High-Throughput Metabolomics Integrated Network Pharmacology Reveals the Underlying Mechanism of Paeoniae Radix Alba Treating Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Drug Administration
2.3. Evaluation of Swelling and Pathological Characteristics
2.4. Urine Sample Collection and Preparation
2.5. Metabolomics Analysis Conditions
2.6. Data Processing and Multivariate Data Analysis
2.7. Network Pharmacology and Molecular Docking Analysis Methods
3. Results
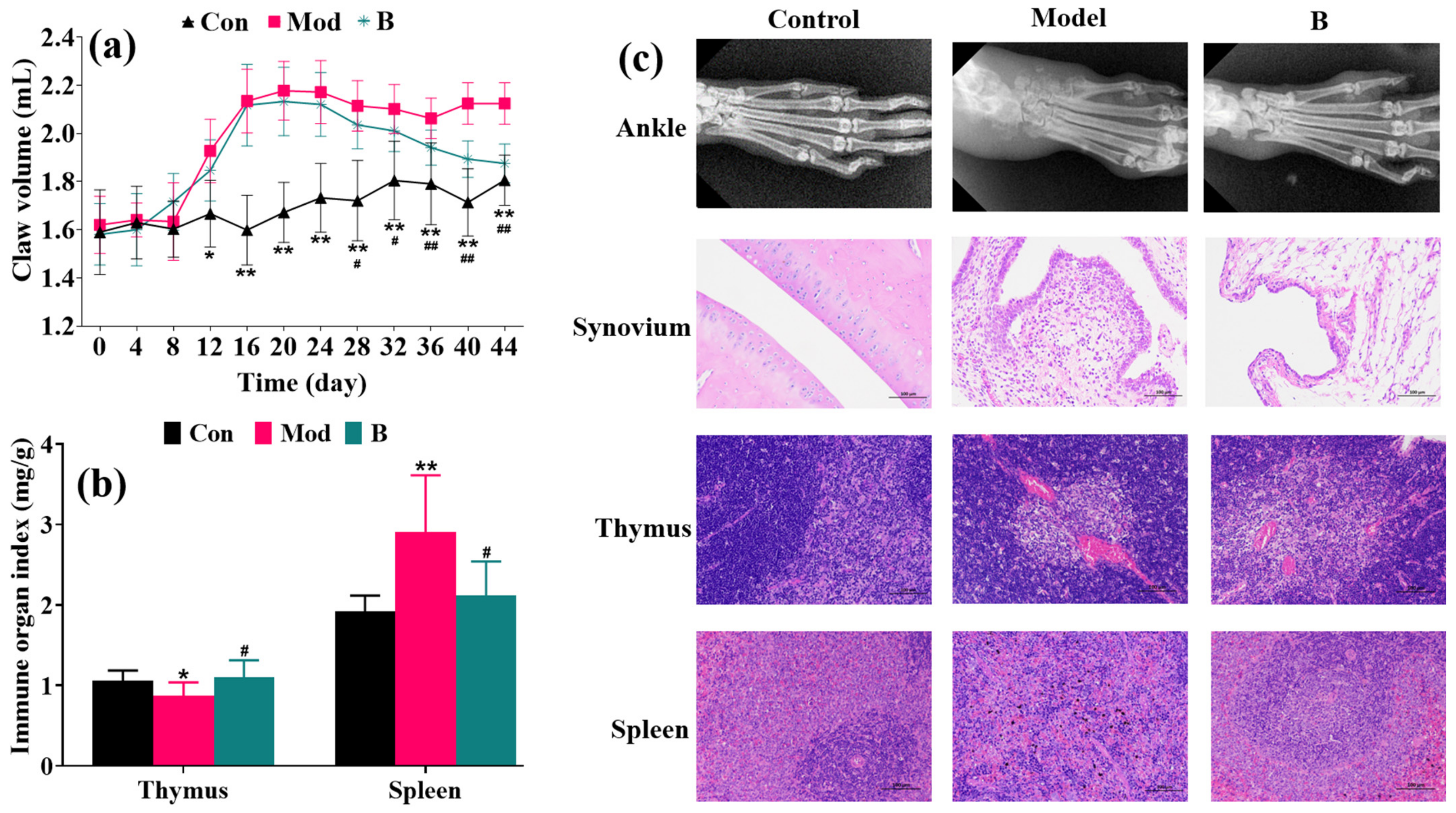
3.1. Inhibitory Effect of B on Rat AIA Paw Swelling
3.2. Multivariate Statistical Analysis and Biomarker Characterization of the Metabolic Profile
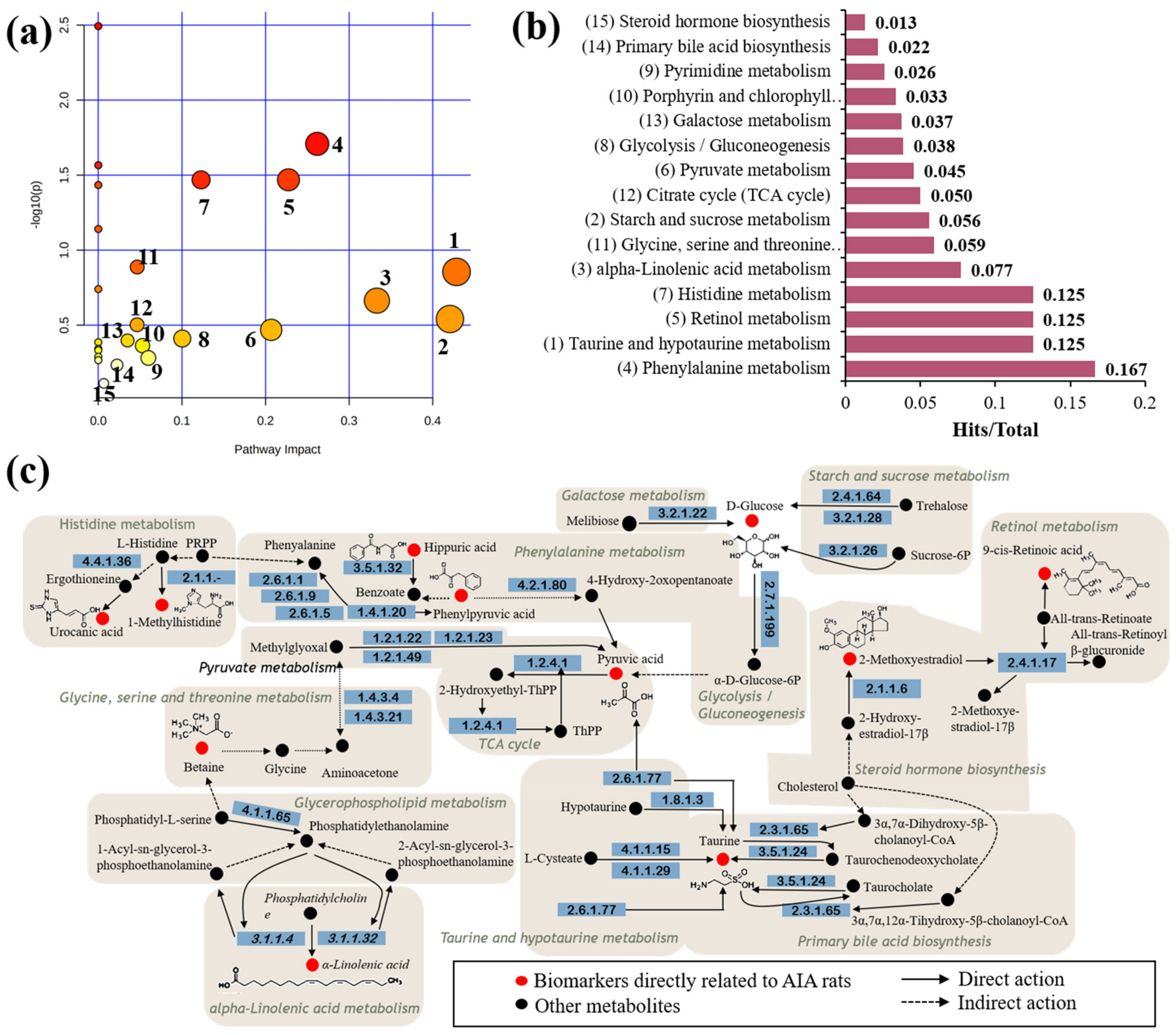
3.3. Metabolic Pathway Integration Enrichment Analysis
3.4. B Regulates Biomarkers and Metabolic Trajectories to Play a Therapeutic Role
3.5. Predicting the Target and Binding Mode of B
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Tan, Y.-Q.; Chen, H.-W.; Li, J.; Wu, Q.-J. Efficacy, Chemical Constituents, and Pharmacological Actions of Radix Paeoniae Rubra and Radix Paeoniae Alba. Front. Pharmacol. 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Shen, M.; Fang, J.; Wei, D.; Qin, L. Advancement in the chemical analysis of Paeoniae Radix (Shaoyao). J. Pharm. Biomed. Anal. 2018, 160, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, J.; Wang, L.; Wang, S.; Nie, X.; Chen, Y.; Fu, Q.; Jiang, M.; Fu, C.; He, Y. Total glucosides of paeony: A review of its phytochemistry, role in autoimmune diseases, and mechanisms of action. J. Ethnopharmacol. 2020, 258, 112913. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, J.; Zhu, H.; Xiong, X.G.; Liang, Q.H.; Zhang, Y.; Zhang, Y.; Wang, Y.; Yang, B.; Huang, X. UPLC-PDA determination of paeoniflorin in rat plasma following the oral administration of Radix Paeoniae Alba and its effects on rats with collagen-induced arthritis. Exp. Ther. Med. 2014, 7, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Xu, J.; Wang, C.; Li, J.; Wang, Q.; Kuang, H.; Yang, B.; Chen, R.; Luo, Z. Paeoniae radix alba polysaccharides obtained via optimized extraction treat experimental autoimmune hepatitis effectively. Int. J. Biol. Macromol. 2020, 164, 1554–1564. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Han, X.; Yang, W.; Wang, G.; Wang, J.; Jiang, X.; Sen, M.; Li, X.; Yu, G.; et al. Mechanism of Paeoniae Radix Alba in the Treatment of Non-alcoholic Fatty Liver Disease Based on Sequential Metabolites Identification Approach, Network Pharmacology, and Binding Affinity Measurement. Front. Nutr. 2021, 8, 677659. [Google Scholar] [CrossRef]
- Jia, X.-Y.; Chang, Y.; Sun, X.-J.; Wu, H.-X.; Wang, C.; Xu, H.-M.; Zhang, L.; Zhang, L.-L.; Zheng, Y.-Q.; Song, L.-H.; et al. Total glucosides of paeony inhibit the proliferation of fibroblast-like synoviocytes through the regulation of G proteins in rats with collagen-induced arthritis. Int. Immunopharmacol. 2014, 18, 1–6. [Google Scholar] [CrossRef]
- Lin, J.; Xiao, L.; Ouyang, G.; Shen, Y.; Huo, R.; Zhou, Z.; Sun, Y.; Zhu, X.; Zhang, J.; Shen, B.; et al. Total glucosides of paeony inhibits Th1/Th17 cells via decreasing dendritic cells activation in rheumatoid arthritis. Cell. Immunol. 2012, 280, 156–163. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, S.; Zhan, F. One of the active ingredients in Paeoniae Radix Alba functions as JAK1 inhibitor in rheumatoid arthritis. Front Pharm. 2022, 13, 3976. [Google Scholar] [CrossRef]
- Guo, R.; Luo, X.; Liu, J.; Liu, L.; Wang, X.; Lu, H. Omics strategies decipher therapeutic discoveries of traditional Chinese medicine against different diseases at multiple layers molecular-level. Pharm. Res. 2020, 152, 104627. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, A.H.; Zhao, Q.Q.; Yan, G.L.; Sun, H.; Wang, X.J. Discovery of quality-marker ingredients of Panax quinquefolius driven by high-throughput chinmedomics approach. Phytomedicine 2020, 74, 152928. [Google Scholar] [CrossRef]
- Ren, J.L.; Dong, H.; Han, Y.; Yang, L.; Zhang, A.H.; Sun, H.; Li, Y.; Yan, G.; Wang, X.J. Network pharmacology combined with metabolomics approach to investigate the protective role and detoxification mechanism of Yunnan Baiyao formulation. Phytomedicine 2020, 77, 153266. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, H.; Wang, Y.; Yan, G.; Sun, H.; Zhou, X.; Wang, Y.; Liu, L.; Wang, X.J.R.A. Discovery and verification of the potential targets from bioactive molecules by network pharmacology-based target prediction combined with high-throughput metabolomics. RSC Adv. 2017, 7, 51069–51078. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Wong, Y.F.; Zhou, H.; Liu, Z.Q.; Xie, Y.; Jiang, Z.H.; Bian, Z.X.; Xu, H.X.; Liu, L. Manipulation of the induction of adjuvant arthritis in Sprague-Dawley rats. Inflamm. Res. 2006, 55, 368–377. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, L.; Zhou, L.L.; Shan, J.J.; Chen, L.T.; Hui-Qin, X.U.; Liu-Qing, D.I. Effect of different compatibility of Daphnes Giraldii Cortex and Glycyrrhizae Radix et Rhizoma on adjuvant-induced arthritis in rats. Chin. Tradit. Herb. Drugs 2014, 45, 1418–1426. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef]
- Holmdahl, R.; Lorentzen, J.C.; Lu, S.; Olofsson, P.; Wester, L.; Holmberg, J.; Pettersson, U. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol. Rev. 2001, 184, 184–202. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, N.; Bhatt, L.K.; Prabhavalkar, K.S. Experimental animal models for rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 193–200. [Google Scholar] [CrossRef]
- Pang, Z.; Wang, G.; Ran, N.; Lin, H.; Wang, Z.; Guan, X.; Yuan, Y.; Fang, K.; Liu, J.; Wang, F. Inhibitory Effect of Methotrexate on Rheumatoid Arthritis Inflammation and Comprehensive Metabolomics Analysis Using Ultra-Performance Liquid Chromatography-Quadrupole Time of Flight-Mass Spectrometry (UPLC-Q/TOF-MS). Int. J. Mol. Sci. 2018, 19, 2894. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.R.; Chokesuwattanaskul, S.; Phelan, M.M.; Welting, T.J.M.; Lian, L.Y.; Peffers, M.J.; Wright, H.L. 1H NMR Metabolomics Identifies Underlying Inflammatory Pathology in Osteoarthritis and Rheumatoid Arthritis Synovial Joints. J. Proteome Res. 2018, 17, 3780–3790. [Google Scholar] [CrossRef]
- de Oliveira, P.G.; Farinon, M.; Sanchez-Lopez, E.; Miyamoto, S.; Guma, M. Fibroblast-Like Synoviocytes Glucose Metabolism as a Therapeutic Target in Rheumatoid Arthritis. Front. Immunol. 2019, 10, 1743. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Huang, J.; Fan, H.; He, D.; Zhao, S.; Shu, Y.; Li, H.; Liu, L.; Lu, S.; Xiao, C.; et al. Treatment of Rheumatoid Arthritis Using Combination of Methotrexate and Tripterygium Glycosides Tablets-A Quantitative Plasma Pharmacochemical and Pseudotargeted Metabolomic Approach. Front. Pharmacol. 2018, 9, 1051. [Google Scholar] [CrossRef] [Green Version]
- Souto-Carneiro, M.; Tóth, L.; Behnisch, R.; Urbach, K.; Klika, K.D.; Carvalho, R.A.; Lorenz, H.M. Differences in the serum metabolome and lipidome identify potential biomarkers for seronegative rheumatoid arthritis versus psoriatic arthritis. Ann. Rheum. Dis. 2020, 79, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, R.; Coras, R.; Rosenthal, S.B.; Sweeney, S.R.; Lodi, A.; Tiziani, S.; Boyle, D.; Kavanaugh, A.; Guma, M. Serum metabolomic profiling predicts synovial gene expression in rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 164. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Dai, Y.; Wen, J.L.; Wang, L.X. ¹H NMR-based metabolomic study of metabolic profiling for systemic lupus erythematosus. Lupus 2011, 20, 1411–1420. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Hu, C.; Xie, Z.; Li, H.; Wei, S.; Wang, D.; Wen, C.; Xu, G. Exploration of the serum metabolite signature in patients with rheumatoid arthritis using gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2016, 127, 60–67. [Google Scholar] [CrossRef]
- Urbaniak, B.; Plewa, S.; Klupczynska, A.; Sikorska, D.; Samborski, W.; Kokot, Z.J. Serum free amino acid levels in rheumatoid arthritis according to therapy and physical disability. Cytokine 2019, 113, 332–339. [Google Scholar] [CrossRef]
- Li, J.; Che, N.; Xu, L.; Zhang, Q.; Wang, Q.; Tan, W.; Zhang, M. LC-MS-based serum metabolomics reveals a distinctive signature in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 1493–1502. [Google Scholar] [CrossRef]
- Zabek, A.; Swierkot, J.; Malak, A.; Zawadzka, I.; Deja, S.; Bogunia-Kubik, K.; Mlynarz, P. Application of 1H NMR-based serum metabolomic studies for monitoring female patients with rheumatoid arthritis. J. Pharm. Biomed. Anal. 2016, 117, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Kayacelebi, A.A.; Willers, J.; Pham, V.V.; Hahn, A.; Schneider, J.Y.; Rothmann, S.; Frölich, J.C.; Tsikas, D. Plasma homoarginine, arginine, asymmetric dimethylarginine and total homocysteine interrelationships in rheumatoid arthritis, coronary artery disease and peripheral artery occlusion disease. Amino Acids 2015, 47, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Jaźwińska-Kozuba, A.; Martens-Lobenhoffer, J.; Kruszelnicka, O.; Rycaj, J.; Chyrchel, B.; Surdacki, A.; Bode-Böger, S.M. Opposite associations of plasma homoarginine and ornithine with arginine in healthy children and adolescents. Int. J. Mol. Sci. 2013, 14, 21819–21832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, D.A. Low free serum histidine concentration in rheumatoid arthritis. A measure of disease activity. J. Clin. Investig. 1975, 55, 1164–1173. [Google Scholar] [CrossRef] [Green Version]
- Tetlow, L.C.; Woolley, D.E. Histamine stimulates the proliferation of human articular chondrocytes in vitro and is expressed by chondrocytes in osteoarthritic cartilage. Ann. Rheum. Dis. 2003, 62, 991–994. [Google Scholar] [CrossRef] [Green Version]
- Breedveld, F.C.; Dayer, J.M. Leflunomide: Mode of action in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2000, 59, 841–849. [Google Scholar] [CrossRef]
- Liu, S.; Neidhardt, E.A.; Grossman, T.H.; Ocain, T.; Clardy, J. Structures of human dihydroorotate dehydrogenase in complex with antiproliferative agents. Structure 2000, 8, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Singh, A.K.; Ahmed, S. MicroRNA-17 Suppresses TNF-α Signaling by Interfering with TRAF2 and cIAP2 Association in Rheumatoid Arthritis Synovial Fibroblasts. J. Immunol. 2016, 197, 2219–2228. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Zhang, X.; Zeng, Y.; Huang, X.; Chen, H.; Wang, S.; Wu, J.; Li, Q.; Zhu, W.; Li, H.; et al. A Novel Phytochemical, DIM, Inhibits Proliferation, Migration, Invasion and TNF-α Induced Inflammatory Cytokine Production of Synovial Fibroblasts From Rheumatoid Arthritis Patients by Targeting MAPK and AKT/mTOR Signal Pathway. Front. Immunol. 2019, 10, 1620. [Google Scholar] [CrossRef] [Green Version]
- Muz, B.; Khan, M.N.; Kiriakidis, S.; Paleolog, E.M. Hypoxia. The role of hypoxia and HIF-dependent signalling events in rheumatoid arthritis. Arthritis Res. 2009, 11, 201. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, Y.; Nakayamada, S.; Kubo, S.; Nakano, K.; Iwata, S.; Miyagawa, I.; Ma, X.; Trimova, G.; Sakata, K.; Tanaka, Y. Th22 Cells Promote Osteoclast Differentiation via Production of IL-22 in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 2901. [Google Scholar] [CrossRef]
- Wang, Q.T.; Zhang, L.L.; Wu, H.X.; Wei, W. The expression change of β-arrestins in fibroblast-like synoviocytes from rats with collagen-induced arthritis and the effect of total glucosides of paeony. J. Ethnopharmacol. 2011, 133, 511–516. [Google Scholar] [CrossRef]
- Liu, R.; Hao, D.; Xu, W.; Li, J.; Li, X.; Shen, D.; Sheng, K.; Zhao, L.; Xu, W.; Gao, Z.; et al. β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm. Biol. 2019, 57, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Li, N.; Liu, Y.; Xu, Q.; Liu, Q.; You, Y.; Wei, Z.; Jiang, Y.; Liu, M.; Guo, T.; et al. Kaempferol inhibits the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes by blocking activation of the MAPK pathway. Int. Immunopharmacol. 2018, 55, 174–182. [Google Scholar] [CrossRef]
- Fechtner, S.; Singh, A.; Chourasia, M.; Ahmed, S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1β signaling in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharmacol. 2017, 329, 112–120. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, T.; Dong, H.; Wang, X. High-Throughput Metabolomics Integrated Network Pharmacology Reveals the Underlying Mechanism of Paeoniae Radix Alba Treating Rheumatoid Arthritis. Molecules 2022, 27, 7014. https://doi.org/10.3390/molecules27207014
Liu L, Li T, Dong H, Wang X. High-Throughput Metabolomics Integrated Network Pharmacology Reveals the Underlying Mechanism of Paeoniae Radix Alba Treating Rheumatoid Arthritis. Molecules. 2022; 27(20):7014. https://doi.org/10.3390/molecules27207014
Chicago/Turabian StyleLiu, Lei, Taiping Li, Hui Dong, and Xijun Wang. 2022. "High-Throughput Metabolomics Integrated Network Pharmacology Reveals the Underlying Mechanism of Paeoniae Radix Alba Treating Rheumatoid Arthritis" Molecules 27, no. 20: 7014. https://doi.org/10.3390/molecules27207014



