Asymmetric Synthesis of Contact Sex Pheromone of Tetropium fuscum and Its Enantiomer
Abstract
1. Introduction
2. Results and Discussion
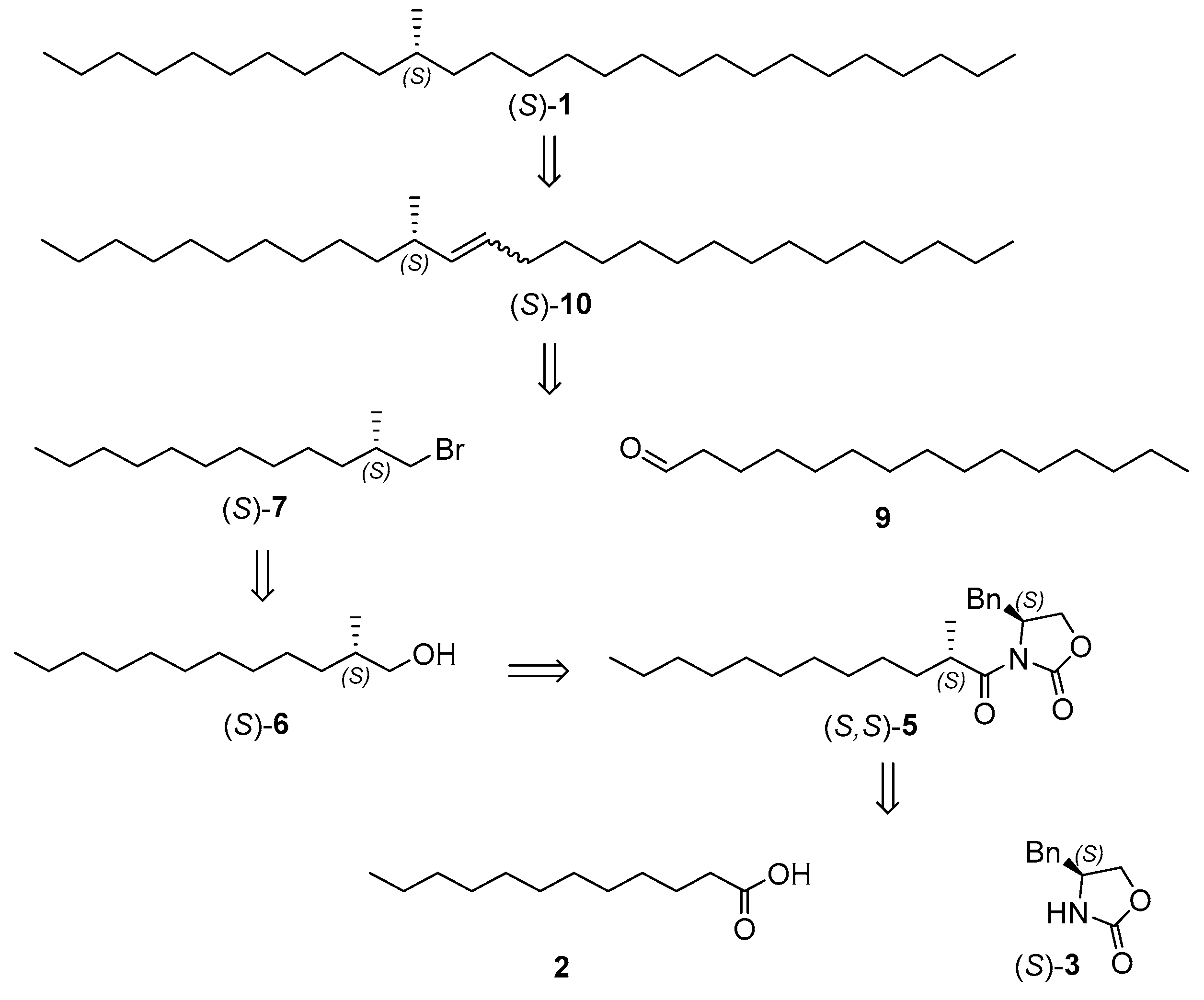
2.1. Retrosynthetic Analysis
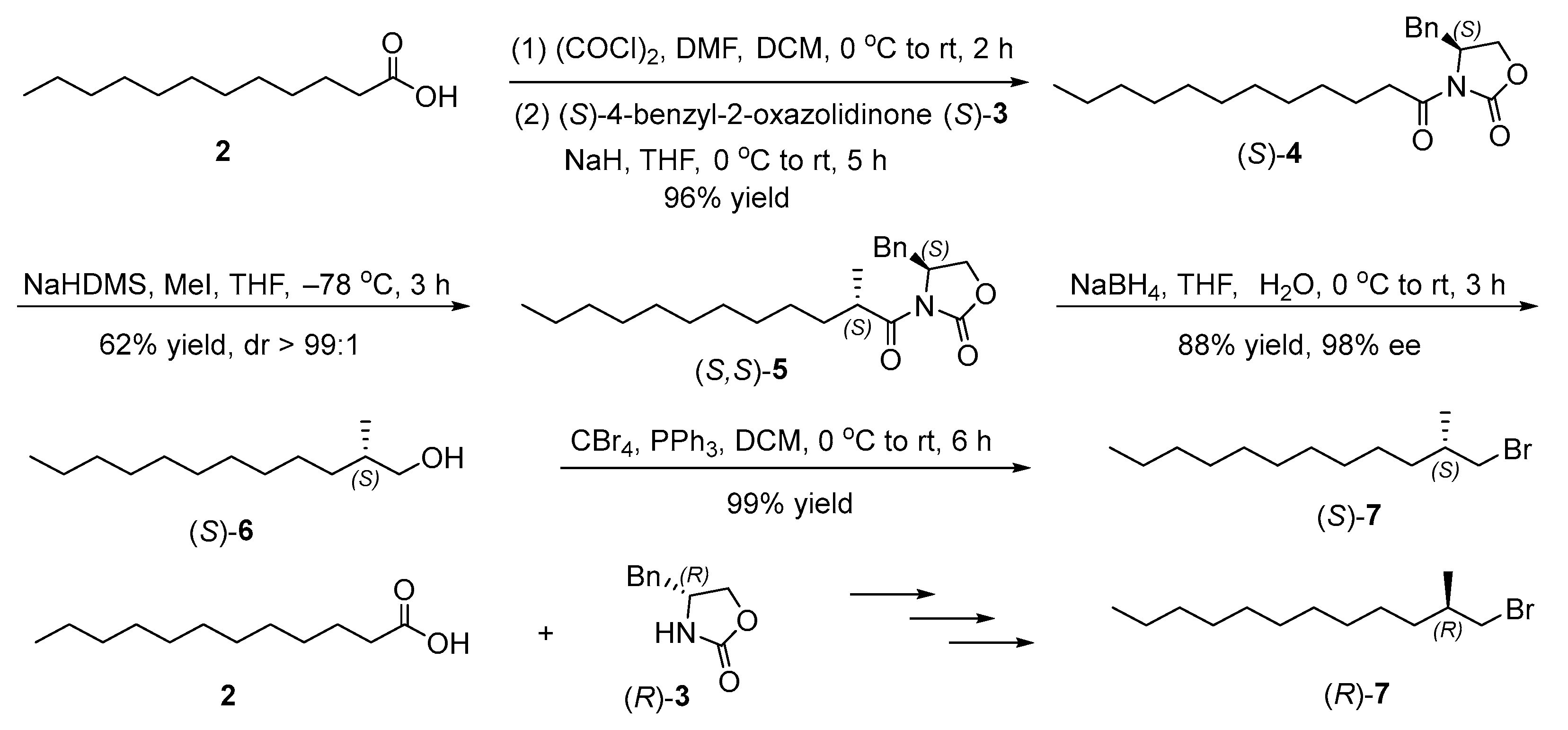
2.2. Synthesis of Chiral Primary Bromides
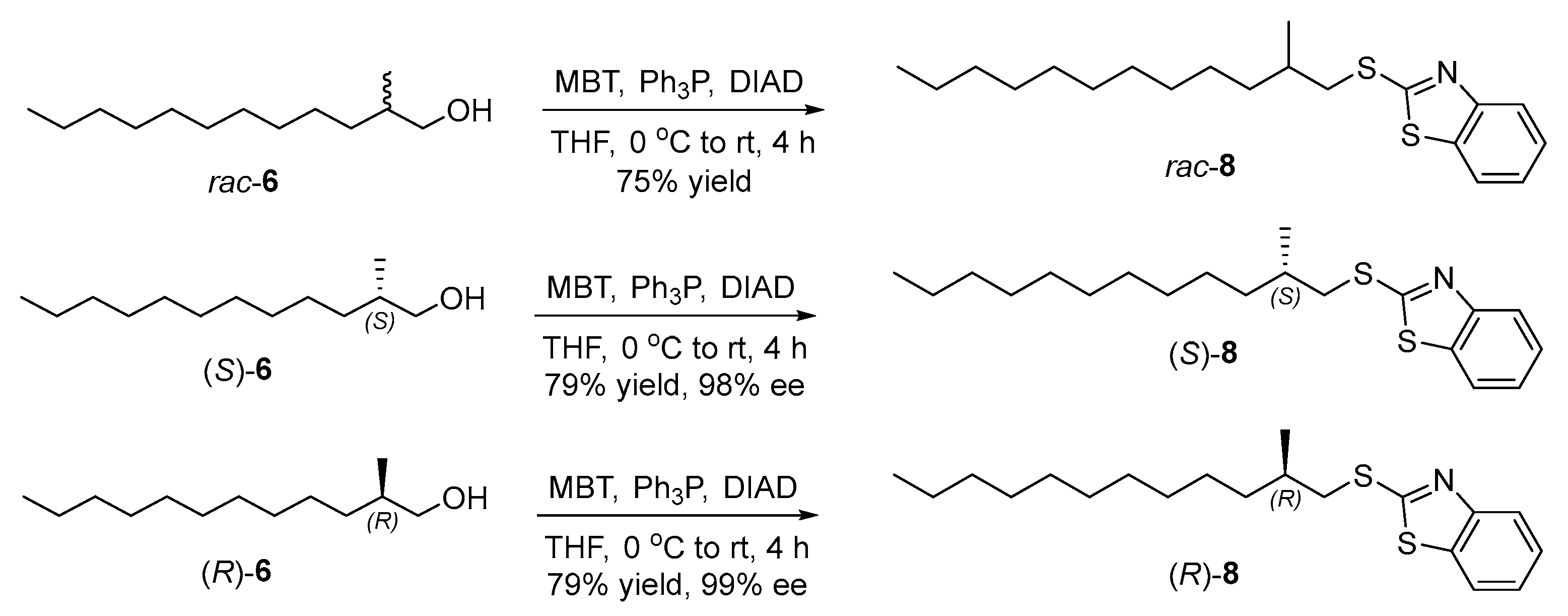
2.3. Research on the Enantiomeric Purity of Chiral Alcohols
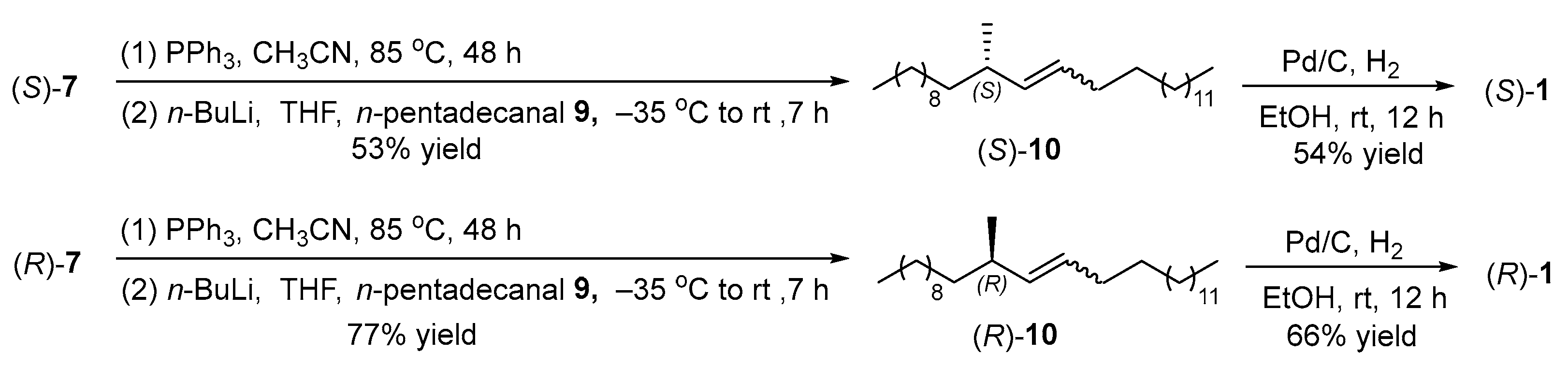
2.4. Synthesis of the Target Compounds
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (S)-4-Benzyl-3-dodecanoyloxazolidin-2-one ((S)-4) (CAS 198649-20-6)
3.3. Synthesis of (S)-4-Benzyl-3-((S)-2-methyldodecanoyl)oxazolidin-2-one ((S,S)-5) (CAS 2771300-96-8)
3.4. Synthesis of (S)-2-Methyldodecan-1-ol ((S)-6) (CAS 57289-26-6)
3.5. Synthesis of (S)-1-Bromo-2-methyldodecane ((S)-7) (CAS 1333499-05-0)
3.6. Synthesis of (R)-4-Benzyl-3-dodecanoyloxazolidin-2-one ((R)-4) (CAS 185803-85-4)
3.7. Synthesis of (R)-4-Benzyl-3-((R)-2-methyldodecanoyl)oxazolidin-2-one ((R,R)-5) (CAS 185803-86-5)
3.8. Synthesis of (R)-2-Methyldodecan-1-ol ((R)-6) (CAS 109034-03-9)
3.9. Synthesis of (R)-1-Bromo-2-methyldodecane ((R)-7) (CAS 1643618-98-7)
3.10. Synthesis of 2-((2-Methyldodecyl)thio)benzo[d]thiazole (rac-8) (New Compound)
3.11. Synthesis of (S)-2-((2-Methyldodecyl)thio)benzo[d]thiazole ((S)-8) (New Compound)
3.12. Synthesis of (R)-2-((2-Methyldodecyl)thio)benzo[d]thiazole ((R)-8) (New Compound)
3.13. Synthesis of (S)-11-Methylheptacos-9-ene ((S)-10) (New Compound)
3.14. Synthesis of (S)-11-Methylheptacosane ((S)-1) (CAS 1370709-05-9)
3.15. Synthesis of (R)-11-Methylheptacos-9-ene ((R)-10) (New Compound)
3.16. Synthesis of (R)-11-Methylheptacosane ((R)-1) (CAS 1370709-06-0)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, J.L.; Heard, S.B.; Sweeney, J.; Pureswaran, D.S. Mate choice errors may contribute to slow spread of an invasive Eurasian longhorn beetle in North America. NeoBiota 2022, 71, 71–89. [Google Scholar] [CrossRef]
- Lemay, M.A.; Silk, P.J.; Sweeney, J. Calling behavior of Tetropium fuscum (Coleoptera: Cerambycidae: Spondylidinae). Can. Entomol. 2010, 142, 256–260. [Google Scholar] [CrossRef]
- Schroeder, M.; Cocos, D.; Johansson, H.; Sweeney, J. Attraction of the cerambycid beetles Tetropium gabrieli, T. castaneum and T. fuscum to pheromones and host tree volatiles. Agr. Forest Entomol. 2021, 23, 203–211. [Google Scholar] [CrossRef]
- Smith, G.; Hurley, J.E. First North American record of the Palearctic species Tetropium fuscum (Fabricius) (Coleoptera: Cerambycidae). Coleopts. Bull. 2000, 54, 540. [Google Scholar] [CrossRef]
- Sweeney, J.; De Groot, P.; MacDonald, L.; Smith, S.; Cocquempot, C.; Kenis, M.; Gutowski, J.M. Host volatile attractants and traps for detection of Tetropium fuscum (F.), Tetropium castaneum L., and other longhorned beetles (Coleoptera: Cerambycidae). Environ. Entomol. 2004, 33, 844–854. [Google Scholar] [CrossRef]
- Dearborn Kenneth, W.; Heard Stephen, B.; Sweeney, J.; Pureswaran Deepa, S. Displacement of Tetropium cinnamopterum (Coleoptera: Cerambycidae) by its invasive congener Tetropium fuscum. Environ. Entomol. 2016, 45, 848–854. [Google Scholar] [CrossRef]
- Heustis, A.; Heard, S.B.; Moise, E.R.D.; Johns, R.; Pureswaran, D.S. Impact of an invasive longhorned beetle, Tetropium fuscum (Coleoptera: Cerambycidae), on community structure of subcortical and wood-associated insects in Eastern Canada. Environ. Entomol. 2018, 47, 39–47. [Google Scholar] [CrossRef]
- MacKay, C.A.; Sweeney, J.D.; Hillier, N.K. Olfactory receptor neuron responses of a longhorned beetle, Tetropium fuscum (Fabr.) (Coleoptera: Cerambycidae), to pheromone, host, and non-host volatiles. J. Insect Physiol. 2015, 83, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.; Silk, P.J.; Rhainds, M.; Mackay, W.; Hughes, C.; Van Rooyen, K.; Mackinnon, W.; Leclair, G.; Holmes, S.; Kettela, E.G. First report of mating disruption with an aggregation pheromone: A case study with Tetropium fuscum (Coleoptera: Cerambycidae). J. Econ. Entomol. 2017, 110, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Mushrow, L.; Morrison, A.; Sweeney, J.; Quiring, D. Heat as a phytosanitary treatment for the brown spruce longhorn beetle. Forest. Chron. 2004, 80, 224–228. [Google Scholar] [CrossRef]
- Petkevicius, K.; Lofstedt, C.; Borodina, I. Insect sex pheromone production in yeasts and plants. Curr. Opin. Biotechnol. 2020, 65, 259–267. [Google Scholar] [CrossRef]
- Yew, J.Y.; Chung, H. Insect pheromones: An overview of function, form, and discovery. Prog. Lipid. Res. 2015, 59, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Silk, P.J.; Sweeney, J.; Wu, J.; Sopow, S.; Mayo, P.D.; Magee, D. Contact sex pheromones identified for two species of longhorned beetles (Coleoptera: Cerambycidae) Tetropium fuscum and T. cinnamopterum in the subfamily Spondylidinae. Environ. Entomol. 2011, 40, 714–726. [Google Scholar] [CrossRef]
- Fujiwara-Tsujii, N.; Yasui, H. Improving contagion and horizontal transmission of entomopathogenic fungi by the white-spotted longicorn beetle, Anoplophora malasiaca, with help of contact sex pheromone. Insects 2021, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, Y.; Akasaka, K.; Masuda, Y.; Konishi, S.; Yang, C.Y.; Takikawa, H.; Mori, K. Pheromone synthesis. Part 265: Synthesis and stereochemical composition of two pheromonal compounds of the female Korean apricot wasp, Eurytoma maslovskii. Tetrahedron 2020, 76, 131410. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, X.; Guaciaro, M.; Molino, B.F. Asymmetric synthesis and absolute configuration of streptophenazine G. J. Org. Chem. 2012, 77, 3191–3196. [Google Scholar] [CrossRef]
- Tsakos, M.; Clement, L.L.; Schaffert, E.S.; Olsen, F.N.; Rupiani, S.; Djurhuus, R.; Yu, W.; Jacobsen, K.M.; Villadsen, N.L.; Poulsen, T.B. Total synthesis and biological evaluation of rakicidin A and discovery of a simplified bioactive analog. Angew. Chem. Int. Ed. 2016, 55, 1030–1035. [Google Scholar] [CrossRef]
- Evans, D.A.; Ennis, M.D.; Mathre, D.J. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of α-substituted carboxylic acid derivatives. J. Am. Chem. Soc. 1982, 104, 1737–1739. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, X.; Tang, C.-J.; Chen, Z.-L. An expeditious access to enantiomerically pure cis-dialkyl-substituted γ- and δ-lactones. Helv. Chim. Acta. 2001, 84, 3428–3432. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, X.; Tang, C.-J.; Chen, Z.-L.; Hu, Q.; Shi, W. Synthesis of natural fragrant molecules cis-3-methyl-4-decanolide and aerangis lactone. General enantioselective routes to β,γ-cis-disubstituted γ-lactones and γ,δ-cis-disubstituted δ-lactones. J. Org. Chem. 2002, 67, 3802–3810. [Google Scholar] [CrossRef] [PubMed]
- Schlamp, K.K.; Gries, R.; Khaskin, G.; Brown, K.; Khaskin, E.; Judd, G.J.; Gries, G. Pheromone components from body scales of female Anarsia lineatella induce contacts by conspecific males. J. Chem. Ecol. 2005, 31, 2897–2911. [Google Scholar] [CrossRef]
- Appel, R. Tertiary phosphane/tetrachloromethane, a versatile reagent for chlorination, dehydration, and phosphorus-nitrogen linking. Angew. Chem. Int. Ed. 1975, 14, 801–811. [Google Scholar] [CrossRef]
- Favaro, C.F.; Millar, J.G.; Zarbin, P.H.G. Identification and synthesis of the male-produced sex pheromone of the stink bug, Pellaea stictica. J. Chem. Ecol. 2015, 41, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Sun, Z.F.; Zhou, L.N.; Liu, L.; Zhang, T.; Du, Z.T. Synthesis of the sex pheromone of the tea tussock moth based on a resource chemistry strategy. Molecules 2018, 23, 1347. [Google Scholar] [CrossRef] [PubMed]
- Mitsunobu, O.; Wada, M.; Sano, T. Stereospecific and stereoselective reactions. I. Preparation of amines from alcohols. J. Am. Chem. Soc. 1972, 94, 679–680. [Google Scholar] [CrossRef]
- Woo, S.H.; Frechette, S.; Khalil, E.A.; Bouchain, G.; Vaisburg, A.; Bernstein, N.; Moradei, O.; Leit, S.; Allan, M.; Fournel, M.; et al. Structurally simple trichostatin A-like straight chain hydroxamates as potent histone deacetylase inhibitors. J. Med. Chem. 2002, 45, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Wittig, G.; Schollkopf, U. Triphenylphosphinemethylene as an olefin-forming reagent. I. Chem. Ber. 1954, 97, 1318–1330. [Google Scholar] [CrossRef]
- Bruns, H.; Herrmann, J.; Mueller, R.; Wang, H.; Wagner Doebler, I.; Schulz, S. Oxygenated N-acyl alanine methyl esters (NAMEs) from the marine bacterium Roseovarius tolerans EL-164. J. Nat. Prod. 2018, 81, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.S.; Cheng, T.-J.R.; Zulueta, M.M.L.; Yang, S.-T.; Lico, L.S.; Hung, S.-C. Total synthesis of tetraacylated phosphatidylinositol hexamannoside and evaluation of its immunomodulatory activity. Nat. Commun. 2015, 6, 7239. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.V.; Srivastava, A.; Hazra, D. Total synthesis of potential antitumor agent, (–)-dictyostatin. Org. Lett. 2007, 9, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Perlmutter, P. “One-pot” reductive lactone alkylation provides a concise asymmetric synthesis of chiral isoprenoid targets. Org. Lett. 2013, 15, 4327–4329. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, J.; Ma, F.; Bian, Q.; Wang, M.; Zhong, J. Asymmetric Synthesis of Contact Sex Pheromone of Tetropium fuscum and Its Enantiomer. Molecules 2022, 27, 6897. https://doi.org/10.3390/molecules27206897
Wang X, Wang J, Ma F, Bian Q, Wang M, Zhong J. Asymmetric Synthesis of Contact Sex Pheromone of Tetropium fuscum and Its Enantiomer. Molecules. 2022; 27(20):6897. https://doi.org/10.3390/molecules27206897
Chicago/Turabian StyleWang, Xueyang, Jianan Wang, Fengbo Ma, Qinghua Bian, Min Wang, and Jiangchun Zhong. 2022. "Asymmetric Synthesis of Contact Sex Pheromone of Tetropium fuscum and Its Enantiomer" Molecules 27, no. 20: 6897. https://doi.org/10.3390/molecules27206897
APA StyleWang, X., Wang, J., Ma, F., Bian, Q., Wang, M., & Zhong, J. (2022). Asymmetric Synthesis of Contact Sex Pheromone of Tetropium fuscum and Its Enantiomer. Molecules, 27(20), 6897. https://doi.org/10.3390/molecules27206897




