Application of the CRISPR/Cas System in Pathogen Detection: A Review
Abstract
1. Introduction
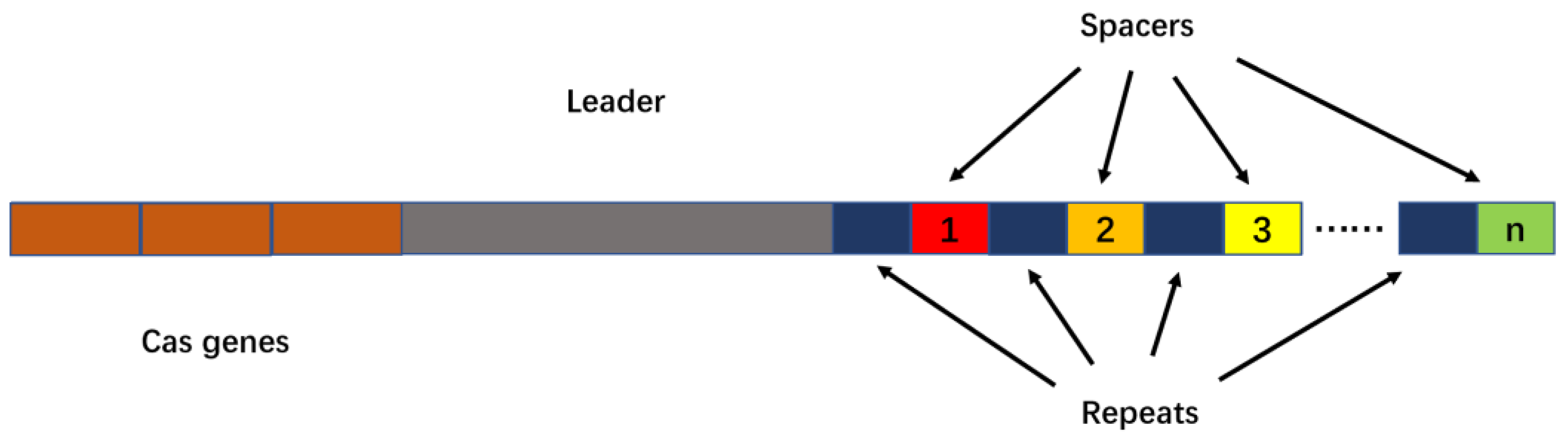
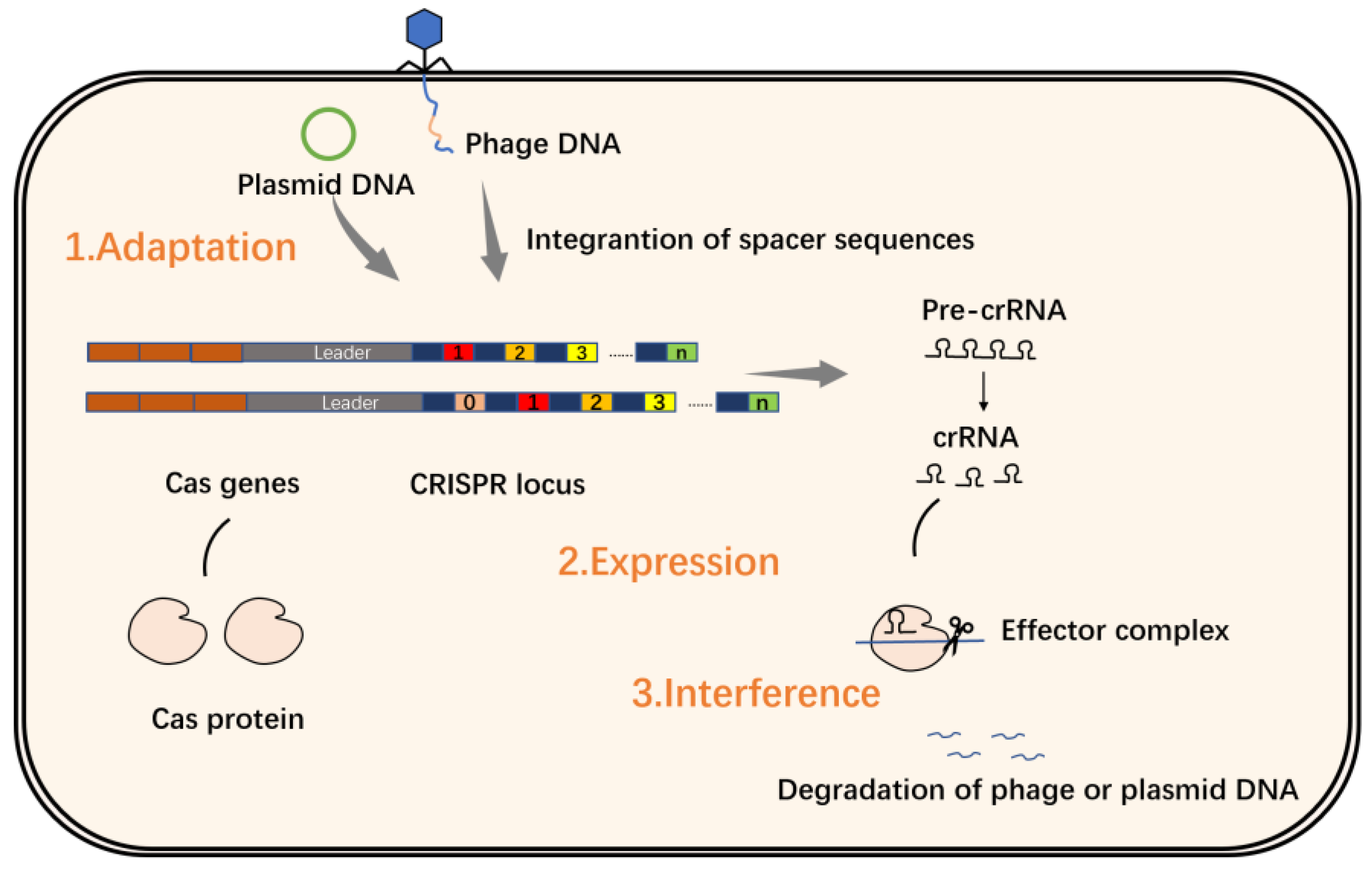
2. Introduction to the CRISPR/Cas System
3. In Vitro Pathogen Detection Based on the CRISPR/Cas System
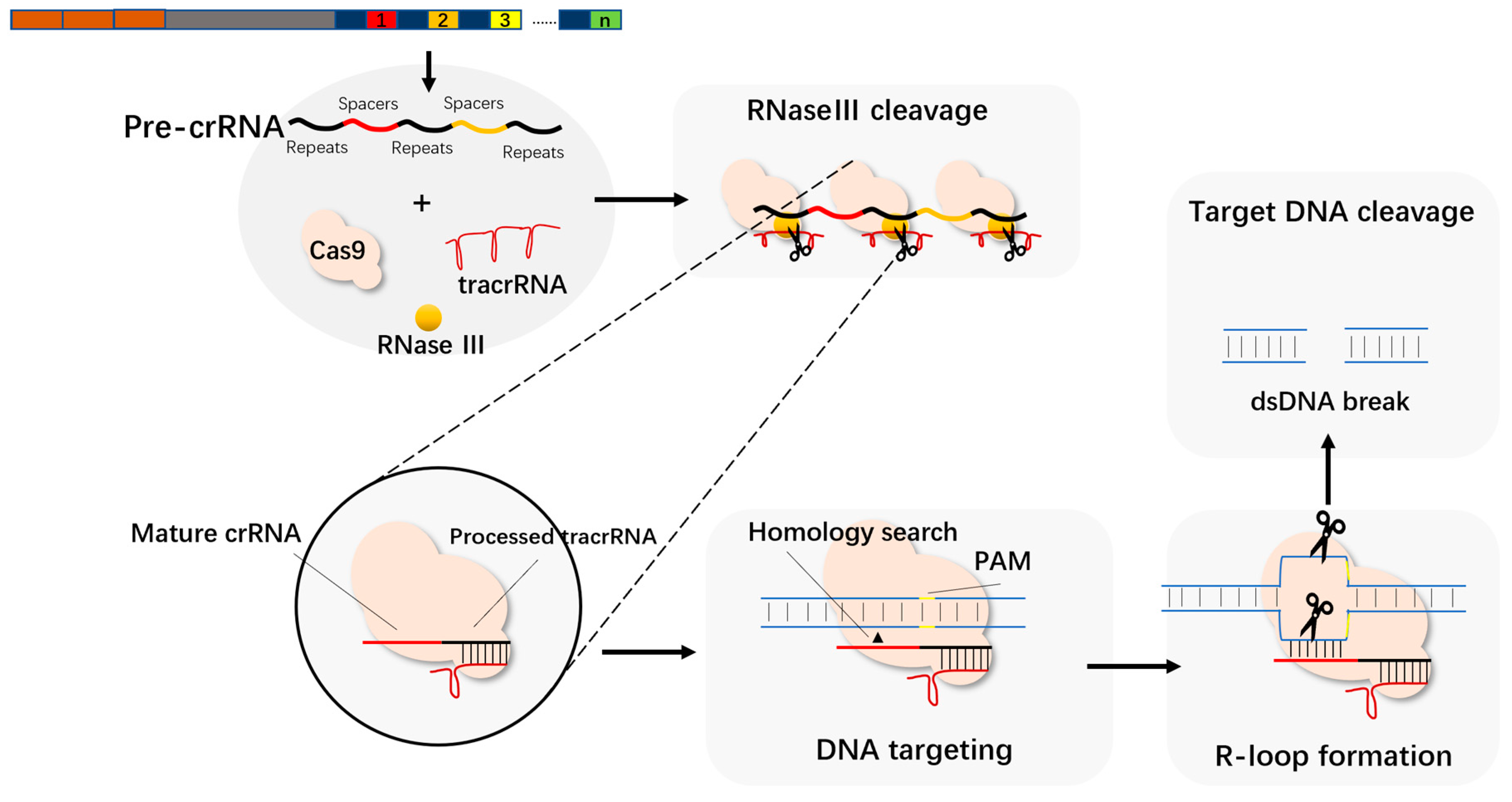
3.1. Cas9
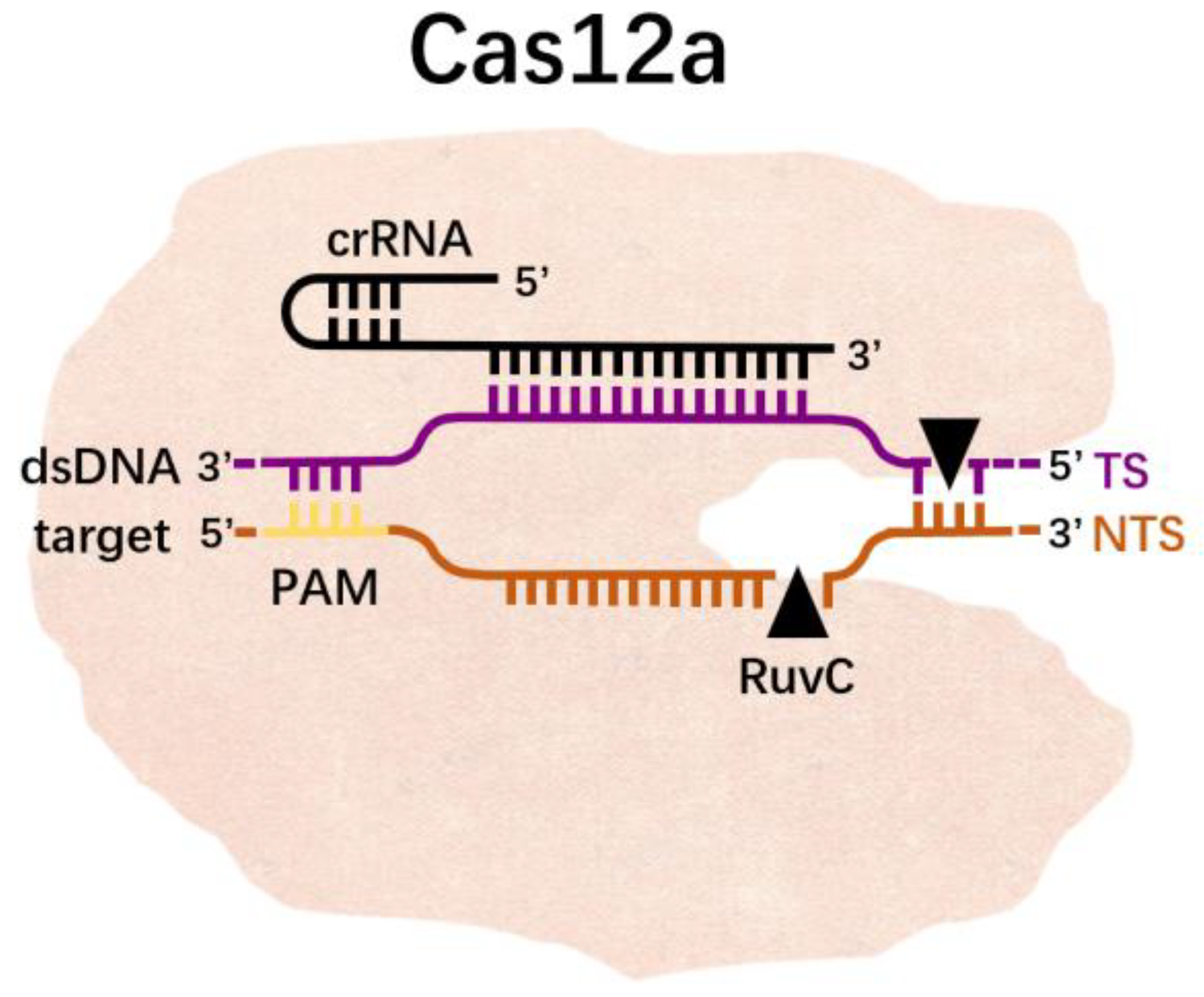
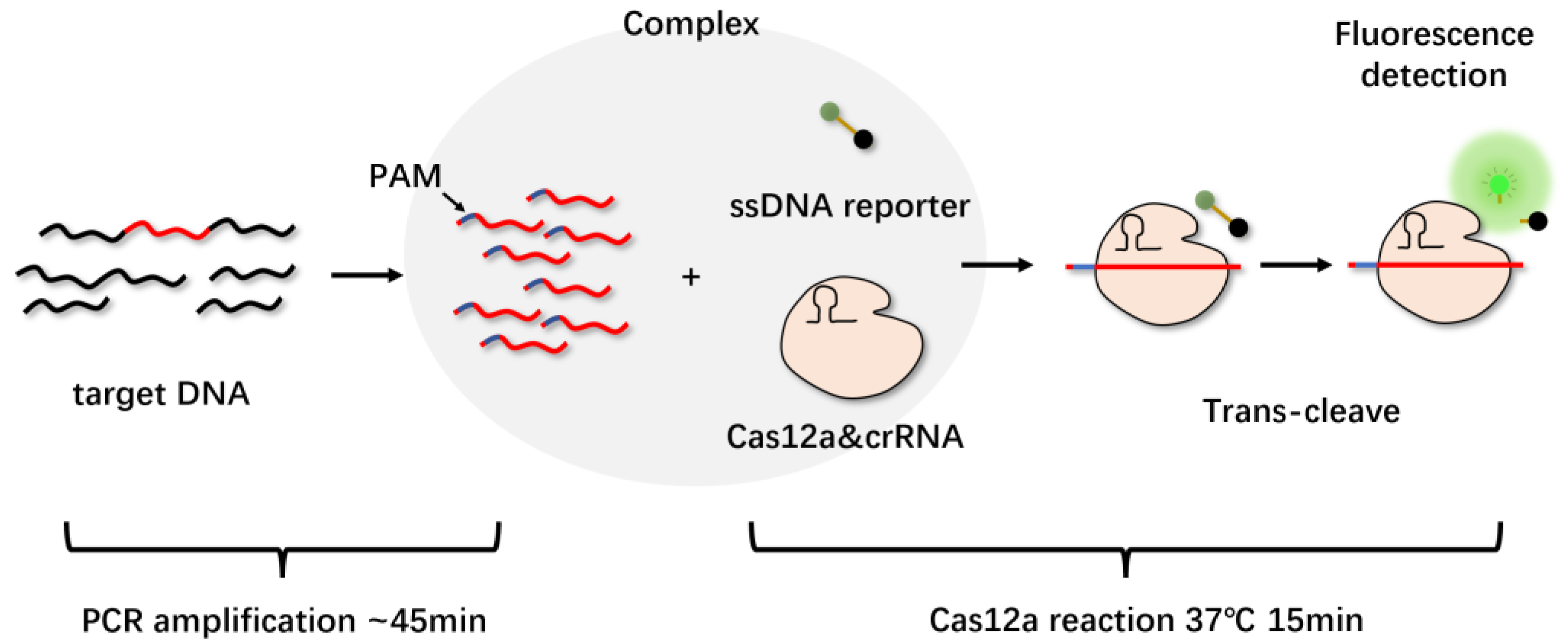
3.2. Cas12a
3.3. Cas12b
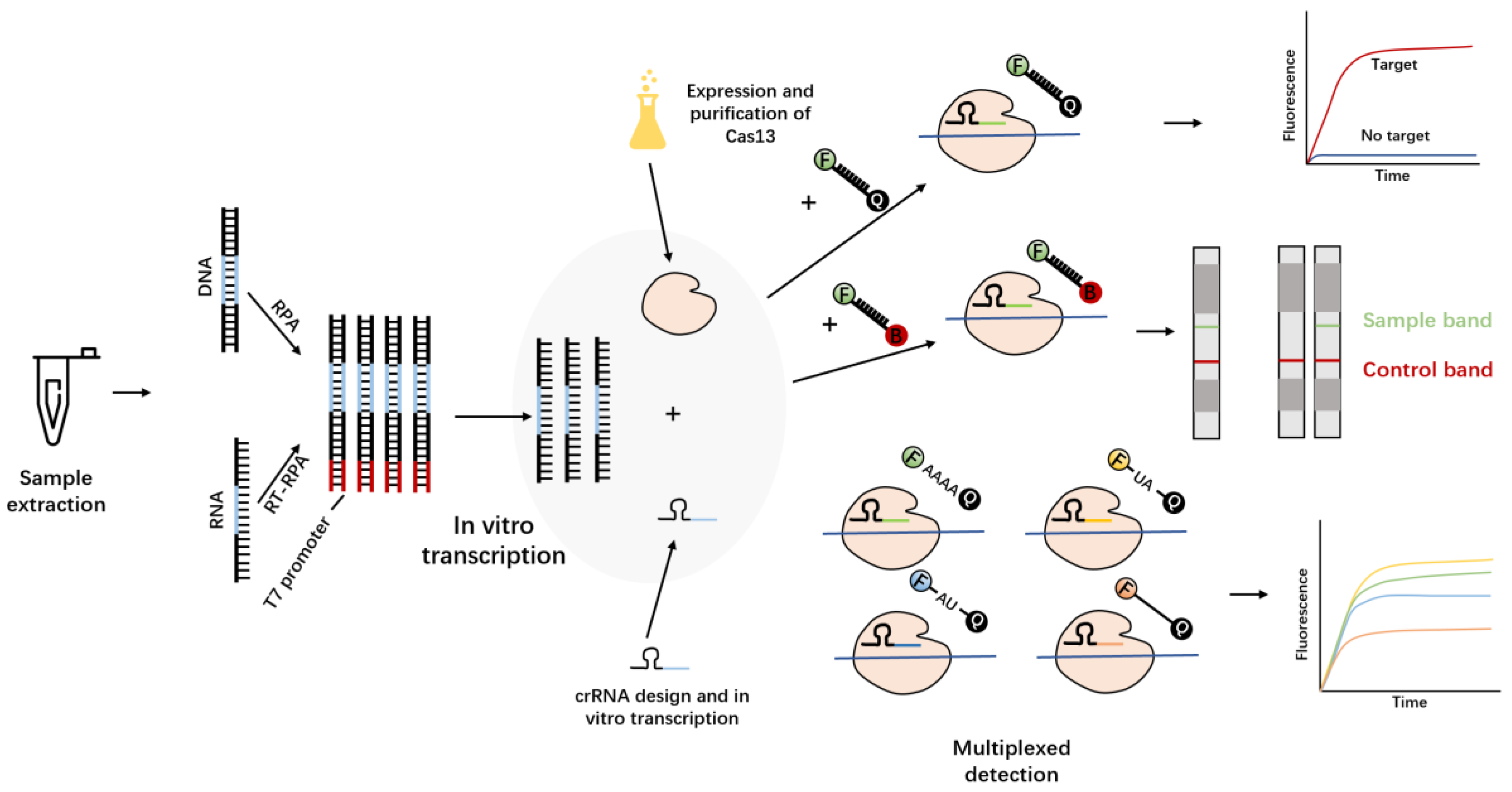
3.4. Cas13a
| Cas Protein | Pathogen | Platform Name | Amplification Methods | Visualization | Sensitivity | Time | References |
|---|---|---|---|---|---|---|---|
| LwCas13a | PPRRSV | SHERLOCK | RPA | Eye/LFD | 172 copies/μL | <1 h | [64] |
| LwCas13a | BVDV | SHERLOCK | RT-RPA | Fluorescence | 103 pM | - | [65] |
| LwCas13a | Staphylococcus aureus | CCB-Detection | PCR/T7transcription | Fluorescence | 1 CFU/mL | <4 h | [66] |
| LwCas13a | H7N9 | - | RT-RPA | Fluorescence | 1 fM | 50 min | [67] |
| LwCas13a | Feline calicivirus (FCV) | - | RPA | Fluorescence/LFD | 5.5 copies/μL | - | [68] |
| LwCas13a | TMUV | - | RPA | Fluorescence | 100 copies/μL | 50 min | [69] |
| LwCas13a | ASFV | CRISPR/Cas13a–LFD | RAA | LFD | 10 copies/μL | <2 h | [70] |
| LwCas13a | EMCV | - | RAA | LFD | 10 copies/μL | <1 h | [71] |
| LwCas13a | P. vivax/P. falciparum | SHERLOCK | RPA | Fluorescence | 10 aM | - | [72] |
| LwCas13a | HBV | - | RCA/PCR | Fluorescence | 1 copies/μL | - | [73] |
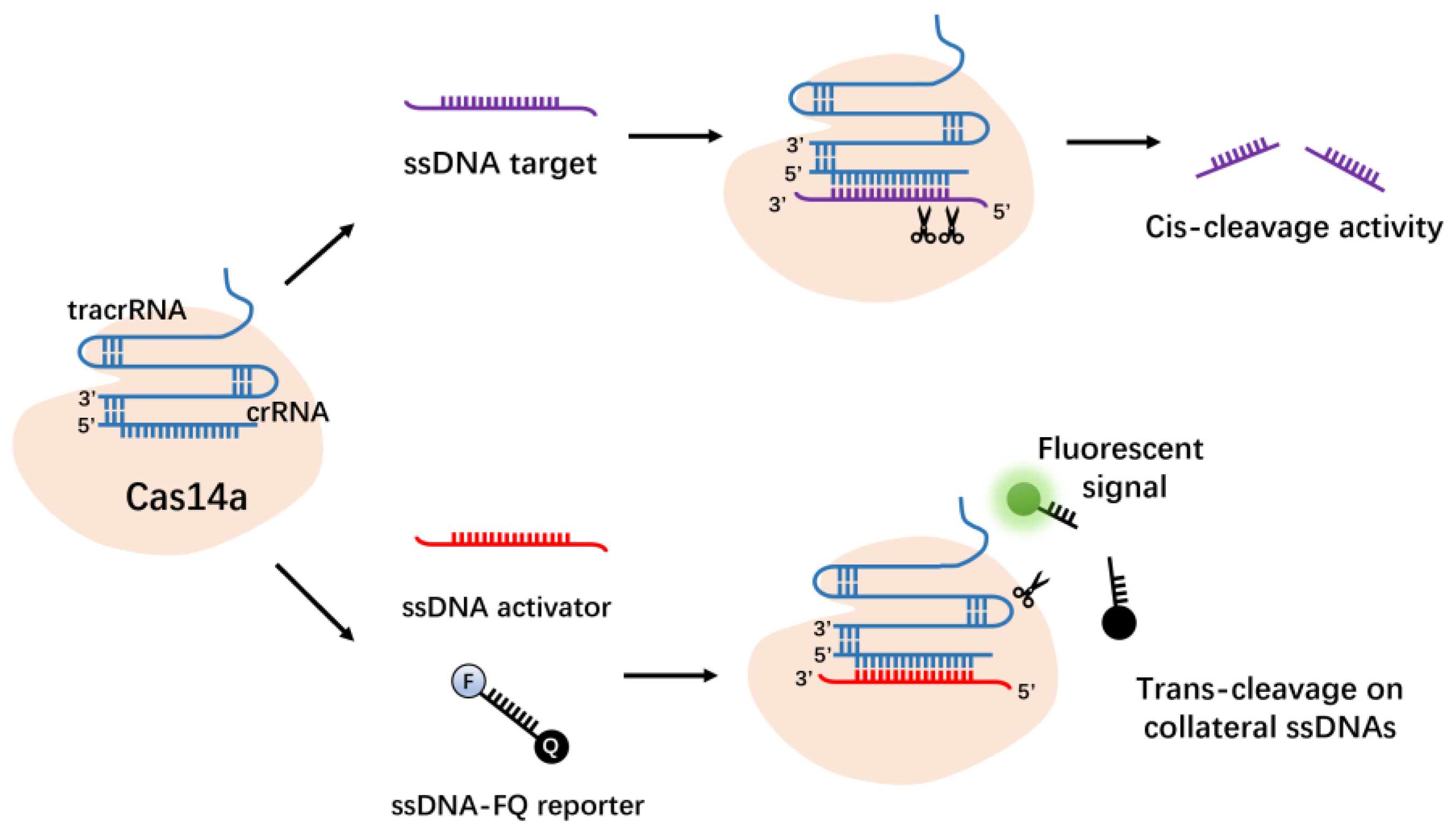
3.5. Cas14a
3.6. Application of the CRISPR System in Pathogen Detection
4. Challenge
4.1. Sequence Restriction
4.2. Multiplexing and Quantitative Detection
4.3. Sample Pre-Treatment
4.4. Contamination during Operation
4.5. On-Site Deployment
4.6. The Lack of a Uniform Standard
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canene-Adams, K. General PCR. Methods Enzym. 2013, 529, 291–298. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Dao Thi, V.L.; Herbst, K.; Boerner, K.; Meurer, M.; Kremer, L.P.; Kirrmaier, D.; Freistaedter, A.; Papagiannidis, D.; Galmozzi, C.; Stanifer, M.L.; et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci. Transl. Med. 2020, 12, eabc7075. [Google Scholar] [CrossRef]
- Reid, M.S.; Le, X.C.; Zhang, H. Exponential Isothermal Amplification of Nucleic Acids and Assays for Proteins, Cells, Small Molecules, and Enzyme Activities: An EXPAR Example. Angew. Chem. (Int. Ed. Engl.) 2018, 57, 11856–11866. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Cui, J.; Huang, L.; Du, B.; Chen, L.; Xue, G.; Li, S.; Zhang, W.; Zhao, L.; Sun, Y.; et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 2020, 26, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Shin, Y.; Chung, S.; Hwang, K.S.; Yoon, D.S.; Lee, J.H. Simple and Highly Sensitive Molecular Diagnosis of Zika Virus by Lateral Flow Assays. Anal. Chem. 2016, 88, 12272–12278. [Google Scholar] [CrossRef]
- Xiong, E.; Jiang, L.; Tian, T.; Hu, M.; Yue, H.; Huang, M.; Lin, W.; Jiang, Y.; Zhu, D.; Zhou, X. Simultaneous Dual-Gene Diagnosis of SARS-CoV-2 Based on CRISPR/Cas9-Mediated Lateral Flow Assay. Angew. Chem. (Int. Ed. Engl.) 2021, 60, 5307–5315. [Google Scholar] [CrossRef]
- Shuryaeva, A.K.; Malova, T.V.; Tolokonceva, A.A.; Karceka, S.A.; Gordukova, M.A.; Davydova, E.E.; Shipulin, G.A. Development and application of LAMP assays for the detection of enteric adenoviruses in feces. Microbiol. Spectr. 2022, 10, e0051622. [Google Scholar] [CrossRef]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. Trends. Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Safavieh, M.; Ahmed, M.U.; Ng, A.; Zourob, M. High-throughput real-time electrochemical monitoring of LAMP for pathogenic bacteria detection. Biosens. Bioelectron. 2014, 58, 101–106. [Google Scholar] [CrossRef]
- Kim, H.E.; Schuck, A.; Lee, S.H.; Lee, Y.; Kang, M.; Kim, Y.-S. Sensitive electrochemical biosensor combined with isothermal amplification for point-of-care COVID-19 tests. Biosens. Bioelectron. 2021, 182, 113168. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; van Embden, J.D.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Miaowen, C.; Wei, L.; Yao, D. CRISPR-CAS system mediated new generation gene targeted modification technology and its application in industrial microorganisms. J. Microbiol. 2017, 57, 1621–1633. [Google Scholar] [CrossRef]
- Bhaya, D.; Davison, M.; Barrangou, R. CRISPR-Cas systems in bacteria and archaea: Versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 2011, 45, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, C.; Salvignol, G.; Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 2005, 151, 653–663. [Google Scholar] [CrossRef]
- Jackson, S.A.; McKenzie, R.E.; Fagerlund, R.D.; Kieper, S.N.; Fineran, P.C.; Brouns, S.J.J. CRISPR-Cas: Adapting to change. Science 2017, 356, eaal5056. [Google Scholar] [CrossRef]
- Barrangou, R.; Horvath, P. A decade of discovery: CRISPR functions and applications. Nat. Microbiol. 2017, 2, 17092. [Google Scholar] [CrossRef]
- Huan, Z.; Yanna, S.; Juan, W.; Qingping, W.; Yu, D. Research progress of nucleic acid detection based on CRISPR/Cas technology. J. Microbiol. 2021, 61, 3856–3869. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.J.; Charpentier, E.; Haft, D.H.; et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.; Artegiani, B.; Hu, H.; Chuva de Sousa Lopes, S.; Clevers, H. Establishment of human fetal hepatocyte organoids and CRISPR-Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat. Protoc. 2021, 16, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Sun, H.H.; Yin, B.C.; Ye, B.C. An RNA-Guided Cas9 Nickase-Based Method for Universal Isothermal DNA Amplification. Angew. Chem. 2019, 131, 5436–5440. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xu, L.; Lou, C.; Ouyang, Q.; Qian, L. Paired dCas9 design as a nucleic acid detection platform for pathogenic strains. Methods 2022, 203, 70–77. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, E.; Tian, T.; Cheng, M.; Lin, W.; Wang, H.; Zhang, G.; Sun, J.; Zhou, X. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9-Mediated Lateral Flow Nucleic Acid Assay. ACS Nano 2020, 14, 2497–2508. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Zhang, L.; Liu, S.; Zhang, M.; Wang, J.; Ning, B.; Peng, Y.; He, J.; Hu, Y.; et al. CRISPR-Cas9 Triggered Two-Step Isothermal Amplification Method for O157:H7 Detection Based on a Metal-Organic Framework Platform. Anal. Chem. 2020, 92, 3032–3041. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.; Wei, W.; Wang, Y.; Wang, B.; Lin, P.; Liu, W.; Xu, L.; Li, X.; Liu, D.; et al. Paired Design of dCas9 as a Systematic Platform for the Detection of Featured Nucleic Acid Sequences in Pathogenic Strains. ACS Synth. Biol. 2017, 6, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Guk, K.; Keem, J.O.; Hwang, S.G.; Kim, H.; Kang, T.; Lim, E.K.; Jung, J. A facile, rapid and sensitive detection of MRSA using a CRISPR-mediated DNA FISH method, antibody-like dCas9/sgRNA complex. Biosens Bioelectron 2017, 95, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Kim, D.E.; Kweon, J.; Jin, C.E.; Kim, S.H.; Kim, Y.; Shin, Y. CRISPR/dCas9-mediated biosensor for detection of tick-borne diseases. Sens. Actuators B Chem. 2018, 273, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, T.; Gao, J.; Lin, N.; Li, W.; Xia, X.; Wang, J. CRISPR-Assisted DNA Detection: A Novel dCas9-Based DNA Detection Technique. CRISPR J. 2020, 3, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.; Seo, H.W.; Kang, B.; Moon, J.; Lee, K.G.; Yong, D.; Kang, H.; Jung, J.; Lim, E.-K.; et al. Clustered Regularly Interspaced Short Palindromic Repeats-Mediated Surface-Enhanced Raman Scattering Assay for Multidrug-Resistant Bacteria. ACS Nano 2020, 14, 17241–17253. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef]
- Fonfara, I.; Richter, H.; Bratovič, M.; Le Rhun, A.; Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 2016, 532, 517–521. [Google Scholar] [CrossRef]
- Yamano, T.; Nishimasu, H.; Zetsche, B.; Hirano, H.; Slaymaker, I.M.; Li, Y.; Fedorova, I.; Nakane, T.; Makarova, K.S.; Koonin, E.V.; et al. Crystal Structure of Cpf1 in Complex with Guide RNA and Target DNA. Cell 2016, 165, 949–962. [Google Scholar] [CrossRef]
- Li, S.Y.; Cheng, Q.X.; Wang, J.M.; Li, X.Y.; Zhang, Z.L.; Gao, S.; Cao, R.B.; Zhao, G.P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Li, F.; Ye, Q.; Chen, M.; Zhou, B.; Zhang, J.; Pang, R.; Xue, L.; Wang, J.; Zeng, H.; Wu, S.; et al. An ultrasensitive CRISPR/Cas12a based electrochemical biosensor for Listeria monocytogenes detection. Biosens. Bioelectron. 2021, 179, 113073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.S.; Pan, J.; Li, F.; Zhu, M.; Xu, M.; Zhu, H.; Yu, Y.; Su, G. Reverse Transcription Recombinase Polymerase Amplification Coupled with CRISPR-Cas12a for Facile and Highly Sensitive Colorimetric SARS-CoV-2 Detection. Anal. Chem. 2021, 93, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, P.; Fan, H.; Dang, L.; Wan, W.; Liu, S.; Li, Y.; Yu, W.; Li, X.; Ma, X.; et al. CRISPR/Cas12a technology combined with immunochromatographic strips for portable detection of African swine fever virus. Commun. Biol. 2020, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-F.; Zhao, K.-R.; Liu, Z.-J.; Wang, L.; Ye, S.-Y.; Liang, G.-X. Cas12a-based electrochemiluminescence biosensor for target amplification-free DNA detection. Biosens. Bioelectron. 2021, 176, 112954. [Google Scholar] [CrossRef]
- He, Q.; Yu, D.; Bao, M.; Korensky, G.; Chen, J.; Shin, M.; Kim, J.; Park, M.; Qin, P.; Du, K. High-throughput and all-solution phase African Swine Fever Virus (ASFV) detection using CRISPR-Cas12a and fluorescence based point-of-care system. Biosens. Bioelectron. 2020, 154, 112068. [Google Scholar] [CrossRef]
- Yang, B.; Shi, Z.; Ma, Y.; Wang, L.; Cao, L.; Luo, J.; Wan, Y.; Song, R.; Yan, Y.; Yuan, K.; et al. LAMP assay coupled with CRISPR/Cas12a system for portable detection of African swine fever virus. Transbound. Emerg. Dis. 2022, 69, e216–e223. [Google Scholar] [CrossRef]
- You, Y.; Zhang, P.; Wu, G.; Tan, Y.; Zhao, Y.; Cao, S.; Song, Y.; Yang, R.; Du, Z. Highly Specific and Sensitive Detection of by Portable Cas12a-UPTLFA Platform. Front. Microbiol. 2021, 12, 700016. [Google Scholar] [CrossRef]
- Liu, S.; Tao, D.; Liao, Y.; Yang, Y.; Sun, S.; Zhao, Y.; Yang, P.; Tang, Y.; Chen, B.; Liu, Y.; et al. Highly Sensitive CRISPR/Cas12a-Based Fluorescence Detection of Porcine Reproductive and Respiratory Syndrome Virus. ACS Synth. Biol. 2021, 10, 2499–2507. [Google Scholar] [CrossRef]
- Li, F.; Ye, Q.; Chen, M.; Xiang, X.; Zhang, J.; Pang, R.; Xue, L.; Wang, J.; Gu, Q.; Lei, T.; et al. Cas12aFDet: A CRISPR/Cas12a-based fluorescence platform for sensitive and specific detection of Listeria monocytogenes serotype 4c. Anal. Chim. Acta 2021, 1151, 338248. [Google Scholar] [CrossRef]
- Jiang, H.J.; Tan, R.; Jin, M.; Yin, J.; Gao, Z.X.; Li, H.B.; Shi, D.Y.; Zhou, S.Q.; Chen, T.J.; Yang, D.; et al. Visual Detection of using Combined CRISPR/Cas12a and Recombinase Polymerase Amplification. Biomed. Environ. Sci. 2022, 35, 518–527. [Google Scholar] [CrossRef]
- Ma, Q.-N.; Wang, M.; Zheng, L.-B.; Lin, Z.-Q.; Ehsan, M.; Xiao, X.-X.; Zhu, X.-Q. RAA-Cas12a-Tg: A Nucleic Acid Detection System for Based on CRISPR-Cas12a Combined with Recombinase-Aided Amplification (RAA). Microorganisms 2021, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, X.; Xu, J.; Nan, L.; Liu, F.; Duan, G.; Yang, H. Rapid and Ultrasensitive Detection of Methicillin-Resistant Based on CRISPR-Cas12a Combined With Recombinase-Aided Amplification. Front. Microbiol. 2022, 13, 903298. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ren, H.; Hu, P.; Wang, Y.; Wang, H.; Li, Y.; Feng, K.; Wang, C.; Cao, Q.; Guo, Y.; et al. Ultra-Sensitive and Rapid Detection of Pathogenic Based on the CRISPR/Cas12a Nucleic Acid Identification Platform. Foods 2022, 11, 2160. [Google Scholar] [CrossRef] [PubMed]
- Jirawannaporn, S.; Limothai, U.; Tachaboon, S.; Dinhuzen, J.; Kiatamornrak, P.; Chaisuriyong, W.; Bhumitrakul, J.; Mayuramart, O.; Payungporn, S.; Srisawat, N. Rapid and sensitive point-of-care detection of Leptospira by RPA-CRISPR/Cas12a targeting lipL32. PLoS Negl. Trop. Dis. 2022, 16, e0010112. [Google Scholar] [CrossRef]
- Shen, Y.; Jia, F.; He, Y.; Fu, Y.; Fang, W.; Wang, J.; Li, Y. A CRISPR-Cas12a-powered magnetic relaxation switching biosensor for the sensitive detection of Salmonella. Biosens. Bioelectron. 2022, 213, 114437. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, S.; Wu, N.; Wu, J.; Wang, G.; Zhao, G.; Wang, J. HOLMESv2: A CRISPR-Cas12b-Assisted Platform for Nucleic Acid Detection and DNA Methylation Quantitation. ACS Synth Biol. 2019, 8, 2228–2237. [Google Scholar] [CrossRef]
- Sam, I.K.; Chen, Y.Y.; Ma, J.; Li, S.Y.; Ying, R.Y.; Li, L.X.; Ji, P.; Wang, S.J.; Xu, J.; Bao, Y.J.; et al. TB-QUICK: CRISPR-Cas12b-assisted rapid and sensitive detection of Mycobacterium tuberculosis. J. Infect. 2021, 83, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gu, D.; Xue, H.; Yu, J.; Tang, Y.; Huang, J.; Zhang, Y.; Jiao, X. Rapid and Accurate Detection With CRISPR-Cas12b Based on Newly Identified -Specific and -Conserved Genomic Signatures. Front. Microbiol. 2021, 12, 649010. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Wang, J.; Wang, M.; Chen, P.; Yin, M.; Li, J.; Sheng, G.; Wang, Y. Two Distant Catalytic Sites Are Responsible for C2c2 RNase Activities. Cell 2017, 168, 121–134.e12. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.T.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 2016, 353, aaf5573. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Deng, Y.; Li, T.; Wang, J.; Wang, T.; Tan, F.; Li, X.; Tian, K. Visual detection of porcine reproductive and respiratory syndrome virus using CRISPR-Cas13a. Transbound. Emerg. Dis. 2020, 67, 564–571. [Google Scholar] [CrossRef]
- Yao, R.; Xu, Y.; Wang, L.; Wang, D.; Ren, L.; Ren, C.; Li, C.; Li, X.; Ni, W.; He, Y.; et al. CRISPR-Cas13a-Based Detection for Bovine Viral Diarrhea Virus. Front. Vet. Sci. 2021, 8, 603919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yin, L.; Dong, Y.; Peng, L.; Liu, G.; Man, S.; Ma, L. CRISPR-Cas13a based bacterial detection platform: Sensing pathogen Staphylococcus aureus in food samples. Anal. Chim. Acta 2020, 1127, 225–233. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Liu, C.; Peng, L.; Khan, H.; Cui, L.; Huang, R.; Wu, C.; Shen, S.; Wang, S.; et al. CRISPR-Cas13a Nanomachine Based Simple Technology for Avian Influenza A (H7N9) Virus On-Site Detection. J. Biomed. Nanotechnol. 2019, 15, 790–798. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; He, Y.; Yang, X.; Li, Y. CRISPR-Cas13a Based Visual Detection Assays for Feline Calicivirus Circulating in Southwest China. Front. Vet. Sci. 2022, 9, 913780. [Google Scholar] [CrossRef]
- He, D.; Liu, G.; Yang, J.; Jiang, X.; Wang, H.; Fan, Y.; Gong, S.; Wei, F.; Diao, Y.; Tang, Y. Specific High-Sensitivity Enzymatic Molecular Detection System Termed RPA-Based CRISPR-Cas13a for Duck Tembusu Virus Diagnostics. Bioconjug. Chem. 2022, 33, 1232–1240. [Google Scholar] [CrossRef]
- Wei, N.; Zheng, B.; Niu, J.; Chen, T.; Ye, J.; Si, Y.; Cao, S. Rapid Detection of Genotype II African Swine Fever Virus Using CRISPR Cas13a-Based Lateral Flow Strip. Viruses 2022, 14, 179. [Google Scholar] [CrossRef]
- Wei, N.; Xiong, J.; Ma, J.; Ye, J.; Si, Y.; Cao, S. Development of efficient, sensitive, and specific detection method for Encephalomyocarditis virus based on CRISPR/Cas13a. J. Virol Methods 2022, 309, 114592. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.H.; Hennelly, C.M.; Lin, J.T.; Ubalee, R.; Boyce, R.M.; Mulogo, E.M.; Hathaway, N.; Thwai, K.L.; Phanzu, F.; Kalonji, A.; et al. A novel CRISPR-based malaria diagnostic capable of Plasmodium detection, species differentiation, and drug-resistance genotyping. EBioMedicine 2021, 68, 103415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Y.; Xu, L.; Fan, Z.; Cao, Y.; Ma, Y.; Li, H.; Ren, F. CRISPR/Cas13-assisted hepatitis B virus covalently closed circular DNA detection. Hepatol. Int. 2022, 16, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Fozouni, P.; Son, S.; Díaz de León Derby, M.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I.; et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell 2021, 184, 323–333.e9. [Google Scholar] [CrossRef]
- Cao, G.; Huo, D.; Chen, X.; Wang, X.; Zhou, S.; Zhao, S.; Luo, X.; Hou, C. Automated, portable, and high-throughput fluorescence analyzer (APHF-analyzer) and lateral flow strip based on CRISPR/Cas13a for sensitive and visual detection of SARS-CoV-2. Talanta 2022, 248, 123594. [Google Scholar] [CrossRef]
- Katzmeier, F.; Aufinger, L.; Dupin, A.; Quintero, J.; Lenz, M.; Bauer, L.; Klumpe, S.; Sherpa, D.; Dürr, B.; Honemann, M.; et al. A low-cost fluorescence reader for in vitro transcription and nucleic acid detection with Cas13a. PLoS ONE 2019, 14, e0220091. [Google Scholar] [CrossRef]
- Heo, W.; Lee, K.; Park, S.; Hyun, K.-A.; Jung, H.-I. Electrochemical biosensor for nucleic acid amplification-free and sensitive detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA via CRISPR/Cas13a trans-cleavage reaction. Biosens. Bioelectron. 2022, 201, 113960. [Google Scholar] [CrossRef]
- Doudna, J.A.; Harrington, L.B.; Burstein, D.; Chen, J.S.; Paez-Espino, D.; Ma, E.; Witte, I.P.; Cofsky, J.C.; Kyrpides, N.C.; Banfield, J.F. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 2018, 362, 839–842. [Google Scholar] [CrossRef]
- Aquino-Jarquin, G. CRISPR-Cas14 is now part of the artillery for gene editing and molecular diagnostic. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 428–431. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, S.; Zhu, X.; Wang, X.; Huang, J.; Yang, X.; Tai, J. LAMP-CRISPR-Cas12-based diagnostic platform for detection of Mycobacterium tuberculosis complex using real-time fluorescence or lateral flow test. Mikrochim. Acta 2021, 188, 347. [Google Scholar] [CrossRef]
- Ackerman, C.M.; Myhrvold, C.; Thakku, S.G.; Freije, C.A.; Metsky, H.C.; Yang, D.K.; Ye, S.H.; Boehm, C.K.; Kosoko-Thoroddsen, T.-S.F.; Kehe, J.; et al. Massively multiplexed nucleic acid detection with Cas13. Nature 2020, 582, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.L.; Zhu, M.; Hua, C.; Weller, J.; Mirhashemi, M.E.; Nguyen, T.G.; Mantena, S.; Bauer, M.R.; Shaw, B.M.; Ackerman, C.M.; et al. Multiplexed CRISPR-based microfluidic platform for clinical testing of respiratory viruses and identification of SARS-CoV-2 variants. Nat. Med. 2022, 28, 1083–1094. [Google Scholar] [CrossRef]
- Shinoda, H.; Taguchi, Y.; Nakagawa, R.; Makino, A.; Okazaki, S.; Nakano, M.; Muramoto, Y.; Takahashi, C.; Takahashi, I.; Ando, J.; et al. Amplification-free RNA detection with CRISPR-Cas13. Commun. Biol. 2021, 4, 476. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, J.; Ren, L.; Jiang, W.; Wang, M.; Zhuang, L.; Zheng, Q.; Yang, R.; Zeng, Y.; Luu, L.D.W.; et al. A one-step, one-pot CRISPR nucleic acid detection platform (CRISPR-top): Application for the diagnosis of COVID-19. Talanta 2021, 233, 122591. [Google Scholar] [CrossRef] [PubMed]
| Cas Protein | Pathogen Type | Pathogen | Visualization | Sensitivity | Time | References |
|---|---|---|---|---|---|---|
| Cas9 | Viruses | Zika | Colorimetry | 1 fM | 2–3h | [26] |
| Cas9 | Bacteria | Listeria monocytogenes | LFA | 150–200 copies/μL | 2 h | [29] |
| Cas9 | Bacteria | E. coli | SDA/RCA | 40 CFU/mL | 2–3 h | [30] |
| Cas9n | Bacteria | S. typhimurium | Fluorescence | 2 copies/μL | <1 h | [27] |
| dCas9 | Bacteria | Mycobacterium tuberculosis | Bioluminescence | 5 × 10−5 nmol/mL | <1 h | [31] |
| dCas9 | Bacteria | Methicillin-resistant Staphylococcus aureus (MRSA) | Fluorescence | 10 CFU/mL | <0.5 h | [32] |
| dCas9 | Bacteria | Scrub typhus (ST)/severe fever with thrombocytopenia syndrome (SFTS) | SMR biosensor | 0.54 aM/0.63 aM | 0.5 h | [33] |
| dCas9 | Viruses | HPV | Microplate reader/eye | - | <0.5 h | [34] |
| dCas9 | Bacteria | Acinetobacter baumannii/Klebsiella pneumoniae | Spectrometry | 10−5 mol/L | 1 h | [35] |
| Cas Protein | Pathogen | Platform Name | Amplification Methods | Visualization | Sensitivity | Time | References |
|---|---|---|---|---|---|---|---|
| LbCas12a | ASFV | POC | RPA/LAMP | Fluorescence | 100 fmol | <2 h | [45] |
| Cas12a | ASFV | LAMP-CRISPR | LAMP | Fluorescence | 7 copies/μL | <1 h | [46] |
| Cas12a | Yersinia pestis | Cas12a-UPTLFA | RPA | UPT-LFA | 3 aM | <1 h | [47] |
| Cas12a | PRRSV | - | RT-RPA | ssDNA-FQ | 1 copies/μL | 25 min | [48] |
| Cas12a | Listeria monocytogenes | Cas12aFDet | PCR/RAA | Fluorescence | 0.64 aM | 15 min | [49] |
| Cas12a | Vibrio parahaemolyticus | - | RPA | Eye | 10−18 M | <30 min | [50] |
| Cas12a | Toxoplasma gondii | RAA-Cas12a-Tg | RAA | ssDNA-FQ | 1 fM | 1 h | [51] |
| Cas12a | Staphylococcus aureus | RAA-Cas12a | RAA | Fluorescence | 10 copies/μL | 1 h | [52] |
| Cas12a | pathogenic Yersinia enterocolitica | - | RPA | Eye | 1.7 CFU/mL | 45 min | [53] |
| Cas12a | Leptospira | - | RPA | Fluorescence/LFDA | 100 cells/mL | <2 h | [54] |
| Cas Protein | Detection Platform | Guide RNA | Target Type | Trans-Cleavage Activity | Amplification Methods | Sensitivity | |
|---|---|---|---|---|---|---|---|
| Cas9 | sgRNA | DNA | No | CAS-EXPAR | aM (10−18) | ||
| Cas12 | Cas12a | HOLMES | crRNA | DNA | Yes | PCR/RT-PCR | aM |
| Cas12a | DETECTR | crRNA | DNA | Yes | RPA | aM | |
| Cas12b | HOLMESv2 | sgRNA | DNA | Yes | LAMP | aM | |
| Cas13 | Cas13a | SHERLOCK | crRNA | RNA | Yes | RPA | aM |
| Cas13b | SHERLOCKv2 | crRNA | RNA | Yes | RPA | zM (10−21) | |
| Cas14 | Cas14a | DETECTR | sgRNA | ssDNA | Yes | RPA | aM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Yuan, C.; Li, L.; Long, M.; Chen, Z. Application of the CRISPR/Cas System in Pathogen Detection: A Review. Molecules 2022, 27, 6999. https://doi.org/10.3390/molecules27206999
Yuan B, Yuan C, Li L, Long M, Chen Z. Application of the CRISPR/Cas System in Pathogen Detection: A Review. Molecules. 2022; 27(20):6999. https://doi.org/10.3390/molecules27206999
Chicago/Turabian StyleYuan, Bowei, Congcong Yuan, Lulu Li, Miao Long, and Zeliang Chen. 2022. "Application of the CRISPR/Cas System in Pathogen Detection: A Review" Molecules 27, no. 20: 6999. https://doi.org/10.3390/molecules27206999
APA StyleYuan, B., Yuan, C., Li, L., Long, M., & Chen, Z. (2022). Application of the CRISPR/Cas System in Pathogen Detection: A Review. Molecules, 27(20), 6999. https://doi.org/10.3390/molecules27206999






