Biowaste-Derived, Highly Efficient, Reusable Carbon Nanospheres for Speedy Removal of Organic Dyes from Aqueous Solutions
Abstract
1. Introduction
2. Results
2.1. OPL 1000 Characterization
2.2. Effect of Carbonization Temperature of CNSs
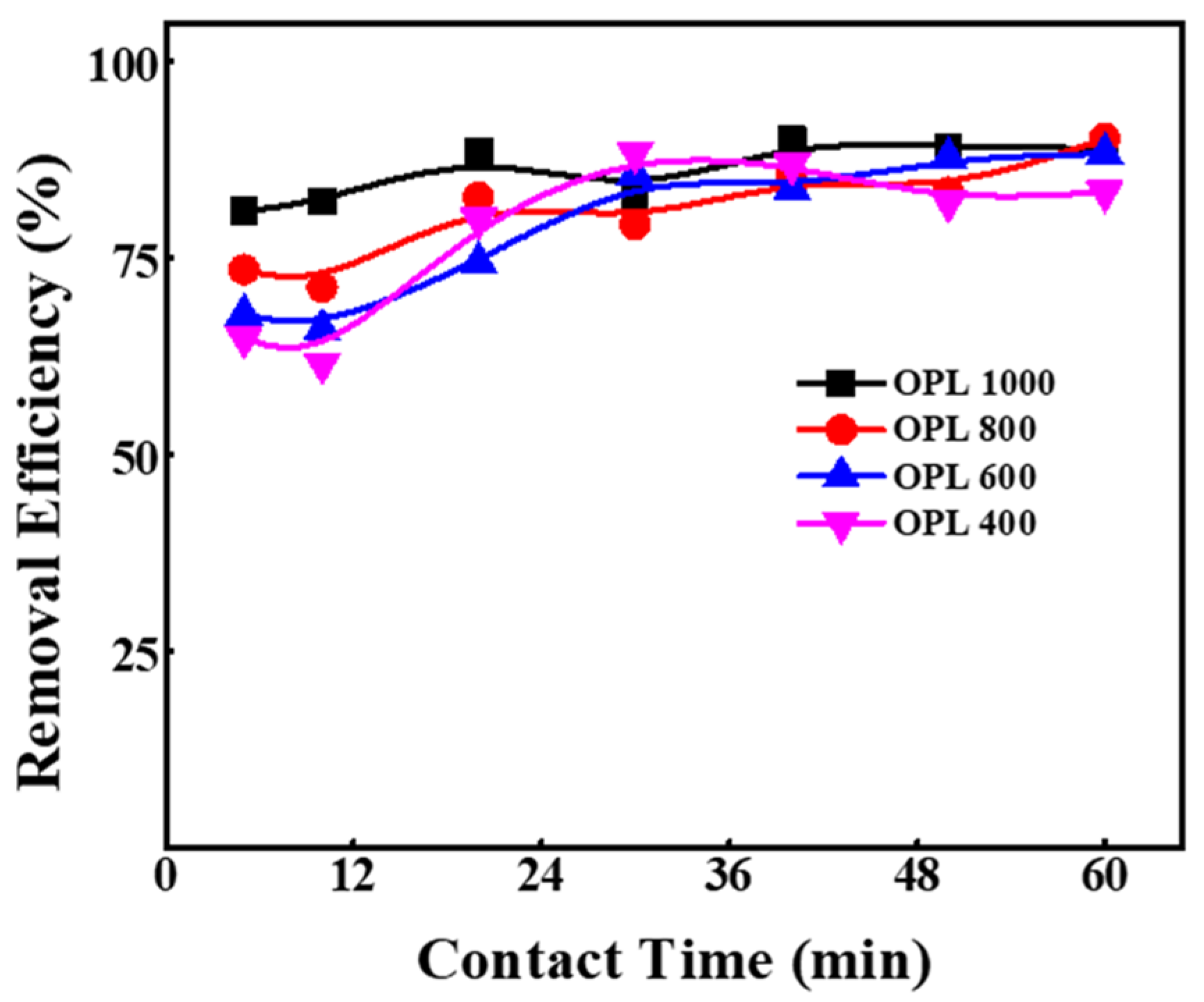
2.3. Effect of Contact Time
2.4. Effect of Adsorbent Dosage
2.5. Effect of Initial Dye Concentration
2.6. Effect of pH of the Dye Solution
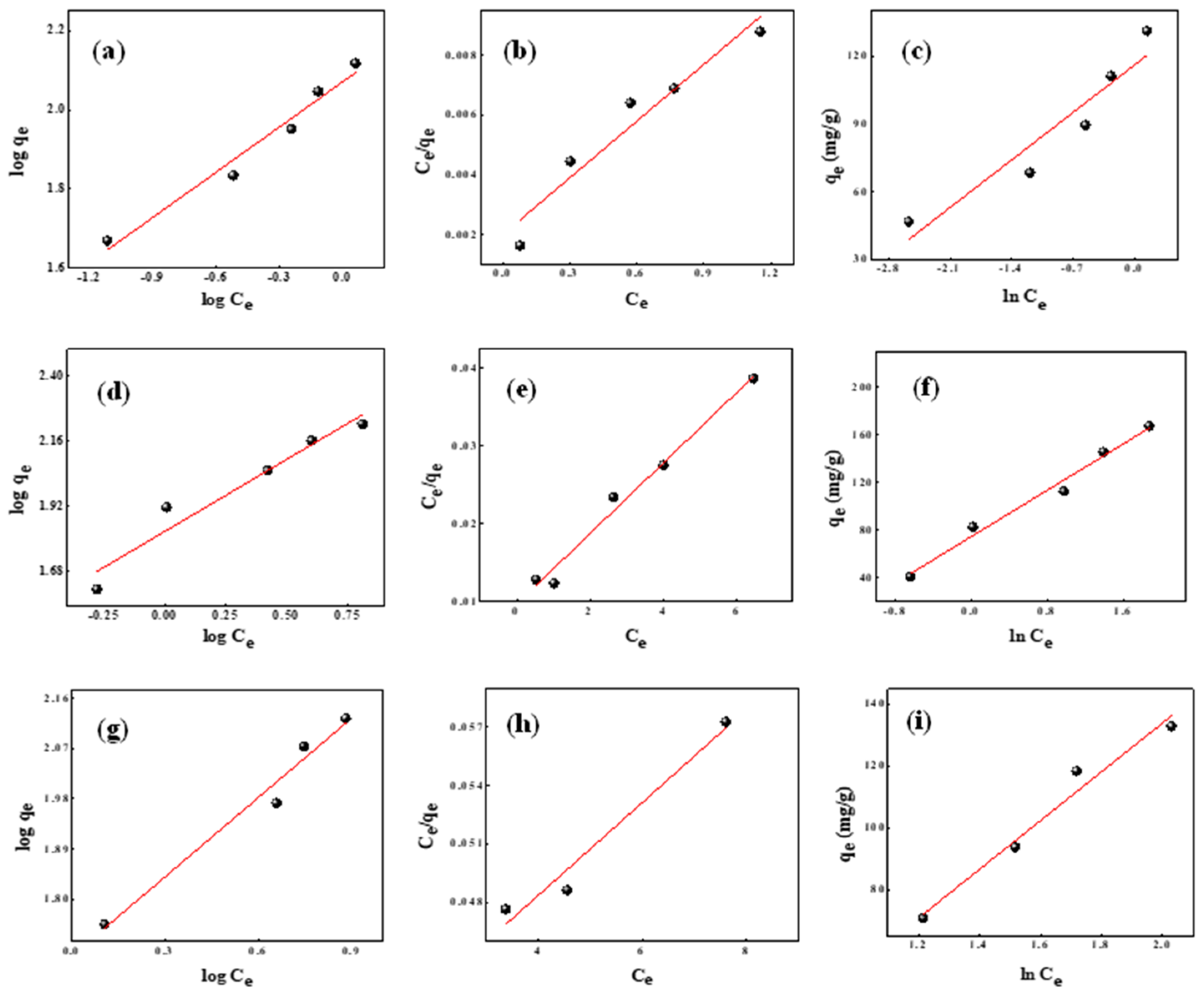
2.7. Adsorption Isotherms
3. Discussion
- Synthesis
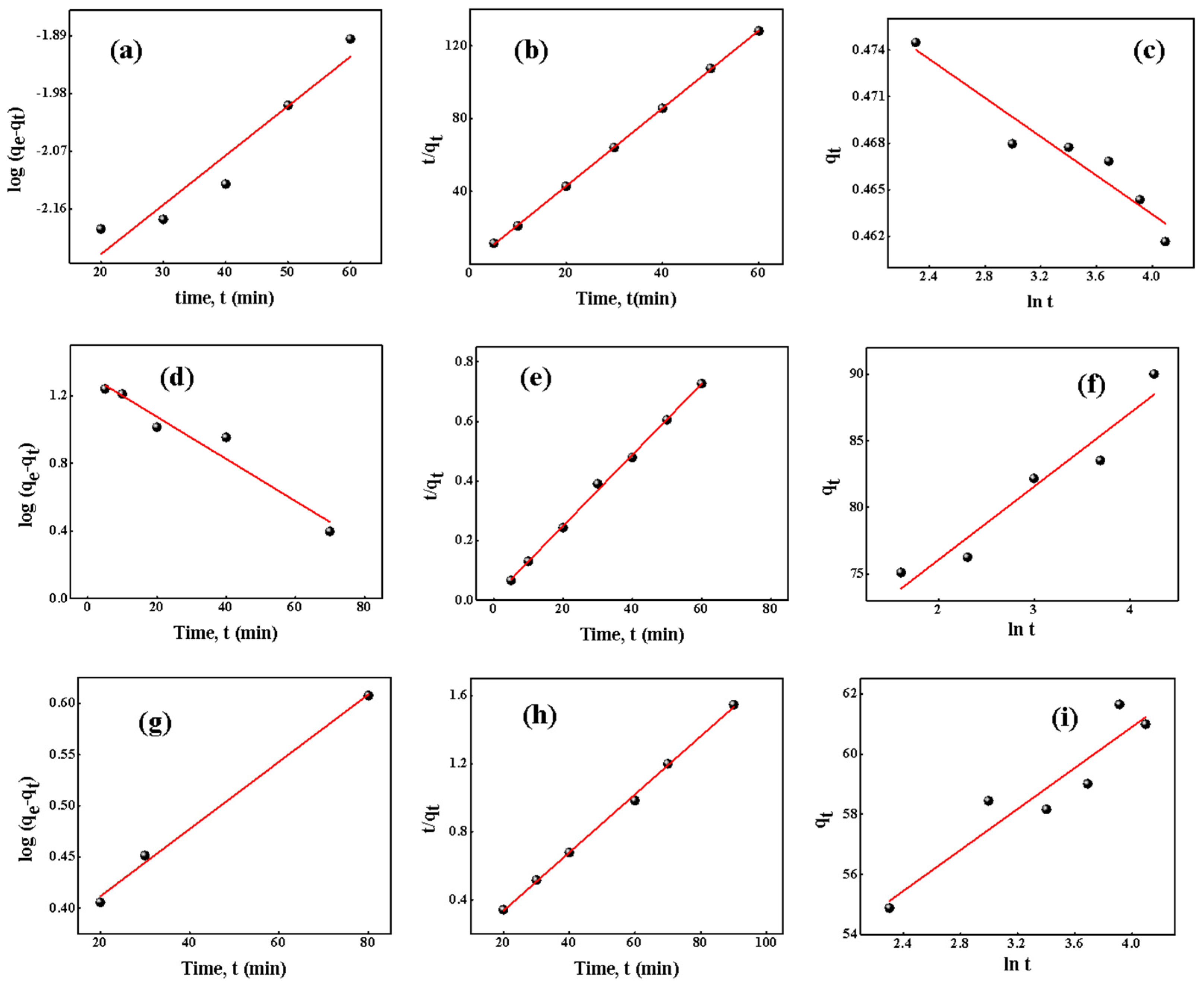
- Kinetics of adsorption
- Reusability
4. Materials and Methods
- Materials & Characterization
- Batch adsorption experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Imran, M.; Islam, A.U.; Tariq, M.A.; Siddique, M.H.; Shah, N.S.; Khan, Z.U.H.; Amjad, M.; Din, S.U.; Shah, G.M.; Naeem, M.A.; et al. Synthesis of Magnetite-Based Nanocomposites for Effective Removal of Brilliant Green Dye from Wastewater. Environ. Sci. Pollut. Res. 2019, 26, 24489–24502. [Google Scholar] [CrossRef] [PubMed]
- Sukla Baidya, K.; Kumar, U. Adsorption of Brilliant Green Dye from Aqueous Solution onto Chemically Modified Areca Nut Husk. S. Afr. J. Chem. Eng. 2021, 35, 33–43. [Google Scholar] [CrossRef]
- Nandi, B.K.; Patel, S. Effects of Operational Parameters on the Removal of Brilliant Green Dye from Aqueous Solutions by Electrocoagulation. Arab. J. Chem. 2017, 10, S2961–S2968. [Google Scholar] [CrossRef]
- Ragab, A.; Ahmed, I.; Bader, D. The Removal of Brilliant Green Dye from Aqueous Solution Using Nano Hydroxyapatite/Chitosan Composite as a Sorbent. Molecules 2019, 24, 847. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Mesoporous Activated Carbons of Low-Cost Agricultural Bio-Wastes with High Adsorption Capacity: Preparation and Artificial Neural Network Modeling of Dye Removal from Single and Multicomponent (Binary and Ternary) Systems. J. Mol. Liq. 2018, 269, 217–228. [Google Scholar] [CrossRef]
- Mohammadi, A.; Daemi, H.; Barikani, M. Fast Removal of Malachite Green Dye Using Novel Superparamagnetic Sodium Alginate-Coated Fe3O4 Nanoparticles. Int. J. Biol. Macromol. 2014, 69, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Krishna Murthy, T.P.; Gowrishankar, B.S.; Chandra Prabha, M.N.; Kruthi, M.; Hari Krishna, R. Studies on Batch Adsorptive Removal of Malachite Green from Synthetic Wastewater Using Acid Treated Coffee Husk: Equilibrium, Kinetics and Thermodynamic Studies. Microchem. J. 2019, 146, 192–201. [Google Scholar] [CrossRef]
- Mondal, N.K.; Kar, S. Potentiality of Banana Peel for Removal of Congo Red Dye from Aqueous Solution: Isotherm, Kinetics and Thermodynamics Studies. Appl. Water Sci. 2018, 8, 157. [Google Scholar] [CrossRef]
- Afkhami, A.; Moosavi, R. Adsorptive Removal of Congo Red, a Carcinogenic Textile Dye, from Aqueous Solutions by Maghemite Nanoparticles. J. Hazard. Mater. 2010, 174, 398–403. [Google Scholar] [CrossRef]
- Raj, R.A.; Manimozhi, V.; Saravanathamizhan, R. Adsorption Studies on Removal of Congo Red Dye from Aqueous Solution Using Petroleum Coke. Pet. Sci. Technol. 2019, 37, 913–924. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Removal of Brilliant Green Dye from Water by Modified Bambusa Tulda: Adsorption Isotherm, Kinetics and Thermodynamics Study. Int. J. Environ. Sci. Technol. 2019, 16, 1649–1662. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, J.; Wang, L. CO2-Activated Porous Carbon Derived from Cattail Biomass for Removal of Malachite Green Dye and Application as Supercapacitors. Chem. Eng. J. 2017, 317, 493–502. [Google Scholar] [CrossRef]
- Ahmad, R.; Ansari, K. Chemically Treated Lawsonia Inermis Seeds Powder (CTLISP): An Eco-Friendly Adsorbent for the Removal of Brilliant Green Dye from Aqueous Solution. Groundw. Sustain. Dev. 2020, 11, 100417. [Google Scholar] [CrossRef]
- Yang, Z.; Asoh, T.A.; Uyama, H. Removal of Cationic or Anionic Dyes from Water Using Ion Exchange Cellulose Monoliths as Adsorbents. Bull. Chem. Soc. Jpn. 2019, 92, 1453–1461. [Google Scholar] [CrossRef]
- Obiora-Okafo, I.A.; Onukwuli, O.D. Characterization and Optimization of Spectrophotometric Colour Removal from Dye Containing Wastewater by Coagulation-Flocculation. Pol. J. Chem. Technol. 2018, 20, 49–59. [Google Scholar] [CrossRef]
- Ejraei, A.; Aroon, M.A.; Ziarati Saravani, A. Wastewater Treatment Using a Hybrid System Combining Adsorption, Photocatalytic Degradation and Membrane Filtration Processes. J. Water Process Eng. 2019, 28, 45–53. [Google Scholar] [CrossRef]
- Molavi, H.; Shojaei, A.; Pourghaderi, A. Rapid and Tunable Selective Adsorption of Dyes Using Thermally Oxidized Nanodiamond. J. Colloid Interface Sci. 2018, 524, 52–64. [Google Scholar] [CrossRef]
- Boudissa, F.; Mirilà, D.; Arus, V.A.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.D.; Roy, R.; Azzouz, A. Acid-Treated Clay Catalysts for Organic Dye Ozonation—Thorough Mineralization through Optimum Catalyst Basicity and Hydrophilic Character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef]
- Rosales, E.; Anasie, D.; Pazos, M.; Lazar, I.; Sanromán, M.A. Kaolinite Adsorption-Regeneration System for Dyestuff Treatment by Fenton Based Processes. Sci. Total Environ. 2018, 622–623, 556–562. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent Advances Based on the Synergetic Effect of Adsorption for Removal of Dyes from Waste Water Using Photocatalytic Process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef]
- Konnerth, H.; Matsagar, B.; Chen, S.; Prechtl, M.; Shieh, F.-K.; Wu, C.-W. Metal-Organic Framework (MOF)-Derived Catalysts for Fine Chemical Production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Giri, B.S.; Gun, S.; Pandey, S.; Trivedi, A.; Kapoor, R.T.; Singh, R.P.; Abdeldayem, O.M.; Rene, E.R.; Yadav, S.; Chaturvedi, P.; et al. Reusability of Brilliant Green Dye Contaminated Wastewater Using Corncob Biochar and Brevibacillus Parabrevis: Hybrid Treatment and Kinetic Studies. Bioengineered 2020, 11, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Zeng, W.-J.; Jiang, T.-T.; Chen, X.; Zhang, X.-L. Incorporating Attapulgite Nanorods into Graphene Oxide Nanofiltration Membranes for Efficient Dyes Wastewater Treatment. Sep. Purif. Technol. 2019, 214, 21–30. [Google Scholar] [CrossRef]
- Du, J.; Chen, A.; Yu, Y.; Zhang, Y.; Lv, H.; Liu, L. Mesoporous Carbon Sheets Embedded by Vesicles for Enhanced Supercapacitor Performance. J. Mater. Chem. A Mater. 2019, 7, 15707–15713. [Google Scholar] [CrossRef]
- El-Sharkawy, R.G. Anchoring of Green Synthesized Silver Nanoparticles onto Various Surfaces for Enhanced Heterogeneous Removal of Brilliant Green Dye from Aqueous Solutions with Error Analysis Study. Colloids Surf. A Phys. Eng. Asp. 2019, 583, 123871. [Google Scholar] [CrossRef]
- Boudechiche, N.; Fares, M.; Ouyahia, S.; Yazid, H.; Trari, M.; Sadaoui, Z. Comparative Study on Removal of Two Basic Dyes in Aqueous Medium by Adsorption Using Activated Carbon from Ziziphus lotus Stones. Microchem. J. 2019, 146, 1010–1018. [Google Scholar] [CrossRef]
- Shojaei, S.; Shojaei, S.; Pirkamali, M. Application of Box–Behnken Design Approach for Removal of Acid Black 26 from Aqueous Solution Using Zeolite: Modeling, Optimization, and Study of Interactive Variables. Water Conserv. Sci. Eng. 2019, 4, 13–19. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Removal of a Cationic Dye from Aqueous Solution by Natural Clay. Groundw. Sustain. Dev. 2018, 6, 255–262. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, N.; Giri, B.S.; Chowdhary, P.; Chaturvedi, P. Removal of Methylene Blue Dye Using Rice Husk, Cow Dung and Sludge Biochar: Characterization, Application, and Kinetic Studies. Bioresour. Technol. 2020, 306, 123202. [Google Scholar] [CrossRef]
- Atun, G.; Ayar, N.; Kurtoğlu, A.E.; Ortaboy, S. A Comparison of Sorptive Removal of Anthraquinone and Azo Dyes Using Fly Ash from Single and Binary Solutions. J. Hazard. Mater. 2019, 371, 94–107. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, L.; Yang, Y.; Liu, X. Porous Carbon Nanospheres Aerogel Based Molecularly Imprinted Polymer for Efficient Phenol Adsorption and Removal from Wastewater. Sep. Purif. Technol. 2021, 274, 119029. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Chang, Y.-J.; Lee, D.-J.; Hsu, J.-P. Water Stable Metal-Organic Framework as Adsorbent from Aqueous Solution: A Mini-Review. J. Taiwan Inst. Chem. Eng. 2018, 93, 176–183. [Google Scholar] [CrossRef]
- Kazachenko, A.; Miroshnikova, A.; Tarabanko, V.; Skripnikov, A.; Malyar, Y.; Borovkova, V.; Sychev, V.; Taran, O. Thermal Conversion of Flax Shives in Sub- and Supercritical Ethanol in the Presence of Ru/C Catalyst. Catalysts 2021, 11, 970. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A.; Abdi, J.; Hayati, B.; Shekarchi, A.A. Bio-Based Magnetic Metal-Organic Framework Nanocomposite: Ultrasound-Assisted Synthesis and Pollutant (Heavy Metal and Dye) Removal from Aqueous Media. Appl. Surf. Sci. 2019, 480, 288–299. [Google Scholar] [CrossRef]
- Kok, B.; Tan, K.; Vakili, M.; Horri, B.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of Dyes by Nanomaterials: Recent Developments and Adsorption Mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Kim, J.; Ide, Y.; Kim, J.H.; Hossain, S.; Bando, Y.; Yamauchi, Y.; Wu, K.C.-W. 3D Network of Cellulose-Based Energy Storage Devices and Related Emerging Applications. Mater. Horiz. 2017, 4, 522. [Google Scholar] [CrossRef]
- Masoudian, N.; Rajabi, M.; Ghaedi, M. Titanium Oxide Nanoparticles Loaded onto Activated Carbon Prepared from Bio-Waste Watermelon Rind for the Efficient Ultrasonic-Assisted Adsorption of Congo Red and Phenol Red Dyes from Wastewaters. Polyhedron 2019, 173, 114105. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Tyagi, A.; Ashfaq, M.; Gupta, R.K. Temperature Dependent, Shape Variant Synthesis of Photoluminescent and Biocompatible Carbon Nanostructures from Almond Husk for Applications in Dye Removal. RSC Adv. 2016, 6, 29545–29553. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Biomass-Based Adsorbents for Removal of Dyes from Wastewater: A Review. Front. Env. Sci. 2021, 9, 558. [Google Scholar] [CrossRef]
- Sharma, A.; Siddiqui, Z.M.; Dhar, S.; Mehta, P.; Pathania, D. Adsorptive Removal of Congo Red Dye (CR) from Aqueous Solution by Cornulaca Monacantha Stem and Biomass-Based Activated Carbon: Isotherm, Kinetics and Thermodynamics. Sep. Sci. Technol. 2019, 54, 916–929. [Google Scholar] [CrossRef]
- Rehman, M.S.U. Adsorption of Brilliant Green Dye on Biochar Prepared From Lignocellulosic Bioethanol Plant Waste. Clean Soil Air Water 2015, 44, 55–62. [Google Scholar] [CrossRef]
- Kumar, A.; Hegde, G.; Manaf, S.A.B.A.; Ngaini, Z.; Sharma, K.V. Catalyst Free Silica Templated Porous Carbon Nanoparticles from Bio-Waste Materials. Chem. Commun. 2014, 50, 12702–12705. [Google Scholar] [CrossRef] [PubMed]
- Yallappa, S.; Manaf, S.A.A.; Hegde, G. Synthesis of a Biocompatible Nanoporous Carbon and Its Conjugation with Florescent Dye for Cellular Imaging and Targeted Drug Delivery to Cancer Cells. Xinxing Tan Cailiao New Carbon Mater. 2018, 33, 162–172. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Manaf, S.A.A.; Chong, K.F.; Hegde, G. Superior Supercapacitive Performance in Porous Nanocarbons. J. Energy Chem. 2016, 25, 734–739. [Google Scholar] [CrossRef][Green Version]
- Onoja, E.; Chandren, S.; Razak, F.I.A.; Wahab, R.A. Extraction of Nanosilica from Oil Palm Leaves and Its Application as Support for Lipase Immobilization. J. Biotechnol. 2018, 283, 81–96. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Romero, R.; Romero, A.; Valverde, J.L. Carbon Nanospheres: Synthesis, Physicochemical Properties and Applications. J. Mater. Chem. 2011, 21, 1664–1672. [Google Scholar] [CrossRef]
- Bhat, V.S.; Supriya, S.; Jayeoye, T.J.; Rujiralai, T.; Sirimahachai, U.; Chong, K.F.; Hegde, G. Influence of Surface Properties on Electro-chemical Supercapacitors Utilizing Callerya atropurpurea Pod Derived Porous Nanocarbons: Structure Property Relationship between Porous Structures to Energy Storage Devices. Nano. Sel. 2020, 1, 226–243. [Google Scholar] [CrossRef]
- Kanagavalli, P.; Pandey, G.R.; Bhat, V.S.; Veerapandian, M.; Hegde, G. Nitrogenated-Carbon Nanoelectrocatalyst Advertently Processed from Bio-Waste of Allium Sativum for Oxygen Reduction Reaction. J. Nanostruct. Chem. 2021, 11, 343–352. [Google Scholar] [CrossRef]
- Xia, L.; Huang, H.; Fan, Z.; Hu, D.; Zhang, D.; Khan, A.S.; Usman, M.; Pan, L. Hierarchical Macro-/Meso-/Microporous Oxygen-Doped Carbon Derived from Sodium Alginate: A Cost-Effective Biomass Material for Binder-Free Supercapacitors. Mater. Des. 2019, 182, 108048. [Google Scholar] [CrossRef]
- Adebayo, M.A.; Adebomi, J.I.; Abe, T.O.; Areo, F.I. Removal of Aqueous Congo Red and Malachite Green Using Ackee Apple Seed–Bentonite Composite. Colloid Interface Sci. Commun. 2020, 38, 100311. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Xu, Q.; He, L.; Chen, Z. Controllable Route to Solid and Hollow Monodisperse Carbon Nanospheres. J. Phys. Chem. C 2009, 113, 10085–10089. [Google Scholar] [CrossRef]
- Bhat, V.S.; Hegde, G.; Nasrollahzadeh, M. A Sustainable Technique to Solve Growing Energy Demand: Porous Carbon Nanoparticles as Electrode Materials for High-Performance Supercapacitors. J. Appl. Electrochem. 2020, 50, 1243–1255. [Google Scholar] [CrossRef]
- Supriya, S.; Sriram, G.; Ngaini, Z.; Kavitha, C.; Kurkuri, M.; de Padova, I.P.; Hegde, G. The Role of Temperature on Physical–Chemical Properties of Green Synthesized Porous Carbon Nanoparticles. Waste Biomass Valoriz. 2020, 11, 3821–3831. [Google Scholar] [CrossRef]
- Xing, Z.; Ju, Z.; Zhao, Y.; Wan, J.; Zhu, Y.; Qiang, Y.; Qian, Y. One-Pot Hydrothermal Synthesis of Nitrogen-Doped Graphene as High-Performance Anode Materials for Lithium Ion Batteries. Sci. Rep. 2016, 6, 26146. [Google Scholar] [CrossRef]
- Ma, G.; Huang, K.; Zhuang, Q.; Ju, Z. Superior Cycle Stability of Nitrogen-Doped Graphene Nanosheets for Na-Ion Batteries. Mater. Lett. 2016, 174, 221–225. [Google Scholar] [CrossRef]
- Ou, J.; Zhang, Y.; Chen, L.; Zhao, Q.; Meng, Y.; Guo, Y.; Xiao, D. Nitrogen-Rich Porous Carbon Derived from Biomass as a High Performance Anode Material for Lithium Ion Batteries. J. Mater. Chem. A Mater. 2015, 3, 6534–6541. [Google Scholar] [CrossRef]
- Knipe, S.W.; Mycroft, J.R.; Pratt, A.R.; Nesbitt, H.W.; Bancroff, G.M. X-Ray Photoelectron Spectroscopic Study of Water Adsorption on Iron Sulphide Minerals. Geochim. Cosmochim. Acta 1995, 59, 1079–1090. [Google Scholar] [CrossRef]
- Gupta, K.; Khatri, O.P. Reduced Graphene Oxide as an Effective Adsorbent for Removal of Malachite Green Dye: Plausible Adsorption Pathways. J. Colloid Interface Sci. 2017, 501, 11–21. [Google Scholar] [CrossRef]
- Almarri, M.; Ma, X.; Song, C. Role of Surface Oxygen-Containing Functional Groups in Liquid-Phase Adsorption of Nitrogen Compounds on Carbon-Basedadsorbents. Energy Fuels 2009, 23, 3940–3947. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Campos, L.C. Role of the Functional Groups in the Adsorption of Bisphenol A onto Activated Carbon: Thermal Modification and Mechanism. J. Water Supply Res. Technol. AQUA 2017, 66, 105–115. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, L.; Zhao, Y.; Yu, Z. Fabrication of Zinc Oxide/Polypyrrole Nanocomposites for Brilliant Green Removal from Aqueous Phase. Arab. J. Sci. Eng. 2019, 44, 111–121. [Google Scholar] [CrossRef]
- Pal, J.; Deb, M.K. Efficient Adsorption of Congo Red Dye from Aqueous Solution Using Green Synthesized Coinage Nanoparticles Coated Activated Carbon Beads. Appl. Nanosci. 2014, 4, 967–978. [Google Scholar] [CrossRef]
- Ojedokun, A.T.; Bello, O.S. Kinetic Modeling of Liquid-Phase Adsorption of Congo Red Dye Using Guava Leaf-Based Activated Carbon. Appl. Water Sci. 2017, 7, 1965–1977. [Google Scholar] [CrossRef]
- Shafiqul Alam, M. Removal of Congo Red Dye from Industrial Wastewater by Untreated Sawdust. Am. J. Environ. Prot. 2015, 4, 207. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, H.; Yang, H.; Wan, Y.; Hu, H.; Zhai, Z.; Qin, J. Graphene Oxide Caged in Cellulose Microbeads for Removal of Malachite Green Dye from Aqueous Solution. J. Colloid Interface Sci. 2015, 437, 277–282. [Google Scholar] [CrossRef]
- Pavan, F.A.; Dias, S.L.P.; Lima, E.C.; Benvenutti, E.V. Removal of Congo Red from Aqueous Solution by Anilinepropylsilica Xerogel. Dyes Pigments 2008, 76, 64–69. [Google Scholar] [CrossRef]
- Chan, S.L.; Tan, Y.P.; Abdullah, A.H.; Ong, S.T. Equilibrium, Kinetic and Thermodynamic Studies of a New Potential Biosorbent for the Removal of Basic Blue 3 and Congo Red Dyes: Pineapple (Ananas comosus) Plant Stem. J. Taiwan Inst. Chem. Eng. 2016, 61, 306–315. [Google Scholar] [CrossRef]
- Chowdhury, A.K.; Sarkar, A.D.; Bandyopadhyay, A. Rice Husk Ash as a Low Cost Adsorbent for the Removal of Methylene Blue and Congo Red in Aqueous Phases. Clean 2009, 37, 581–591. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of Cellulose-Based Wastes for Adsorption of Dyes from Aqueous Solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Guo, W.; Tian, Y. Removal of Malachite Green and Crystal Violet Cationic Dyes from Aqueous Solution Using Activated Sintering Process Red Mud. Appl. Clay Sci. 2014, 93–94, 85–93. [Google Scholar] [CrossRef]
- Nasiri Azad, F.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Goudarzi, A.; Jamshidi, M. Enhanced Simultaneous Removal of Malachite Green and Safranin O by ZnO Nanorod-Loaded Activated Carbon: Modeling, Optimization and Adsorption Isotherms. New J. Chem. 2015, 39, 7998–8005. [Google Scholar] [CrossRef]
- Khan, T.A.; Rahman, R.; Ali, I.; Khan, E.A.; Mukhlif, A.A. Removal of Malachite Green from Aqueous Solution Using Waste Pea Shells as Low-Cost Adsorbent—Adsorption Isotherms and Dynamics. Toxicol. Env. Chem. 2014, 96, 569–578. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Preparation, Characterization and Adsorption Studies of the Chemically Modified Luffa aegyptica Peel as a Potential Adsorbent for the Removal of Malachite Green from Aqueous Solution. J. Mol. Liq. 2019, 274, 315–327. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A.; Gholami, T. Removal of Malachite Green (a Toxic Dye) from Water by Cobalt Ferrite Silica Magnetic Nanocomposite: Herbal and Green Sol-Gel Autocombustion Synthesis. Int. J. Hydrogen Energy 2017, 42, 24846–24860. [Google Scholar] [CrossRef]
- Saif Ur Rehman, M.; Munir, M.; Ashfaq, M.; Rashid, N.; Nazar, M.F.; Danish, M.; Han, J.I. Adsorption of Brilliant Green Dye from Aqueous Solution onto Red Clay. Chem. Eng. J. 2013, 228, 54–62. [Google Scholar] [CrossRef]
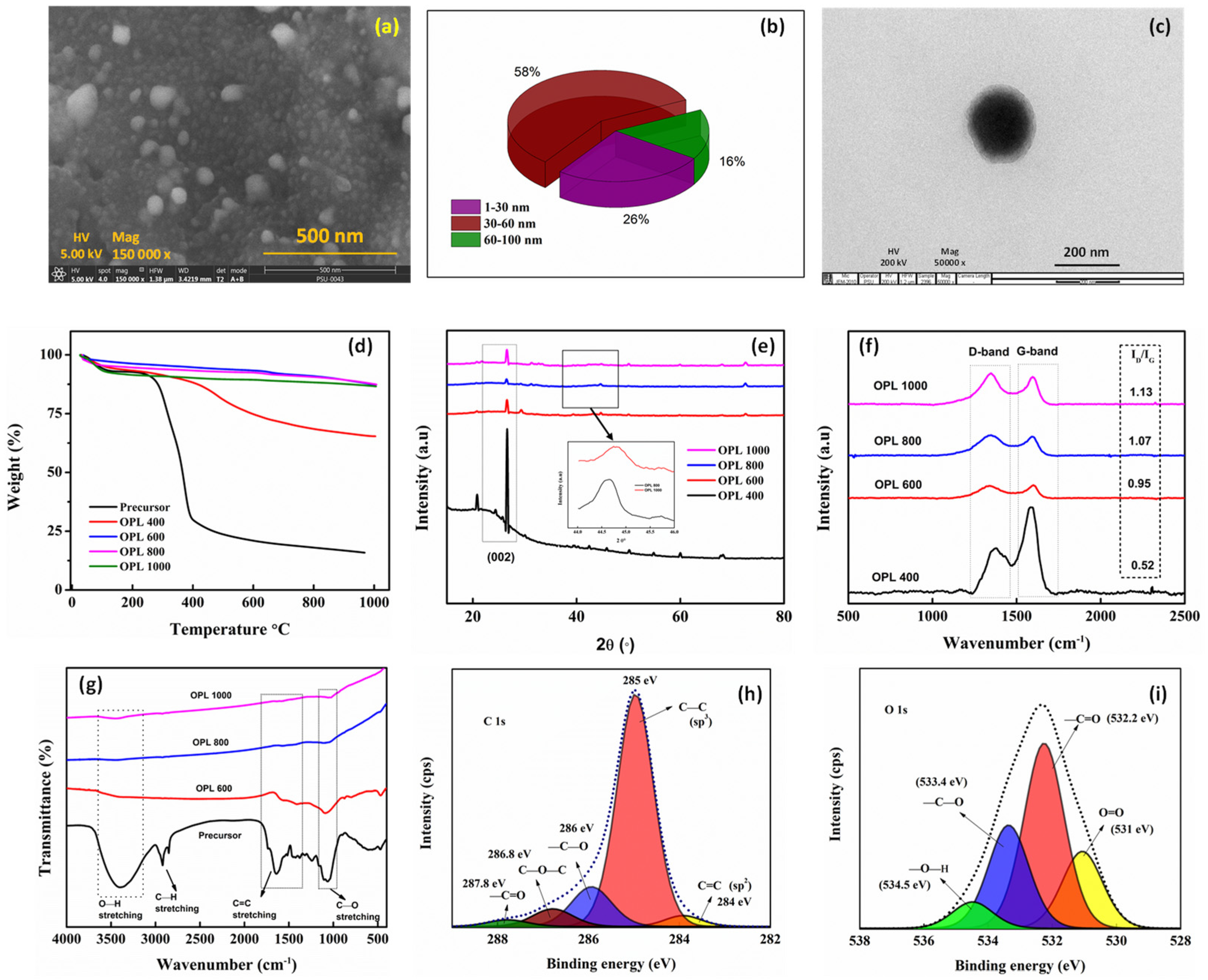






| Material | Elemental Composition (Atomic Percentage) (%) | |||
|---|---|---|---|---|
| Carbon (C) | Oxygen (O) | Silicon (Si) | Calcium (Ca) | |
| OPL precursor | 67.2 | 32.34 | 0.34 | 0.12 |
| OPL 800 | 84.63 | 13.88 | 0.69 | 0.62 |
| OPL 1000 | 87.64 | 12.04 | 0.22 | 0.1 |
| Dyes | Langmuir Isotherm | Freundlich Isotherm | Temkin Isotherm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q0 | b | RL | R2 | KF | 1/n | R2 | KT | B | R2 | |
| (mg/g) | (mg/g) | (L/mg) | (L/g) | (J/mol) | ||||||
| BG | 500.00 | 0.555 | 6.55 | 0.9161 | 117.65 | 0.380 | 0.9597 | 47.643 | 30.05 | 0.8718 |
| MG | 104.16 | 2.133 | 0.05 | 0.9787 | 67.14 | 0.531 | 0.9263 | 4.6519 | 48.67 | 0.9797 |
| CR | 25.77 | 16.869 | 169.69 | 0.9344 | 50.00 | 0.472 | 0.9613 | 0.7388 | 78.91 | 0.9586 |
| Sl. No. | Adsorbent | Contaminant | Adsorption Capacity, mg/g | Reference |
|---|---|---|---|---|
| 1 | Aniline propyl silica xerogel | CR | 22.62 | [59] |
| 2 | Banana peel | CR | 1.727 | [8] |
| 3 | Pineapple plant stem | CR | 11.966 | [60] |
| 4 | Rice husk ash | CR | 7.047 | [61] |
| 5 | Orange peel | CR | 14 | [62] |
| 6 | OPL based CNSs | CR | 25.77 | Present work |
| 7 | Graphene oxide cellulose bead composites | MG | 30.09 | [57] |
| 8 | Super-paramagnetic sodium | MG | 47.84 | [6] |
| 9 | activated sintering process red mud | MG | 60.5 | [63] |
| 10 | ZnO nanorod-loaded activated carbon | MG | 59.17 | [64] |
| 11 | Waste pea shells | MG | 6.20 | [65] |
| 12 | NaOH modified luffa aegyptica peel | MG | 78.79 | [66] |
| 13 | Cobalt ferrite silica magnetic nanocomposite | MG | 75.5 | [67] |
| 14 | OPL based CNSs | MG | 104.16 | Present work |
| 15 | Red clay | BG | 125 | [68] |
| 16 | Modified bambusa tulda | BG | 41.67 | [11] |
| 17 | Magnetite based nanocomposites | BG | 252.17 | [1] |
| 18 | Chemically modified areca nut husk | BG | 18.21 | [2] |
| 19 | Chemically treated Lawsonia inermis seeds powder | BG | 34.96 | [13] |
| 20 | Silver nanoparticles | BG | 27.20 | [25] |
| 21 | Modified pozzolan | BG | 350.6 | [69] |
| 22 | OPL based CNSs | BG | 500 | Present study |
| Dyes | Pseudo First Order | Pseudo Second Order | Elovich Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | qe2 | R2 | α | β | R2 | |
| (min−1) | (mg/g) | (g/mg/min) | (mg/g) | (mg/g/min) | (g/mg) | |||||
| BG | 1298.70 | 0.0927 | 0.8996 | 26.99 | 0.47 | 0.215 | 0.9998 | 9921.04 | 161.29 | 0.9025 |
| MG | 3333.3 | 3.7618 | 0.9315 | 001.10 | 90.90 | 8260 | 0.9979 | 7190.64 | 0.1811 | 0.9091 |
| CR | 6250 | 1.4129 | 0.9924 | 161252.42 | 61.72 | 3419.855 | 0.9979 | 3634.12 | 0.2935 | 0.8559 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnappa, B.; Bhat, V.S.; Ancy, V.; Joshi, J.C.; S, J.M.; Naik, M.; Hegde, G. Biowaste-Derived, Highly Efficient, Reusable Carbon Nanospheres for Speedy Removal of Organic Dyes from Aqueous Solutions. Molecules 2022, 27, 7017. https://doi.org/10.3390/molecules27207017
Krishnappa B, Bhat VS, Ancy V, Joshi JC, S JM, Naik M, Hegde G. Biowaste-Derived, Highly Efficient, Reusable Carbon Nanospheres for Speedy Removal of Organic Dyes from Aqueous Solutions. Molecules. 2022; 27(20):7017. https://doi.org/10.3390/molecules27207017
Chicago/Turabian StyleKrishnappa, Bhavya, Vinay S. Bhat, Vimala Ancy, Jyotsna Clemi Joshi, Jyothi M. S, Maya Naik, and Gurumurthy Hegde. 2022. "Biowaste-Derived, Highly Efficient, Reusable Carbon Nanospheres for Speedy Removal of Organic Dyes from Aqueous Solutions" Molecules 27, no. 20: 7017. https://doi.org/10.3390/molecules27207017
APA StyleKrishnappa, B., Bhat, V. S., Ancy, V., Joshi, J. C., S, J. M., Naik, M., & Hegde, G. (2022). Biowaste-Derived, Highly Efficient, Reusable Carbon Nanospheres for Speedy Removal of Organic Dyes from Aqueous Solutions. Molecules, 27(20), 7017. https://doi.org/10.3390/molecules27207017








