Chemical Synthesis of Trans 8-Methyl-6-Nonenoyl-CoA and Functional Expression Unravel Capsaicin Synthase Activity Encoded by the Pun1 Locus
Abstract
1. Introduction
2. Results
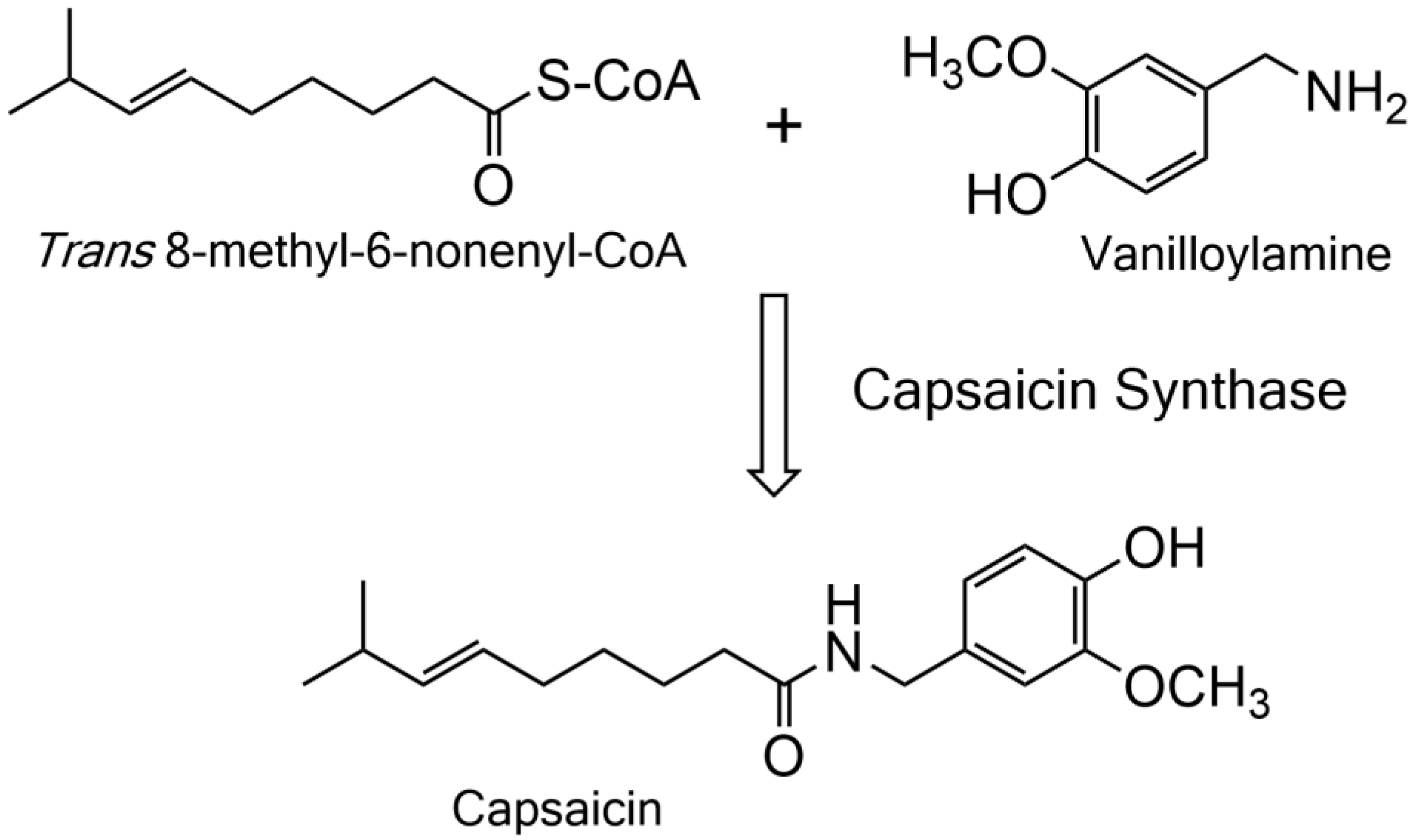
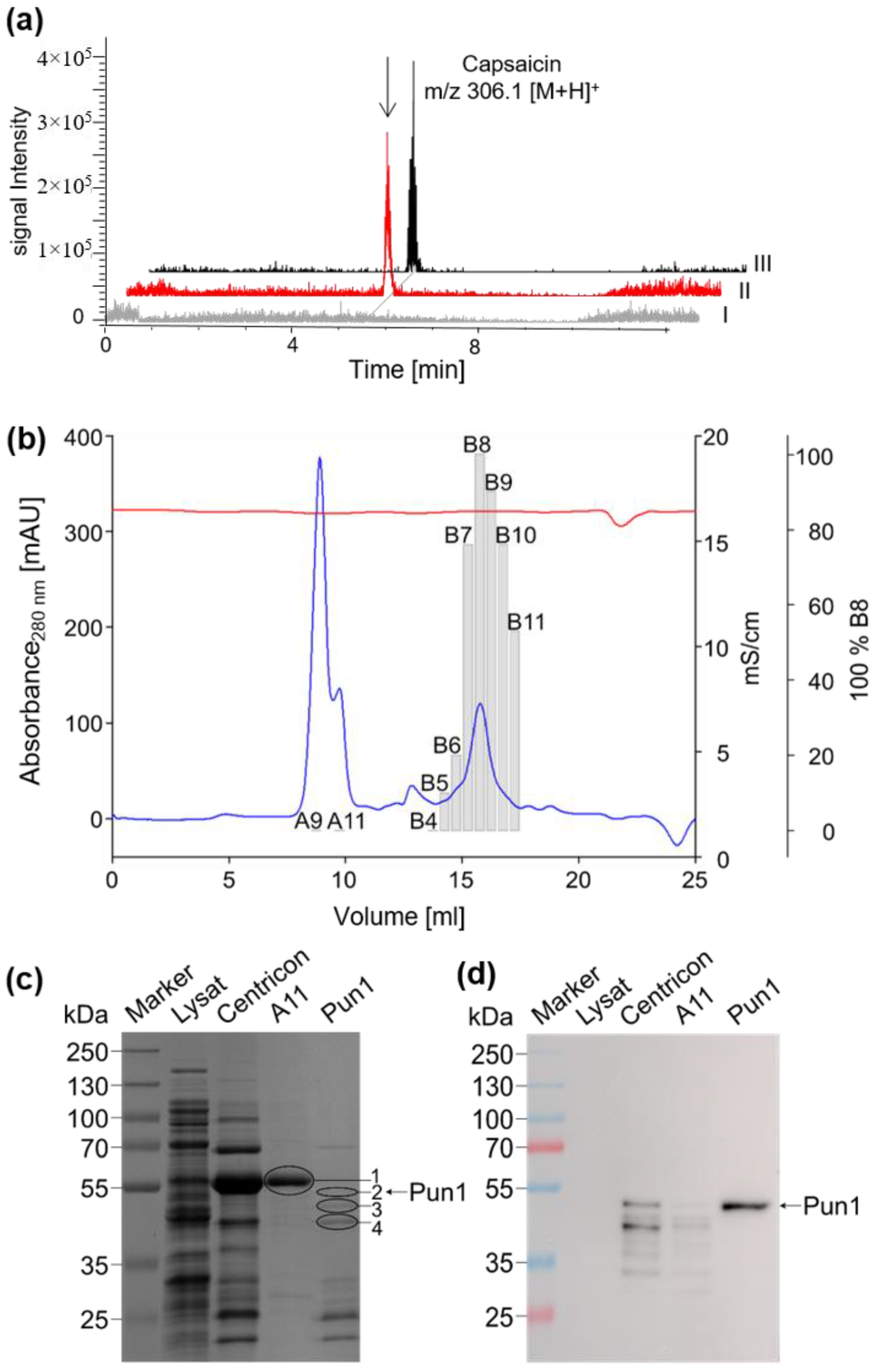
2.1. Detection, Cloning, and Functional Expression of Capsaicin Synthase
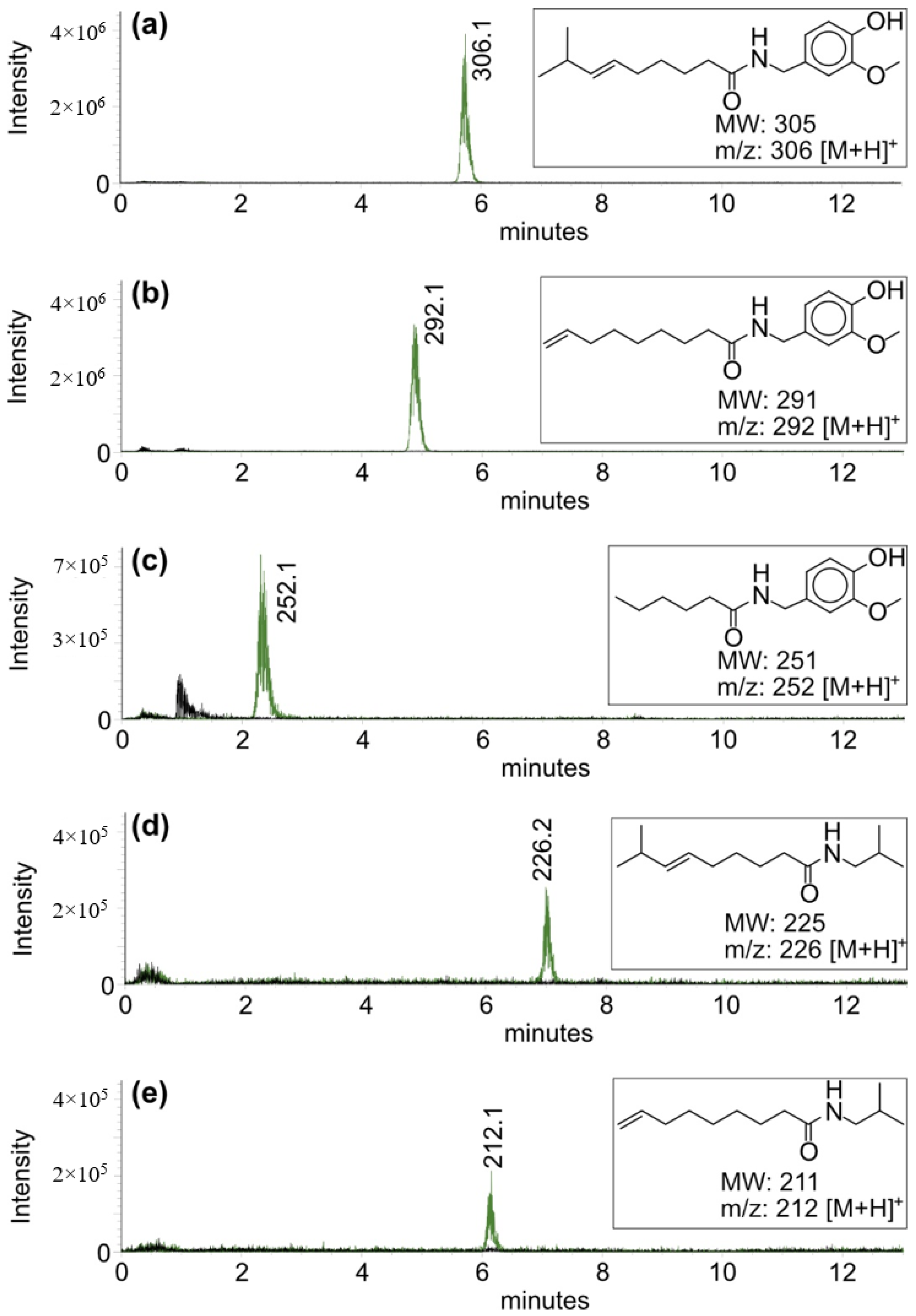
2.2. Properties of Recombinant Capsaicin Synthase
3. Discussion
4. Materials and Methods
4.1. Substrate Synthesis
4.2. Cloning and Functional Expression of Recombinant Capsaicin Synthase
4.3. Protein Digestion and LC-MS/MS-Based Analysis
4.4. Enzyme Assays and Product Analytics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Heiser, C.B. Origin of some cultivated new world plants. Annu. Rev. Ecol. Syst. 1979, 10, 309–326. [Google Scholar] [CrossRef]
- Rowland, B.J.; Villalong, B.; Burns, E.E. Capsaicin production in sweet bell and pungent jalapeño peppers. J. Agric. Food Chem. 1983, 31, 484–487. [Google Scholar] [CrossRef]
- Caterina, M.; Schumacher, M.; Tominaga, M.; Rosen, T.; Levine, J.; Julius, D. The capsaicin receptor: A heat-activated ion channelin the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef]
- Aza-Gonzáles, C.; Nunes-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsacionoid biosynthesis in hot chili pepper (Capsicum spp.). Plant Cell Rep. 2011, 30, 695–706. [Google Scholar] [CrossRef]
- Mazourek, M.; Pujar, A.; Borovsky, Y.; Paran, I.; Mueller, L.; Jahn, M.M. A dynamic interface for capsaicinoid systems biology. Plant Physiol. 2009, 150, 1806–1821. [Google Scholar] [CrossRef] [PubMed]
- Arce-Rodriguez, M.L.; Ochoa-Alejo, N. Biochemistry and molecular biology of capsaicinoid biosynthesis: Recent advances and perspectives. Plant Cell Rep. 2019, 38, 1017–1030. [Google Scholar] [CrossRef]
- Stewart, C.; Kang, B.C.; Liu, K.; Mazourek, M.; Moore, S.L.; Yoo, E.Y.; Kim, B.D.; Paran, I.; Jahn, M.M. The Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant J. 2005, 42, 675–688. [Google Scholar] [CrossRef]
- Kim, S.; Park, M.; Yeom, S.; Kim, Y.; Lee, J.M.; Lee, H.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.; et al. Genome sequence of the hot pepper provides insight into evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef]
- Qin, C.; Yu, C.; Shen, Y.; Fang, X.; Chen, L.; Min, J.; Cheng, J.; Zhao, S.; Xu, M.; Luo, Y.; et al. Whole genome sequencing of cultivated and wild peppers provides insights into domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef]
- Ogawa, K.; Murota, K.; Shimura, H.; Furuya, M.; Togawa, Y.; Matsumura, T.; Masuta, T. Evidence of capsaicin synthase activity of the Pun1-encoded protein and its role as a determinant of capsaicinoid accumulation in pepper. BMC Plant Biol. 2015, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, H.; Yu, O. Methods for Using Capsaicin Synthase for the Microbial Production of Capsaicinoids. U.S. Patent 9,951,358 B2, 24 April 2018. [Google Scholar]
- Muratovska, N.; Grey, C.; Carlquist, M. Engineering Saccharomyces cerevisiae for production of the capsaicinoid nonivamide. Micobial. Cell Fact. 2022, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Werner, V.; Petersen, M. A BAHD hydroxycinnamoyltransferase from Actaea racemosa catalyses the formation of fukinolic and cimicifugic acids. Planta 2019, 250, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.W.; Jirschitzka, J.; Porta, T.; Reichelt, M.; Luck, K.; Torre, J.C.P.; Dolke, F.; Varesio, E.; Hopfgartner, G.; Gershenzon, J.; et al. The last step in cocaine biosynthesis is catalyzed by a BAHD acyltransferase. Plant Physiol. 2015, 167, 89–101. [Google Scholar] [CrossRef]
- Schnabel, A.; Athmer, B.; Manke, K.; Schumacher, F.; Cotinguiba, F.; Vogt, T. A piperic acid Identification and characterization of piperine synthase from black pepper, Piper nigrum L. Comm. Biol. 2021, 4, 445. [Google Scholar] [CrossRef]
- Petersen, M. Hydroxycinnamoyltransferases in plant metabolism. Phytochem. Rev. 2015, 15, 699–727. [Google Scholar] [CrossRef]
- D’Auria, J.C. Acyltransferases in plants: A good time to be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Jäckel, L.; Schnabel, A.; Stellmach, H.; Klauß, U.; Matschi, S.; Hause, G.; Vogt, T. The terminal enzymatic step in piperine biosynthesis is co-localized with the product piperine in specialized cells of black pepper (Piper nigrum L.). Plant J. 2022, 111, 731–747. [Google Scholar] [CrossRef]
- Møller, B. Dynamic metabolons. Science 2010, 330, 1328–1329. [Google Scholar] [CrossRef]
- Laursen, T.; Borch, J.; Knudsen, C.; Bavishi, K.; Torta, F.; Martens, H.J.; Silvestro, D.; Hatzakis, N.S.; Wenk, M.R.; Dafforn, T.R.; et al. Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 2016, 354, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Watchi, M.; Nemoto, W.; Goto, T.; Yoshida, Y.; Yasuba, K.I.; Ohno, S.; Doi, M. Capsaicinoid biosynthesis in the pericarp of chili pepper fruits is associated with a placental septum-like transcriptome profile and tissue structure. Plant Cell Rep. 2021, 40, 1859–1874. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Escobedo, L.; Garcia-Amezquita, L.E.; Olivas, G.I.; Ornelas-Paz, J.J.; Sepulveda, D.R. Capsaicinoids content and proximate composition of Mexican chili peppers (Capsicum spp.) cultivated in the State of Chihuahua. CyTA-J. Food 2013, 11, 179–184. [Google Scholar] [CrossRef]
- Beaudoin, G.A.W.; Facchini, P.J. Benzylalkaloid biosynthesis in opium poppy. Planta 2014, 240, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Kulagina, N.; Méteignier, L.-V.; Papon, N.; O’Connor, S.E.; Courdavault, V. More than a Catharanthus plant: A multicellular and pluri-organelle alkaloid-producing factory. Curr. Opin. Plant Biol. 2022, 67, 102200. [Google Scholar] [CrossRef]
- Uzaki, M.; Yamamoto, K.; Murakami, A.; Fuji, Y.; Ohnishi, M.; Ishizaki, K.; Fukaki, H.; Hirai, M.Y.; Mimura, T. Differential regulation of fluorescent alkaloid metabolism between idioblast and lacticifer cells during leaf development in Catharanthus roseus seedlings. J. Plant Res. 2022, 135, 473–483. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, H.B.; Jeong, H.J.; Kim, H. Agrobacterium-Mediated Capsicum annuum gene editing in two cultivars, hot pepper CM334 and bell pepper Dempsey. Int. J. Mol. Sci. 2021, 22, 3921. [Google Scholar] [CrossRef]
- Peter, D.M.; Vögeli, B.; Socorro Cortina, N.; Erb, M. A chemoenzymatic road map to the synthesis of CoA Esters. Molecules 2016, 21, 517. [Google Scholar] [CrossRef]
- Dippe, M.; Bauer, A.K.; Porzel, A.; Funke, E.; Müller, A.O.; Schmidt, J.; Beier, M.; Wessjohann, L. A coenzyme A-conjugated cinnamic acids–enzymatic synthesis of a CoA-Ester library and application in biocatalytic cascades to vanillin derivatives. Adv. Synth. Catal. 2019, 36, 5346–5350. [Google Scholar] [CrossRef]
- Stöckigt, J.; Zenk, M.H. Chemical properties and syntheses of hydroxycinnamoyl-CoA derivatives. Z. Naturforsch. 1975, 30c, 352–358. [Google Scholar] [CrossRef]
- Schnabel, A.; Cotinguiba, F.; Athmer, B.; Yang, C.; Westermann, B.; Schaks, A.; Porzel, A.; Brandt, W.; Schumacher, F.; Vogt, T. A piperic acid CoA ligase produces a putative precursor of piperine, the pungent principle from black pepper fruits. Plant J. 2020, 102, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Majovsky, P.; Naumann, C.; Lee, C.W.; Lassowskat, I.; Trujillo, M.; Dissmeyer, N.; Hoehenwarter, W. Targeted proteomics analysis of protein degradation in plant signaling on an LTQ-Orbitrap mass spectrometer. J. Proteome Res. 2014, 13, 4246–4258. [Google Scholar] [CrossRef] [PubMed]
| Accession/Organism | Annotation | Peptide Score | Total Peptides | Calculated Mass [kDa]/pI | Total Mascot Score |
|---|---|---|---|---|---|
| Q58VT1 C. frutescens | Acyltransferase Pun1 | 25.868 | 9 | 49.3/6.95 | 837 |
| A0A2G3D6U1 C. chinense | Acyltransferase Pun1 | 22.082 | 8 | 49.2/6.77 | 769 |
| D2Y3X2 C. annuum | Acyltransferase Pun1 | 11.364 | 5 | 49.3/7.72 | 492 |
| P00761 | Trypsin | 6.894 | 3 | 24.4/7.18 | 207 |
| Substrate Combination | Agmatine | Isobutylamine | Vanilloylalcohol | Vanilloylamine |
|---|---|---|---|---|
| 8-Methyl-6-nonenoyl-CoA | n.d. | 6 | n.d. | 100 |
| 8-Nonenoyl-CoA | n.d. | 4 | n.d. | 112 |
| Hexanoyl-CoA | n.d. | n.d. | n.d. | 22 |
| Myristoyl-CoA | n.d. | n.d. | n.d. | n.d. |
| 4-Coumaroyl-CoA | (−) | n.d. | (−) | n.d. |
| Feruolyl-CoA | (−) | n.d. | (−) | n.d. |
| Benzoyl-CoA | (−) | n.d. | (−) | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milde, R.; Schnabel, A.; Ditfe, T.; Hoehenwarter, W.; Proksch, C.; Westermann, B.; Vogt, T. Chemical Synthesis of Trans 8-Methyl-6-Nonenoyl-CoA and Functional Expression Unravel Capsaicin Synthase Activity Encoded by the Pun1 Locus. Molecules 2022, 27, 6878. https://doi.org/10.3390/molecules27206878
Milde R, Schnabel A, Ditfe T, Hoehenwarter W, Proksch C, Westermann B, Vogt T. Chemical Synthesis of Trans 8-Methyl-6-Nonenoyl-CoA and Functional Expression Unravel Capsaicin Synthase Activity Encoded by the Pun1 Locus. Molecules. 2022; 27(20):6878. https://doi.org/10.3390/molecules27206878
Chicago/Turabian StyleMilde, Raika, Arianne Schnabel, Toni Ditfe, Wolfgang Hoehenwarter, Carsten Proksch, Bernhard Westermann, and Thomas Vogt. 2022. "Chemical Synthesis of Trans 8-Methyl-6-Nonenoyl-CoA and Functional Expression Unravel Capsaicin Synthase Activity Encoded by the Pun1 Locus" Molecules 27, no. 20: 6878. https://doi.org/10.3390/molecules27206878
APA StyleMilde, R., Schnabel, A., Ditfe, T., Hoehenwarter, W., Proksch, C., Westermann, B., & Vogt, T. (2022). Chemical Synthesis of Trans 8-Methyl-6-Nonenoyl-CoA and Functional Expression Unravel Capsaicin Synthase Activity Encoded by the Pun1 Locus. Molecules, 27(20), 6878. https://doi.org/10.3390/molecules27206878








