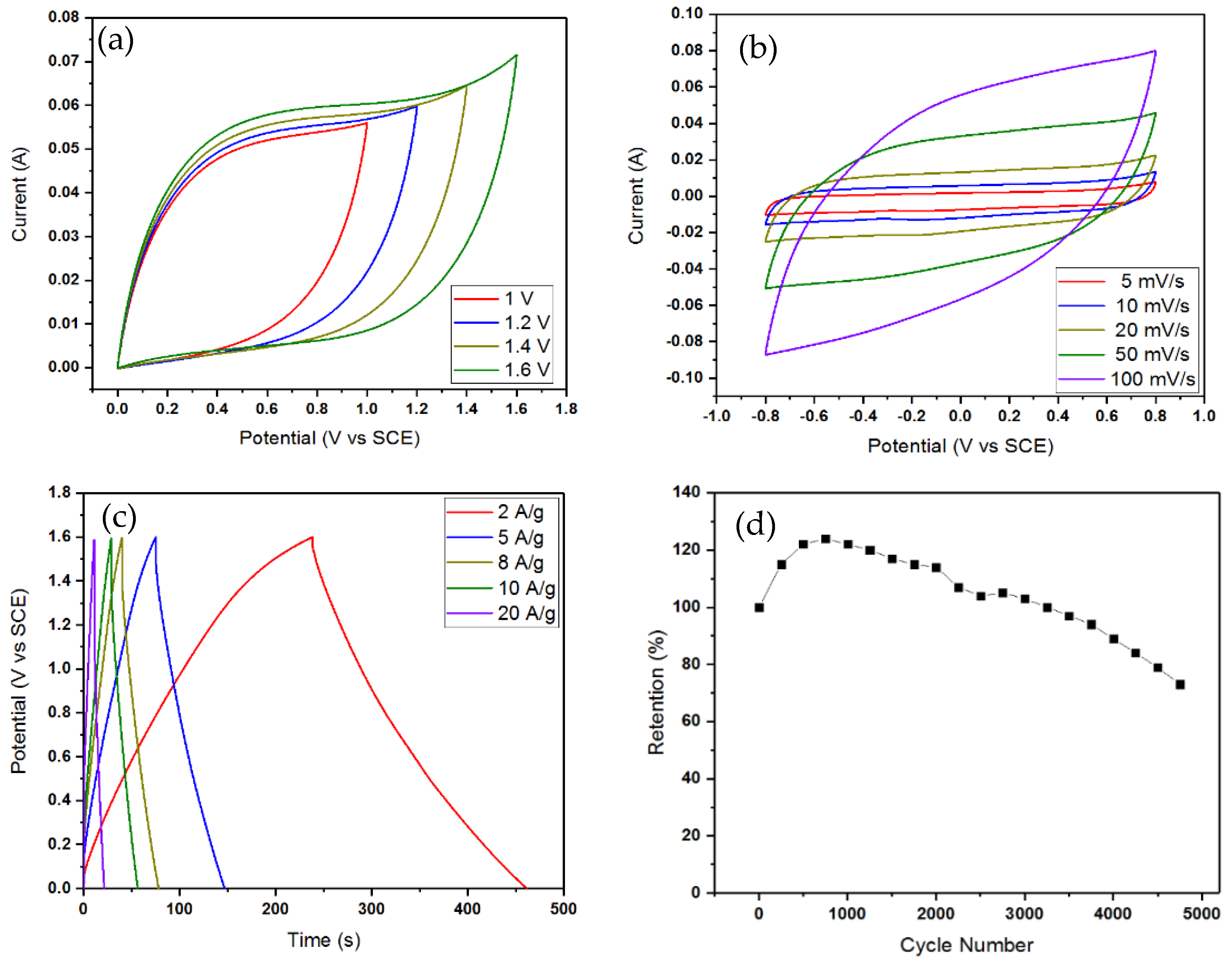
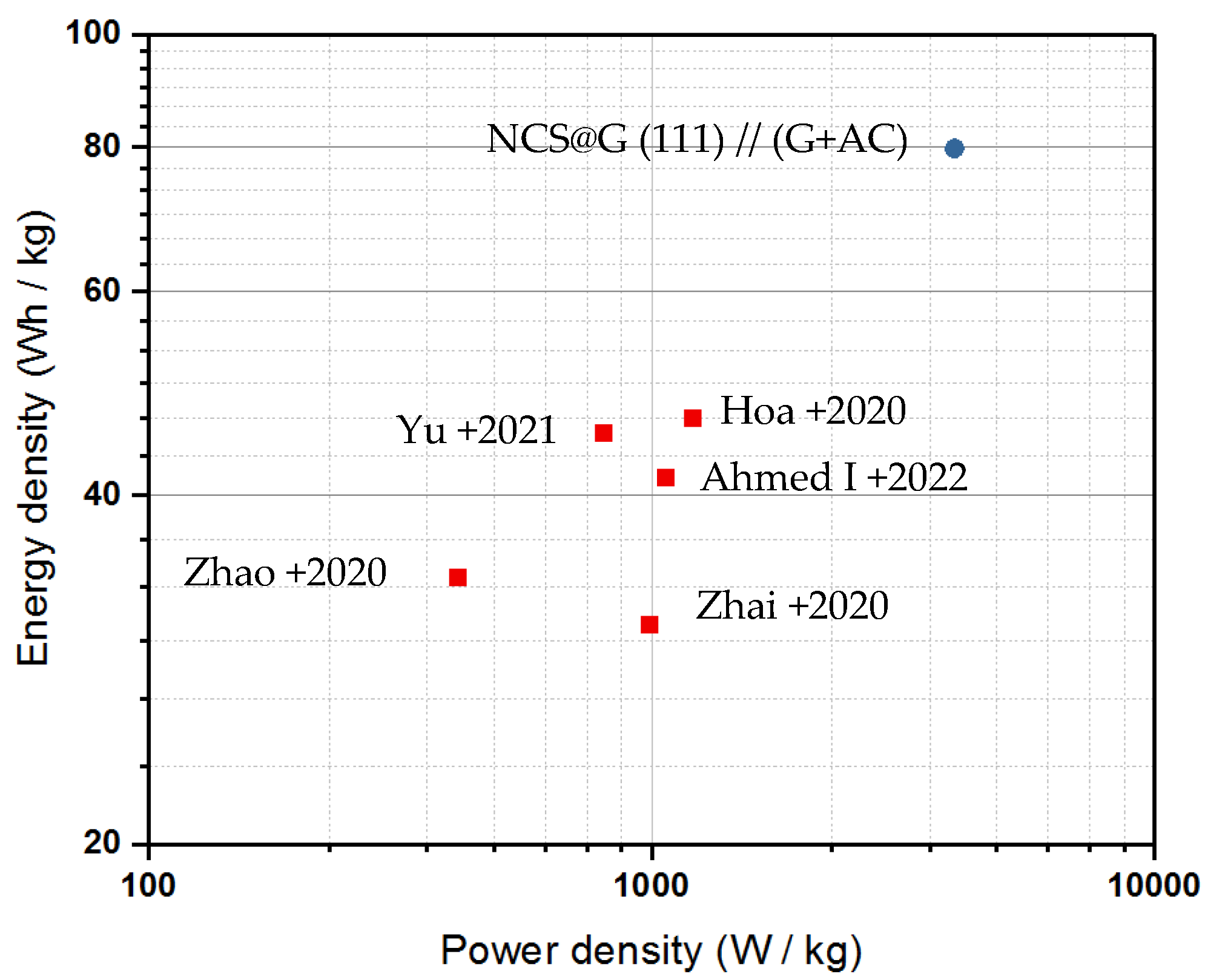
2.6. Assembly and Performance of a Solid-State Supercapattery Cell
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the NCS@G Hybrids
3.3. Material Characterizations
3.4. Experimental Design and Data Analysis
3.5. Preparation of NCS@G/Ni Electrodes and (G + AC)/Ni Electrodes
3.6. Electrochemical Measurements of the Single Electrode and Supercapattery Cell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Holechek, J.L.; Geli, H.M.E.; Sawalha, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Mohanty, A.; Balasingam, K.S.; Kim, S.J.; Ramadoss, A. Comprehensive Insight into the Mechanism, Material Selection and Performance Evaluation of Supercapatteries. Nano-Micro Lett. 2020, 12, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshetu, G.G.; Zhang, H.; Judez, X.; Adenusi, H.; Armand, M.; Passerini, S.; Figgemeier, E. Production of high-energy Li-ion batteries comprising silicon-containing anodes and insertion-type cathodes. Nat. Commun. 2021, 12, 5459. [Google Scholar] [CrossRef]
- Chen, G.Z.; Akinwolemiwa, B. Fundamental Consideration for Electrochemical Engineering of Supercapattery. J. Braz. Chem. Soc. 2018, 29, 960–972. [Google Scholar]
- Chen, W.; Zhang, X.; Mo, L.E.; Zhang, Y.; Chen, S.; Zhang, X.; Hu, L. NiCo2S4 quantum dots with high redox reactivity for hybrid supercapacitors. Chem. Eng. J. 2020, 388, 124109. [Google Scholar]
- Ismail, M.M.; Hong, Z.Y.; Arivanandhan, M.; Yang, T.C.K.; Pan, G.T.; Huang, C.M. In-situ binder-free and hydrothermal growth of nanostructured NiCo2S4/Ni electrodes for solid-state hybrid supercapacitors. Energies 2021, 14, 7114. [Google Scholar] [CrossRef]
- DongSu, Y.L.; Sang, Z.; Su, X.; Chen, H.; Yan, X. High-performance layered NiCo2S4@rGO/rGO film electrode for flexible electrochemical energy storage. Electrochim. Acta 2019, 328, 135088. [Google Scholar]
- Thalji, M.R.; Ali, G.A.M.; Liu, P.; Zhong, Y.L.; Chong, K.F. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2021, 40, 128216. [Google Scholar] [CrossRef]
- Folorunso, O.; Onibonoje, M.O.; Hamam, Y.; Sadiku, R.; Ray, S.S. Fabrication and Model Characterization of the Electrical Conductivity of PVA/PPy/rGO Nanocomposite. Molecules 2022, 27, 3696. [Google Scholar] [CrossRef]
- Yu, J.; Li, H.; Shi, X.; Sun, Z. Advanced Asymmetric Supercapacitor Based on Graphene/Single-Walled Carbon Nanotube and Mesoporous Hollow NiCo2S4 Sub-microsphere Electrodes with High Energy Density. J. Electron. Mater. 2021, 50, 3095–3104. [Google Scholar] [CrossRef]
- He, D.; Li, F.; Xiao, Y.; Chen, S.; Zhu, Z.; Chen, H.; Hu, X.; Peng, W.; Xin, S.; Bai, Y. Electrostatic self-assembly assisted hydrothermal synthesis of bimetallic NiCo2S4@N, S co-doped graphene for high performance asymmetric supercapacitors. Electrochim. Acta 2022, 40, 139751. [Google Scholar] [CrossRef]
- Huang, C.M.; Chen, L.C.; Yang, H.C.; Li, M.H.; Pan, T.C. Preparation of acrylic acid-modified chitin improved by an experimental design and its application in absorbing toxic organic compounds. J. Hazard. Mater. 2012, 241–242, 190–196. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Wang, B. Optimization of encapsulation efficiency and average particle size of Hohenbuehelia serotina polysaccharides nanoemulsions using response surface methodology. Food Chem. 2017, 229, 479–486. [Google Scholar] [CrossRef]
- Manojkumar, N.; Muthukumaran, C.; Sharmila, G. A comprehensive review on the application of response surface methodology for optimization of biodiesel production using different oil sources. JKSUES 2022, 34, 198–208. [Google Scholar] [CrossRef]
- Quanhong, L.; Caili, F. Application of response surface methodology for extraction optimization of germinant pumpkin seeds protein. Food Chem. 2005, 92, 701–706. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, R.; Zhang, C.; Xi, S.; Jones, M.W.M.; Tesfamichael, T.; Du, A.; Gui, K.; Ostrikov, K.; Wang, H. Plasma-induced on-surface sulfur vacancies in NiCo2S4 enhance energy storage performance of supercapatteries. J. Mater. Chem. A 2020, 8, 9278–9291. [Google Scholar] [CrossRef]
- Yu, L.; Chen, G.Z. Supercapatteries as High-Performance Electrochemical Energy Storage Devices. Electrochem. Energy Rev. 2020, 3, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Erusappan, E.; Pan, G.T.; Chung, H.Y.; Chong, S.; Thiripuranthagan, S.; Yang, T.C.K.; Huang, C.M. Hierarchical Nickel-Cobalt Oxide and Glucose-based Carbon Electrodes for Asymmetric Supercapacitor with High Energy Density. J. Taiwan Inst. Chem. Eng. 2020, 112, 330–336. [Google Scholar] [CrossRef]
- Abdel-Salam, A.I.; Attia, S.Y.; El-Hosiny, F.I.; Sadek, M.A.; Mohamed, S.G.; Rashad, M.M. Facile one-step hydrothermal method for NiCo2S4/rGO nanocomposite synthesis for efficient hybrid supercapacitor electrodes. Mater. Chem. Phys. 2022, 277, 125554. [Google Scholar] [CrossRef]
- Zhai, R.; Xiao, Y.; Ding, T.; Wu, Y.; Chen, S.; Wei, W. Construction of NiCo2S4 heterostructure based on electrochemically exfoliated graphene for high-performance hybrid supercapacitor electrode. J. Alloys Compd. 2020, 845, 156164. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Lin, Y.; Chen, J.; Li, K.; Chen, A. Design and construction of nickel-cobalt-sulfide nanoparticles in-situ grown on graphene with enhanced performance for asymmetric supercapacitors. Diam. Relat. Mater. 2020, 108, 107925. [Google Scholar] [CrossRef]
- Hoa, N.V.; Dat, P.A.; Hieu, N.V.; Le, T.N.; Minh, N.C.; Tang, N.V.; Nga, D.T.; Ngoc, T.Q. Rapid and efficient synthesis of high-porous reduced graphene oxide/NiCo2S4 nanocomposites for supercapacitor application. Diam. Relat. Mater. 2020, 106, 107850. [Google Scholar]








| Trials | Independent Variables | Response Value | ||
|---|---|---|---|---|
| X1 G/NCS (%) | X2 Time (h) | X3 S/Ni | Specific Capacitance [F g−1] | |
| 1 | −1 | −1 | −1 | 1040 |
| 2 | −1 | −1 | +1 | 840 |
| 3 | −1 | +1 | −1 | 801 |
| 4 | −1 | +1 | +1 | 1033 |
| 5 | +1 | −1 | −1 | 1704 |
| 6 | +1 | −1 | +1 | 1920 |
| 7 | +1 | +1 | −1 | 1500 |
| 8 | +1 | +1 | +1 | 2380 |
| 9 | −1.682 | 0 | 0 | 450 |
| 10 | +1.682 | 0 | 0 | 1660 |
| 11 | 0 | −1.682 | 0 | 1570 |
| 12 | 0 | +1.682 | 0 | 1460 |
| 13 | 0 | 0 | −1.682 | 1070 |
| 14 | 0 | 0 | +1.682 | 1640 |
| 15 | 0 | 0 | 0 | 844 |
| 16 | 0 | 0 | 0 | 780 |
| 17 | 0 | 0 | 0 | 1040 |
| 18 | 0 | 0 | 0 | 980 |
| 19 | 0 | 0 | 0 | 910 |
| 20 | 0 | 0 | 0 | 912 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 835.51 | 9 | 92.83 | 49.71 | <0.0001 | significant |
| X1 | 514.01 | 1 | 514.01 | 275.24 | <0.0001 | |
| X2 | 0.0410 | 1 | 0.0410 | 0.0220 | 0.8851 | |
| X3 | 50.50 | 1 | 50.50 | 27.04 | 0.0004 | |
| X1X2 | 1.29 | 1 | 1.29 | 0.6885 | 0.4260 | |
| X1X3 | 18.06 | 1 | 18.06 | 9.67 | 0.0111 | |
| X2X3 | 26.72 | 1 | 26.72 | 14.31 | 0.0036 | |
| X12 | 3.54 | 1 | 3.54 | 1.90 | 0.1985 | |
| X22 | 157.17 | 1 | 157.17 | 84.16 | <0.0001 | |
| X32 | 88.98 | 1 | 88.98 | 47.64 | <0.0001 | |
| Residual | 18.67 | 10 | 1.87 | |||
| Lack of fit | 6.79 | 5 | 1.36 | 0.5714 | 0.7230 | not significant |
| Pure error | 11.88 | 5 | 2.38 | |||
| Cor total | 854.18 | 19 |
| Optimum Conditions | Coded Levels | Actual Levels |
|---|---|---|
| G/NCS (%) | 1.00 | 6 |
| Hydrothermal time (h) | 1.00 | 10 |
| S/Ni | 1.00 | 6 |
| Response | Predicted values | Experimental values |
| Specific capacitance | 2317 | 2376 ± 60 |
| Independent Variable | Coded Levels | ||||
|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | |
| G/NCS (%) | 0.6 | 2.0 | 4.0 | 6.0 | 7.4 |
| Time (h) | 4.6 | 6.0 | 8.0 | 10.0 | 11.4 |
| S/Ni | 3.3 | 4.0 | 5.0 | 6.0 | 6.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Z.-Y.; Chen, L.-C.; Li, Y.-C.M.; Hsu, H.-L.; Huang, C.-M. Response Surface Methodology Optimization in High-Performance Solid-State Supercapattery Cells Using NiCo2S4–Graphene Hybrids. Molecules 2022, 27, 6867. https://doi.org/10.3390/molecules27206867
Hong Z-Y, Chen L-C, Li Y-CM, Hsu H-L, Huang C-M. Response Surface Methodology Optimization in High-Performance Solid-State Supercapattery Cells Using NiCo2S4–Graphene Hybrids. Molecules. 2022; 27(20):6867. https://doi.org/10.3390/molecules27206867
Chicago/Turabian StyleHong, Zhong-Yun, Lung-Chuan Chen, Yu-Chu M. Li, Hao-Lin Hsu, and Chao-Ming Huang. 2022. "Response Surface Methodology Optimization in High-Performance Solid-State Supercapattery Cells Using NiCo2S4–Graphene Hybrids" Molecules 27, no. 20: 6867. https://doi.org/10.3390/molecules27206867
APA StyleHong, Z.-Y., Chen, L.-C., Li, Y.-C. M., Hsu, H.-L., & Huang, C.-M. (2022). Response Surface Methodology Optimization in High-Performance Solid-State Supercapattery Cells Using NiCo2S4–Graphene Hybrids. Molecules, 27(20), 6867. https://doi.org/10.3390/molecules27206867








