Abstract
Tin oxide as a semiconductor metal oxide has revealed great potential in the field of gas sensing due to its porous structure and reduced size. Especially for tin oxide and its composites, inherent properties such as high surface areas and their unique semiconducting properties with tunable band gaps make them compelling for sensing applications. In combination with the general benefits of metal oxide nanomaterials, the incorporation of metal oxides into metal oxide nanoparticles is a new approach that has dramatically improved the sensing performance of these materials due to the synergistic effects. This review aims to comprehend the sensing mechanisms and the synergistic effects of tin oxide and its composites in achieving high selectivity, high sensitivity and rapid response speed which will be addressed with a full summary. The review further vehemently highlights the advances in tin oxide and its composites in the gas sensing field. Further, the structural components, structural features and surface chemistry involved in the gas sensing are also explained. In addition, this review discusses the SnO2 metal oxide and its composites and unravels the complications in achieving high selectivity, high sensitivity and rapid response speed. The review begins with the gas sensing mechanisms, which are followed by the synthesis methods. Further key results and discussions of previous studies on tin metal oxide and its composites are also discussed. Moreover, achievements in recent research on tin oxide and its composites for sensor applications are then comprehensively compiled. Finally, the challenges and scope for future developments are discussed.
1. Introduction
Metal oxide nanoparticles represent a field of materials chemistry and have attracted considerable attention due to their potential applications in domestic, industrial and commercial fields as sensors due to their many significant features such as easy production, low cost and compact size [1,2]. The potential implications of metal oxides in fields such as energy storage, catalysis, medicine, informational technology and gas sensing have driven much research attention to the development of synthetic pathways towards their nanostructure fabrication. Due to the reduced size and increased surface-to-volume ratio, the nano-sized compounds have shown applications in different fields such as gas sensing [3,4,5,6], catalysis [7,8,9,10,11], lithium-ion batteries [12,13,14] and dye-sensitized solar cells [15,16,17,18]. Among various applications of nanomaterials, gas sensing has attracted much attention from the scientific community due to the increased demand for efficient sensors for defense, environmental applications [19], exhaust gas determination in automobiles [20], leakage determination in chemical plants [21], product quality assurance in food companies [22], etc. One of the most prominent applications of gas sensors is the detection of harmful gases present in our environment and their precise monitoring beyond a certain limit, which has become the most challenging aspect for humankind in this ever-polluted environment. Thus, the preparation of gas sensors is ever-increasingly demanded, particularly for those sensors which can sense dreadful gases even at very small ppm levels that are very toxic not only to humans but also to other organisms. Among the various classes of nanomaterials such as metal sulfides, metal phosphides and metal oxides, the metal oxides as gas sensors are efficient as compared to their counterparts. The metal oxides have fast response, excellent sensitivity and recovery and above all low cost, while the sulfides and phosphides are lacking in these properties. Several advanced nanomaterials are known for nanocatalysis [23,24,25,26], and rich fabrication techniques are known [27,28,29] for their stabilization. The focus in designing the metal oxide nanostructures and their composites is on enhancing their properties of gas sensing such as response speed, selectivity and sensitivity [30].
The performance of tin oxide nanoparticles as sensors is greatly influenced by structure and morphology which otherwise create a great hindrance to achieving highly sensitive properties of gas sensors based on bulk materials. The close intimate link between the chosen synthetic pathway and morphology could allow the preparation of energy-efficient metal oxide nanoparticles whose properties can be exploited in technologically important areas of sensors [31,32,33,34,35]. N-type metal oxides are the most dynamic metal oxides used as gas sensors and are being thoroughly investigated. Among them, SnO2 as an n-type semiconductor has received many considerations in environmental monitoring, catalysis, transparent conducting films, lithium rechargeable batteries, biochemical sensors, ultra-sensitive gas sensors and dye-sensitized solar cells [36,37,38,39,40,41,42]. In addition, SnO2 as an alcohol sensor has useful applications in fields such as the food industry and breath analysis that are very beneficial for human health [43]. Nowadays, much work is being performed for synthesizing different types of nanostructures with a wide range of morphologies such as hollow spheres, core–shell, microspheres, nanospheres, nanotubes and nanowires for the sensing of ethanol, H2 and methanol [44,45,46,47,48]. Among these morphologically different nanostructures, ethanol shows better sensing results as compared to H2 and methanol. For example, Vuong et al. synthesized SnO2 microspheres, nanorods and nanoflowers via a simple hydrothermal route [49]. Likewise, Zhang et al. studied mesoporous tin dioxide nanopowder-based sensors to selectively detect ethanol vapor [50]. Similarly, Sahay et al. prepared monocrystalline SnO2 nanostructures and studied AC transport properties [51].
An important parameter and a distinguishing feature of an efficient metal oxide gas sensor is its sensing property, which includes sensitivity, selectivity and response speed, whose details are largely unexplored and represent our focus in this review. The sensing property is remarkably known for a large number of gas detecting systems in various types of control processes and laboratory analysis [52,53,54]. Additionally, even if a number of reviews have already been written on metal oxides as gas sensors [55,56,57], much still needs to be done with respect to their particle size and morphology in order to enhance their sensing properties, which we will address in this comprehensive review. Further, it has been concluded that a large surface-to-volume ratio of SnO2 and its different composites will ensure enhanced surface area available for the target gas interactions, as a result of which the realization of high sensitivity even at low temperatures can be obtained. As a consequence of this, the materials with high specific surface area and specific surface morphology will manifest the presence of sufficient active sites that can selectively and effectively interact with the target gas, which can consequently improve the sensing performance of a material, including its sensitivity and selectivity [58]. Although many reviews have already been written on the metal oxides as gas sensors, no systematic and comprehensive effort has been made towards their lack of stability and limited selectivity which has resulted in the gas response’s “long-term spoiling”. Hence, in this review, our main focus will be on these two parameters. Even though various types of laboratory analysis and control processes have been employed for gas detecting systems [52,53,54], high-selectivity, rapid-response speed and high-sensitivity gas sensors are still the need of the hour to improve the levels of gas sensing even at very minute levels. Hence, in this review, we will provide a holistic overview of metal oxide nanostructures and composites for gas sensing applications with precise monitoring, keenly observing the changes incorporated by doping, particle size and morphology [59,60,61,62]. Herein, we comprehensively review the literature of a particular metal oxide, i.e., SnO2, and its composites to understand the fundamental gas sensing mechanisms and current progress in the field of gas detection. In addition, we will discuss the necessary structural components, sensing mechanisms and the development of composite architectures for the better development of sensing pathways which will have a better and insightful enhancement in their sensing response. Hence in this review, we will implement a holistic and integrated approach and consider the inroads made in the earlier approaches to metal oxide nanoparticles and the forays to develop and obtain a deep insight into the metal oxide gas sensors with challenges and future directions to unravel the complications in achieving high selectivity, high sensitivity and rapid response speed of metal oxides by various synthetic procedures [62].
2. Gas Sensing Mechanism
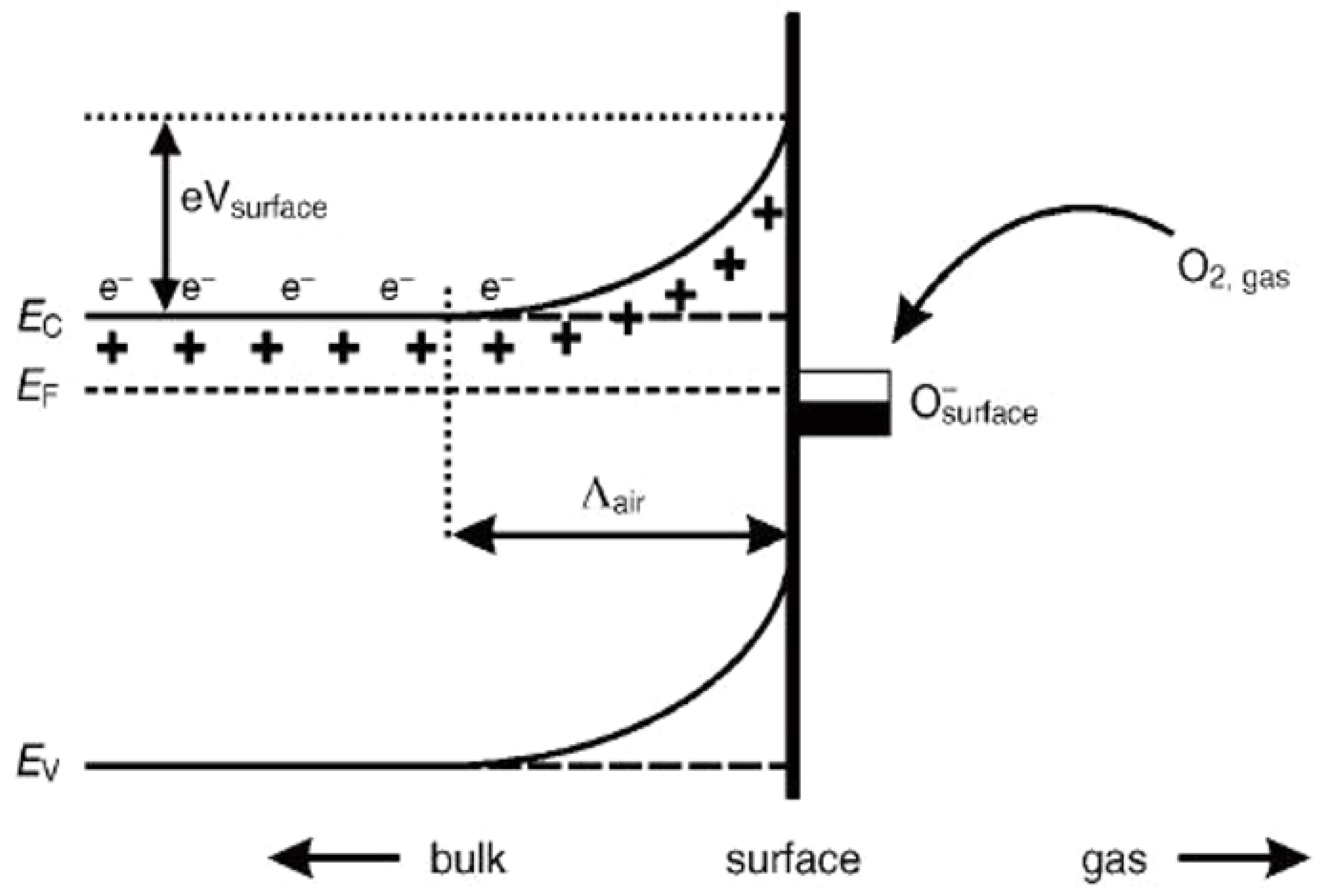
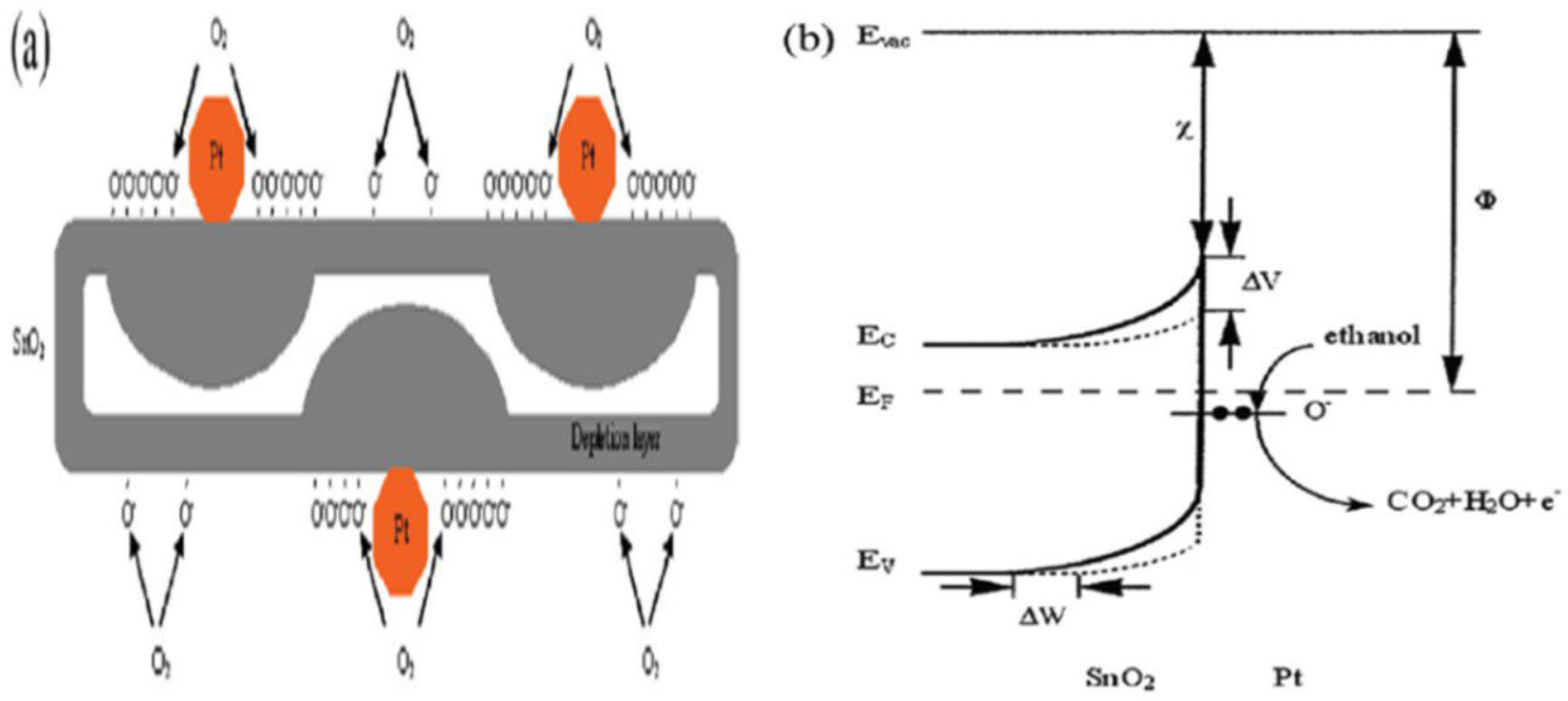
In order to design highly efficient metal oxide gas sensors, it is necessary to understand the underlying sensing mechanism responsible for the sensing property of the materials. Besides understanding the mechanism, it is also important to have an in-depth knowledge of the factors which influence these sensing properties of the materials. Although the causes of gas response and its exact fundamental mechanism are still controversial, essentially the trapping of electrons at adsorbed molecules and the band bending induced due to the effect of charged molecules are the prime factors responsible for a change in conductivity. Here, the sensing mechanism of metal oxides with n-type in air is given with reference to SnO2. Usually, the adsorption of oxygen in air takes place on the surface of the SnO2 sensing film. The oxygen species adsorbed on the surface of SnO2 can pull electrons from the inner surface of the SnO2 film. At an operating temperature of 300–450 °C [63], the O− is believed to be dominant, and this range is considered as its working temperature. As shown in Figure 1 [64], the conduction band (Ec) electrons are extracted by the adsorption of O2 molecules which are present on the metal oxide surface, and these electrons in the form of ions are then trapped at the surface, which leads to the formation of a region which is devoid of electrons, generally called an electron-depleted region, and a band bending in which the electron-deficient region is the so-called space charge layer whose length of thickness is the region of band bending. Consequently, there is a decrease in the reaction between reducing gases and oxygen species that results in the reversal of the band bending, which increases the conductivity of gas sensors for most metal oxides. Figure 1 depicts the mechanism of conduction when depletion regions are smaller than the grain size, suitable only for n-type semiconducting metal oxides.
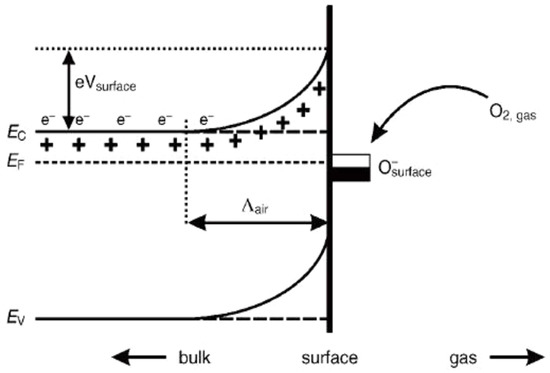
Figure 1.
Simplified model illustrating band bending in a wide band gap semiconductor after chemisorption of charged species (here the ionosorption of oxygen) on surface sites. EC, EV and EF denote the energy of the conduction band, valence band and Fermi level, respectively, while Ʌair denotes the thickness of the space charge layer, and eVsurface denotes the potential barrier. The conducting electrons are represented by e−, and + represents the donor sites. (Reprinted with permission from [64], Copyright 2003, Elsevier.).
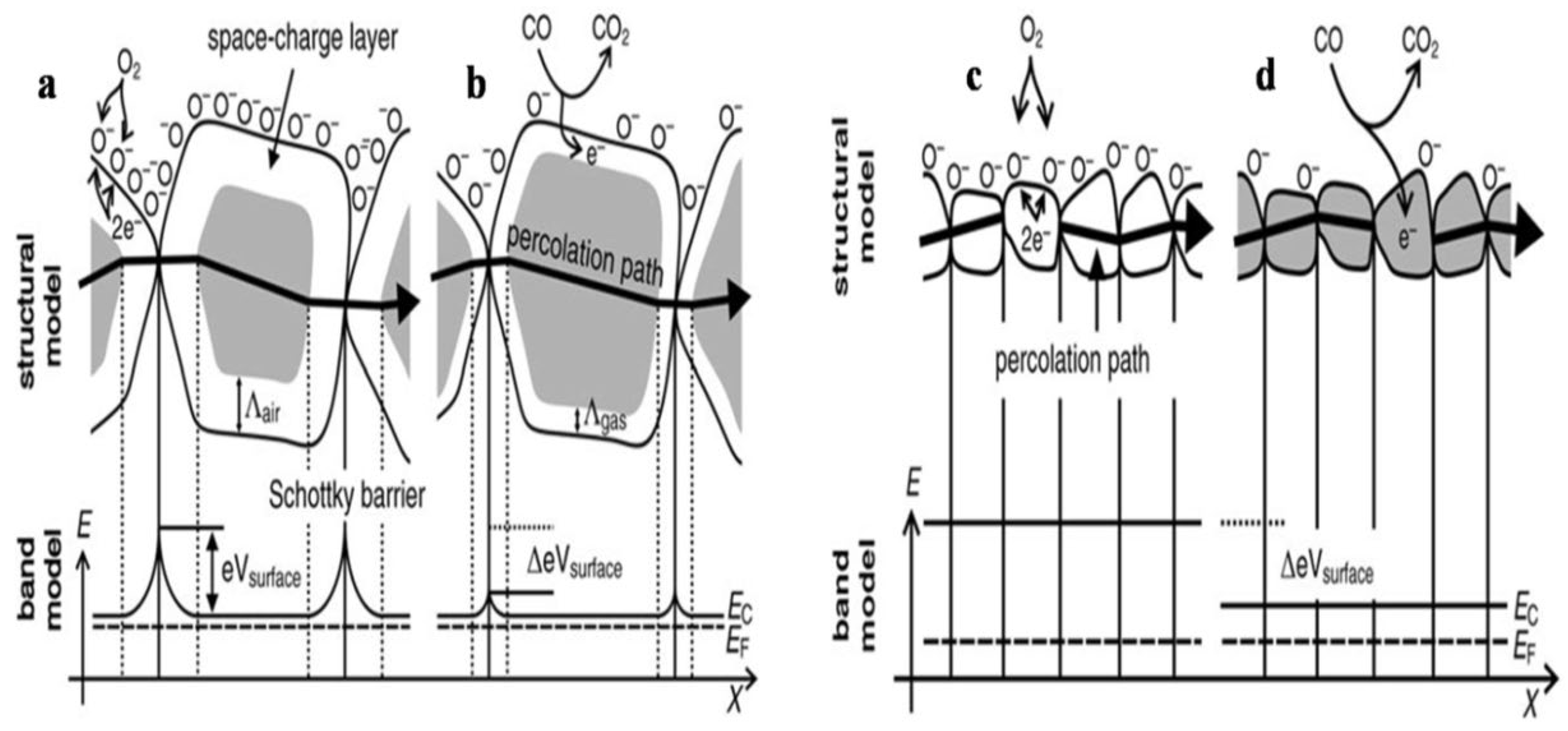
The stoichiometry of the overall metal oxide surface plays a decisive role in the surface conductivity phenomena of gas sensors. The increase in surface conductivity is a result of donors which are oxygen vacancies, and when the electrons adsorb oxygen ions, the surface conductivity diminishes and acts as a surface acceptor. Further when reference gas is exposed with or without CO, the CO is oxidized by O− upon exposure of gas sensors to the reference gas with CO and thus results in the release of electrons to the bulk medium. Moreover, the space charge layer decreases in thickness as a result of a fall in the surface O– number, and because of that, the Schottky barrier between the two grains is lowered, and ultimately the electrons can conduct in sensing layers through different grains. The conductive mechanism based on the structural and band model is illustrated by Figure 2a–d.
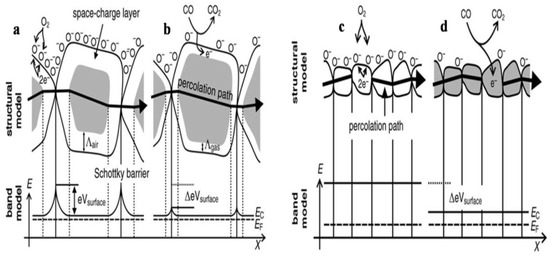
Figure 2.
Structural and band model showing the role of intergranular contact regions in determining the conductance over a polycrystalline metal oxide semiconductor: (a) initial state and (b) effect of CO on Λair and eVsurface for large grains; structural and band model for particles with D <2 Λair leading to the so-called “flat-band” case: (c) the initial state and (d) the effect of CO on the position of the conduction band Ec (Reprinted with permission from [64] Copyright 2003, Elsevier).
3. Synthesis of Tin Oxide and Its Composites
With the advent of nanochemistry, research in the fields of synthesis and characterization of nanoparticles and nanostructures has received considerable attention, and the high-technological applicability of nanoparticles and nanostructures makes them potentially relevant in different fields. Nowadays, nanomaterials are widely used not only for biological and environmental applications but also for sensing and electronic applications. Among the various classes of nanomaterials such as metal sulfides, metal phosphides and metal oxides, the metal oxide nanostructures possess some superior and peculiar characteristics due to their unique chemical and physical properties as a result of which highly efficient nanodevices are fabricated from them and they are surprisingly valuable in gas sensing applications. The metal oxide nanomaterials, being superior, find their use in different fields such as catalysis, medicine, sensing and electronic devices. Due to their large number of applications, researchers have developed various synthetic strategies to produce metal oxide nanostructures with tailored geometries [65,66,67,68] and utilize them for a wide variety of applications. This section of the review will have a simplistic and systematic overview of the different synthetic strategies involved and the effect of these approaches on the reaction conditions and the final product obtained. Synthesis of different nanomaterials such as metal oxides, metal phosphides and metal sulfides, particularly the metal oxides with desired size, shape and crystal morphology, has been the cause of concern ever since the inception of different synthetic methods and has been regarded as very crucial in different fields according to applicability; therefore, many routes or methods have been developed by different researchers in order to obtain the best metal oxides with enhanced sensing properties. It has been observed that physical and chemical conditions and the method of synthesis play a decisive role in determining the shape, crystal structure, size, morphology and other properties of the final product. Thus, on the basis of the present literature survey, various types of chemical methods such as hydrothermal, solvothermal, polymeric citrate precursor, co-precipitation, microemulsion and sol–gel methods and other methods such as ion exchange, photo deposition, electrochemical and other solid state techniques have been employed to synthesize different types of metal oxides for varied purposes. Some of the commonly employed routes for the synthesis of metal oxides are discussed in this review.
4. Hydrothermal Method
The hydrothermal method is one of the most rapidly and commonly growing methods for the synthesis of metal oxide nanoparticles, especially tin oxide nanoparticles. This method of synthesis has received more attention and increased in significance in the last few years because of certain added advantages such as the ease of controlling reaction conditions (e.g., pressure, temperature, pH), and metal oxides of varied morphologies are produced by altering the reaction conditions. Sun et al. [69] used a facile one-step hydrothermal route to synthesize SnO2 nanostructures by calcining precipitates composed of 2D nanosheets with high porosity. The morphological evolution of SnO2 samples with time and the entire possible growth mechanism were ascribed to the self-assembly and nucleation of building blocks. The final morphology, i.e., random porous structures in the nanosheets of the product, was attributed to the concentration of the precursor. The experimental results reveal a possible growth mechanism for the as-prepared SnO2 nanostructures. Moreover, it was observed that the SnO2 nanostructures which were annealed at 600 °C for 2 h showed higher gas sensing properties as compared to the sensor SnO2 nanoparticles prepared conventionally. This change in sensing was ascribed to the unique flower-like structures, as shown in Figure 3a–c, which usually facilitate mass transportation and gas diffusion in sensing materials. Figure 3d shows the pictorial representation process for the formation of SnO2 hierarchical nanosheets.

Figure 3.
(a) SEM image of SnO2 porous 3D architectures after annealing the precipitates at 600 °C for 2 h; (b,c) magnified SEM images; (d) schematic representation of the process of formation of SnO2 hierarchical nanosheets. (Reprinted with permission from [69]. Copyright 2011, the Royal Society of Chemistry, London).
Chiu et al. [70] developed a hydrothermal method for the synthesis of nanocrystalline SnO2 nanoparticles with an average grain size of 3.0 ± 0.5 nm. As-synthesized SnO2 particles were annealed at 300 °C for 1 h under 10% H2/Ar. An atomic ratio of 2.3/1 (O/Sn) was found for the SnO2 nanoparticles prepared thermally, and an atomic ratio of 1.2/1 was found for the as-synthesized SnO2 nanoparticles. For the thermally treated SnO2, a surface area of about 92 m2/g was observed as compared to the 130 m2/g for the SnO2 nanoparticles synthesized at 150 °C with rough undefined morphology. In the as-synthesized SnO2, the oxygen ions are replaced by the chloride ions; the thermally treated SnO2 acts as a better sensor for ethanol compared to the as-synthesized SnO2. For the as-synthesized SnO2 sensor, the sensitivity of SnO2 nanoparticles can be enhanced by heating simply for 5 min at 350 °C to remove Cl− partly. Consequently, efficient gas sensing in response to ethanol was revealed for the SnO2 gas sensor with a detection limit of as low as 1.7 ppm. Figure 4 shows the comparative response of SnO2 nanoparticles to ethanol before annealing and after annealing.
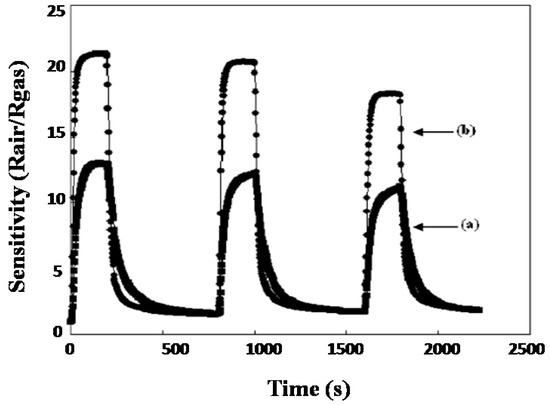
Figure 4.
Repetitive response of the (a) as-synthesized and (b) thermally treated SnO2 to ethanol (25 ppm) at 220 °C. (Reprinted with permission from [70]. Copyright 2007, American Chemical Society, Washington, DC, USA).
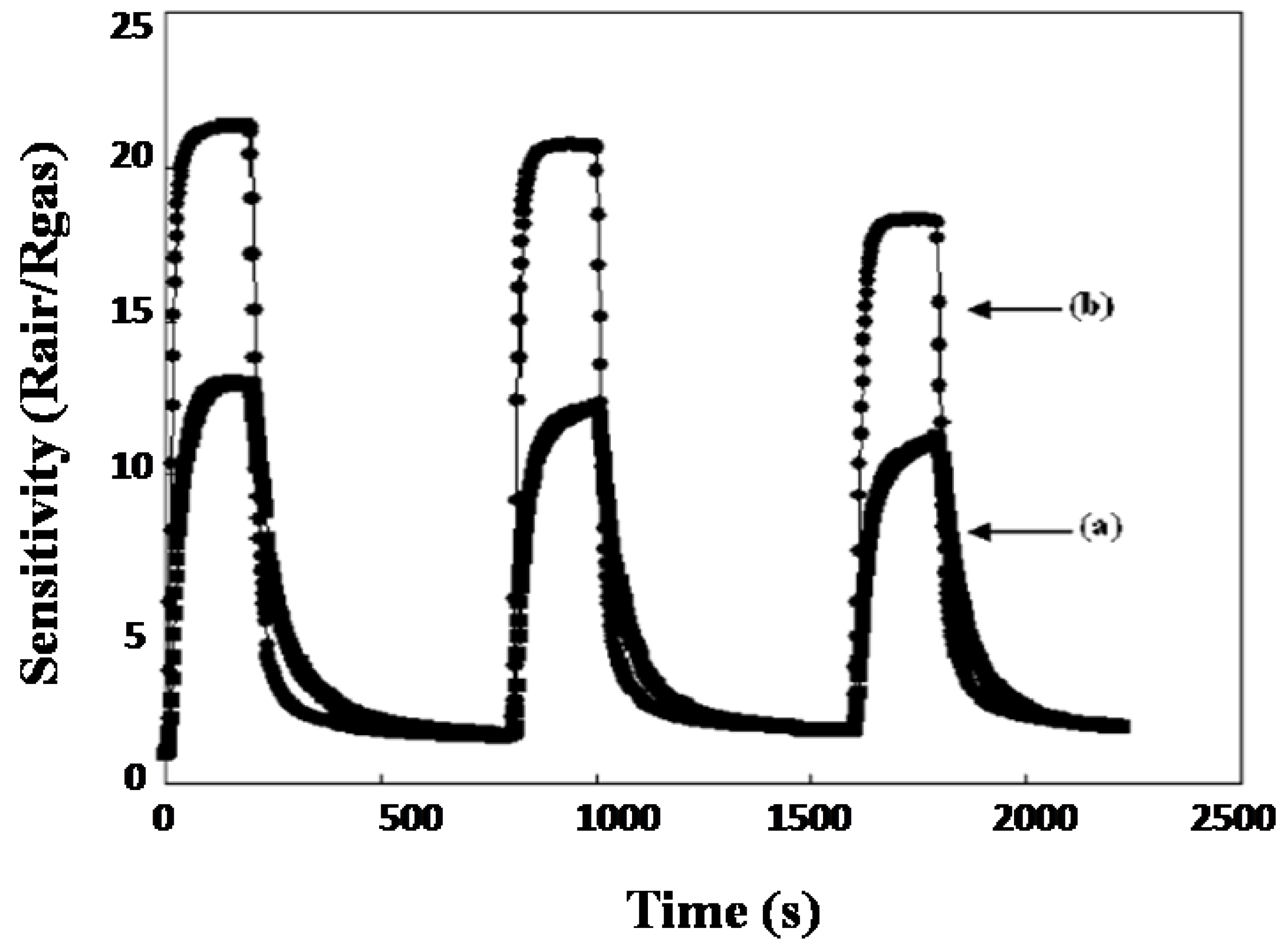
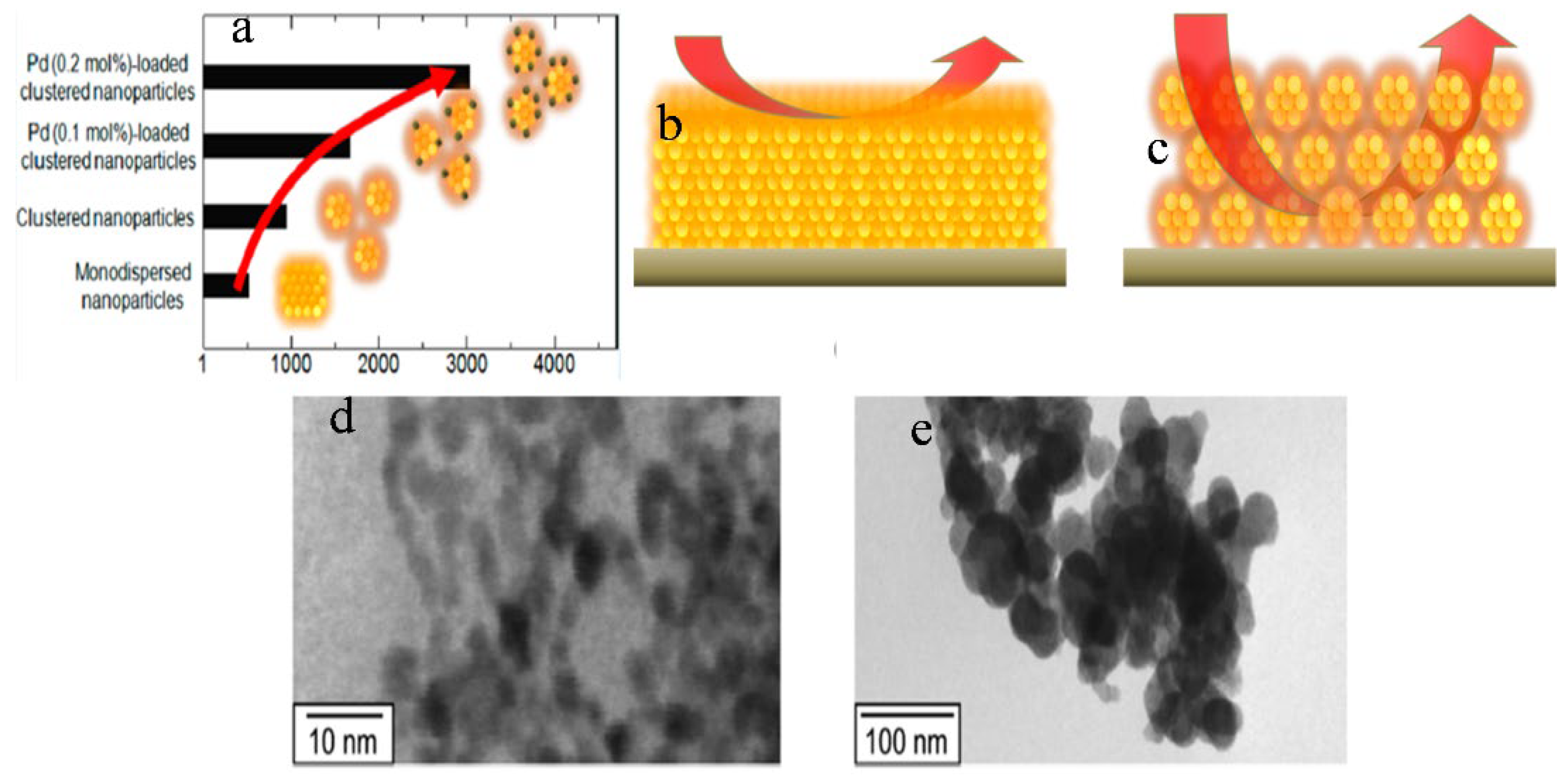
Suematsu et al. [71] used a hydrothermal route to synthesize SnO2 clustered nanoparticles from SnO2.nH2O where preformed nanocrystals (ca. 5 nm) agglomerated at pH 9.3 to form large secondary nanocrystals (ca. 45 nm). Adding Pd-[(NH3)2(NO2)2] to the precursor solution resulted in the formation of Pd-loaded clusters of SnO2 nanoparticles. Toluene was sensed by the application of highly fabricated films of clustered SnO2 nanoparticles with spherical morphology. The spin coating method was used to develop highly porous gas sensing films by loading Pd with the clustered SnO2 particles. An improved response of sensors at 300 °C to H2 and CO was observed for the sensor devices using the porous films. The sensor response was increased by increasing the film porosity, which enhanced the diffusivity through the sensing films. Note that the sensor response was further increased by loading Pd onto the clustered nanospheres as a result of electrical and catalytic sensitization effects. More importantly, the Pd-loaded SnO2 nanoparticles showed increased toluene sensitivity due to an increase in gas diffusivity through the sensing film which helped in its detection at very low ppb levels, as shown in Figure 5a–c. From TEM results, it was concluded that the agglomeration of nanoparticles was a result of a decrease in the pH of the solution forming larger secondary nanospheres, as shown in Figure 5d,e. Tan et al. [38] demonstrated a simple hydrothermal method to synthesize SnO2 hollow sphere nanoparticles using carbon microspheres as templates with the decomposition of SnCl4. The obtained nanospheres with hollow spherical morphology have a size of 100 nm, and a significant number of SnO2 nanospheres have a diameter of nearly 6 nm. The obtained sensitivity of SnO2-based hollow nanospheres was as high as 75 to 1000 ppm for ethanol, and the response and recovery times were 4 and 10 s, respectively as shown in Figure 6. The increase in sensing property can be ascribed to the porous structure of hollow SnO2 nanospheres and the small size of nanospheres. It was revealed that SnO2 nanospheres can act as promising materials with fast response and recovery for the fabrication of gas sensors.
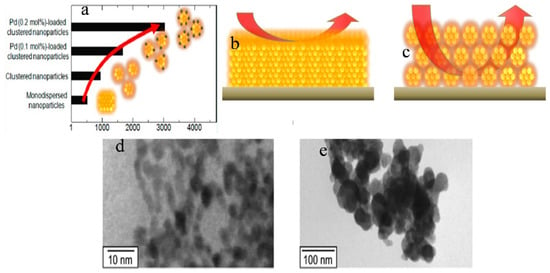
Figure 5.
(a) Sensor response of different nanoparticles to toluene; (b,c) represent the gas diffusion in films made with monodispersed nanoparticles and clustered nanoparticles, respectively; (d) and (e) are the TEM images of SnO2 nanoparticles at pH 10.6 and pH 9.3, respectively. (Reprinted with permission from [71]. Copyright 2014, American Chemical Society, Washington, DC, USA).
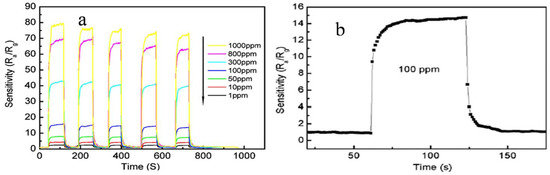
Figure 6.
(a) Schematic representation of the different sensing characteristics of SnO2 hollow spheres upon exposure to different ethanol concentrations ranging from 1 to 1000 ppm; (b) sensor response and recovery times of different SnO2 hollow spheres to 100 ppm of ethanol for one cycle (Reprinted with permission from [38]. Copyright 2008, Elsevier).
Shi et al. [72] prepared SnO2 nanotubes on a hard template of polycarbonate by employing a hydrothermal route at low temperatures. The obtained nanostructures with nanotube morphology were found to be a few nanometers in size with fine particles, as shown in Figure 7a,b. It was observed that as the reaction temperature increased gradually, the size of the SnO2 nanostructures also showed an increase. The SnO2 nanocrystals showed that the band gap of these nanostructures increased from 3.75 eV with a particle size of 5.6 nm to 3.99 eV with a particle size of 3.3 nm. The results showed that the SnO2 nanotubes can have potential applications for gas sensors with enhanced gas sensitivity.
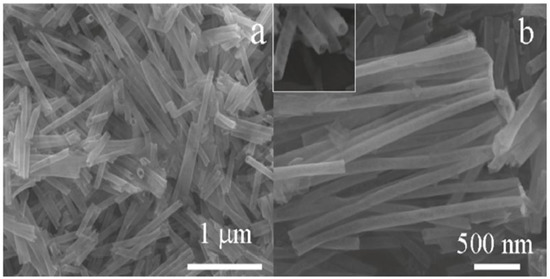
Figure 7.
SEM images of the as-prepared SnO2 nanotubes: (a) overview and (b) magnified image showing the open ends of nanotubes. (Reprinted with permission from [72]. Copyright 2011, American Chemical Society, Washington, DC, USA).
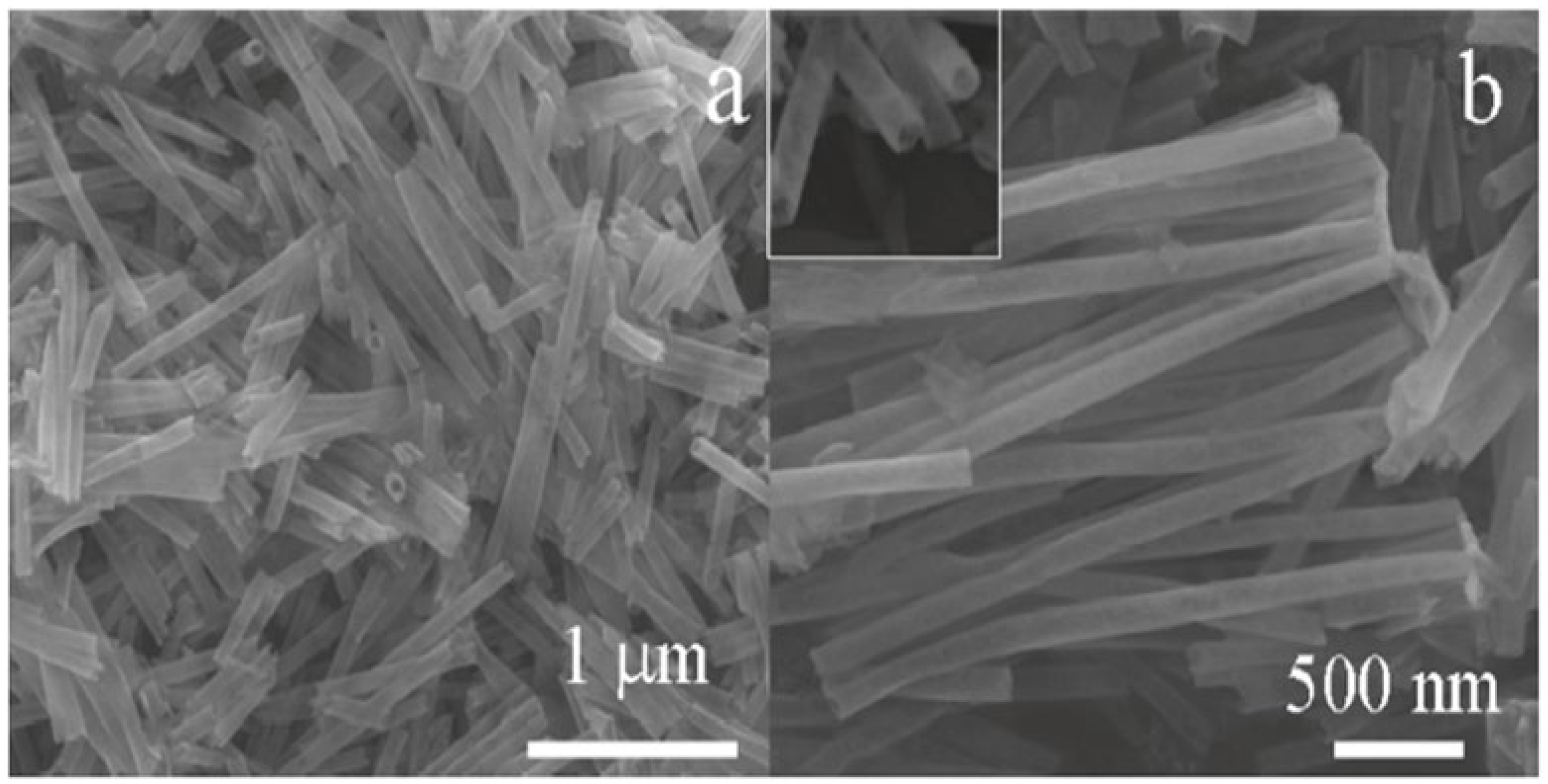
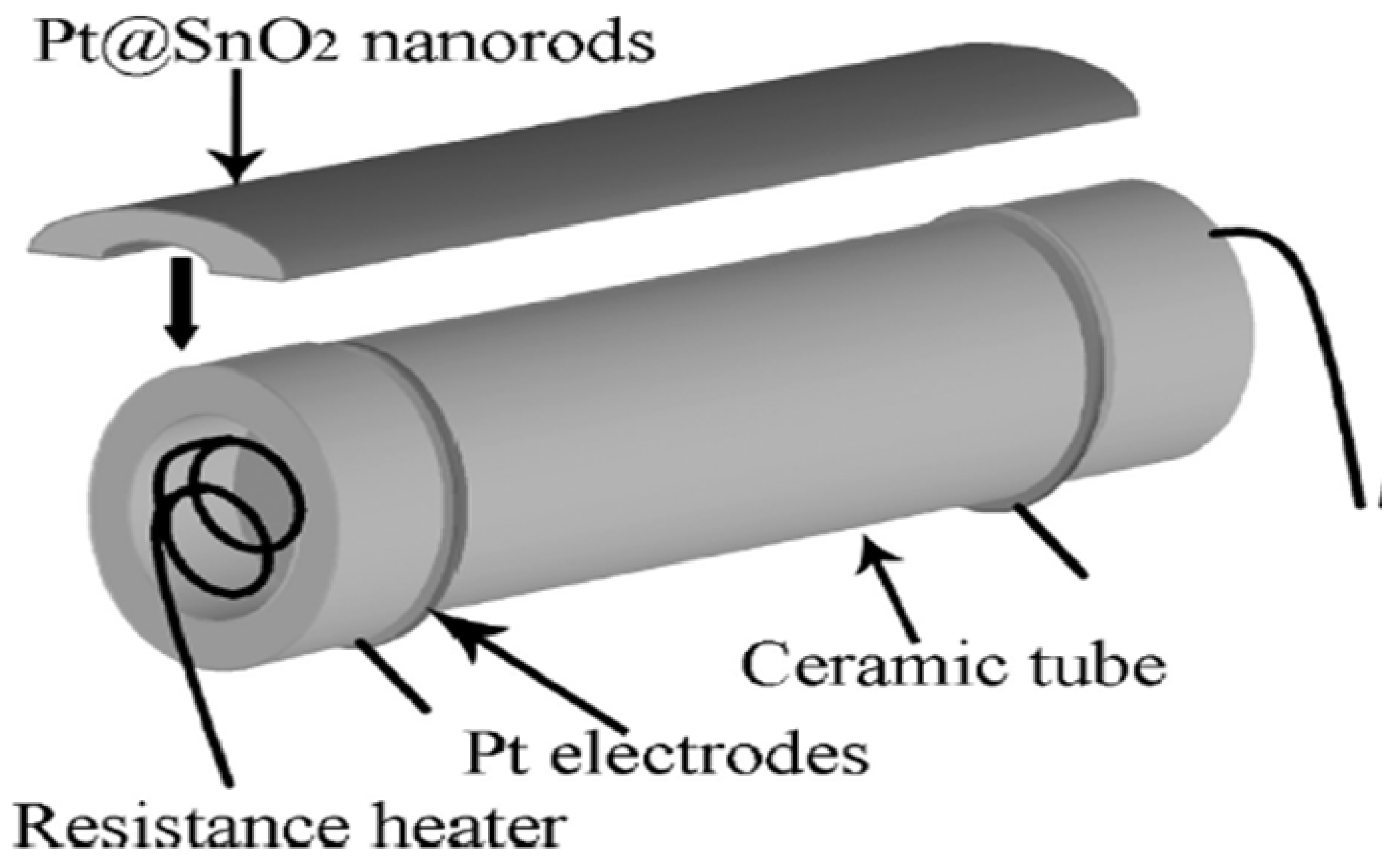
Xue et al. [73] employed a simple one-step hydrothermal route and successfully synthesized a highly sensitive Pt@SnO2 nanorod-based gas sensor, as represented pictorially in Figure 8. The morphology of synthesized nanoparticles obtained were nanorods with microstructures. At a high temperature of about 300 °C, upon exposure to 200 ppm of ethanol, the sensor sensitivity reached 39.5. This can be attributed to the influence of electrical and chemical contributions of Pt, because of which the sensor displays high gas sensitivity. Further, when the sensor is heated to 200 °C, opposite variations of resistances are observed, which may be attributed to the surface oxygen ions having different temperature-dependence. The results reveal that the synthesized nanostructures are potential sensors with high performance capacity.
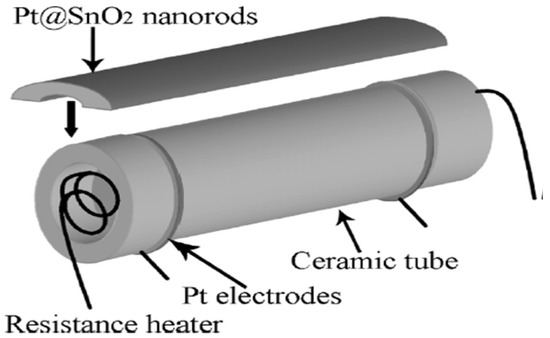
Figure 8.
Schematic diagram showing Pt@SnO2 nanorod-based gas sensor. (Reprinted with permission from [73]. Copyright 2010, American Chemical Society, Washington, DC, USA).
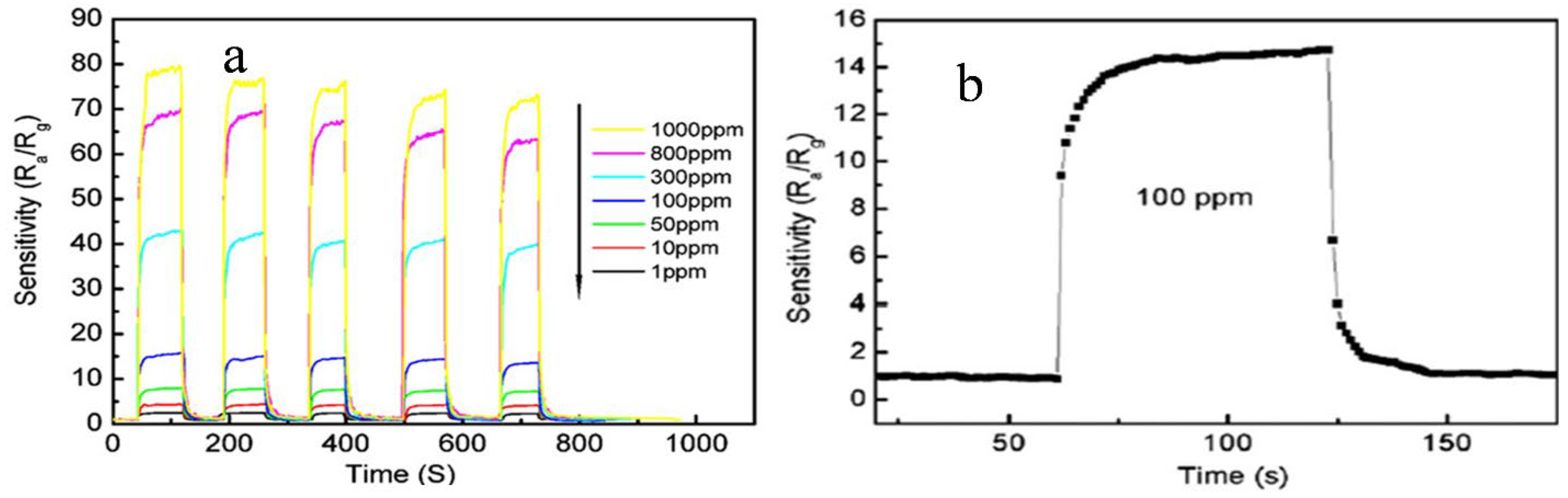
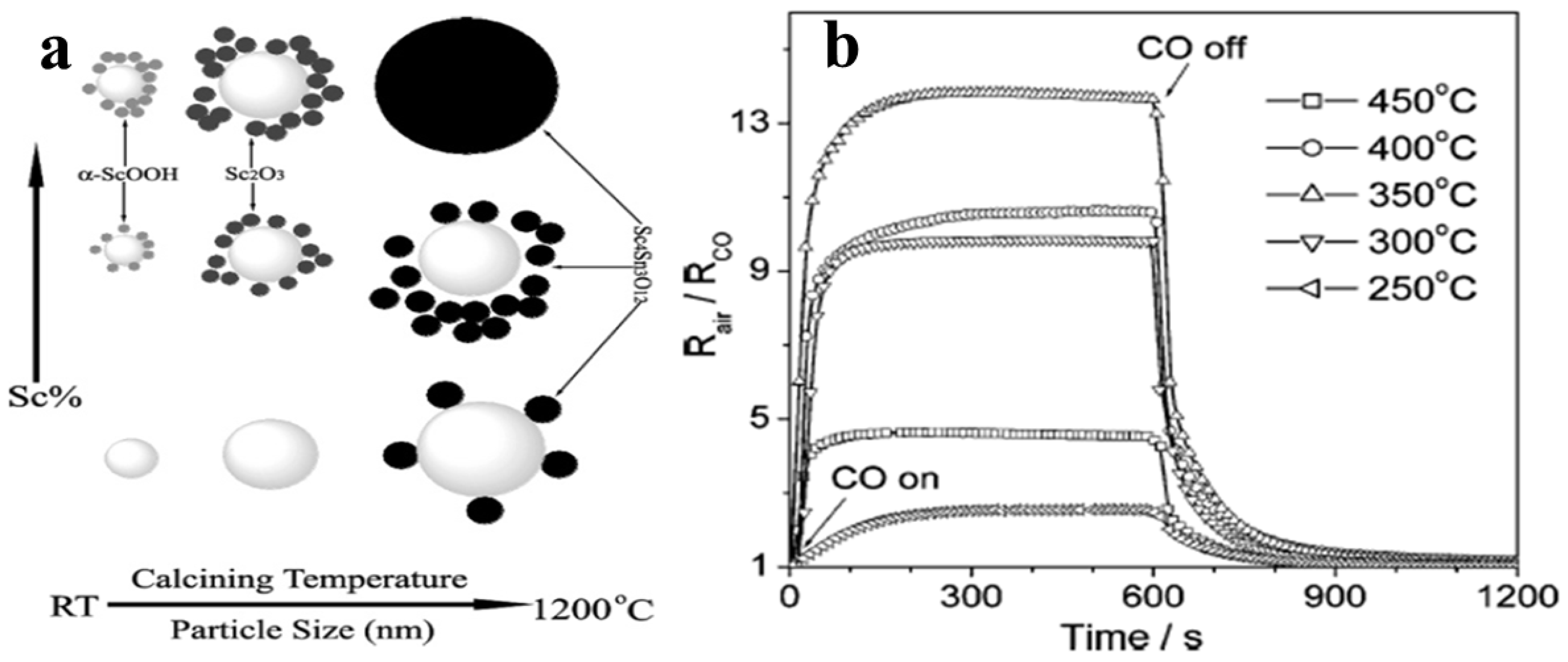
Xu et al. [74] for the first time synthesized Scandia-doped tin oxide powders in the presence of urea by a two-step hydrothermal method (produces weakly agglomerated nanocrystallites) followed by calcination between 500 and 1200 °C. When Sc2O3 is added as a dopant in appropriate amounts in nanosized SnO2, as was revealed by the textural studies, it can retard grain growth and stabilize the surface area to withstand high calcination temperatures below 1000 °C with discrete undefined morphology. The sensing measurements of CO gas reveal that sensitivity of SnO2 can improve significantly when Sc is incorporated at the surface of the nanocrystal, and at 800 °C, a pellet sample with 10 mol% of Scandia content displays enhanced sensing properties in response to CO in the operating temperature range of 300–400 °C. Figure 9a,b shows the variation of phases with Sc percentage and calcining temperature and the sensitivity of as-synthesized Sc doped SnO2 at different operating temperatures, respectively.
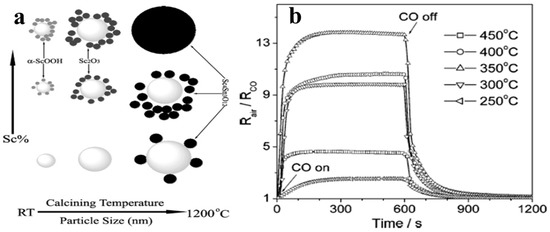
Figure 9.
(a) Phasic dependence of the (SnO2)1−X (Sc2O3)X (X = 0.002–0.6) nanocrystallites upon the calcination temperature/particle size; (b) effect of operation temperature on the sensitivity of CO in 1000 ppm of the as-sintered (SnO2)0.90 (Sc2O3)0.10 pellet. (Reprinted with permission from [74]. Copyright 2005, American Chemical Society, Washington, DC, USA).
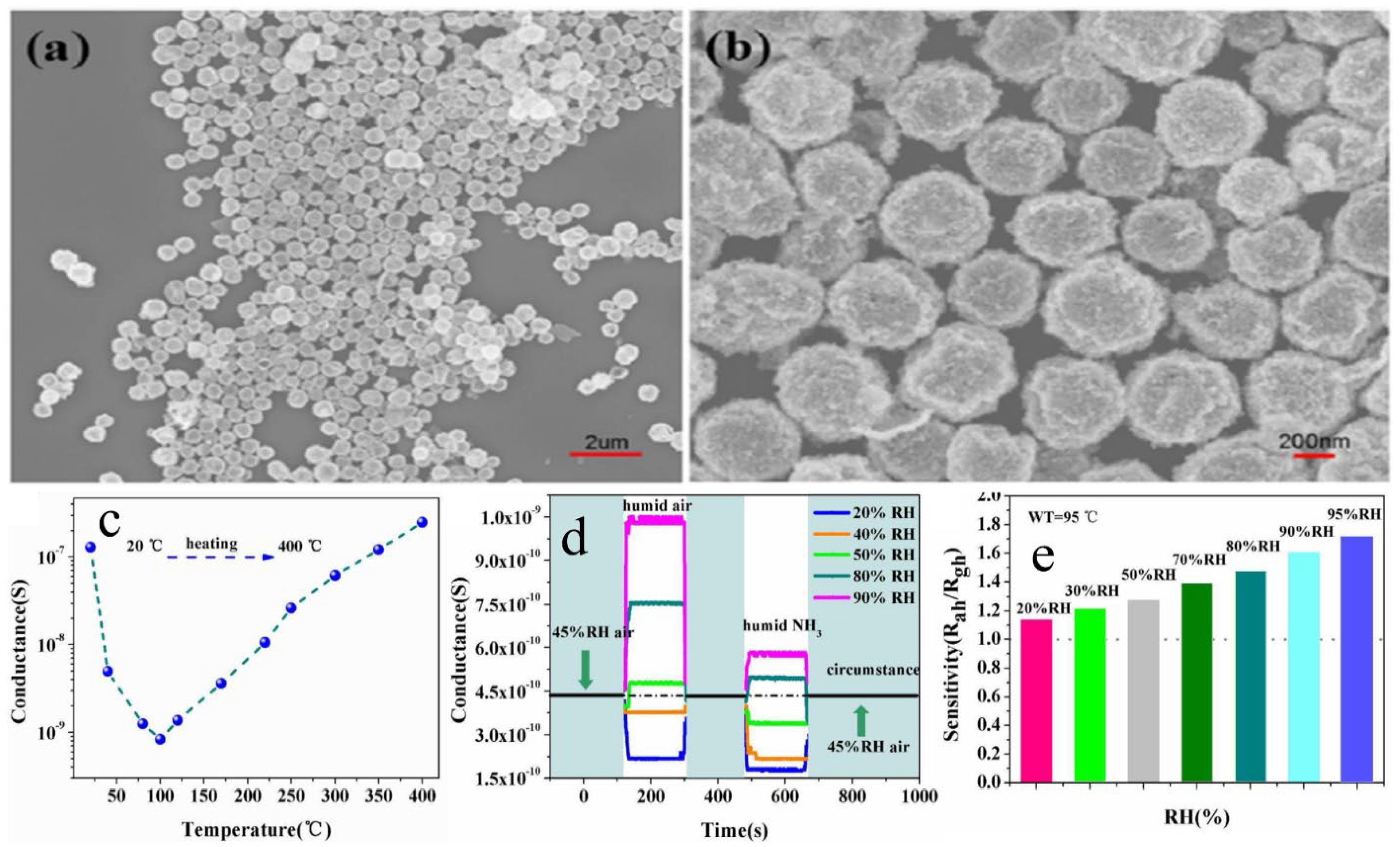
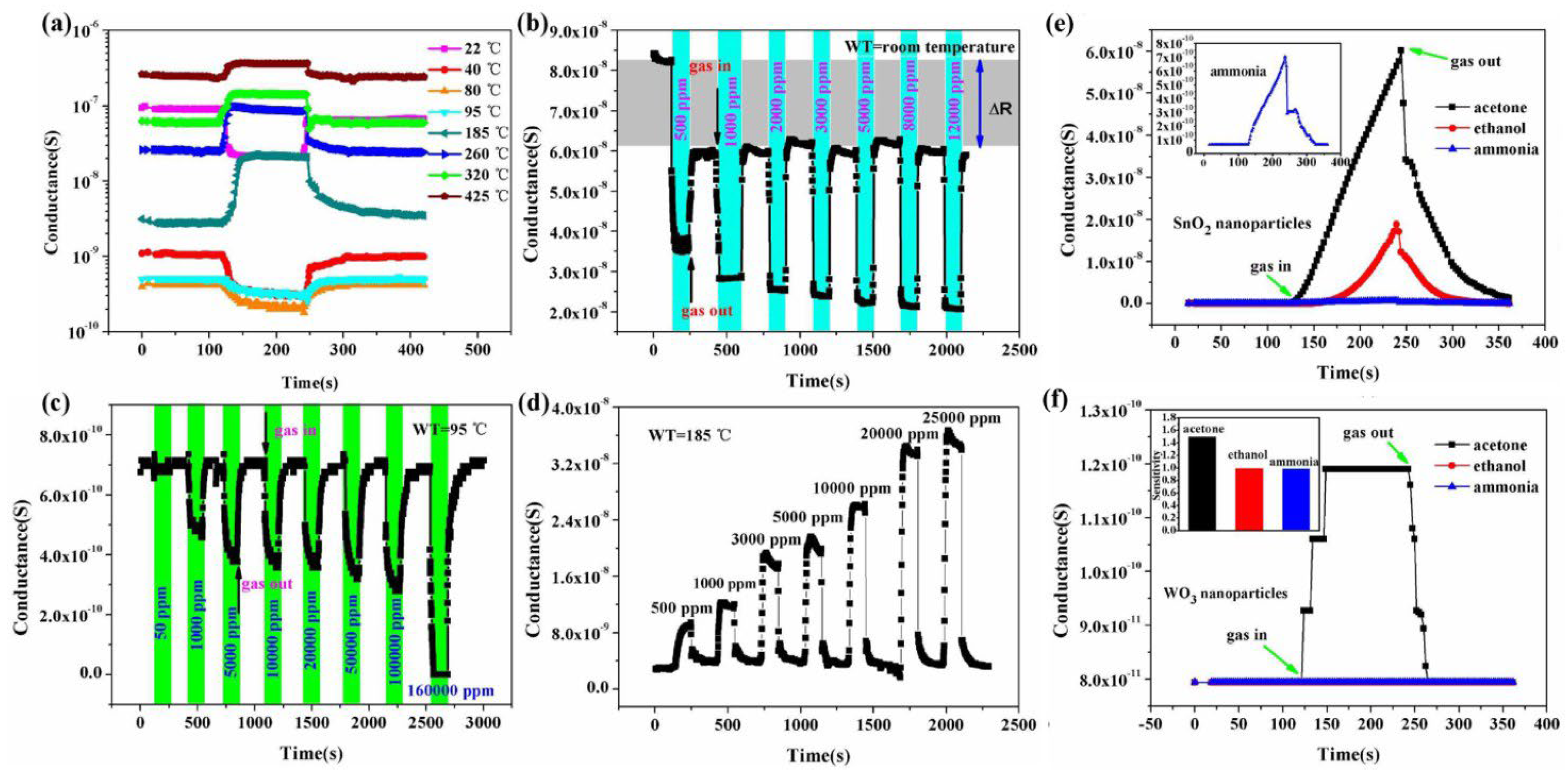
Li et al. [75] synthesized WO3 and SnO2 hollow spheres by a simple hydrothermal method followed by calcination. For synthesis, 0.5 mmol of Na2SnO3, 5 g of glucose and 1 mmol of Na2WO4 were mixed in 50 mL of distilled water. After this, the solution was then transferred into a stainless Teflon-lined autoclave and stirred for several minutes, sealed and heated at a temperature of 200 °C for 20 h. In this approach, extrinsic sensing behaviors with WO3 and SnO2 hollow-sphere-based gas sensors were observed. These responses obtained were treated as pseudo-sensing responses, and these results were considered as the interactions between adsorbed water and target gas. With the increase in temperature, the response of the pseudo-p-type sensor to ethanol can be converted into normal n-type as a result of transitions of sensing mechanisms. The WO3-SnO2 composites showed an enhanced sensing property which accounts for the extrinsic sensing behaviors. The results reveal that humidity has a counterproductive effect on gas sensing, and they open up a new promising way to produce gas sensors with reduced operation temperature or synthesize gas sensors at room temperature with little power consumption. SEM results reveal that the morphology of the synthesized samples was hollow spherical, roughly uniform with good monodispersity as shown in Figure 10a,b, whereas Figure 10c–e reveal the temperature dependence, effect of humidity on ammonia sensing and improvement in gas sensitivity to 5000 ppm ethanol of the as-developed SnO2/WO3 based sensor. Similarly, Figure 11 shows the mechanism of gas sensing over the surface of SnO2/WO3 at different operating temperatures.
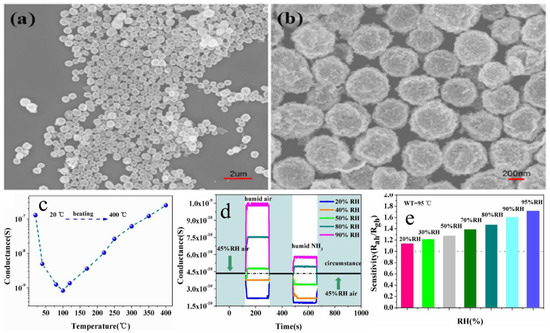
Figure 10.
(a,b) SEM of the WO3−SnO2 hollow nanospheres. (c) Temperature−dependent conductance of the gas sensor. (d) Effect of humidity on the NH3 sensing at 95 °C. (e) Improvement in gas sensitivity to 5000 ppm ethanol by increasing surrounding humidity from 20 to 95% RH. (Reprinted with permission from [75]. Copyright 2015, American Chemical Society, Washington, DC, USA).
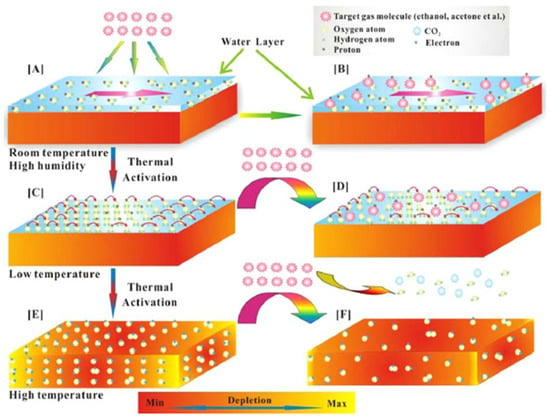
Figure 11.
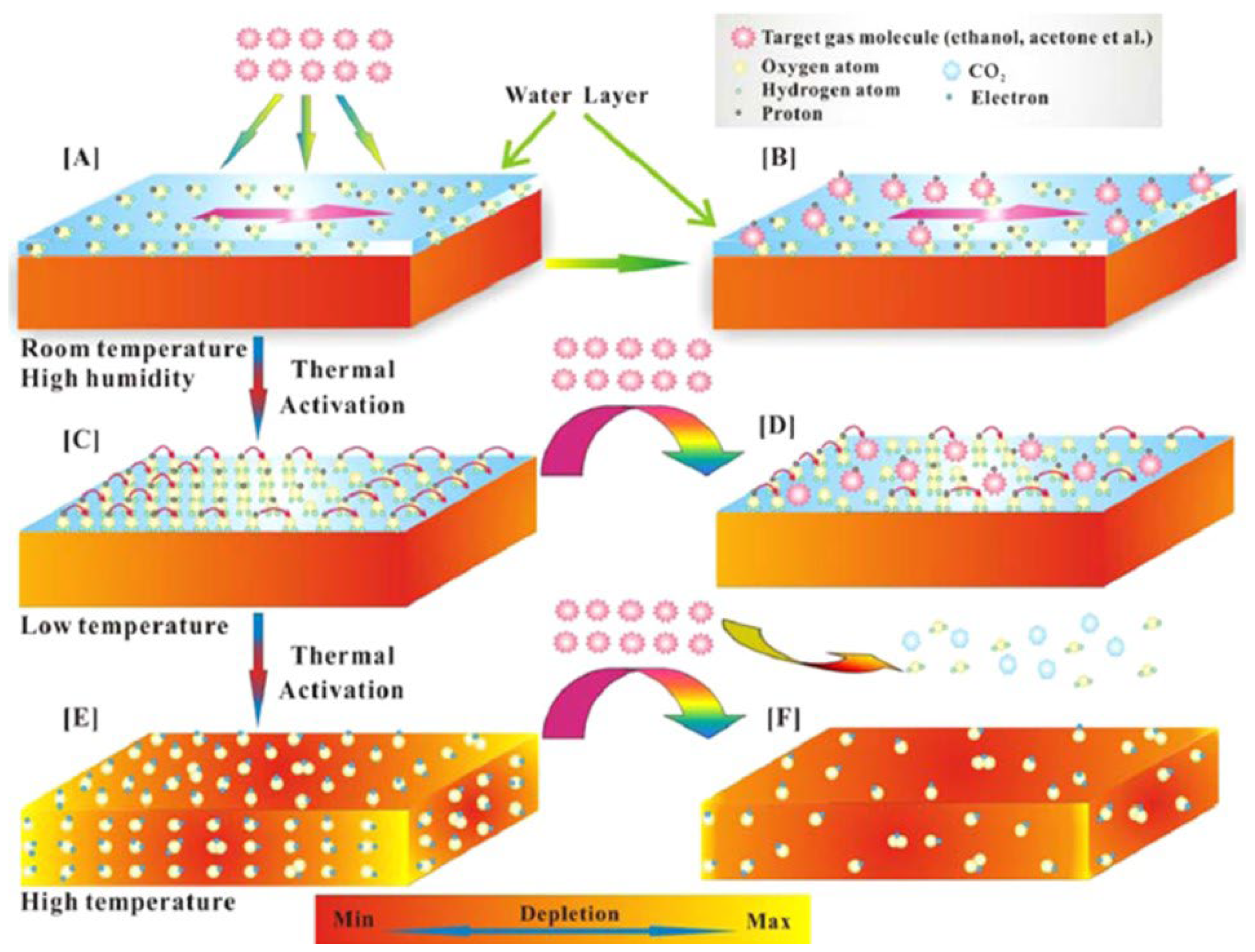
Schematic diagram showing the sensing mechanism of sensors at different working conditions: (A,B) high temperature and high humidity, (C,D) low temperature (a slight thermal shock to the sensor) and (E,F) high temperature. (Reprinted with permission from [75]. Copyright 2015, American Chemical Society, Washington, DC, USA).
5. Polymeric Citrate Precursor Method
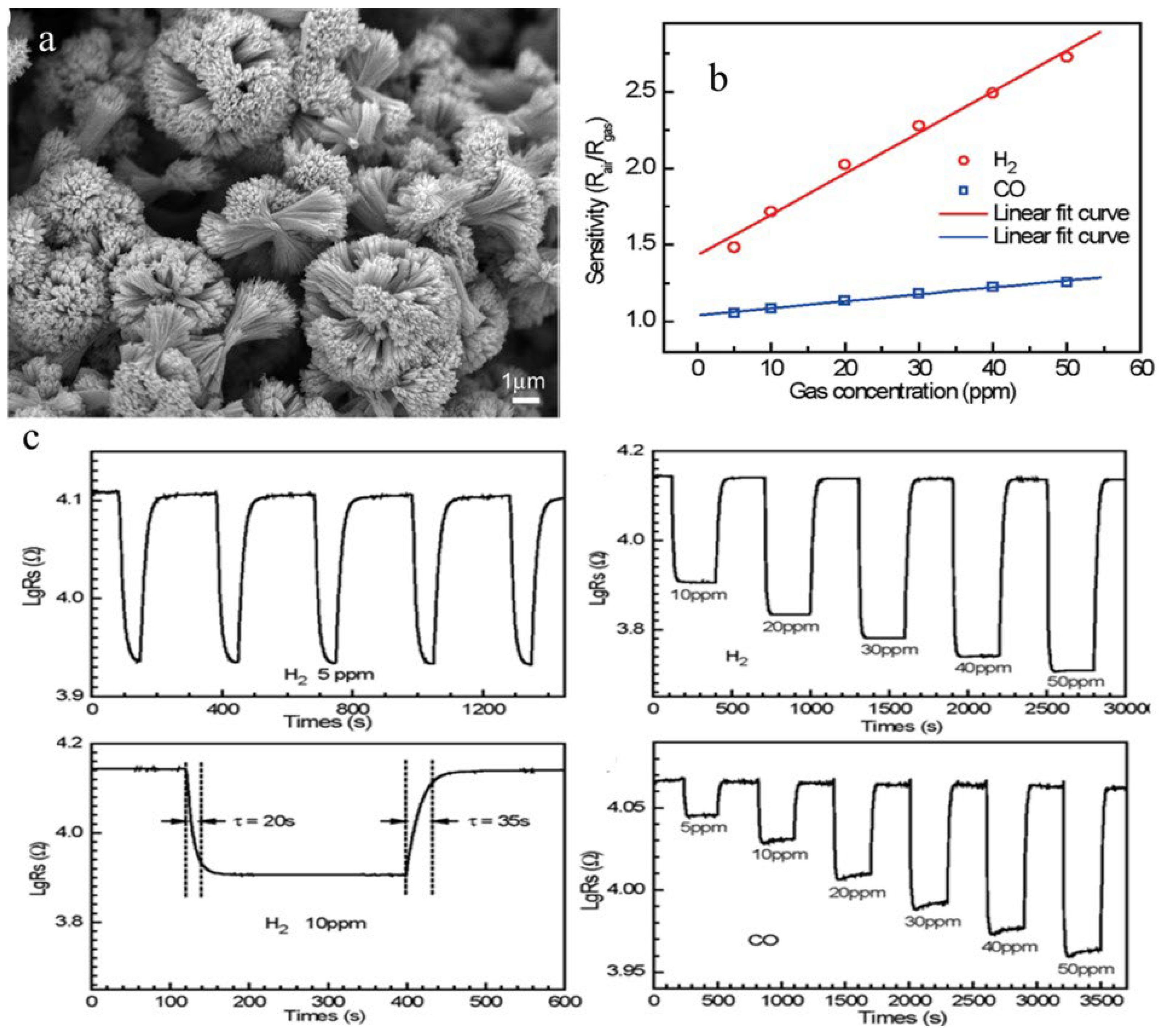
Another method used to synthesize metal oxides is a low-temperature polymeric citrate precursor (PCP) method. In this method, a multifunctional organic acid (e.g., malic acid, citric acid) chelates with the metal ion and results in the formation of a stable metal complex along with a diol such as ethylene glycol which is called gel. Random distribution of cations starts occurring in the starting solution as a result of gel formation. When this solution is heated to a high temperature, the organic moieties are removed from the gel component, which results in the formation of highly crystalline, very fine, homogeneous oxide powders at lesser temperatures as compared to other solid-state techniques. Jiang et al. [76] employed a simple PCP route for the synthesis of SnO2 microstructures followed by a suitable thermal treatment through a surfactant-assisted and solvent-induced assembly technique. In this method, 1.46 g of H2C2O4 was dispersed into a mixed solution containing 40 mL of polyethylene glycol (PEG-600) and 120 mL of ethanol under constant stirring. Further, 1.78 g of SnCl2·2H2O was added to the solution after H2C2O4 was completely dissolved, followed by dropwise addition of 16 mL of deionized water. After centrifugation and stirring, the obtained product was washed with distilled water and ethanol several times. The products obtained were annealed at 200 °C for 2 h to transform them into flower-like SnO2 nano/microstructure morphology. Gas sensing applications of as-obtained SnO2 microstructures towards CO and H2 reveal excellent sensing properties with an extremely low detecting limit (5 ppm) and notable sensitivity with short response/recovery times and good reproducibility, as shown in Figure 12b,c, which is ascribed to the unique flower-like structure as shown in Figure 12a with three-dimensional geometry of SnO2 nanostructures and NPs which is considered as an important constituent for improved gas sensing performance.
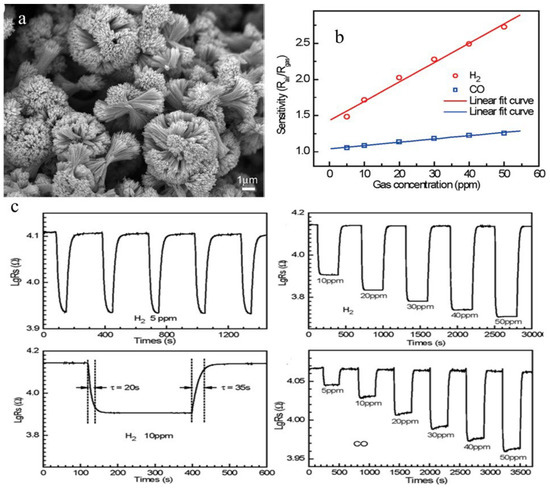
Figure 12.
(a) SEM image of SnO2 microstructure. (b) Linear dependence relation between resistance response sensitivity and gas concentration. (c) Exposure of SnO2 microstructure to H2 for gas sensing depicts the change in concentration of H2 and its effect on sensor response, the response and recovery times for H2 at 10 ppm, and the variation of sensor response with change in concentration of CO. (Reprinted with permission from [76]. Copyright 2009, American Chemical Society, Washington, DC, USA).
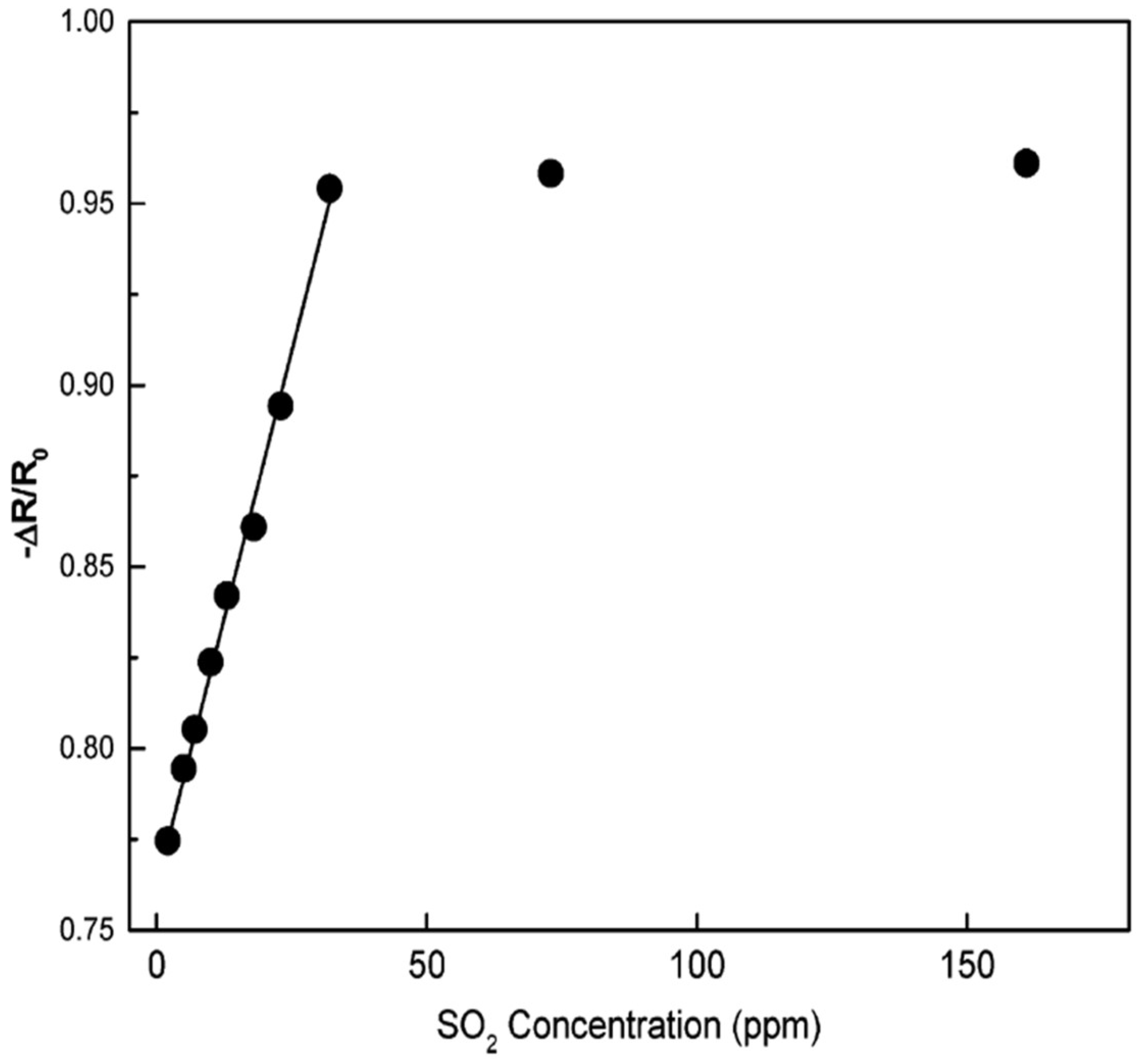
Hidalgo et al. [77] demonstrated a simple polymeric precursor method for the synthesis of SnO2-NiO nanopowders with different compositions. In this method, cationic precursors were added to a citric acid and ethylene glycol solution. Sn2-(C6O7H4).H2O (tin citrate was prepared by SnCl2.H2O) and Fe(NO3)3.9H2O were used as the precursors, and HNO3 was added to this system to obtain the desired solubilization of citrate ions in the whole system. To obtain the desired molar concentrations, the appropriate amounts of precursors were calculated. After this, the solution was heated at 180–200 °C to promote the polyesterification between ethylene glycol and citric acid resulting in the formation of a polymer chain with sites available to react with the present ions. Then the liquid precursor was heated at 450 °C for 4 h, and a powder rich in carbon was obtained, further ground and then again heat treated at 500 °C for 5 h to guarantee total carbon elimination from the compound with nearly spherical morphology. In the SnO2-NiO system, the separation of Ni is used to obtain a rapid sensor response to SO2. Compared to pure SnO2, response to SO2 is enhanced in sensitivity and speed with reliable operation at room temperature for SnO2 films containing 1 mol% of Ni. The sensor measurements and drift results showed a completely reversible reaction at this composition with an enhanced electrical response at this temperature. The calibration plots show that the sensor is applicable for detecting SO2 with concentrations as low as 25 ppm, as shown in Figure 13.
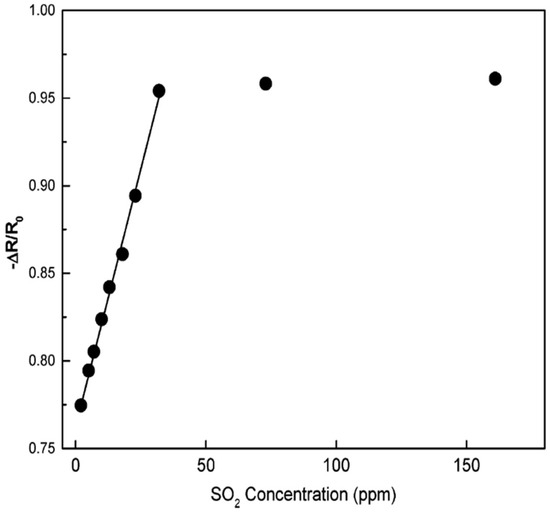
Figure 13.
Calibration curve of SnO2-1 mol% Ni sensor for relating SO2 content to the electrical response. SO2 was diluted with dry N2 to achieve the desired concentrations. The electrical responses were measured after 4.5 min of injection. (Reprinted with permission from [77]. Copyright 2005, American Chemical Society, Washington, DC, USA).
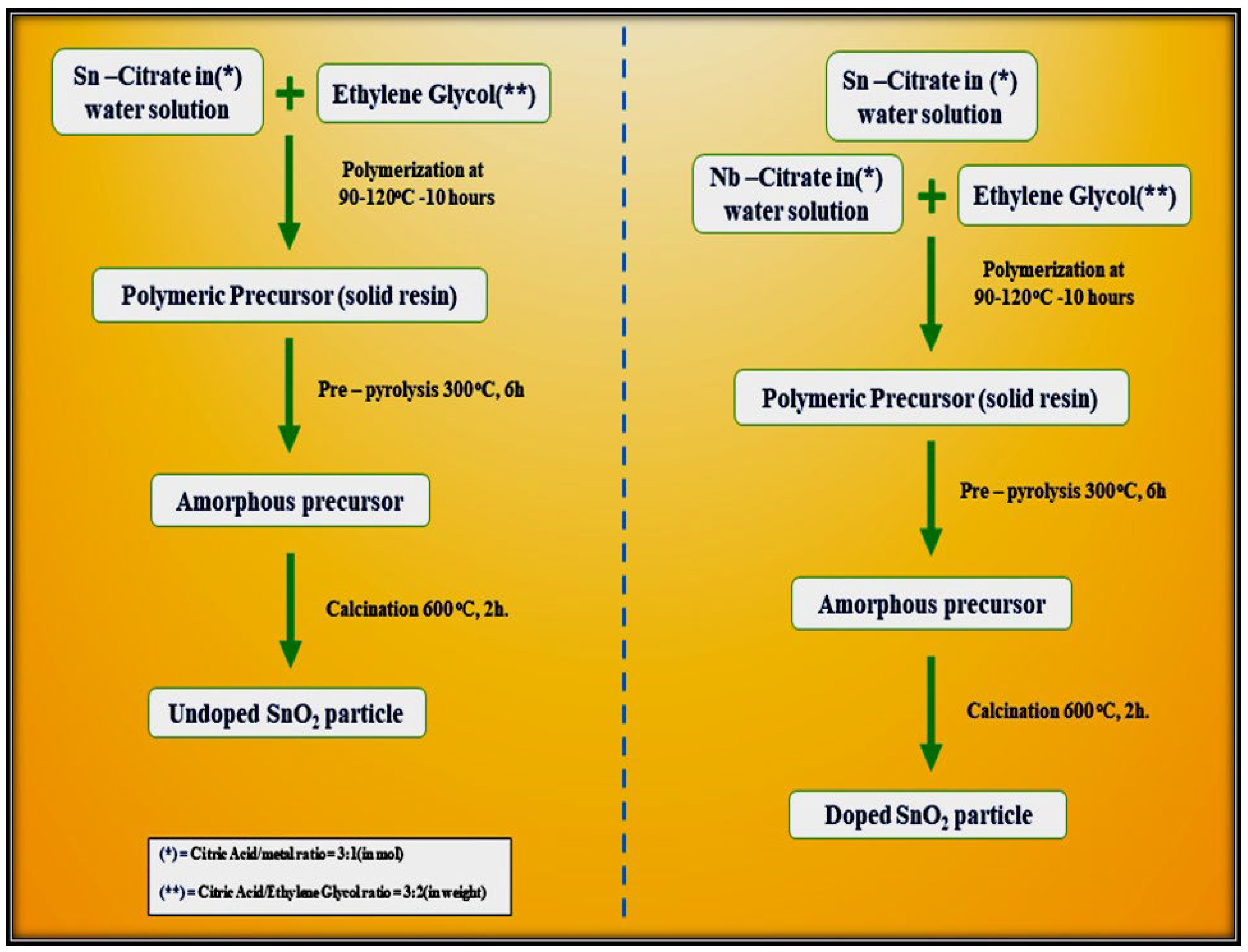
Leite et al. [78] demonstrated a simple polymeric route for the synthesis of undoped and Nb2O5-doped tin oxide. This method is based on the chelation of cations by a hydro carboxylic acid such as citric acid. The citrate solution is then mixed with ethylene glycol through a polyesterification reaction to enhance polymerization. The reaction occurs after the water has been eliminated at a temperature ranging from 90 °C to 120 °C. Figure 14 outlines the steps required for the synthesis of both undoped SnO2 particles and SnO2 doped with 5 mol.% Nb2O5. Both the precursors were calcined in two steps, initially at 300 °C for 6 h to promote pre-pyrolysis and then at 600 °C for 2 h to allow complete oxidation of the precursor and to promote crystallization of the SnO2 phase, as shown in the flow chart below.
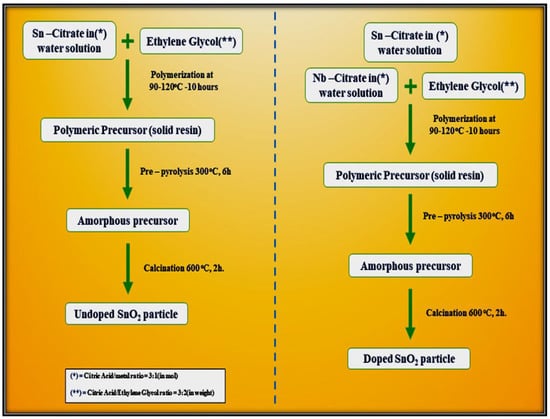
Figure 14.
Mechanistic steps involved in the synthesis of undoped SnO2 and Nb2O5–doped SnO2 by polymeric precursor method.
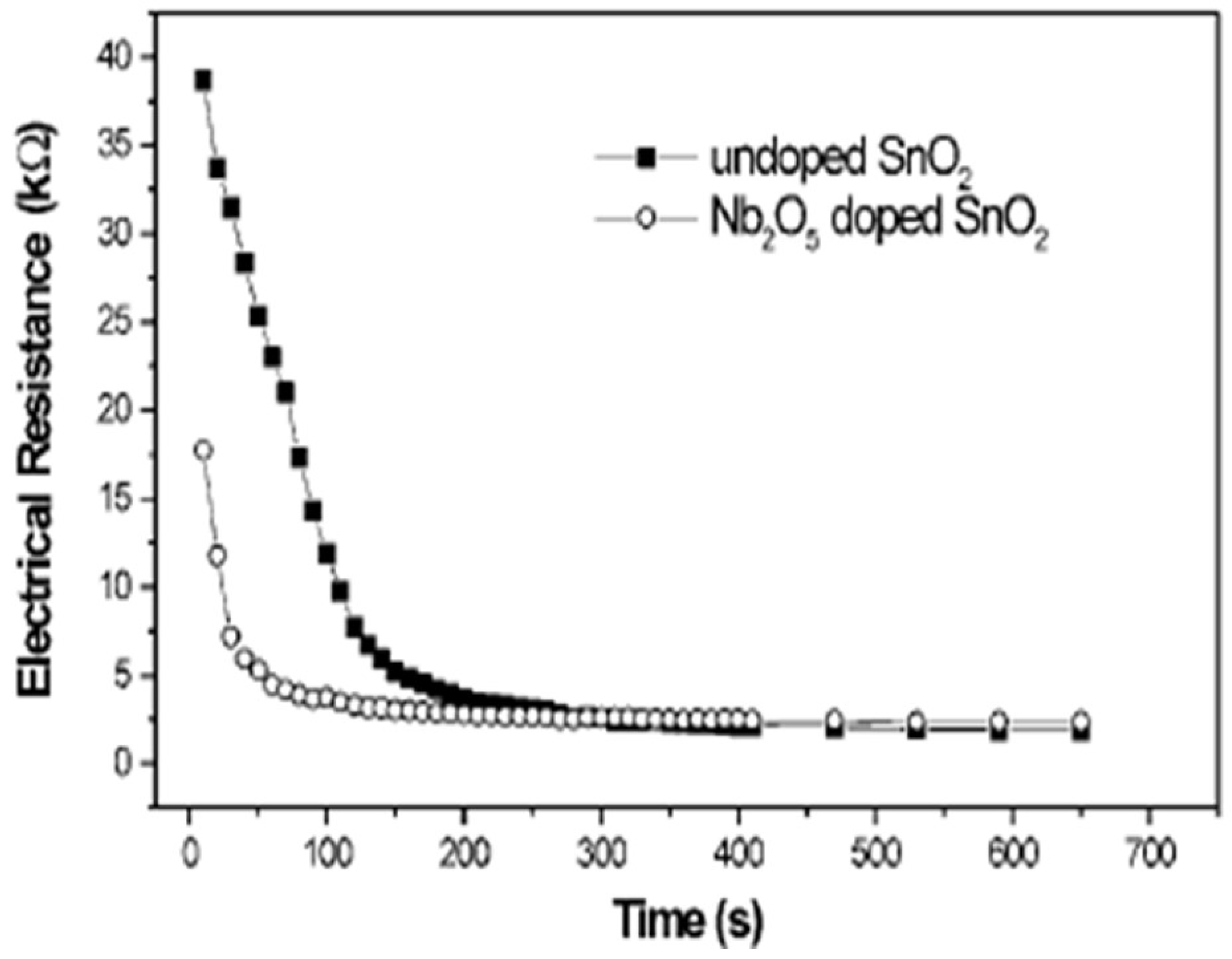
Preliminary gas sensing measurements with doped SnO2 and undoped SnO2 thin films were tested for ethanol. The prepared suspensions were deposited on an alumina substrate by spin coating and were then sintered at 500 °C. Gas sensing tests showed that both powders offer a good response with roughly spherical morphology. However, the sensor response time of doped SnO2 was shorter, as shown in Figure 15. The preliminary results depict that doped SnO2 has good sensing properties. In other words, Nb2O5 can be used to control particle size during the synthesis process, which will produce a material with good potential applications in sensor technology.
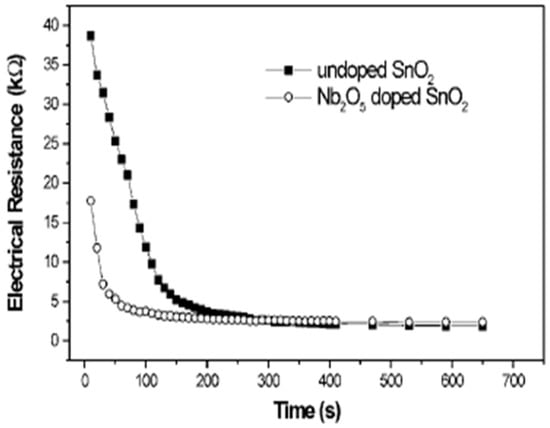
Figure 15.
Time response of the Nb2O5-doped SnO2 and undoped particles deposited by spin coating on alumina substrates with ethanol as the testing gas at 100 ppm. (Reprinted with permission from [78]. Copyright 2000, John Wiley and Sons).
6. Microemulsion or Reverse Micellar Method
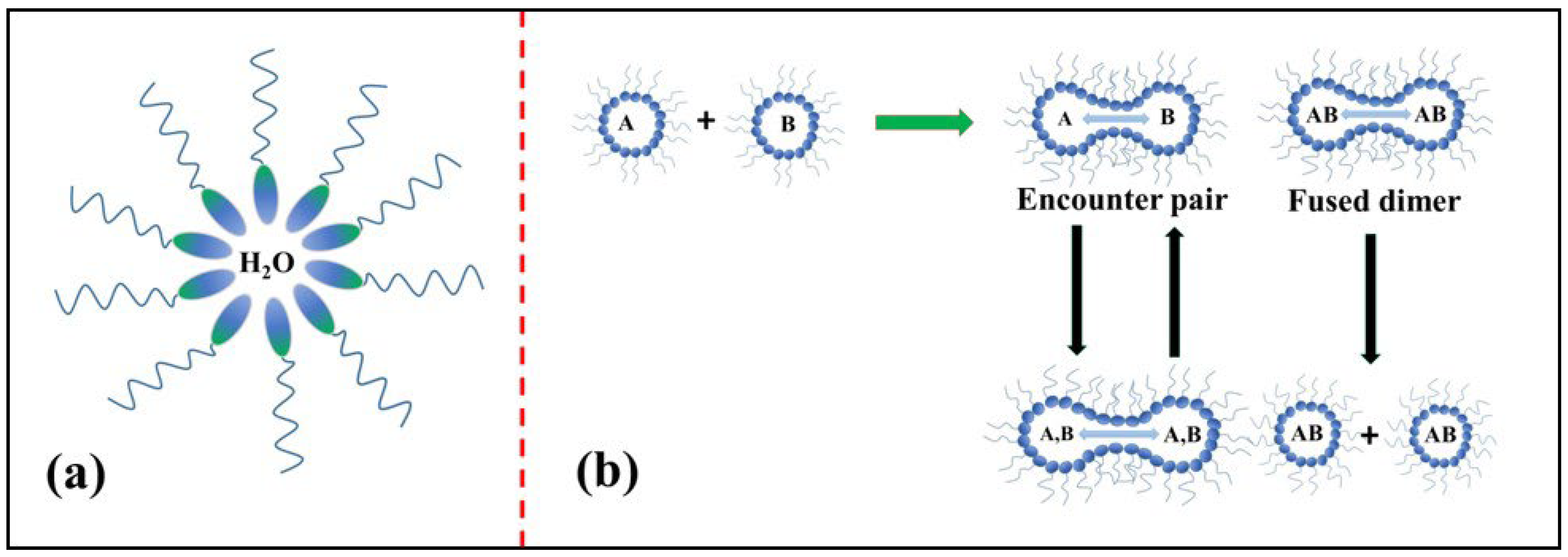
The microemulsion method is another significant procedure for the synthesis of metal oxides; in this method, polar and nonpolar solvents of two immiscible liquids are mixed together by the addition of surfactant in a vessel, which results in the formation of an oil-in-water (O/W) microemulsion [79]. Figure 16a depicts the structure of a microemulsion consisting of a phase encapsulated by a hydrophilic polar head group of a surfactant directed inwards and a chain of long hydrophobic hydrocarbons (which are nonpolar) directed outwards towards the oil phase [80]. In the reverse micelle or microemulsion method, the polar head which is water soluble forms a water pool content that is characterized by a W0 ratio, i.e., the concentration of water to the concentration of surfactant. When W0 ˂ 15, reverse micelles are formed, and when W0 > 15, a microemulsion is formed. The water pool has a size range of 10–15 nm. These reverse micelles have great control over the shape and size of nanoparticles and thus can act as uniformly sized nanoreactors. The important feature of this method is that we can obtain different shapes of micro emulsions in the phase diagram, and the morphologies of the final material, as shown in Figure 16b, can be chosen at different positions in the phase diagram below.
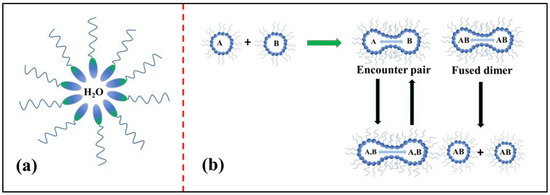
Figure 16.
(a) Schematic of W/O microemulsion or reverse micelle; (b) mechanistic steps involved in the reverse micellar method.
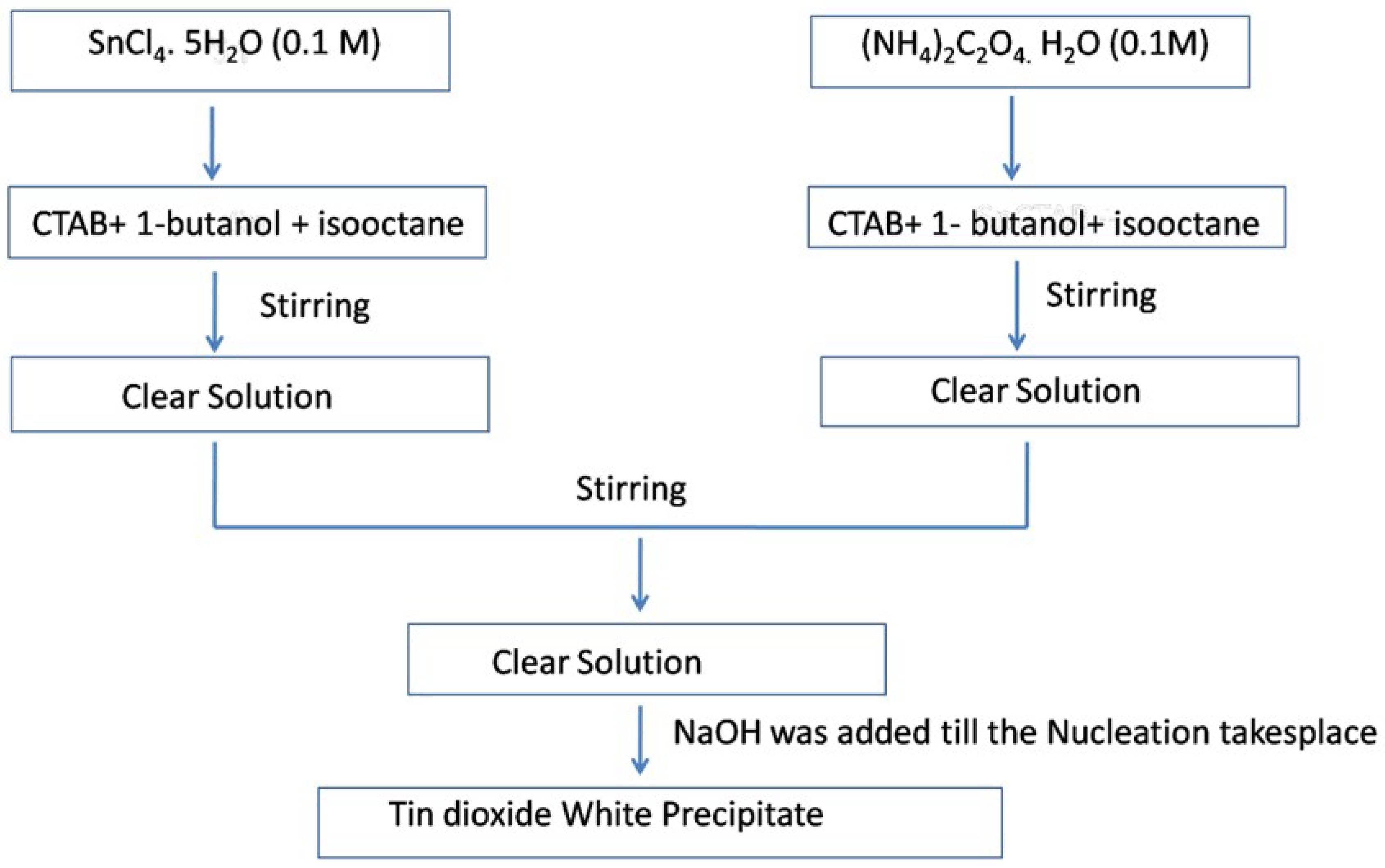
Ahmed et al. [81] used the reverse micellar route to synthesize SnO2 nanoparticles, using CTAB as the surfactant. Monophasic tin oxide nanoparticles with rough undefined morphology were found to be crystalline after heating at 500 °C and using NH3 as a precipitating agent. Gas sensing measurements of the as-prepared SnO2 showed enhanced sensitivity towards n-butanol as compared to the other solution phase techniques such as the co-precipitation method used for the preparation of SnO2 polycrystalline samples. The presence of a high oxygen vacancy level in the nanoparticles resulted in enhanced gas sensitivity, as reported in the literature [81]. The schematic procedure for the synthesis of SnO2 nanoparticles using reverse micelles is shown in Figure 17.
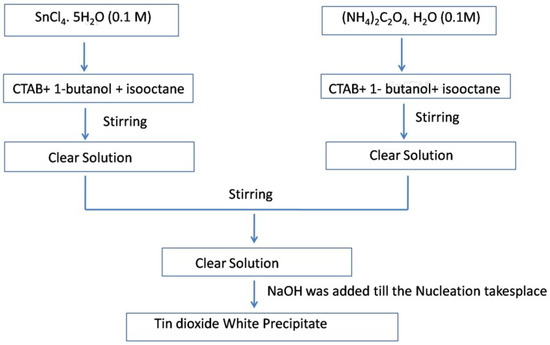
Figure 17.
Flow chart for the synthesis of SnO2 nanoparticles at 500 °C.
Gyger et al. [5] synthesized SnO2 nanoparticles by a simple microemulsion method. The preparation of samples was performed via water-in-oil microemulsion (w/o) by mixing 5 mL of hexanol as a co-surfactant and 1.82 gm CTAB as a surfactant in 50 mL of n-dodecane as the nonpolar phase, and further, demineralized water and 2 mL of a 5:1 mixture of methanol were added. In addition, 10 mL of a 0.01 M solution of Sn(Ot-Bu)4 (ABCR, 99.99%) in dodecane was added dropwise to this micellar system, and the reaction mixture was left for 12 h to react. This resulted in the hydrolysis at the liquid–liquid phase boundary of the tin alcoholate, due to which polar-phase hollow spheres were established. The reaction was concluded by adding 20 mL of ethylene glycol. The resulting colorless precipitate was washed several times with ethanol followed by centrifugation, and the colorless SnO2 nanoparticles were obtained. The nanoparticles obtained with hollow spherical morphology revealed a good sensor response to CO in a concentration range of 50 to 300 ppm that is relevant to the sensing applications. Zhang et al. [82] employed a reverse-micelle-mediated solution route for the synthesis of SnO2 one-dimensional nanocrystals. A typical assembly-mediated process was employed for the formation of SnO2 hollow spheres. The crystal habit of the SnO2 rutile phase and the reverse micelle nature are believed to be responsible for the growth behavior of 1-D SnO2 nanocrystals. The temperature range of 220–240 °C resulted in the growth and formation of the morphology of SnO2 nanowires, while the morphology of nanorods was obtained when the temperature of the reaction was below 220 °C. The aggregates of SnO2 nanocrystals between dendrites and hollow spheres were regarded as the intermediates for the nanowires. The CO gas sensing measurements revealed that the hollow-structured products had a good sensitivity and stability. Compared with SnO2 nanorod dendrites and nanowires, the specific surface area is believed to be the dominating factor for enhanced sensing activity. Liangyuan et al. [83] synthesized nanocrystalline ZnO-SnO2 nanocomposites as gas sensor materials by successfully employing a microemulsion synthesis route for controlled morphology and grain size. In this method, a water-in-oil microemulsion that contained a maximal amount of water and a minimal amount of surfactant was created. Then CTAB, n-octane, water and n-pentanol were taken in appropriate amounts to form a solution, and the ratio of water or CTAB to alcohol, the precursor salt concentration and the effects of the alcohol chain length on the stability and formation of microemulsions were studied. The morphology of the synthesized nanoparticles was spherical. The performance of the obtained nanocomposites was characterized for gas sensing measurements. The gas sensing results revealed that the obtained nanocomposites are selective for the detection of NO2 and CO and are highly sensitive, with sensor response depending on the composite concentration, operating temperature, calcination temperature and concentration of gas in air, as shown in Figure 18a–c. Further, adding surface coatings or dopants of metals or other oxides resulted in a dramatic increase in sensing performance.
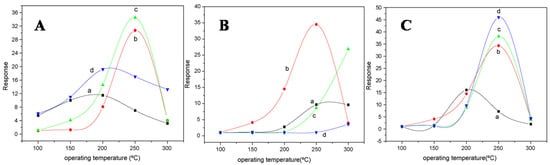
Figure 18.
(A) Responses of (a) 0, (b) 20, (c) 40 and (d) 60 mol% ZnO nanocomposites calcined at 600 °C to 500 ppm NO2; (B) responses of 40% ZnO–60% SnO2 nanocomposite calcined at (a) 400 °C, (b) 600 °C, (c) 800 °C and (d) 1000 °C to 500 ppm NO2; (C) responses of 40% ZnO–60% SnO2 nanocomposites to (a) 200 ppm, (b) 500 ppm, (c) 800 ppm and (d) 1000 ppm NO2. (Reprinted with permission from [83]. Copyright 2008, Elsevier).
7. Sol–Gel Method
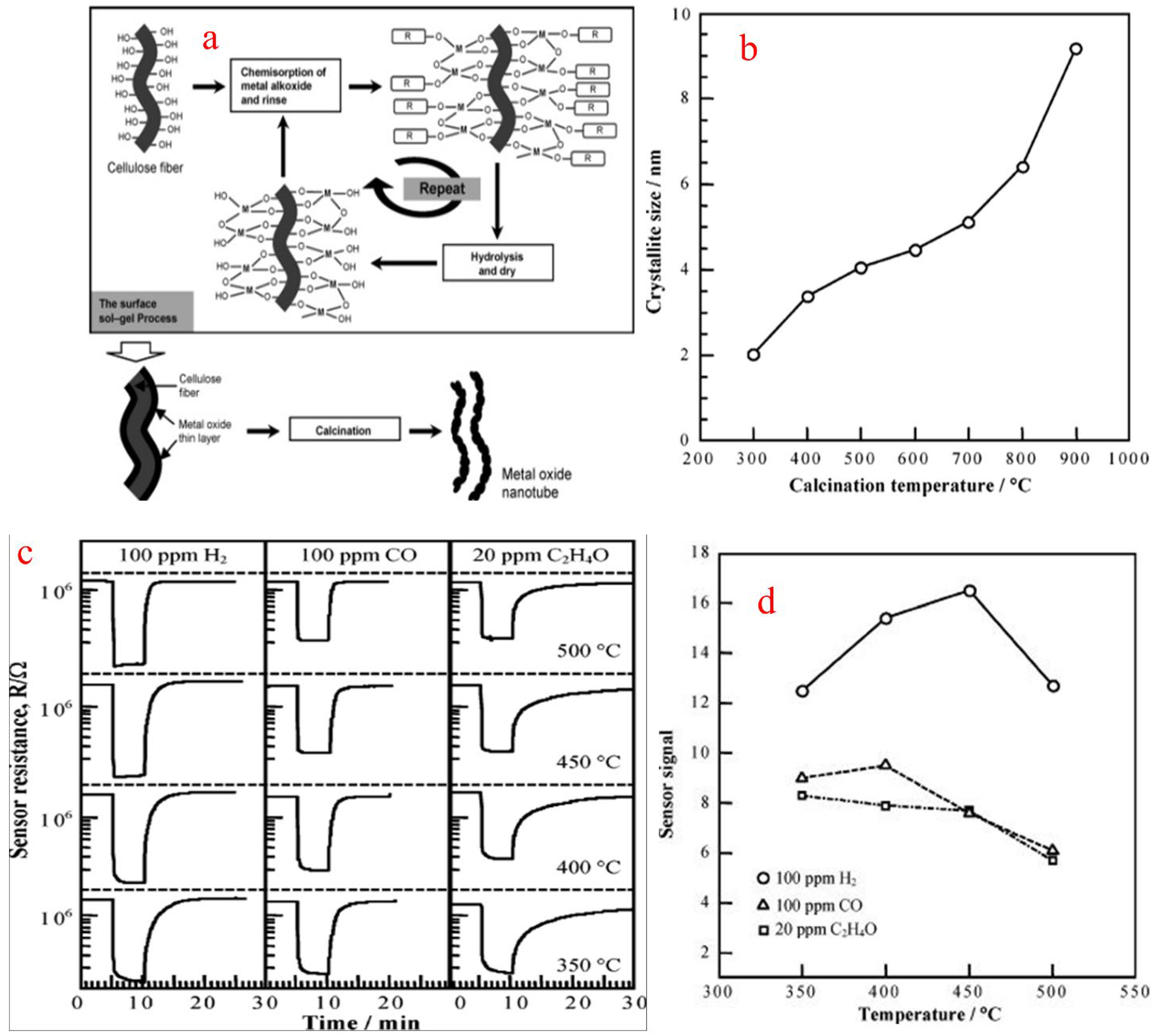
A sol is a colloidal suspension of a particle in a liquid. In a typical sol–gel process, hydrolysis reactions of a precursor form a colloidal suspension which includes metal–organic compounds such as metal alkoxides or generally inorganic metal salts. The sol–gel process involves the homogeneous solution of one or more selected alkoxides as the starting material because metal alkoxides on hydrolysis give oxide as the colloidal product which remains in the suspension rather than precipitation. In a sol–gel method, the formation of a concentrated suspension, called sol, of a metallic hydroxide is evaporated by dehydration and results in the formation of a semi-solid mass called gel. A varied number of mixed and pure oxides can be obtained by controlled heating of a gelatin material. This method has good control over the particle size and gel. The sol is heated to form gel, and gel on calcination gives the final product. Huang et al. [84] synthesized SnO2 nanotubular materials by employing a sol–gel route using a template of a natural cellulosic substance. SnO2 gel layers were first coated by a surface sol–gel process using a precursor tetraisopropoxytin-2-propanol adduct to give SnO2 with the morphology of nanotubular materials, after calcination in air to form natural hollow replicas of cellulose fibers as shown in Figure 19a. The obtained nanotubes were then calcined at different temperatures, resulting in the formation of different shapes and sizes of nanotubes, as shown in Figure 19b. To obtain pure SnO2, a calcination temperature of above 500 °C is needed. The sensor performance for H2, CO and ethylene oxide was measured from a sensor setup which was fabricated from the SnO2 nanotube sheet, and the sensor signal (S) in response to 100 ppm H2 was 16.5 at 450 °C, as shown in Figure 19c,d, and was found to be comparable to that of the SnO2 conventional sensor.
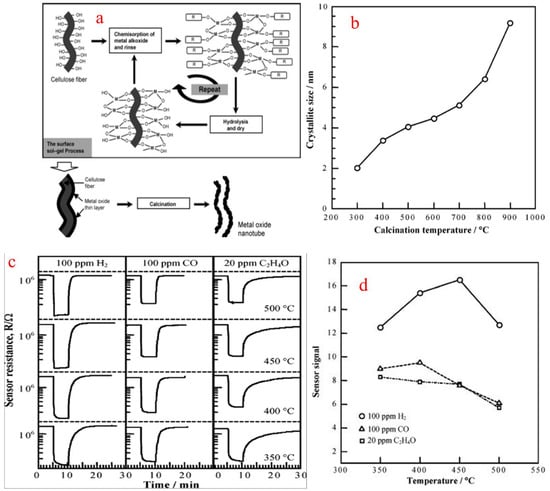
Figure 19.
(a) Sol–gel synthesis of metal oxide nanotubes using cellulose fibers. (b) Dependence of SnO2 crystallite size on calcination temperature. (c) Response transients of SnO2 nanotubes for 100 ppm H2, 100 ppm CO and 20 ppm C2H4O at various temperatures. (d) Temperature dependence of SnO2 nanotube sensor in response to 100 ppm H2, 100 ppm CO and 20 ppm C2H4O. (Reprinted with permission from [84]. American Chemical Society, Washington, DC, USA).
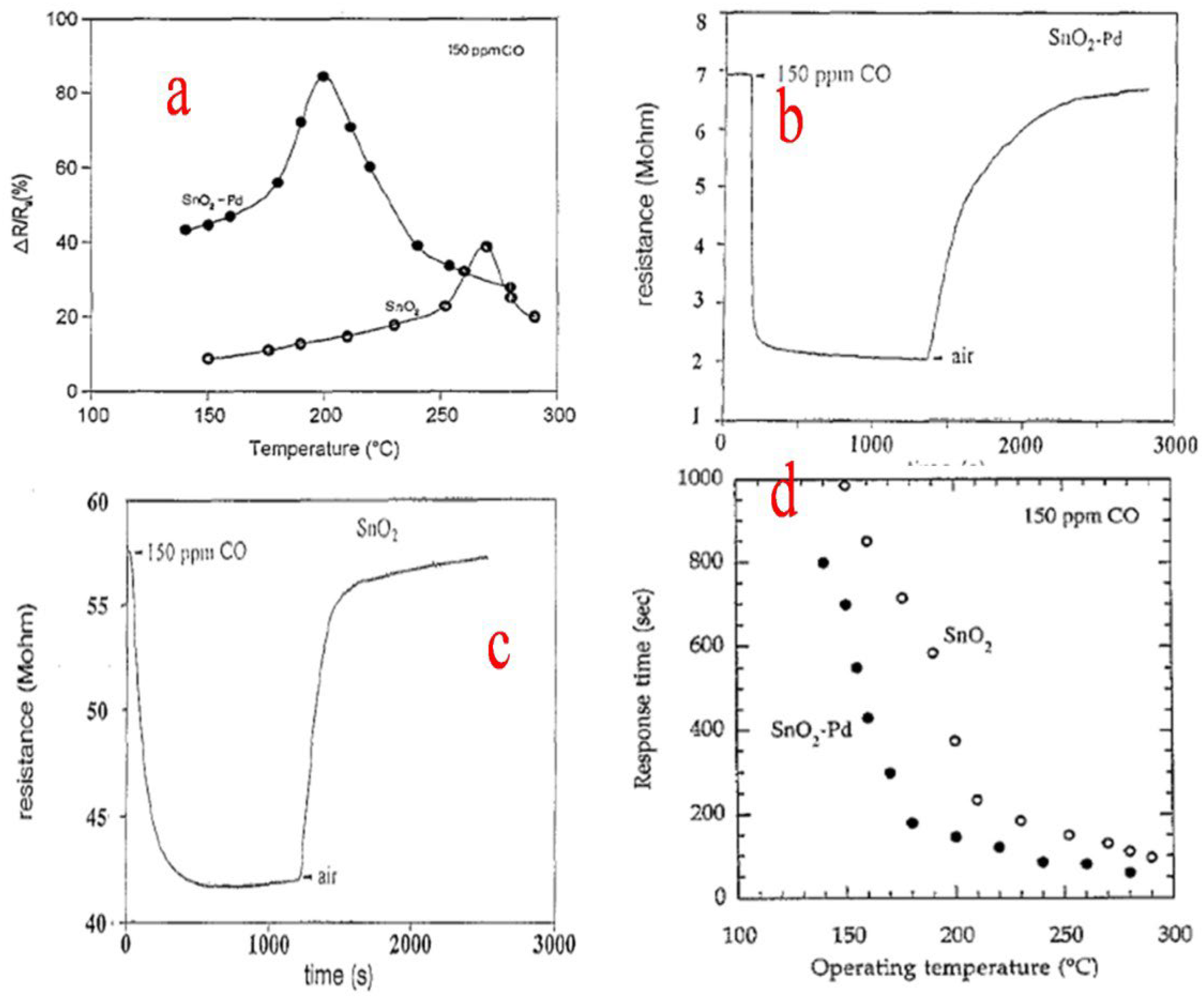
Zhang et al. [85] used granulated tin to synthesize nanocrystalline SnO2 particles by a simple sol–gel method. During the process, the granulated tin in HNO3 solution obtained by dissolution is mixed with the citric acid which acts as a stabilizer and slows down the process of condensation and hydrolysis. SnO2 nanocrystals obtained ranged from 2.8 to 5.1 nm in size and had a specific surface area ranging from 289 to 143 m2g−1 when different heating temperatures were employed. The obtained nanocrystallites displayed a reduction in particle size as well as lattice expansion. Further, the precursor condenses and hydrolyzes in an uncontrolled manner in the absence of citric acid, which results in the formation of larger and broader nanocrystals with roughly spherical morphology. This route can be employed to synthesize tin oxide doped nanocrystallites and also serves beneficial purposes in fields such as electronics and engineering and, above all, gas sensing. Rella et al. [86] used a sol–gel technique to synthesize SnO2-based thin films. During the process, thin films based on Pd-doped SnO2 and undoped SnO2 were prepared. Sensing measurements revealed that the sensitivity of the sensor towards CO is increased by the palladium doping, along with a decrease in the sensitivity maximum temperature. The surface area of SnO2 is enhanced by Pd, which also helps in catalyzing the oxidation of CO. The changes that occurred in the different aspects of doped and undoped SnO2 are shown in Figure 20a–d. Consequently, the increase in the active area to volume ratio and the higher roughness of the modified films led to the better sensing performance of the films loaded with Pd.
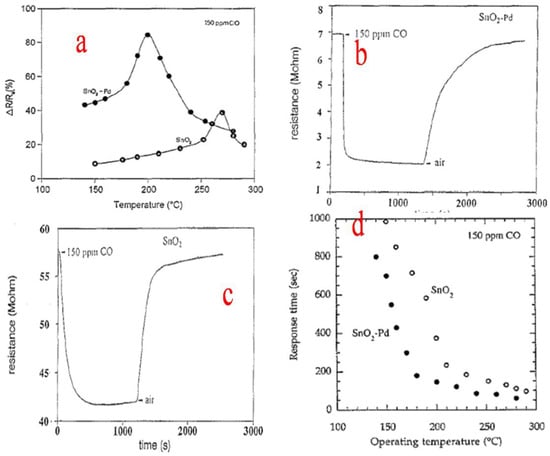
Figure 20.
(a) Sensitivity as a function of the operating temperature for Pd-modified and pure SnO2 thin films. (b) Variation in the electrical resistance for a typical Pd-doped SnO2 thin film. (c) Variation in the electrical resistance for a pure SnO2 film. (d) Response time versus the operating temperature for both pure and Pd−doped SnO2 sensors. (Reprinted with permission from [86]. Copyright 1997, Elsevier).
8. Sensing Applications of Pure and SnO2-Based Composites
The change in resistance upon exposure to a particular target gas forms the basis of the underlying working principle mechanism of gas sensors. For an n-type SnO2 semiconductor, there is a formation of a depletion layer that is devoid of electrons as oxygen is chemisorbed on the semiconductor surface, which results in the formation of semiconducting resistive and core–shell structures [75,87]; when exposed to the reducing gases, the former is oxidized by the oxygen species carrying negative charge which are adsorbed on the SnO2 surface, and thus the sensor shows an increase in conductivity response as a result of the production of more electrons by oxidation reactions. During the process, the first and foremost prerequisite is the development of active materials for gas sensors with ample porosity and large surface areas with ease in the access of analyte gases which increases the sensitivity of sensors. In the case of nanoparticles with dense aggregates, the target gas response is limited by the small pore sizes as compared to the porous nanostructures which have a fast gas response and high surface areas to offer. In general, the packing arrangement of the individual atoms, along with the dimensions of the building blocks and the resultant porosity, plays an important role in the sensing performance of such nanostructures. Moreover, a sufficient surface area is provided by the SnO2 hierarchical building blocks for the interfacial chemical reactions to occur as well as the effective diffusion of target gases towards the sensing interface. Further, loading metal onto the surface of prepared porous films results in the enhancement of the selectivity and sensitivity of the gas sensors.
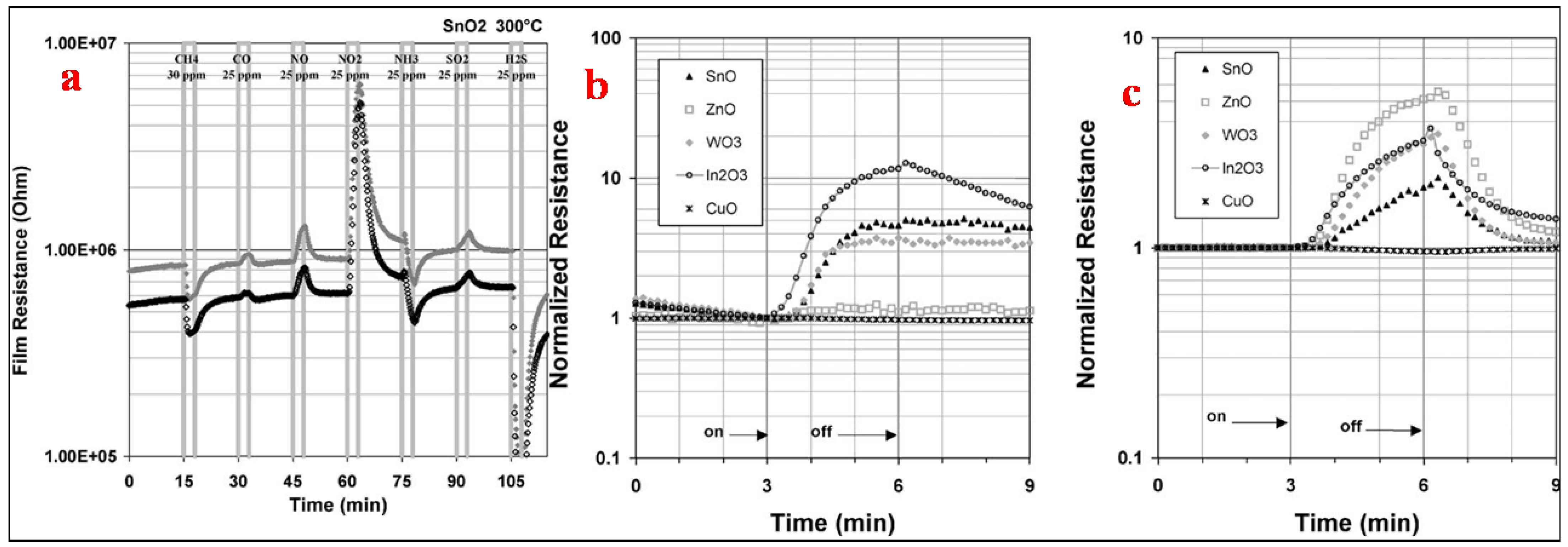
Tomchenko et al. [1] used five thick film semi-conducting metal oxides, namely In2O3, CuO, SnO2, ZnO and WO3, to study their gas sensing relative to NH3, SO2, H2S, CH4, CO, NO and NO2. For thick film sensors, to determine the optimum operating temperatures, the films were studied successively at 200, 300 and 400 °C. The tests of the gas hit sequence used are shown in Figure 21a. In order to maintain steady flow rates in the system, the delivered gas concentration was set at 25 ppm for all gases except for CH4, which was kept at 30 ppm. Followed by 12 min of air purge, each gas has a 3 min long exposure. In order to assess the short-term repeatability of the sensor response, this exercise was repeated six times. The obtained maximum sensitivities for this test stage are shown in Figure 21a. All target gases under investigation responded to all types of sensors, thereby showing that the sensors are nonselective in principle. The semiconductors of n-type such as ZnO, WO3, In2O3 and SnO2 revealed a decrease in resistance when exposed to CH4 and H2S and a dramatic increase in resistance when exposed to NO and NO2. In contrast, the p-type semiconductors such as CuO showed an increase in resistance for H2S and a decrease for NO and NO2. The sensitivity even to these active gases was very low for CuO sensors. At 25 ppm of H2S, the CuO sensor response was nearly around 1.2 at 300 °C and 400 °C. The magnitudes of other gas responses for CuO were 1.1 (towards NO2 at 300 °C) or lower. The target gases were found to be more active towards n-type semiconductors with high sensing of NOX and H2S along with the response to other gases of significant interest as well. At 200 °C, a maximum in sensitivity was attained towards particular gases, as can be seen from Table 1. For sensor arrays, 300 °C temperature was chosen as the working temperature because of a compromise between the response speed of the sensor, which became adequate only at 300 °C, and the sharp sensitivity drop observed at temperatures above 300 °C. A comparison of the sensors’ normalized response at 200 °C and 400 °C to NO2 is shown in Figure 21b,c which shows that SnO2, In2O3 and WO3 sensors demonstrated very slow recovery towards NO2 at 200 °C but high sensitivity performance. Remarkable recovery was observed when the same sensors were heated at 400 °C after NO2 gas hit them (about 3 min (Figure 21a)), with less sensing sensitivity towards this gas. Taking all these factors and influences into consideration, the operating temperature of 300 °C was selected for sensor materials and other gases of interest with the same tendency for the investigated sensor arrays.
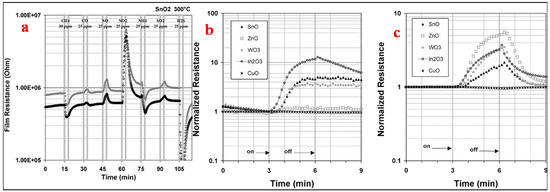
Figure 21.
(a) Gas exposure sequence used at each temperature to determine the optimal operating temperature and (b,c) normalized response of SMO sensors to NO2 at (a) −200 °C and (b) −400 °C. (Reprinted with permission from [1]. Copyright 2003, Elsevier).

Table 1.
Maximum sensitivities during the experiments on sensor operating temperatures. Here a negative value means that the sensor’s resistance decreased during the gas hit. If the resistance increased, the corresponding sensitivity is shown as a positive number.
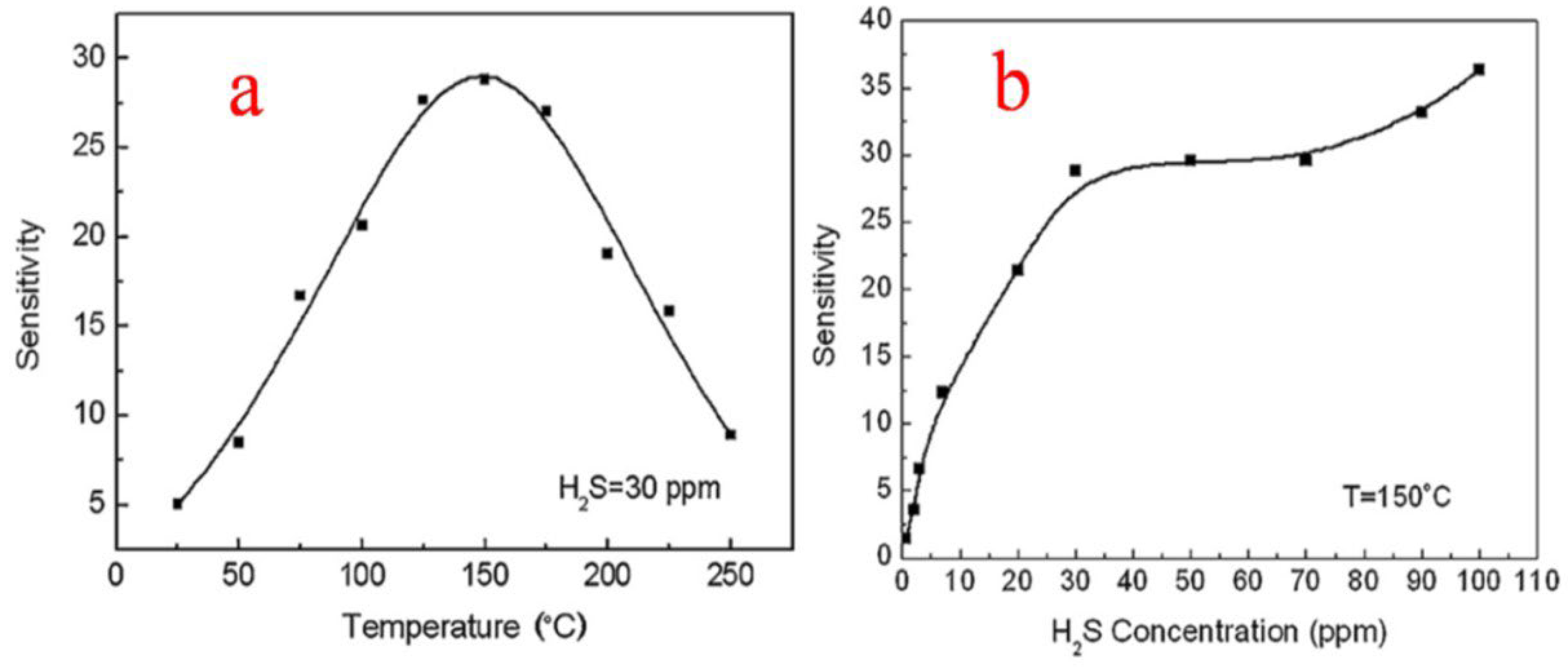
Liu et al. [37] studied the responses of the thick sensor films at temperatures ranging from room temperature (25 °C) to 250 °C. The sensitivity towards 30 ppm H2S was calculated and plotted, and it was observed that at 25 °C for 30 ppm H2S, the sensor showed a response with a maximum of around 28.8 at 150 °C, as shown in Figure 22a, and then as the temperature was increased, the sensor response showed a decrease. The enhanced performance of the as-reported SnO2 nanoparticles is attributed to the electrical and structural stabilities of the uniform porous micro-structured network of thick film, with more analyte gas penetration and enhanced surface area. This study of thick film sensors thus revealed that the nanoparticles of SnO2 with no specific additives were more selective and sensitive in response to even low concentrations (30 ppm) of H2S both at room temperature and at optimal detection temperature of 150 °C, as depicted in Figure 22b, showing the high attractiveness and practical applications of SnO2 in H2S gas sensing.
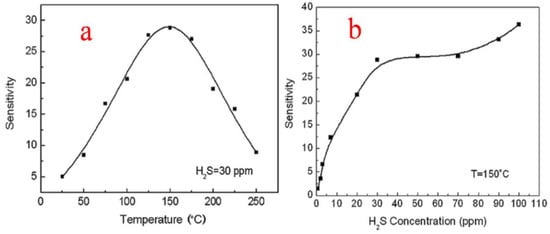
Figure 22.
(a) Response sensitivity at different operation temperatures for H2S at 30 ppm. (b) Response sensitivity to different concentrations of H2S gas at optimal temperature. (Reprinted with permission from [37]. Copyright 2009, Elsevier).
Tan et al. [38] observed SnO2 hollow spheres for gas sensing applications with the aid of templates of carbon microspheres. The as-obtained nanospheres with a size of 100 nm have a significant number of SnO2 nanoparticles of nearly 6 nm diameter. The response sensitivity of SnO2 hollow-sphere-based sensors to 1000 ppm ethanol is as high as 75, and the recovery and response times are only 10 and 4 s; the sensitivities and recovery times for other ethanol concentrations were lower. Such excellent and enhanced sensing properties can be attributed to the small size of the SnO2 hollow spheres and the porous structure of SnO2 nanocrystals. On the surface of sensing materials, adsorption and desorption of oxygen cause metal-oxide-based gas sensors to change in resistance, which results in the creation of a depletion layer of electrons on the surface of SnO2 nanoparticles. The dependence of the width on the surface depletion layer which operates on the space charge model greatly affects the sensitivity. The surface depletion layer width (L) can be shown in Equations (1) and (2).
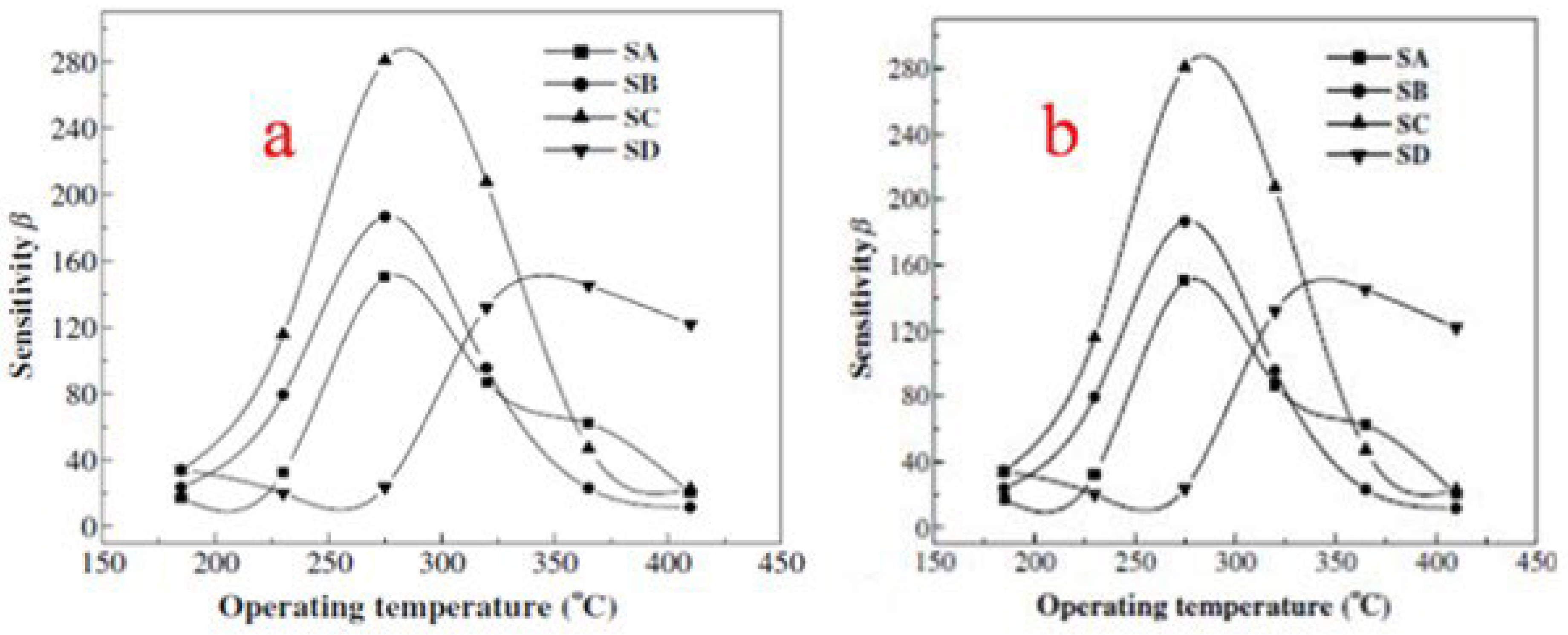
where Ld is the Debye length, eV is the barrier height, kT is the thermal energy and nC is the carrier concentration. According to the above equation, the calculated Ld is about 3 nm for SnO2 nanoparticles in this report. The SnO2 nanocrystals have a diameter of about 6 nm, which is close to 2Ld. Now here in the case of the SnO2 nanocrystal, due to the adsorption of oxygen in air, the electrons are completely depleted. Consequently, when these sensors are exposed to the reducing gases, the depleted region electrons are released back to the conduction band, due to which the resistance of sensors is sharply changed. This is the reason why theSnO2 sensor exhibits high sensitivity. One more reason behind its high sensitivity is that gas molecules diffuse more rapidly in porous structures as compared to denser structures, which balances the desorption and adsorption of target gases quickly and shortens the recovery and response times. The obtained SnO2 hollow spheres have a fast response and high sensitivity and are believed to be promising materials for gas sensing applications. Wang et al. [45] prepared mesostructured tin oxide for sensing applications with high specific surface areas of about 368, 343 and 134 m2/g for calcination at 300, 350 and 400 °C, respectively. First, in this study, the effect of the operating temperature on the gas sensing properties was studied. As depicted in Figure 23, the operating temperature has a profound effect on the sensors’ sensitivity to 1000 ppm H2 (a) and C2H5OH (b). The sensors obtained from mesostructured tin oxide displayed the highest sensing performance in response to H2 at 300 °C. However, the highest sensing performance was observed at 345 °C, as shown in Figure 23a, for the polycrystalline tin oxide based sensor. The sensitivities to H2 at 300 °C operating temperature were 13, 13.9 and 23.5 for materials calcined at 300, 350 and 400 °C, respectively. For polycrystalline tin oxide, a maximum sensitivity of 7.3 at 345 °C was achieved, which is lower as compared to the mesostructured tin oxide sensors, which is ascribed to the desorption and adsorption mechanism of gas on SnO2 [88,89]. In addition, both the ionic forms of molecular oxygen i.e., O2ˉ and Oˉ, are absorbed by the n-type metal oxides. As a result of this fascinating process, the sensor material in presence of the oxygen ion becomes more sensitive to the presence of reducing gases. When the temperature is preferentially low, the surface selectively adsorbs O2ˉ, and consequently the sensitivity of the material is small. On contrary, when the temperature is high, the adsorption of Oˉ increases and the sensitivity of the material shows an increase too, as revealed in Figure 23b where the symbols SA, SB, SC and SD represent the sensors based on mesostructured tin oxide calcined at 300, 350 and 400 °C and polycrystalline tin oxide, respectively. On the other hand, there is a steady adsorption of all the oxygen ionic species which were adsorbed earlier when the temperature is increased too much accompanied by a decrease in sensitivity [90]. In addition, the sensing properties are greatly affected by the surface area of SnO2 nanoparticles. Consequently, a higher sensitivity to H2 was observed for SnO2 sensors with higher surface area [91]. In the present investigation, at an operating temperature of 300 °C, the surface area of 136 m2/g for mesostructured tin oxide has the highest sensitivity, as shown in Figure 23b. The result obtained was different as compared to the previous studies [92] which suggested that residues of carbon and surfactant on the surface of sensing materials affect the adsorption of reducing gases. The sensing results revealed that mesostructured tin oxide can act as a potential candidate for efficient gas sensing materials.
L = Ld(2eV/kT)½
Ld = (εε0kT/2e2nc)½
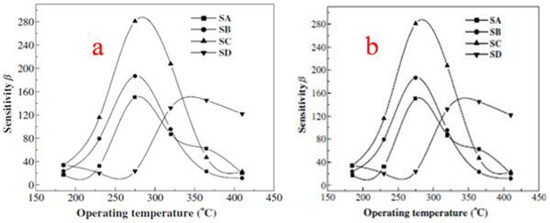
Figure 23.
(a) The sensitivity of sensors with the effect of different operating temperatures at (a) 1000 ppm H2 and (b) 1000 ppm C2H5OH. (Reprinted with permission from [45]. Copyright 2003, Elsevier).
Chiu et al. [70] studied the gas sensing properties of SnO2 nanoparticles with an average particle size of about 3.0 ± 0.5 nm. At 25 ppm of ethanol, the sensing measurements of SnO2 nanoparticles were carried out to determine the optimum temperature range between 100 and 400 °C. Both the thermally treated and as-synthesized SnO2 nanoparticles exhibited different sensing properties due to the different ratios of O/Sn. Further, ethanol was sensed more efficiently by the thermally treated SnO2 as compared to the as-reported SnO2 in which oxygen sites are occupied by the chloride ions. For the as-synthesized SnO2, with the heat treatment of 350 °C for 5 min, the sensing performance can be improved by the exposure of more sites of oxygen and by partly removing Clˉ ions from the nanoparticles. Thus, SnO2 nanoparticles treated thermally showed enhanced sensing performance for alcohol at a minimal detection limit of as low as 1.7 ppm. Further, the long carbon chain of alcohol was ascribed as the reason for the enhanced sensor signal. Shi et al. [72] studied the gas sensing property of SnO2 nanotubes. It was revealed that the as-prepared SnO2 nanotubes with fine grain size and hollow tubular structure may enhance the interaction of the detected gas molecules and the SnO2 surface, resulting in full and fast gas access to the nanocrystals of SnO2. Hence, the sensor response is expected to show an increase. The selected SnO2 nanowires were prepared at 45 °C, and SnO2 nanotubes were prepared at 45 °C and 90 °C, and all three samples were then analyzed and evaluated to reveal the gas sensing performance. The gas sensing performance of all three samples showed a gradual increase as the ethanol gas concentration was increased, as revealed in Figure 24. However, the gas sensing measurements revealed that the SnO2 nanotubes showed better sensing performance than SnO2 nanowires when exposed to ethanol under the same conditions. Further, the improved sensing performance of SnO2 nanotubes prepared at 45°C as compared to those prepared at 90 °C was ascribed to the smaller grain size of the SnO2 nanotubes obtained at 45 °C. The smaller grain size and hollow tubular surface morphology of SnO2 nanotubes as compared to the nanowires were recognized as the reasons for their higher sensing performance. Subsequently, superior gas sensing performance was observed for as-prepared SnO2 nanotubes, which proved that these nanotubes can act as efficient and potential candidates for gas sensing applications.
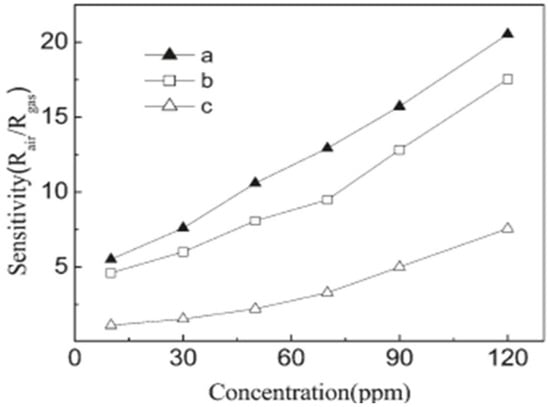
Figure 24.
Room temperature sensitivity towards ethanol of (a) SnO2 nanotubes prepared at 45 °C, (b) SnO2 nanotubes prepared at 90 °C and (c) SnO2 nanowires prepared at 45 °C. (Reprinted with permission from [72]. Copyright 2011, American Chemical Society, Washington, DC, USA).
Keeping in view the sensing applications of pristine SnO2 nanoparticles, different modifications such as loading of metals on the surface of SnO2, doping with different metals and development of different metal oxide/SnO2 nanocomposites have been introduced to enhance the sensing activity of SnO2 nanoparticles. Suematsu et al. [71] observed that loading Pd onto the preformed SnO2 nanocrystals is another significant route for improving the sensing performance of SnO2 nanoparticles. Pd-loaded SnO2 clustered nanoparticles as sensor devices showed enhanced sensing performance in response to CO and H2 when compared with monodispersed nanoparticles. The increase in sensitivity was ascribed to the enhancement in film porosity, as was deduced by the measurements of pore size which revealed that using clustered SnO2 nanoparticles results in an increase in the peak pore size of the sensing films, as a result of which the diffusivity of the gas through the sensing films showed an increase. As a consequence, electrical and catalytic sensitization effects produced by the loading of Pd onto the clustered nanoparticles result in an increase in sensor response. More importantly, SnO2 nanoparticle clusters loaded with Pd displayed significantly high toluene sensitivity due to the enhanced diffusivity of the analyte gas into the sensing film, at a very low concentration limit of 2.5 ppb.
Xue et al. [73] studied uniformly loaded Pt@SnO2 nanorods for gas sensing applications with different concentrations of ethanol, i.e., 10, 50, 100 and 200 ppm, at different working temperatures. The sensitivity measurements of sensors in response to 10, 50, 100 and 200 ppm ethanol reach 3.7, 9.5, 30.1 and 39.5, respectively. Very fast sensor recovery and response times are observed: about 10 and 2 s, respectively. Moreover, good stability and repeatability were observed in the sensors. In addition, the interface between Pt nanoparticles and SnO2 nanorods resulted in the improvement of sensing performance [56,93,94,95], which is possibly due to two factors. Firstly, the region close to the interface experiences the possible chemical influence of the catalytic activity of Pt nanoparticles. Secondly, contact between Pt nanoparticles and SnO2 nanorods produces an electrical contribution. The chemical nature of Pt influences the chemical effects of the Pt/SnO2 interface. Here the role is played by two effects [56,95]. Primarily, oxygen molecules are easily adsorbed on the surface of SnO2 nanorods with the assistance of Pt, which is considered a better dissociation catalyst for oxygen as compared to SnO2, and thus the oxygen adsorbed on the surface can diffuse faster to create surface vacancies which result in the formation of oxygen ions due to the capture of electrons from the conduction band of SnO2 nanorods, as shown in Figure 25a. Moreover, both the molecular-ion conversion rate and the quantity of oxygen adsorbed are increased, which subsequently results in the electron depletion from the SnO2 nanorods occurring more rapidly and at a greater degree. As compared to the pristine SnO2 surface, the interface depletion layer of SnO2/Pt is wider; consequently, at the SnO2/Pt interface, the energy band bends wider (∆W), as depicted in Figure 25b. Secondly, hydrocarbons produce more active radicals due to their catalytic property as Pt breaks them down, which ultimately leads to the reaction between reducing ions and surface-adsorbed oxygen ions. Further, at the interface of the SnO2/Pt region, the electrons are released more readily from the surface reaction back to the conduction band, which results in the increase in conductivity of Pt@SnO2 nanorods in reducing gas atmosphere. Consequently, the sensors are more active in gas sensing in the regions which are close to the SnO2/Pt interface. The regions of contact between the Pt interface and SnO2 nanorods lead to a greater degree of electron depletion as a case of electrical contribution due to which the work function of SnO2 (4.5 eV) is lower than that of Pt (5.65 eV); so, from the SnO2 nanorods, the electrons start moving to Pt, which results in the formation of an additional depletion layer and a Schottky barrier at the interface. In addition, at the SnO2/Pt interface, the energy band bends higher (∆v). In addition, for explaining the high gas sensing performance of sensors, the small size effect of Pt@SnO2 nanorods should be invoked. The depletion layer (Ld) thickness for n-type metal oxides plays a key role in their sensing performance. When the diameter is close to or smaller than 2Ld of metal oxides, it results in high sensitivity as the electrical flow is dominated by the depletion layer. As reported by Xue et al. [73], SnO2 nanorods have a diameter that is about 5–10 nm, which is close to 2Ld for SnO2 materials, for which Ld is about 3 nm. Further, even the relative depletion layer and regions far away from the SnO2 interface are also very wide. Thus, all these factors play a significant role in the sensitivity of Pt@SnO2 nanorods and consequently result in their high sensing performance. The sensing performance measurements of SnO2 nanorods reveal that their sensitivity was enhanced from 21.1 to 39.5 by Pt loading.
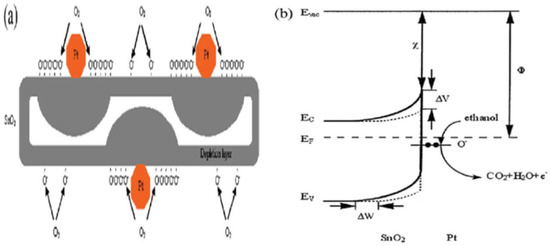
Figure 25.
(a) Processes taking place at the Pt@SnO2 surface, oxygen adsorption on the pristine surface of SnO2 nanorods and dissociation and adsorption of oxygen at the regions close to the SnO2/Pt interface. (b) The energy band diagram of Pt@SnO2 nanorods. The solid line depicts the regions close to the SnO2/Pt interface, which are wider (∆W) and higher (∆V) than those of the pristine SnO2 surface represented by a dashed line. In ethanol atmosphere, the electrons are released easily from the surface reaction at the SnO2/Pt interface. (Reprinted with permission from [73]. Copyright 2010, American Chemical Society, Washington, DC, USA).
Kalmakov et al. [56] studied the performance of gas sensing before and after incorporation of a Pd catalyst on the individual SnO2 nanobelts and nanowires configured as gas sensors. It was observed that sensing measurements were carried out in the same reaction chamber in which Pd was deposited in situ, which showed that modification in behavior was due to the functionalization of Pd apart from the change in properties from one nanowire to another. Before metal incorporation, the changes in the conductance revealed that Schottky barrier-type junctions were created on the nanowire surface by the Pd nanoparticles which formed electron depletion regions within the nanowire; thus, the conducting effective channel was constricted, which drastically reduced the conductance. Thus, the dramatic increase in the sensing performance upon the incorporation of Pd was attributed to the combined influence of the “spillover effect” in which atomic oxygen formed catalytically on the Pd nanoparticle surface migrates onto the tin oxide, and the back “spillover effect”, in which the weakly attached molecular oxygen migrates back to the Pd and is dissociated catalytically. Consequently, both precursor capture and delivery of activated ionic species from the surface of the SnO2 nanostructure are meticulously enhanced by strategically and catalytically active Pd nanoparticles. Zhang et al. [96] studied Cu-doped and undoped SnO2 porous thin films with large surface areas for gas sensing. High selectivity and sensitivity and short response and recovery times were observed for the Cu-doped SnO2 porous film gas sensors, which had an operating temperature of 180 °C with average recovery and response times of ~42.4 and ~10.1 in response to 100 ppm of H2S, respectively. Further, the sensitivity magnitude of the order of 1 was observed for the Cu-doped SnO2 porous film, and it had remarkably better selectivity than the SnO2 undoped gas sensor because of the conversion mechanism between CuS and CuO. Further, it was found that H2S gas concentration increased linearly from 10 to 100 ppm as the sensor response increased from 2.6 to 25.3. More significantly, these sensors with well-defined porous structures exhibited high reproducibility for sensing due to the precisely controlled process and unique morphology. The results obtained reveal that the fabrication of doped SnO2 porous film gas sensors is an efficient way of producing gas sensors with high performance, a lower rate of power consumption and a low cost for H2S sensing and can be used generally for the fabrication of gas sensors of semiconductor metal oxides with multilayer, porous and easily doped nanostructures which have strategically important properties for gas sensing.
Another important strategy for improving the gas sensing ability of SnO2 is the development of metal oxide nanocomposites. In the literature, several SnO2/metal oxide nanocomposites were developed and their application in gas sensing was evaluated. Li et al. [75] studied WO3-SnO2 hybrid hollow sphere gas sensors with temperature-dependent abnormal p-n transitions. SnO2 and WO3 are generally well-known classes of materials with n-type semi-conducting nature, whereas the hollow spheres of WO3-SnO2 with sensing mechanisms controlled by operation temperature display abnormal sensor behavior. In a surprisingly wide operation temperature range, i.e., from room temperature 25 °C to about 95 °C, the sensor showed abnormal p-type responses, while at higher temperatures, it displayed a normal n-type sensing response to ethanol. At 5000 ppm ethanol, the sensor’s conductance response curves at different temperatures were recorded, as shown in Figure 26a. At the temperature of 95 °C, when the sensor is exposed to ethanol with p-type conductance, the sensing response seems to be decreasing rapidly, and when the temperature is kept above 185 °C, an n-type response is shown. Meanwhile, the p-type responses showed a gradual decrease with the increase in the operation temperature. These responses were measured at different ethanol concentrations at temperatures of 22, 85 and 185 °C, as shown in Figure 26b–d. Both n-type and p-type sensor signals were enhanced after ethanol exposure, thereby revealing the possibility that the sensors can be used over a broad range of temperatures. Moreover, at 95 °C, the sensor performance in response to the gases acetone, ammonia and ethanol was observed for WO3 and SnO2 nanoparticles, as shown in Figure 26e,f. When the sensor was exposed to ammonia and ethanol, normal p-type sensing behavior was displayed, but instead, a higher response to acetone was shown at lower temperatures, thereby showing that SnO2 and WO3 are more active in response to acetone, resulting in hollow-sphere-based gas sensors of WO3-SnO2 leading to n-type responses when exposed to acetone. Further, after applying various complex impedance techniques and measuring the sensors’ behavior with various reducing gases, it was revealed that the abnormal sensing response resulted as a consequence of a reaction on the material surface between adsorbed water and the target gas. The competition between extrinsic and intrinsic sensing behavior leads to the control of temperature-controlled n–p switch, which is because protons from the adsorbed water and target gas react with the adsorbed oxygen ions. The total conductivity as an external part is solely regulated by the conduction of the water layer due to the former one, and the intrinsic conductivity of the sensor can be modulated by altering the sensing material’s electron concentration as a result of the latter one. Further, the abundant oxygen vacancies and large area active sites of the hybrid and hollow nanostructures which facilitate the observation of extrinsic sensing behaviors may possibly lead to enhancement of the adsorption of water. This study thus offers new insights into developing humidity-controllable and practical temperature gas sensors with little power consumption based on the extrinsic properties with new approaches concerted towards their sensing mechanisms.
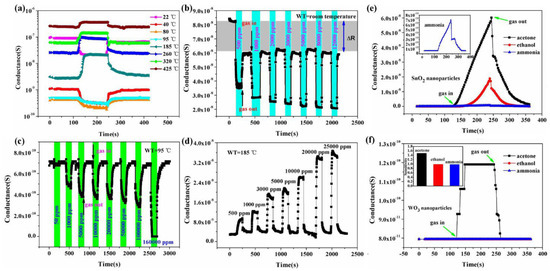
Figure 26.
(a) Responses of WO3−SnO2 hollow−sphere−based gas sensors towards 5000 ppm ethanol at various temperatures; (b−d) the typical sensor response curves to various concentrations of ethanol at room temperature, 95 and 185 °C respectively; (e,f) the sensing response of SnO2 nanoparticles and WO3 nanoparticles at 95 °C. (Reprinted with permission from [75]. Copyright 2015, American Chemical Society, Washington, DC, USA).
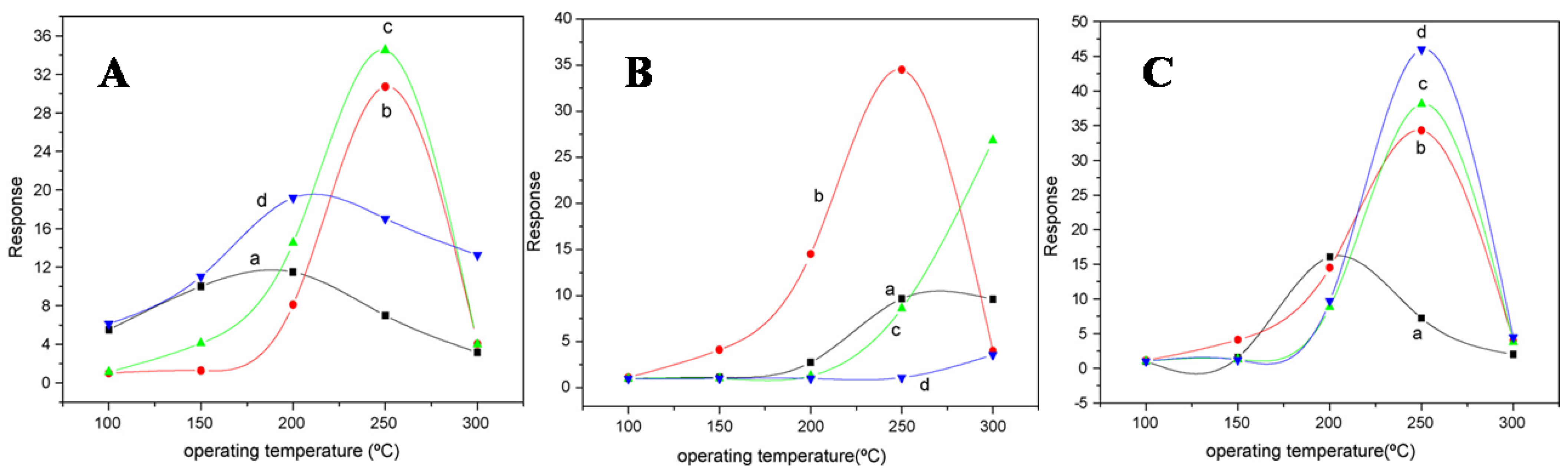
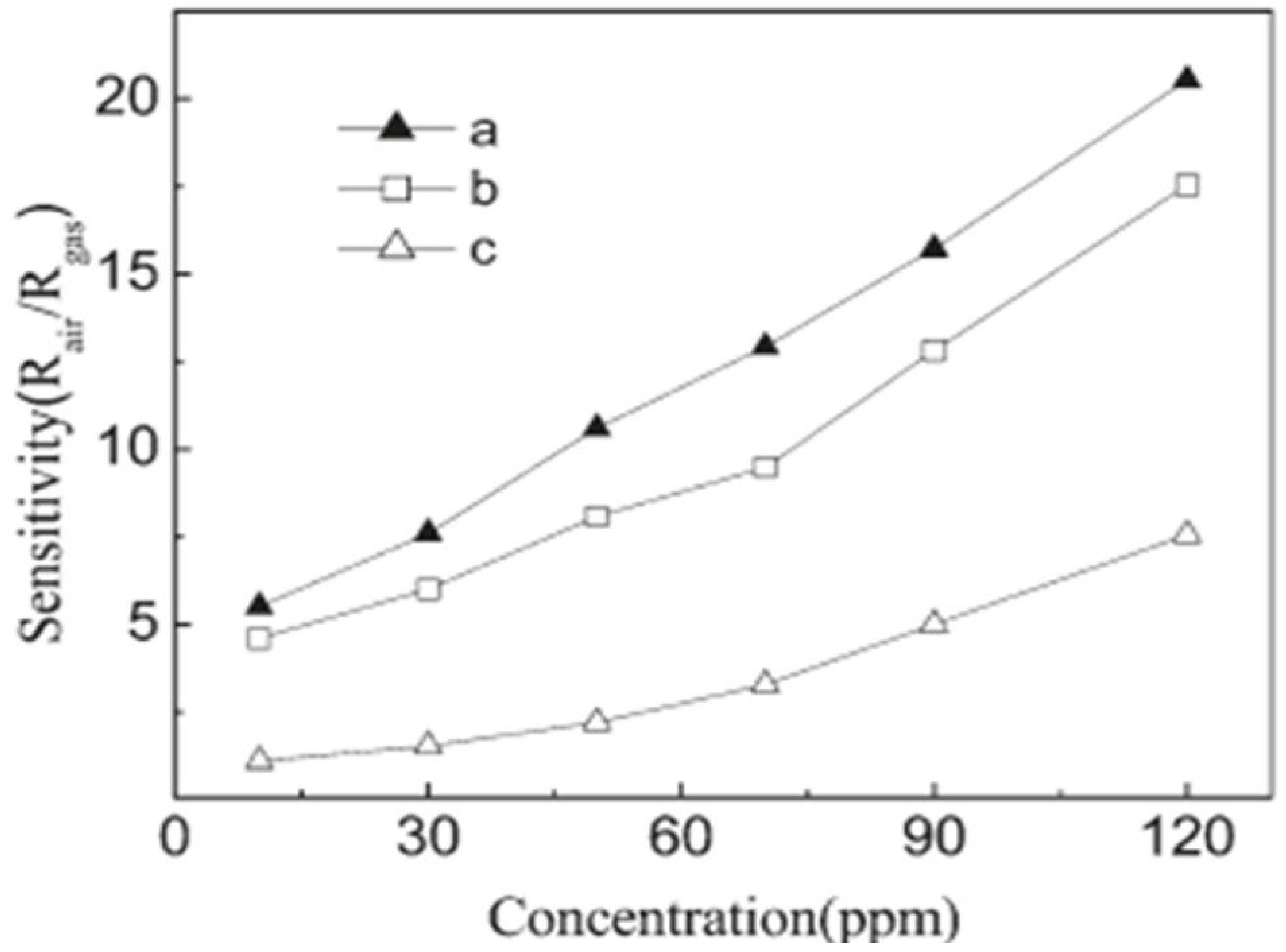
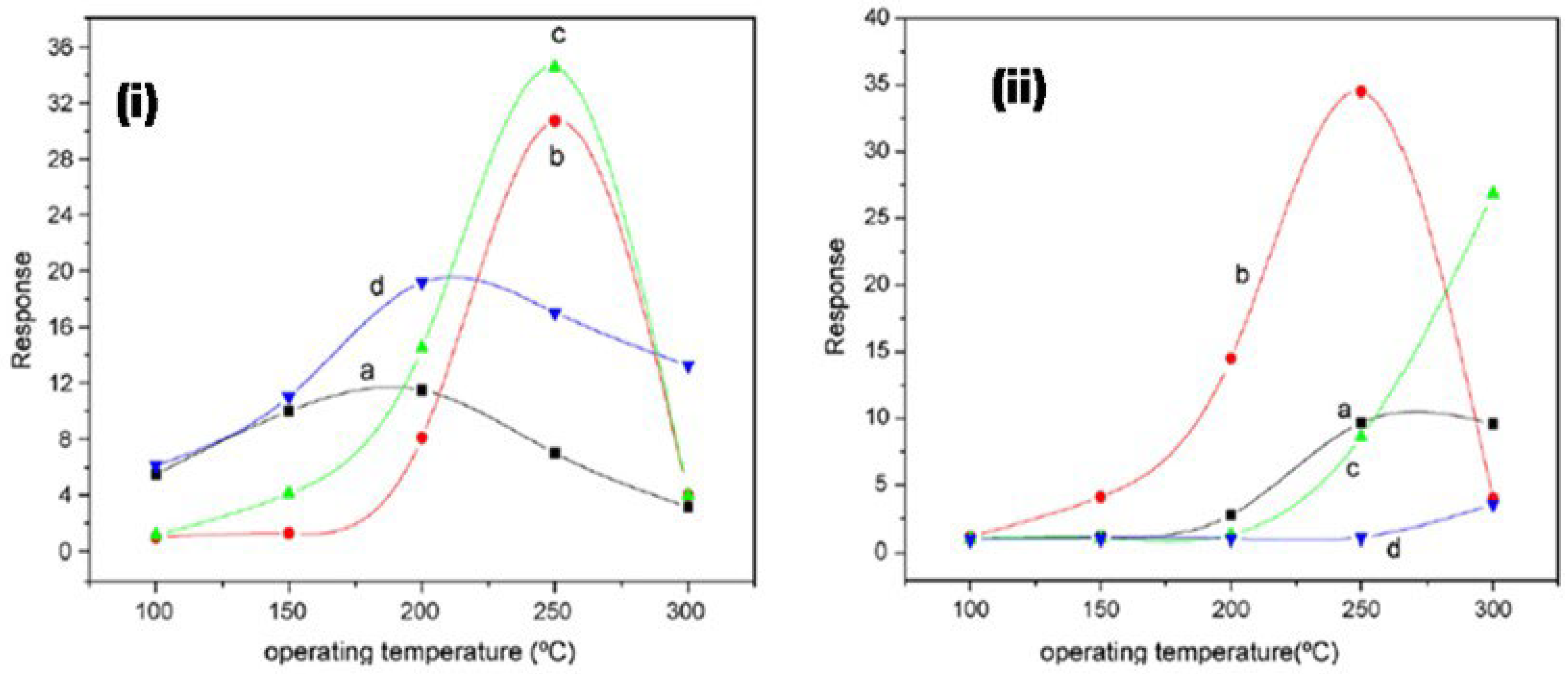
Liangyuan et al. [83] showed that a 40 mol% ZnO–60 mol% SnO2 nanocomposite had the maximum sensor response of 34.5 to NO2 when calcined at 600 °C with an operating temperature range of 250 °C, while pure nanocrystalline SnO2 prepared by the same route had the NO2 response of 7.0, as shown in Figure 27i. The enhanced gas response was attributed to the smaller SnO2 grain size of 5 nm of the 40% nanocomposite of ZnO as compared to the pure SnO2 (19 nm). As the ZnO content in the nanocomposites increased, there was an increase in the NO2 sensing response with less than 40 mol% ZnO, but with the higher ZnO loading (˃40 mol% ZnO) there was a decrease in sensing response which revealed that SnO2 mainly controlled the NO2 response. These results were in concurrence with the studies of surface area measured by BET. The gas response of the nanocomposite showed a decrease with the increase in calcination temperature, which was mainly attributed to the loss ingrain growth size and surface area, as depicted by Figure 27ii. A surface area of around 101 m2g−1 was obtained for the composite with 40% ZnO at 400 °C, which was below the surface area of the sample calcined at a temperature of 600 °C, with lower sensor response, as shown in Figure 27i. Because of this, the nanocomposite does not fit the requirement of the law of crystallinity when heated at 400 °C for sensor applications. On the other hand, there is a decrease in the nanocomposite’s sensor response when it is calcined above 800 °C; this is due to the significant crystal growth of the SnO2 nanocomposite, as a result of which there is a decrease in surface area. Thus, it was observed that ZnO-SnO2 nanocomposite sensing response is profoundly affected by operating temperature, and in a temperature range of 100 and 300 °C, it was found to be sensitive to NO2. At the 250 °C operating temperature, maximum sensing response was observed for NO2.
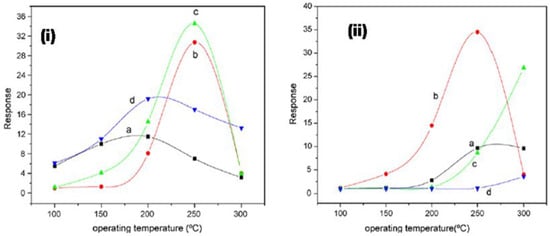
Figure 27.
(i) The response of different nanocomposites with (a) 0 mol%, (b) 20 mol%, (c) 40 mol% and (d) 60 mol% ZnO calcined at 600 °C to 500 ppm NO2; (ii) the response of 40% ZnO–60% SnO2 nanocomposites calcined at (a) 400 °C, (b) 600 °C, (c) 800 °C and (d) 1000 °C to 500 ppm NO2. (Reprinted with permission from [83]. Copyright 2008, Elsevier).
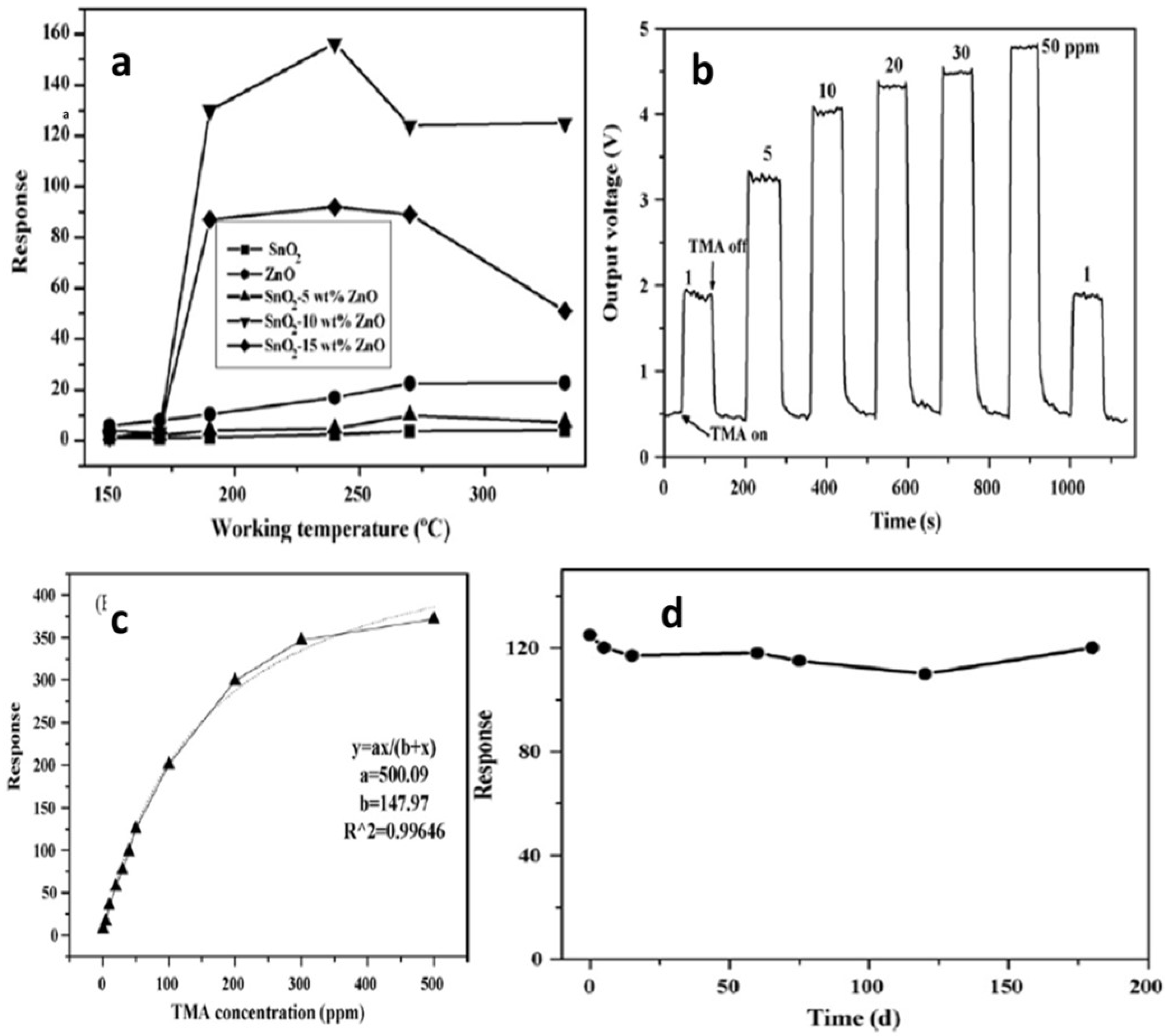
Zhang et al. [97] employed 50 ppm trimethylamine (TMA) at 150–330 °C for measuring sensing response values of SnO2-ZnO nanocomposites, SnO2 nanoparticles and ZnO microrod sensors, as shown in Figure 28a. At a temperature below 170 °C, the sensing measurement values of 10 and 15 wt% ZnO-doped SnO2 were very low, i.e., less than 10, but with the increase in working temperature to 190 °C or above, the sensor response showed a dramatic increase. At 240 °C, a high value of sensor response was obtained for both the types of sensors, i.e., 10 and 15 wt% ZnO-doped SnO2 sensors, with sensing values of 156 and 92, respectively. With the increase in temperature for pure SnO2 nanoparticles, 5 wt% ZnO-doped SnO2 and ZnO microrods, there was a slight increase in sensor response values, which at 330 °C were 4.0, 7.3 and 22.8 respectively. On the other hand, much higher sensor values were obtained at 50 ppm TMA and 190–330 °C for 10 and 15 wt% ZnO-doped SnO2 sensors compared to those of pure SnO2 nanoparticles, and the response of the 10 wt% ZnO-doped SnO2 sensor had the highest value, as shown in Figure 28b–d. By doping with a suitable amount of ZnO microrods, the sensor response to TMA was enhanced greatly for SnO2 nanoparticles. In addition, there was an increase in the charge transportation of nanocomposites caused by the addition of one-dimensional ZnO microrods into the SnO2 nanoparticles, which resulted in an enhanced sensor response. Further, a quick and high response to TMA at 190–330 °C was observed for the SnO2-ZnO nanocomposite sensor. Further, when sensing TMA at 330 °C, the SnO2-ZnO sensor displayed some enhanced sensing features such as excellent sensitivity, high selectivity, prompt response/recovery and strong stability. In determining the freshness of a dead fish, this sensor also displayed peculiar and superb sensing characteristics.
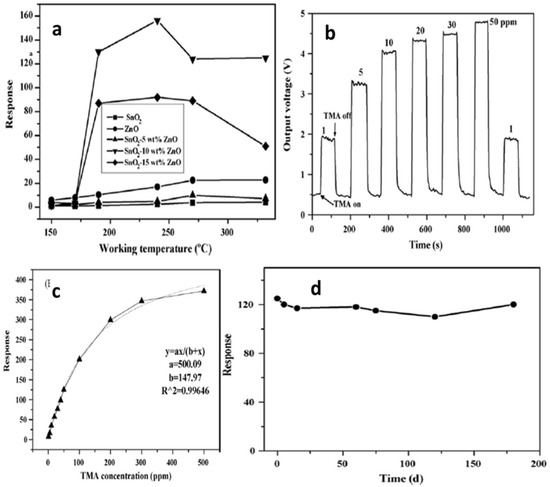
Figure 28.
(a) The response of sensors to 50 ppm TMA at different working temperatures. (b) Response transients and (c) magnitude response of 10 wt% ZnO-doped SnO2 nanocomposite sensor for different concentrations of TMA at 330 °C. (d) The stability of the SnO2-ZnO nanocomposite sensor. (Reprinted with permission from [97]. Copyright 2008, Elsevier).
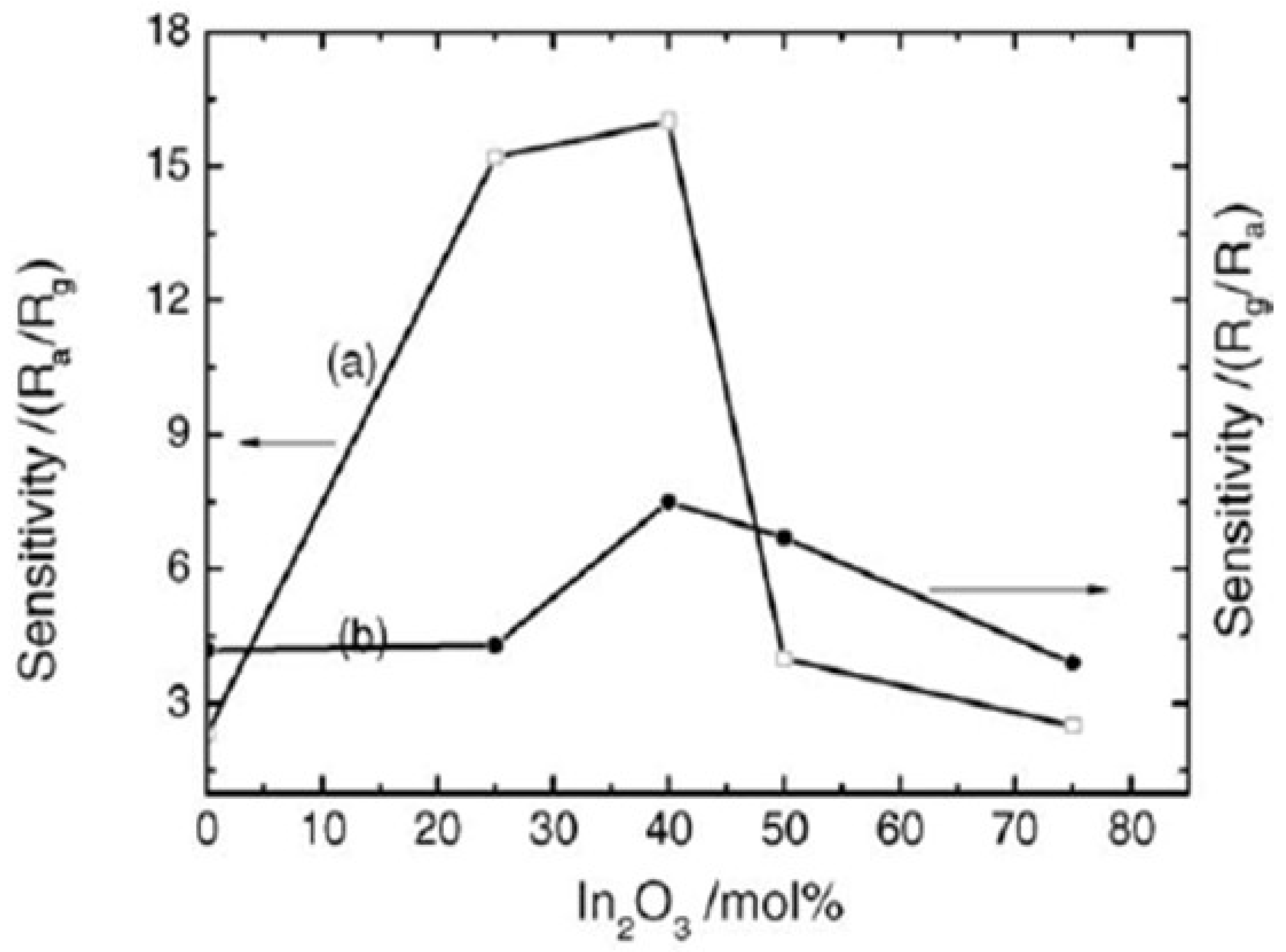
Aifan et al. [98] studied the selectivity and sensitivity of SnO2-In2O3 nanocomposites for CO and NO2 gases. Further, the effects of the operating temperature and oxide composition on gas sensitivity were studied. Figure 29 depicts the sensor values for NO2 at 150 °C and CO at 250 °C with a calcination temperature of 650 °C for different nanocomposites. Nanocomposites containing 40% In2O3 had the highest sensitivity of 16.0 and 7.5 for CO and NO2, respectively, with 0.05 M of total salt concentration and calcination temperature of 600 °C for 4 h. On the other hand, pure nanocrystalline SnO2 prepared by the same methodology had sensitivity values of only 2.3 and 4.2 for CO and NO2. Moreover, much smaller grain size and high analyte gas adsorption on the surface of nanocomposite resulted in an increase in the sensitivity. Further, selectivity and sensitivity are also affected by the operating temperature of the gas sensor. At a temperature range of 100 to 300 °C, the nanocomposites were found to be sensitive to CO and NO2. For CO and NO2, 250 °C and 200 °C as optimum operating temperatures reveal the highest sensing performance, respectively. Not only the operating temperature, but also the concentration of gas corresponds to the sensitivity of the 40% In2O3 nanocomposite. At an operating temperature in air, with the increase in gas concentration, the sensitivity increased linearly. Thus, the above discussion reveals the importance of the application of different gas sensors. It was observed that SnO2 shows great potential for application as a sensor. Using different synthetic approaches, the gas sensing property of SnO2 can be improved. In addition to the synthesis approaches, different techniques such as development of composites of SnO2, doping with different metals and deposition of metals on the surface of SnO2 nanoparticles were discussed. It was revealed that these techniques lead to the improvement of the sensing ability of pure SnO2 to a considerable extent. The detailed reaction conditions and variation of particle size of SnO2 nanoparticles prepared by various methods are represented in Table 2.
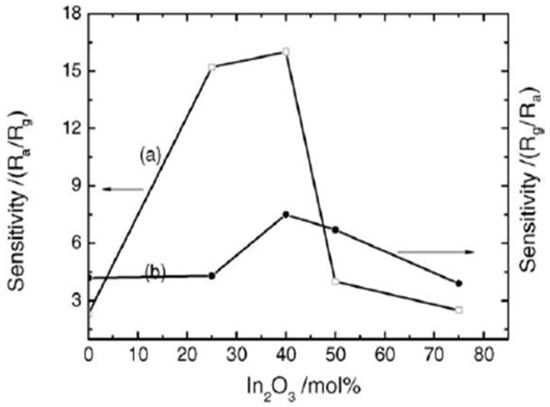
Figure 29.
The sensitivity to (a) 1000 ppm of CO at 250 °C operating temperature and (b) 450 ppm of NO2 at 150 °C operating temperature for different nanocomposites calcined at 600 °C. (Reprinted with permission from [98]. Copyright 2006, Elsevier).

Table 2.
Reaction conditions and variation of particle size of SnO2 nanoparticles prepared by various methods.
9. Sensing of CO, CH4, NO, NO2, NH3, SO2 and H2S by SnO2 Nanostructures
The semiconductor metal oxides’ gas sensing ability is very closely associated with the surface of the sensing materials with respect to their capacity of adsorbing oxygen on their surfaces. Tin oxide is an excellent semiconducting material and possesses good electrical properties, as a result of which it can be used for the detection of even low levels of CO gas. The conductance in SnO2 is predominantly affected by the number of negatively charged oxygen adsorbates on the surface of the sensing materials as a result of the transfer of electrons to the surface of the SnO2 which decreases the electron concentration and increases the resistance of the sensor. At low temperatures, the commonly chemisorbed oxygen ion is O2−, and at higher temperatures, O− and O2− are commonly chemisorbed [55,149]. When SnO2 is brought in contact with an oxidizing gas such as CO, the adsorbed oxygen reacts with the CO gas molecules on the surface of the SnO2 material, as a result of which the electrons in the adsorbed oxygen trapped are released again back to the conduction band of SnO2, thus resulting in an increase in resistance. The surface of SnO2 involves chemical reactions such as the adsorption of CO gas, which is then followed by the desorption of CO2 which causes an increase in the conductance; consequently, the surface oxygen vacancies are then replenished due to the adsorption of molecular oxygen. In the case of SnO2, the concentration of electrons depends on the stoichiometry deviation determined by the number of oxygen vacancies created by the atomic defects. Further, the electrical properties of SnO2 also strongly depend on the surface states which are produced by the oxygen and other gas molecules that are chemisorbed at the grain boundaries due to which space charge appearance and band modulation result in the SnO2. Hence, the change in the chemisorbed molecule density is the main factor supposed to be responsible for the electrical response of the SnO2 while the tin dioxide phase remains chemically stable. Similarly, other gases such as NO2 and NO are adsorbed in the same sensing mechanism in which bridging O vacancies mainly contribute to the sensing. The sensing mechanism in the case of NO2 on the SnO2 surface is mainly dominated by the adsorption of the single molecule of NO2 at the surface of O vacancies. As a result of this, one of the O atoms of NO2 fills the vacancy and results in the desorption of the weakly bonded NO from the surface of SnO2, as reported by Maiti et al. [150]. Similarly, for the H2S gas adsorption on the pure SnO2 surface, first, the adsorption of oxygen with the negative charge results in the formation of an electron depletion layer near the surface. Then the oxidation of H2S gas at the surface (O−ads) with the adsorbed oxygen into SO2 (g) and H2O (g) provides the necessary free electrons to the semiconducting core, thereby increasing the response of the sensor in proportion to the H2S concentration. This process requires the diffusion of H2S and then its subsequent oxidation. As a result, a very short response time is observed, which indicates that both the gas diffusion and the oxidation reaction are very rapid. Further, the doping of SnO2 surface with CuO can show an additional decrease in the sensor response upon exposure to the H2S at the interface between CuO and SnO2 due to the conversion of semiconducting CuO into metallic CuS [151]. Thus, the short response values were not affected by the doping with CuO. This reveals that the conversion into CuS and the diffusion and subsequent oxidation of H2S occur rapidly, which results in the sensing of H2S gas. For the sensing of NH3 gas, Yuan et al. [152] reported that WO3-SnO2 nanosheets can significantly sense NH3 after coating of SnO2 shell layer with WO3 through atomic layer deposition. The sensing mechanism is reported to be based on the formation of the depletion layer as well as the built-in electric field at the interface of the WO3 and SnO2 shell. When the NH3 gas comes in contact with the core–shell nanosheet sensors at the surface, the thickness of the electron-depleted region and heterojunction interface of WO3@SnO2 core–shell nanosheets becomes thinner, thus resulting in decreased resistance and improving the response of the sensor. In brief, the formation of the heterojunction WO3@SnO2 increases the resistance of the sensor in air, but it further decreases the resistance of the sensor in the NH3 atmosphere. As a result of these phenomena, the response of the WO3@SnO2 hetero structured nanosheet sensor is greatly improved. Hence the formation of the heterojunction as well as the electron-depleted region between the WO3 and SnO2 nanosheets results in the enhancement of the NH3 sensing response. The sensing performance comparison parameters of SnO2-based gas sensors using different morphologies are presented in Table 3.

Table 3.
Comparison of sensing performance parameters of SnO2-based gas sensors with different morphologies.
Tin oxide, as a semiconductor metal oxide, can also be employed for the sensing of SO2 gas. The origin of the sensing of SO2 gas arises from the change in the electrical conductivity of the SnO2 sensor as a result of the chemical reactions that take place on the surface of the SnO2 due to the physisorbed oxygen species and the target SO2 molecules. At the grain boundaries, the atmospheric oxygen molecules are adsorbed and trap the electrons from the interior of the SnO2 surface. Hence, at the interface of the SnO2, a space charge layer is created with air or between the adjacent grains, which results in the formation of a potential barrier. As a result of the creation of the potential barrier, the easy flow of electrons is impeded by the potential barrier; thereby, the SnO2 becomes highly resistive and thus gives a high value of initial resistance. In addition, as the temperature increases, the resistance decreases due to the semiconducting nature of SnO2. However, as the temperature is increased to around 160 °C, a small increase in resistance is observed, which is a consequence of the conversion of molecular oxygen (O2−) into atomic oxygen (2O−) and thus the trapping of more free electrons [153,154,155,156]. The interaction with the adsorbed oxygen (O−) by the SO2 gas molecules on the surface of SnO2 releases the trapped charge carriers back. Due to this phenomenon, the resistance of the surface decreases as shown in the following reaction:
SO2 + O− (adsorbed) → SO3 + e_
Thus, in the SnO2, the modulation of space charge layer at the grain boundaries and gas molecules (Fermi control energy mechanism) is thus responsible for the change in the sensor resistance to the SO2 gas [157,158]. On the surface of SnO2, the adsorption of reducing gases such as SO2 results in the creation of a new weak donor energy level within the energy band gap [157,159]. Due to this, the resistance on the SnO2 surface decreases in the presence of target gas SO2. This decrease in the resistance with an increase in the temperature is observed up to a certain temperature and may be due to donor states’ gradual ionization. As the temperature increases, there is an increase in the value of resistance as the rate of desorption of gas molecules is greater than the rate of adsorption. Thus, at higher temperatures, a minimum value of resistance and hence a maximum sensing response is obtained, and this may the possible sensing mechanism for the other types of reducing gases such as NH3, H2 and H2S as well.
The mechanism of CH4 sensing was reported by Bonu et al., who concluded from their study that CH4 being thermodynamically stable is generally detected at temperatures above 300 °C [160]. From their study, they concluded that the sensing mechanism of reducing gas CH4 is mainly attributed to the surface defect density, and the nature of defects and the relative position of defects in the band diagram are considered to be the main factors responsible for the sensing of CH4 in the SnO2 nanostructures. In addition, without having sufficient surface defects, the high surface-to-volume ratio does not affect the sensing process. Further, the in-plane and bridging “O” vacancy defects in the defect state positions explain the temperature-dependent CH4 sensing response. The defect states created close to the conduction band minimum as a result of in-plane “O” vacancies have been found to be very sensitive to the low temperatures due to the very low activation energy as compared to the bridging “O” vacancies. The metal oxides possessing such structures may also exhibit similar effects.
10. Conclusions, Challenges and Future Perspectives
In this review, we vividly discussed the synthesis of porous and hollow nanostructures of SnO2 and their unique surface morphology and modification by loading and doping with other elements. The progress in the synthetic routes of metal oxides and composites and their status in the field of gas sensing applications have been vehemently discussed. In this report, our main focus was on presenting the research status of tin oxide and its composites and giving an idea and outline to develop them for enhanced gas sensing applications, particularly in environmental remediation. The effects of different reaction conditions on the morphology, structure and other physical aspects of tin oxide and its composites and the effect of various synthetic methods employed were also briefly discussed. The hydrothermal and reverse micellar synthetic routes, with some good and innovative results reported, seem to act as great alternatives to high-temperature conventional synthetic routes. Enhanced gas sensing applications of tin oxide and its composites are reported due to these low-temperature methods which offer better control of reaction conditions to create optimal conditions for their synthesis, giving these sensors a better advantage over their counterparts. These routes are only applicable for the synthesis of cost-effective specific tin oxide and its composites, and thus they are not considered as general routes for synthesis; thus, they serve as an added advantage for these sensors in their synthesis due to their cost-effectiveness and ready availability.
With the advent and advancement of fundamental research, gas sensing serves as the main route to solve the environment-related problems of the world. However, due to increased industrialization and rapid increase in the toxic energy effluents in the environment, tin oxide and its composites still face a number of challenges as gas sensors due to their low sensitivity and selectivity towards which our focus should be concerted. An efficient gas sensor should have the capacity to sense the gases even at very low ppm levels so that even a minimal amount of toxic gases can be detected in the environment. So, in this review, our main focus was on the morphology and particle size of tin oxide and its composites which in turn has a profound effect on the sensitivity, selectivity and stability of the gas sensors. Although many efforts have been concerted, much still needs to be done with regard to fully understanding the mechanism that governs the process of gas sensing. With thorough knowledge for understanding the basic mechanism responsible for gas sensing with the aid of new evolved synthetic strategies and the development of sophisticated characterization techniques, a breakthrough in their gas sensing activity could be expected in the coming future.
In terms of future perspectives, there are still tremendous opportunities to investigate metal oxide nanomaterials and their amazing and boundless combinations with metal oxide nanocomposites for sensor applications. Although the synthetic strategies for tin oxide nanostructures have shown significant development, efforts should still be directed towards better understanding the interactions and underlying sensing mechanisms which are not still clear for tin oxide in particular and metal oxides in general, which in turn affects the sensitivity and selectivity of sensors. Moreover, these hidden sensing phenomena have a role in the design of metal oxides and the optimization of their gas sensing performance. Compared to their pure-phase counterparts, doping meticulously with specific components with desired chemical composition can produce some advantageous characteristics. In addition to the development of tin oxide and its composites as gas sensors, the mass production of these sensors for practical applications should place a special focus on cost-effective techniques for sensor immobilization, techniques with an environmentally friendly approach, improvement in the operating temperature range of gas sensors (sensors should operate at a wide range of temperatures and pH values) and high-yield synthesis of tin oxide and its composites with the exposure of specific properties. For gas sensing applications, other technical problems such as sensitivity, lack of stability and selectivity have resulted in long-term spoiling of the gas response and hence act as the limitations faced by the SnO2 sensors and their composites which must be highlighted and addressed as the need of the hour in the sensing field. The presence of various types of structural defects during the synthesis of these materials often led to insufficient quality and low production rates, and hence these materials do not meet the industrial standards for their commercialization. The structural changes introduced during the synthesis can have a large influence on the metal oxides and their composites and consequently on their sensing performance. So, to overcome these limitations of metal oxides and their composites in the gas sensing field, using synthetic chemical methods, desired morphological features can be tuned so that the surface of the material can be easily accessible to the particular target gas, which can in turn have a profound effect on the sensing parameters such as selectivity, sensitivity and stability, and the quality assurance and quality control of the sensors can also be enhanced through these proactive and selective morphological changes being incorporated into the metal oxides and their composites, which can lead to the substantial enhancements in these parameters in the future developments of sensors. The future pathway and strategies should further include the miniaturization of structural and chemical approaches with the incorporation of desired structural changes in a highly precise manner, which is still an unresolved challenge that requires further research, innovation and development. Above all, the present review will further elevate the hope to stimulate the progress and development of tin oxide and its composites with concerted efforts and strategies to be employed by researchers to achieve excellent sensing performance with good stability, high sensitivity and selectivity and environmental friendliness.
In summary, we have reviewed the recent advances in the preparatory methods, in particular, the hydrothermal and reverse micellar synthetic routes, for the tin oxide hybrid materials with enhanced and optimized control over the structural features which further led to the advancement of this unique group of materials. The engineering of unique structural features such as morphology, size, engineered defects, functionalization, crystallinity and doping which consequently leads to the precise miniaturization of the various physicochemical properties has produced miraculous results in using tin oxide and its composites in the field of gas sensing to develop particular specific gas sensors, as has been discussed above. In addition, the structural and compositional features have been effectively probed as a result of the improvements in the integration and characterization methods which have helped in better understanding the structure–property relationships within these materials. Furthermore, we discussed the sensing mechanisms and the processes governing them for the development of sensing platforms, highlighting the basic and fundamental principles governing their response, sensitivity and selectivity. In addition, emphasis has also been placed on how the compositional and structural features and the surface chemistry of the tin oxide and its hybrids play a crucial role in their electrical properties which ultimately are revealed in the form of fascinating applications such as the one in the field of gas sensing. Furthermore, tin oxide and its composites have found very wide applications in the gas sensing area due to their various physical and chemical properties such as large surface-to-volume ratio, tunable band gaps and excellent thermal stability which has been widely harnessed in the sensing field. Last but not least, the advancements in the recent past in the preparatory methods for tin oxide and its composites have been discussed, showing how an even greater degree of control over their structural and morphological features such as crystallinity, size, thickness, functionalization and doping has been achieved. Noteworthily, tin oxide and its hybrids with specific advances in the field of gas sensing were vividly probed and illustrated in the context of three major analyte are as, namely gases, ions and volatile organic compounds, with a detailed discussion of both historical and analytical perspectives.
Author Contributions
N.A.P. is responsible of literature review, data collection and rough draft of the manuscript, whereas T.A. is responsible for conceptualization, supervision and final draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
T.A. thanks the research scheme 01(2897)/17/EMR-II sponsored by CSIR, New Delhi, Government of India, for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
T.A. thanks the research scheme 01(2897)/17/EMR-II sponsored by CSIR, New Delhi, Government of India, for financial support to the Nanochemistry Research Laboratory at Jamia Millia Islamia. NAP thanks UGC, New Delhi for the research fellowship. The authors also acknowledge the DST PURSE program at CIF, Jamia Millia Islamia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tomchenko, A.A.; Harmer, G.P.; Marquis, B.T.; Allen, J.W. Semiconducting Metal Oxide Sensor Array for the Selective Detection of Combustion Gases. Sens. Actuators B Chem. 2003, 93, 126–134. [Google Scholar] [CrossRef]
- Kanan, S.M.; El-Kadri, O.M.; Abu-Yousef, I.A.; Kanan, M.C. Semiconducting Metal Oxide Based Sensors for Selective Gas Pollutant Detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef]
- Phul, R.; Perwez, M.; Ahmed, J.; Sardar, M.; Alshehri, S.M.; Alhokbany, N.; Majeed Khan, M.A.; Ahmad, T. Efficient Multifunctional Catalytic and Sensing Properties of Synthesized Ruthenium Oxide Nanoparticles. Catalysts 2020, 10, 780. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Armelao, L.; Cacciamani, A.; Mari, C.M.; Polizzi, S.; Ruffo, R.; Scotti, R.; Testino, A.; Wahba, L.; Morazzoni, F. One-Step Preparation of SnO2 and Pt-Doped SnO2 as Inverse Opal Thin Films for Gas Sensing. Chem. Mater. 2010, 22, 4083–4089. [Google Scholar] [CrossRef]
- Gyger, F.; Hübner, M.; Feldmann, C.; Barsan, N.; Weimar, U. Nanoscale SnO2 Hollow Spheres and their Application as a Gas-Sensing Material. Chem. Mater. 2010, 22, 4821–4827. [Google Scholar] [CrossRef]
- Pandit, N.A.; Shahazad, M.; Ahmad, T. Structural Characterization and Gas Sensing Applications of Ultrafine ZrO2 nanospheres Using Low Temperature Solution Route. Mater. Today Proc. 2021, 36, 724–729. [Google Scholar] [CrossRef]
- Mehtab, A.; Banerjee, S.; Mao, Y.; Ahmad, T. Type-II CuFe2O4/Graphitic-Carbon Nitride Heterojunctions for High Efficiency Photocatalytic and Electrocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2022, 14, 44317–44329. [Google Scholar] [CrossRef]
- Long, J.; Xue, W.; Xie, X.; Gu, Q.; Zhou, Y.; Chi, Y.; Chen, W.; Ding, Z.; Wang, X. Sn2+ Dopant Induced Visible-Light Activity of SnO2 Nanoparticles for H2 Production. Catal. Commun. 2011, 16, 215–219. [Google Scholar] [CrossRef]
- Jain, S.K.; Fazil, M.; Pandit, N.A.; Ali, S.A.; Naaz, F.; Khan, H.; Mehtab, A.; Ahmed, J.; Ahmad, T. Modified, Solvo thermally Derived Cr-doped SnO2 Nanostructures for Enhanced Photocatalytic and Electrochemical Water Splitting Applications. ACS Omega 2022, 7, 14138–14147. [Google Scholar] [CrossRef]
- Jain, S.K.; Pandit, N.A.; Fazil, M.; Ali, S.A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Chemical Fabrication, Structural Characterization and Photocatalytic Water Splitting Application of Sr-Doped SnO2 Nanoparticles. Nanotechnology 2022, 33, 355706. [Google Scholar] [CrossRef]
- Mehtab, A.; Alshehri, S.M.; Ahmad, T. Photocatalytic and Photoelectrocatalytic Water Splitting by Porous g-C3N4 Nanosheets for Hydrogen Generation. ACS Appl. Nano Mater. 2022, 5, 12656–12665. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, Q.; Zhang, Q.; Li, J. In Situ Growth of Mesoporous SnO2 on Multiwalled Carbon Nanotubes: A Novel Composite with Porous-Tube Structure as Anode for Lithium Batteries. Adv. Funct. Mater. 2007, 17, 2772–2778. [Google Scholar] [CrossRef]
- Zhang, H.X.; Feng, C.; Zhai, Y.C.; Jiang, K.L.; Li, Q.Q.; Fan, S.S. Cross-Stacked Carbon Nanotube Sheets Uniformly Loaded with SnO2 Nanoparticles: A Novel Binder-Free and High-Capacity Anode Material for Lithium-Ion Batteries. Adv. Mater. 2009, 21, 2299–2304. [Google Scholar] [CrossRef]
- Ding, S.; Luan, D.; Boey, F.Y.C.; Chen, J.S.; Lou, X.W. SnO2 Nanosheets Grown on Graphene Sheets with Enhanced Lithium Storage Properties. Chem. Commun. 2011, 47, 7155–7157. [Google Scholar] [CrossRef]
- Qian, J.; Liu, P.; Xiao, Y.; Jiang, Y.; Cao, Y.; Ai, X.; Yang, H. TiO2-Coated Multilayered SnO2 Hollow Microspheres for Dye-Sensitized Solar Cells. Adv. Mater. 2009, 21, 3663–3667. [Google Scholar] [CrossRef]
- Prasittichai, C.; Hupp, J.T. Surface Modification of SnO2Photoelectrodes in Dye-Sensitized Solar Cells: Significant Improvements in Photovoltage via Al2O3 Atomic Layer Deposition. J. Phys. Chem. Lett. 2010, 1, 1611–1615. [Google Scholar] [CrossRef]
- Snaith, H.J.; Stavrinadis, A.; Docampo, P.; Watt, A.A.R. Lead-Sulphide Quantum-Dot Sensitization of Tin Oxide Based Hybrid Solar Cells. Sol. Energy 2011, 85, 1283–1290. [Google Scholar] [CrossRef]
- Kay, A.; Grätzel, M. Dye-Sensitized Core-Shell Nanocrystals: Improved Efficiency of Mesoporous Tin Oxide Electrodes Coated with a Thin Layer of an Insulating Oxide. Chem. Mater. 2002, 14, 2930–2935. [Google Scholar] [CrossRef]
- Kincaid, R.; Johnson, K.; Mount, G.H.; Yonge, D.; Havig, J.; Westberg, H.; Lamb, B.; Rumburg, B. Measurement of Atmospheric Ammonia at a Dairy Using Differential Optical Absorption Spectroscopy in the Mid-Ultraviolet. Atmos. Environ. 2002, 36, 1799–1810. [Google Scholar]
- Sauvan, M.; Tournier, G.; Lalauze, R.; Pupier, C.; Pijolat, C. Gas Detection for Automotive Pollution Control. Sens. Actuators B Chem. 2002, 59, 195–202. [Google Scholar]
- Kohl, D. Function and Applications of Gas Sensors. J. Phys. D Appl. Phys. 2001, 34, R125–R149. [Google Scholar] [CrossRef]
- Ampuero, S.; Bosset, J.O. The Electronic Nose Applied to Dairy Products: A Review. Sens. Actuators B Chem. 2003, 94, 1–12. [Google Scholar] [CrossRef]
- Ahmed, J.; Ahamad, T.; Alhokbany, N.; Ahmad, T.; Hussain, A.; Salman, E.; Al-Farraj, S.; Alshehri, S.M. Molten Salts Derived Copper Tungstate Nanoparticles as Bifunctional Electro-catalysts for Electrolysis of Water and Supercapacitors. ChemElectroChem 2018, 5, 3938–3945. [Google Scholar] [CrossRef]
- Farooq, U.; Phul, R.; Alshehri, S.M.; Ahmed, J.; Ahmad, T. Electrocatalytic and Enhanced Photocatalytic Applications of Sodium Niobate Nanoparticles Developed by Citrate Precursor Route. Sci. Rep. 2019, 9, 4488. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Fazil, M.; Naaz, F.; Pandit, N.A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Silver-Doped SnO2 Nanostructures for Photocatalytic Water Splitting and Catalytic Nitrophenol Reduction. New J. Chem. 2022, 46, 2846–2857. [Google Scholar] [CrossRef]
- Periyasamy, M.; Kar, A. Modulating the Properties of SnO2 Nanocrystals: Morphological Effects on Structural, Photoluminescence, Photocatalytic, Electrochemical and Gas Sensing Properties. J. Mater. Chem. C 2020, 8, 4604–4635. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Rare Earth Doped Metal Oxide Nanoparticles for Photocatalysis: A Perspective. Nanotechnology 2022, 33, 142001. [Google Scholar] [CrossRef]
- Ahmad, T.; Ganguli, A.K. Reverse micellar route to nanocrystalline titanates (SrTiO3, Sr2TiO4 and PbTiO3): Structural aspects and dielectric properties. J. Am. Ceram. Soc. 2006, 89, 1326–1332. [Google Scholar] [CrossRef]
- Ali, S.A.; Ahmad, T. Chemical Strategies in Molybdenum based Chalcogenides Nanostructures for Photocatalysis. Int. J. Hydrogen Energy 2022, 47, 29255–29283. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Liu, J.; Li, H.Y.; Hu, Z.; Luo, X.; Gao, N.; Zhang, B.; Jiang, J.; Zhong, A.; et al. Sensitive H2 Gas Sensors Based on SnO2 Nanowires. Sens. Actuators B Chem. 2021, 345, 130334. [Google Scholar] [CrossRef]
- He, T.; Liu, W.; Lv, T.; Ma, M.; Liu, Z.; Vasiliev, A.; Li, X. MXene/SnO2 Heterojunction Based Chemical Gas Sensors. Sens. Actuators B Chem. 2021, 329, 129275. [Google Scholar] [CrossRef]
- Choi, M.S.; Mirzaei, A.; Na, H.G.; Kim, S.; Kim, D.E.; Lee, K.H.; Jin, C.; Choi, S.W. Facile and Fast Decoration of SnO2 Nanowires with Pd Embedded SnO2-x Nanoparticles for Selective NO2 Gas Sensing. Sens. Actuators B Chem. 2021, 340, 129984. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Synergistic Effects of SnO2 and Au Nanoparticles Decorated on WS2 Nanosheets for Flexible, Room-Temperature CO Gas Sensing. Sens. Actuators B Chem. 2021, 332, 129493. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Myung, J.H. Selective Ppb-Level NO2 Gas Sensor Based on SnO2-Boron Nitride Nanotubes. Sens. Actuators B Chem. 2021, 331, 129464. [Google Scholar] [CrossRef]
- Bai, J.; Wang, C.C.; Liu, K.; Wang, H.; Liu, Y.; Liu, F.; Suo, H.; Liang, X.; Zhang, C.; Liu, F.; et al. Enhanced Gas Sensing Performance Based on the PtCu Octahedral Alloy Nanocrystals Decorated SnO2 Nanoclusters. Sens. Actuators B Chem. 2021, 330, 129375. [Google Scholar] [CrossRef]
- Nejati-Moghadam, L.; Esmaeili Bafghi-Karimabad, A.; Salavati-Niasari, M.; Safardoust, H. Synthesis and Characterization of SnO2 Nanostructures Prepared by a Facile Precipitation Method. J. Nanostr. 2015, 5, 47–53. [Google Scholar]
- Liu, H.; Gong, S.; Hu, Y.; Zhao, J.; Liu, J.; Zheng, Z.; Zhou, D. Tin Oxide Nanoparticles Synthesized by Gel Combustion and Their Potential for Gas Detection. Ceram. Int. 2009, 35, 961–966. [Google Scholar] [CrossRef]
- Tan, Y.; Li, C.; Wang, Y.; Tang, J.; Ouyang, X. Fast-Response and High Sensitivity Gas Sensors Based on SnO2 hollow Spheres. Thin Solid Film. 2008, 516, 7840–7843. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Ma, C.L.; Sun, X.D.; Li, H.D. Preparation of Nanocrystalline Metal Oxide Powders with the Surfactant-Mediated Method. Inorg. Chem. Commun. 2002, 5, 751–755. [Google Scholar] [CrossRef]
- Lou, C.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Atomic Layer Deposition of ZnO on SnO2 Nanospheres for Enhanced Formaldehyde Detection. Sens. Actuators B Chem. 2021, 329, 129218. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Zhao, L.; Liu, F.; Sun, X.; Hu, X.; Lu, G. Preparation of Ce-Doped SnO2 Cuboids with Enhanced 2-Butanone Sensing Performance. Sens. Actuators B Chem. 2021, 341, 130039. [Google Scholar] [CrossRef]
- Singh, S.; Sattigeri, R.M.; Kumar, S.; Jha, P.K.; Sharma, S. Superior Room-Temperature Ammonia Sensing Using a Hydrothermally Synthesized MoS2/SnO2 Composite. ACS Omega 2021, 6, 11602–11613. [Google Scholar] [CrossRef]
- Guo, C.; Cao, M.; Hu, C. A Novel and Low-Temperature Hydrothermal Synthesis of SnO2 nanorods. Inorg. Chem. Commun. 2004, 7, 929–931. [Google Scholar] [CrossRef]
- Hieda, K.; Hyodo, T.; Shimizu, Y.; Egashira, M. Preparation of Porous Tin Dioxide Powder by Ultrasonic Spray Pyrolysis and Their Application to Sensor Materials. Sens. Actuators B Chem. 2008, 133, 144–150. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Li, Y.; Zhou, Z. Mesostructured SnO2 as Sensing Material for Gas Sensors. Solid State Electron. 2004, 48, 627–632. [Google Scholar] [CrossRef]
- Im, J.H.; Lee, J.H.; Park, D.W. Synthesis of Nano-Sized Tin Oxide Powder by Argon Plasma Jet at Atmospheric Pressure. Surf. Coatings Technol. 2008, 202, 5471–5475. [Google Scholar] [CrossRef]
- Wang, L.; Ma, S.; Xu, X.; Li, J.; Yang, T.; Cao, P.; Yun, P.; Wang, S.; Han, T. Oxygen Vacancy-Based Tb-Doped SnO2 Nanotubes as an Ultra-Sensitive Sensor for Ethanol Detection. Sens. Actuators B Chem. 2021, 344, 130111. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Chang, X.; Li, J.; Pan, L.; Jiang, Y.; Gao, W.; Gao, C.; Sun, S. Metal-Organic Framework-Derived Porous SnO2 Nanosheets with Grain Sizes Comparable to Debye Length for Formaldehyde Detection with High Response and Low Detection Limit. Sens. Actuators B Chem. 2021, 347, 130599. [Google Scholar] [CrossRef]
- Vuong, D.D.; Hien, V.X.; Trung, K.Q.; Chien, N.D. Synthesis of SnO2 Micro-Spheres, Nano-Rods and Nano-Flowers via Simple Hydrothermal Route. Phys. E Low-Dimens. Syst. Nanostructures 2011, 44, 345–349. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Tan, R.Q.; Li, Y.; Song, W.J. Solution Route to SnO2 Crystals with Controllable Morphology. Appl. Surf. Sci. 2012, 258, 1958–1963. [Google Scholar] [CrossRef]
- Sahay, P.P.; Mishra, R.K.; Pandey, S.N.; Jha, S.; Shamsuddin, M. AC Transport Properties of Nanocrystalline SnO2 semiconductor. Ceram. Int. 2012, 38, 1281–1286. [Google Scholar] [CrossRef]
- Zampolli, S.; Elmi, I.; Ahmed, F.; Passini, M.; Cardinali, G.C.; Nicoletti, S.; Dori, L. An Electronic Nose Based on Solid State Sensor Arrays for Low-Cost Indoor Air Quality Monitoring Applications. Sens. Actuators B Chem. 2004, 101, 39–46. [Google Scholar] [CrossRef]
- Bon, D.M.; Ulbrich, I.M.; De Gouw, J.A.; Warneke, C.; Kuster, W.C.; Alexander, M.L.; Baker, A.; Beyersdorf, A.J.; Blake, D.; Fall, R.; et al. Measurements of Volatile Organic Compounds at a Suburban Ground Site (T1) in Mexico City during the MILAGRO 2006 Campaign: Measurement Comparison, Emission Ratios, and Source Attribution. Atmos. Chem. Phys. 2011, 11, 2399–2421. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, W.; Zhou, Q.; Zheng, Y.; You, Z. Characterization of the Gas Sensors Based on Polymer-Coated Resonant Microcantilevers for the Detection of Volatile Organic Compounds. Anal. Chim. Acta 2010, 671, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide Semiconductor Gas Sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor Gas Sensors: Dry Synthesis and application. Angew. Chemie-Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef]
- Nascimento, E.P.; Firmino, H.C.T.; Santos, A.M.C.; Sales, H.B.; Silva, V.D.; Macedo, D.A.; Neves, G.A.; Medeiros, E.S.; Menezes, R.R. Facile Synthesis of Hollow F-Doped SnO2 Nanofibers and Their Efficiency in Ethanol Sensing. J. Am. Ceram. Soc. 2021, 104, 1297–1308. [Google Scholar] [CrossRef]
- Yan, W.; Liu, Y.; Shao, G.; Zhu, K.; Cui, S.; Wang, W.; Shen, X. Chemical Surface Adsorption and Trace Detection of Alcohol Gas in Graphene Oxide-Based Acid-Etched SnO2 Aerogels. ACS Appl. Mater. Interfaces 2021, 13, 20467–20478. [Google Scholar] [CrossRef]
- Cai, Z.; Goo, E.; Park, S. Hydrogen sensing performance and its enhanced sensing mechanisms of hollow structured SnO2 nanospheres activated by noble metal nanoparticles. J. Mater. Res. Technol. 2021, 15, 1716–1731. [Google Scholar] [CrossRef]
- Cai, Z.; Goo, E.; Park, S. Synthesis of Tin Dioxide (SnO2) Hollow Nanospheres and Its Ethanol-Sensing Performance Augmented by Gold Nanoparticle Decoration. J. Alloys Compd. 2021, 883, 160868. [Google Scholar] [CrossRef]
- Sohal, M.K.; Mahajan, A.; Gasso, S.; Nahirniak, S.V.; Dontsova, T.A.; Singh, R.C. Modification of SnO2 Surface Oxygen Vacancies through Er Doping for Ultralow NO2 Detection. Mater. Res. Bull. 2021, 133, 111051. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and Practical Aspects in the Design of Nanoscaled SnO2 Gas Sensors: A Status Report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and Metal Oxide Nanoparticles in Chemiresistors: Does the Nanoscale Matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ganguli, A.K. Structural and Dielectric Characterization of Nanocrystalline (Ba,Pb) ZrO3 Developed by Reverse Micellar Synthesis. J. Am. Ceram. Soc. 2006, 89, 3140–3146. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahmed, J.; Ahamad, T.; Alhokbany, N.; Arunachalam, P.; Al-Mayouf, A.M.; Ahmad, T. Synthesis, Characterization, Multifunctional Electrochemical (OGR/ORR/SCs) and Photodegradable Activities of ZnWO4 Nanobricks. J. Solgel Sci. Technol. 2018, 87, 137–146. [Google Scholar] [CrossRef]
- Ahmad, T.; Ganguli, A.K. Synthesis of nanometer-sized particles of bariumorthotitanate prepared through a modified reverse micellar route: Structural characterization, phase stability and dielectric properties. J. Mater. Res. 2004, 19, 2905–2912. [Google Scholar] [CrossRef]
- Ahmad, T.; Ganguly, A.; Ahmed, J.; Ganguli, A.K.; Alhartomy, O.A.A. Nanorods of Transition Metal Oxalates: A Versatile Route to the Oxide Nanoparticles. Arab. J. Chem. 2011, 4, 125–134. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, W.; Cao, Y.; Guan, Y.; Sun, Y.; Lu, G. Porous SnO2 Hierarchical Nanosheets: Hydrothermal Preparation, Growth Mechanism, and Gas Sensing Properties. Cryst. Eng. Comm. 2011, 13, 3718–3724. [Google Scholar] [CrossRef]
- Chiu, H.C.; Yeh, C.S. Hydrothermal Synthesis of SnO2 Nanoparticles and their Gas-Sensing of Alcohol. J. Phys. Chem. C 2007, 111, 7256–7259. [Google Scholar] [CrossRef]
- Suematsu, K.; Shin, Y.; Hua, Z.; Yoshida, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Nanoparticle Cluster Gas Sensor: Controlled Clustering of SnO2 Nanoparticles for Highly Sensitive Toluene Detection. ACS Appl. Mater. Interfaces 2014, 6, 5319–5326. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Lin, H. Preparation of Band Gap Tunable SnO2 Nanotubes and their Ethanol Sensing Properties. Langmuir 2011, 27, 3977–3981. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Chen, Z.; Ma, C.; Xing, L.; Chen, Y.; Wang, Y.; Wang, T. One-Step Synthesis and Gas-Sensing Characteristics of Uniformly Loaded Pt@SnO2 Nanorods. J. Phys. Chem. C 2010, 114, 3968–3972. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.W.; Sun, X.; Xu, C.L.; Yan, C.H. Synthesis, Structure, Texture, and CO Sensing Behavior of Nanocrystalline Tin Oxide Doped with Scandia. J. Phys. Chem. B 2005, 109, 3269–3278. [Google Scholar] [CrossRef]
- Li, H.; Xie, W.; Ye, T.; Liu, B.; Xiao, S.; Wang, C.; Wang, Y.; Li, Q.; Wang, T. Temperature-Dependent Abnormal and Tunable p-n Response of Tungsten Oxide-Tin Oxide Based Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 24887–24894. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Wu, X.L.; Guo, Y.G.; Wan, L.J. SnO2-Based Hierarchical Nanomicrostructures: Facile Synthesis and Their Applications in Gas Sensors and Lithium-Ion Batteries. J. Phys. Chem. C 2009, 113, 14213–14219. [Google Scholar] [CrossRef]
- Hidalgo, P.; Castro, R.H.R.; Coelho, A.C.V.; Gouvêa, D. Surface Segregation and Consequent SO2 Sensor Response in SnO2-NiO. Chem. Mater. 2005, 17, 4149–4153. [Google Scholar] [CrossRef]
- Leite, E.R.; Weber, I.T.; Longo, E.; Varela, J.A. A New Method to Control Particle Size and Particle Size Distribution of SnO2 Nanoparticles for Gas Sensor Applications. Adv. Mater. 2000, 12, 965–968. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Ahmad, T.; Vaidya, S.; Ahmed, J. Microemulsion Route to the Synthesis of Nanoparticles. Pure Appl. Chem. 2008, 80, 2451–2477. [Google Scholar] [CrossRef]
- Ahmad, T.; Ubaidullah, M.; Shahazad, M.; Kumar, D.; Al-Hartomy, O.A. Reverse Micellar Synthesis, Structural Characterization and Dielectric Properties of Sr-doped BaZrO3Nanoparticles. Mater. Chem. Phys. 2017, 185, 31–38. [Google Scholar] [CrossRef]
- Ahmed, J.; Vaidya, S.; Ahmad, T.; Sujatha Devi, P.; Das, D.; Ganguli, A.K. Tin Dioxide Nanoparticles: Reverse Micellar Synthesis and Gas Sensing Properties. Mater. Res. Bull. 2008, 43, 264–271. [Google Scholar] [CrossRef]
- Zhang, D.F.; Sun, L.D.; Xu, G.; Yan, C.H. Size-Controllable One-Dimensional SnO2 Nanocrystals: Synthesis, Growth Mechanism, and Gas Sensing Property. Phys. Chem. Chem. Phys. 2006, 8, 4874–4880. [Google Scholar] [CrossRef] [PubMed]
- Liangyuan, C.; Shouli, B.; Guojun, Z.; Dianqing, L.; Aifan, C.; Liu, C.C. Synthesis of ZnO-SnO2 Nanocomposites by Microemulsion and Sensing Properties for NO2. Sens. Actuators B Chem. 2008, 134, 360–366. [Google Scholar] [CrossRef]
- Huang, J.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N.; Kunitake, T. Nanotubular SnO2 Templated by Cellulose Fibers: Synthesis and Gas Sensing. Chem. Mater. 2005, 17, 3513–3518. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L. Synthesis and Characterization of Nanocrystalline Tin Oxide by Sol-Gel Method. J. Solid State Chem. 2004, 177, 1425–1430. [Google Scholar] [CrossRef]
- Rella, R.; Serra, A.; Siciliano, P.; Vasanelli, L.; De, G.; Licciulli, A. CO Sensing Properties of SnO2 Thin Films Prepared by the Sol-Gel Process. Thin Solid Films 1997, 304, 339–343. [Google Scholar] [CrossRef]
- Han, X.; Jin, M.; Xie, S.; Kuang, Q.; Jiang, Z.; Jiang, Y.; Xie, Z.; Zheng, L. Synthesis of Tin Dioxide Octahedral Nanoparticles with Exposed High-Energy {221} Facets and Enhanced Gas-Sensing Properties. Angew. Chemie-Int. Ed. 2009, 48, 9180–9183. [Google Scholar] [CrossRef]
- Park, J.S.; Jeong, J.K.; Chung, H.J.; Mo, Y.G.; Kim, H.D. Electronic Transport Properties of Amorphous Indium-Gallium-Zinc Oxide Semiconductor upon Exposure to Water. Appl. Phys. Lett. 2008, 92, 95–98. [Google Scholar] [CrossRef]
- Yang, H.X.; Qian, J.F.; Chen, Z.X.; Ai, X.P.; Cao, Y.L. Multilayered Nanocrystalline SnO2 Hollow Microspheres Synthesized by Chemically Induced Self-Assembly in the Hydrothermal Environment. J. Phys. Chem. C 2007, 111, 14067–14071. [Google Scholar] [CrossRef]
- Manno, D.; Micocci, G.; Rella, R.; Serra, A.; Taurino, A.; Tepore, A. Titanium Oxide Thin Films for NH3 monitoring: Structural and Physical Characterizations. J. Appl. Phys. 1997, 82, 54–59. [Google Scholar] [CrossRef]
- Li, G.J.; Kawi, S. Synthesis, Characterization and Sensing Application of Novel Semiconductor Oxides. Talanta 1998, 45, 759–766. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of Tin Oxide Surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Dolbec, R.; El Khakani, M.A. Sub-Ppm Sensitivity towards Carbon Monoxide by Means of Pulsed Laser Deposited SnO2: Pt Based Sensors. Appl. Phys. Lett. 2007, 90, 173114. [Google Scholar] [CrossRef]
- Esfandyarpour, B.; Mohajerzadeh, S.; Famini, S.; Khodadadi, A.; AslSoleimani, E. High Sensitivity Pt-doped SnO2 Gas Sensors Fabricated Using Sol-Gel Solution on Micromachined (1 0 0) Si Substrates. Sens. Actuators B Chem. 2004, 100, 190–194. [Google Scholar] [CrossRef]
- Ménini, P.; Parret, F.; Guerrero, M.; Soulantica, K.; Erades, L.; Maisonnat, A.; Chaudret, B. CO Response of a Nanostructured SnO2 Gas Sensor Doped with Palladium and Platinum. Sens. Actuators B Chem. 2004, 103, 111–114. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, P.; Wang, Y.; Ma, Y.; Zhong, J.; Sun, X. Facile Fabrication of a Well-Ordered Porous Cu-Doped SnO2 thin Film for H2S Sensing. ACS Appl. Mater. Interfaces 2014, 6, 14975–14980. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, W.D. De Fabrication of SnO2-ZnO Nanocomposite Sensor for Selective Sensing of Trimethylamine and the Freshness of Fishes. Sens. Actuators B Chem. 2008, 134, 403–408. [Google Scholar] [CrossRef]
- Chen, A.; Huang, X.; Tong, Z.; Bai, S.; Luo, R.; Liu, C.C. Preparation, Characterization and Gas-Sensing Properties of SnO2-In2O3 Nanocomposite Oxides. Sens. Actuators B Chem. 2006, 115, 316–321. [Google Scholar] [CrossRef]
- Wang, C.; Ge, M.; Xu, X.; Zhou, Y.; Zhang, Z. Large-Scale Synthesis of SnO2 Nanosheets with High Lithium Storage Capacity. J. Am. Chem. Soc. 2010, 132, 46–47. [Google Scholar] [CrossRef]
- Lou, X.W.; Yuan, C.; Archer, L.A. Shell-by-Shell Synthesis of Tin Oxide Hollow Colloids with Nanoarchitectured Walls: Cavity Size Tuning and Functionalization. Small 2007, 3, 261–265. [Google Scholar] [CrossRef]
- Mehrabi Matin, B.; Mortazavi, Y.; Khodadadi, A.A.; Abbasi, A.; Anaraki Firooz, A. Alkaline- and Template-Free Hydrothermal Synthesis of Stable SnO2 Nanoparticles and Nanorods for CO and Ethanol Gas Sensing. Sens. Actuators B Chem. 2010, 151, 140–145. [Google Scholar] [CrossRef]
- Niu, M.; Huang, F.; Cui, L.; Huang, P.; Yu, Y.; Wang, Y.N. Hydrothermal Synthesis, Structural Characteristics, and Enhanced Photocatalysis of SnO2/α-Fe2O3 Semiconductor Nano heterostructures. ACS Nano 2010, 4, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Tan, R.; Cui, P.; Li, Y.; Song, W. Synthesis of SnO2 Nano-Sheets by a Template-Free Hydrothermal Method. Mater. Lett. 2009, 63, 2085–2088. [Google Scholar] [CrossRef]
- Talebian, N.; Jafarinezhad, F. Morphology-Controlled Synthesis of SnO2 Nanostructures Using Hydrothermal Method and Their Photocatalytic Applications. Ceram. Int. 2013, 39, 8311–8317. [Google Scholar] [CrossRef]
- Firooz, A.A.; Mahjoub, A.R.; Khodadadi, A.A. Preparation of SnO2 Nanoparticles and Nanorods by Using a Hydrothermal Method at Low Temperature. Mater. Lett. 2008, 62, 1789–1792. [Google Scholar] [CrossRef]
- Lupan, O.; Chow, L.; Chai, G.; Schulte, A.; Park, S.; Heinrich, H. A Rapid Hydrothermal Synthesis of Rutile SnO2 Nanowires. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2009, 157, 101–104. [Google Scholar] [CrossRef]
- Du, F.; Guo, Z.; Li, G. Hydrothermal Synthesis of SnO2 Hollow Microspheres. Mater. Lett. 2005, 59, 2563–2565. [Google Scholar] [CrossRef]
- Lupan, O.; Chow, L.; Chai, G.; Heinrich, H.; Park, S.; Schulte, A. Synthesis of One-Dimensional SnO2 Nanorods via a Hydrothermal Technique. Phys. E Low-Dimens. Syst. Nanostructures 2009, 41, 533–536. [Google Scholar] [CrossRef]
- Lou, X.W.; Chen, J.S.; Chen, P.; Archer, L.A. One-Pot Synthesis of Carbon-Coated SnO2 Nanocolloids with Improved Reversible Lithium Storage Properties. Chem. Mater. 2009, 21, 2868–2874. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, D.; Yu, G.; Zhang, H.; Yao, K. A Simple Hydrothermal Route for Synthesizing SnO2 Quantum Dots. Nanotechnology 2006, 17, 2386–2389. [Google Scholar] [CrossRef]
- Zhang, D.F.; Sun, L.D.; Yin, J.L.; Yan, C.H. Low-Temperature Fabrication of Highly Crystalline SnO2 Nanorods. Adv. Mater. 2003, 15, 1022–1025. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Rao, M.M.; Venugopal, N.; Kim, K.B. Hydrothermal Synthesis of SnO2-V2O5 Mixed Oxide and Electrochemical Screening of Carbon Nano-Tubes (CNT), V2O5, V2O5-CNT, and SnO2-V2O5-CNT Electrodes for Supercapacitor Applications. J. Power Sources 2007, 166, 578–583. [Google Scholar] [CrossRef]
- Dou, X.; Sabba, D.; Mathews, N.; Wong, L.H.; Lam, Y.M.; Mhaisalkar, S. Hydrothermal Synthesis of High Electron Mobility Zn-Doped SnO2Nanoflowers as Photoanode Material for Efficient Dye-Sensitized Solar Cells. Chem. Mater. 2011, 23, 3938–3945. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yao, L.; Zhang, G.; Dionysiou, D.D.; Li, J.; Du, X. One-Step Hydrothermal Synthesis of High-Performance Visible-Light-Driven SnS2/SnO2NanoheterojunctionPhotocatalyst for the Reduction of Aqueous Cr(VI). Appl. Catal. B Environ. 2014, 144, 730–738. [Google Scholar] [CrossRef]
- Zhao, B.; Shao, G.; Fan, B.; Li, W.; Pian, X.; Zhang, R. Enhanced Electromagnetic Wave Absorption Properties of Ni-SnO2 Core-Shell Composites Synthesized by a Simple Hydrothermal Method. Mater. Lett. 2014, 121, 118–121. [Google Scholar] [CrossRef]
- Xue, X.; Xing, L.; Chen, Y.; Shi, S.; Wang, Y.; Wang, T. Synthesis and H2S Sensing Properties of CuO-SnO2 Core/Shell PN-Junction Nanorods. J. Phys. Chem. C 2008, 4, 12157–12160. [Google Scholar] [CrossRef]
- Qin, L.; Xu, J.; Dong, X.; Pan, Q.; Cheng, Z.; Xiang, Q.; Li, F. The Template-Free Synthesis of Square-Shaped SnO2 Nanowires: The Temperature Effect and Acetone Gas Sensors. Nanotechnology 2008, 19, 185705. [Google Scholar] [CrossRef]
- Wang, J.; Du, N.; Zhang, H.; Yu, J.; Yang, D. Large-Scale Synthesis of SnO2 Nanotube Arrays as High-Performance Anode Materials of Li-Ion Batteries. J. Phys. Chem. C 2011, 115, 11302–11305. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Yu, J.; Qian, Y. Chemical Control Synthesis of Nanocrystalline SnO2 by Hydrothermal Reaction. Mater. Lett. 1999, 40, 23–26. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Dai, L.; Cao, C.; Guo, X. Influence of Surfactants on the Morphology of SnO2 Nanocrystals Prepared via a Hydrothermal Method. J. Solid State Chem. 2012, 189, 49–56. [Google Scholar] [CrossRef]
- Kim, C.; Noh, M.; Choi, M.; Cho, J.; Park, B. Critical Size of a Nano SnO2 Electrode for Li-Secondary Battery. Chem. Mater. 2005, 17, 3297–3301. [Google Scholar] [CrossRef]
- Lin, Y.S.; Duh, J.G.; Hung, M.H. Shell-by-Shell Synthesis and Applications of Carbon-Coated SnO2 Hollow Nanospheres in Lithium-Ion Battery. J. Phys. Chem. C 2010, 114, 13136–13141. [Google Scholar] [CrossRef]
- Ahmad, T.; Khatoon, S.; Coolahan, K. Optical and Magnetic Properties of Sn1-xMnxO2 Dilute Magnetic Semiconductor Nanoparticles. J. Alloys Compd. 2014, 615, 263–269. [Google Scholar] [CrossRef]
- Ahmad, T.; Khatoon, S. Structural Characterization and Properties of Nanocrystalline Sn1-xCoxO2 Based Dilute Magnetic Semiconductors. J. Mater. Res. 2015, 30, 1611–1618. [Google Scholar] [CrossRef]
- Ahmad, T.; Khatoon, S.; Coolahan, K. Structural, Optical, and Magnetic Properties of Nickel-Doped Tin Dioxide Nanoparticles Synthesized by Solvothermal Method. J. Am. Ceram. Soc. 2016, 99, 1207–1211. [Google Scholar] [CrossRef]
- Murase, K.; Morrison, K.L.; Tam, P.Y.; Stafford, R.L.; Jurnak, F.; Weiss, G.A. EF-Tu Binding Peptides Identified, Dissected, and Affinity Optimized by Phage Display GDP Conformation of EF-Tu. Because EF-Tu Is Abundant in the Cell, Other Functions Have Been Suggested, Particularly during Periods of Cellular Stress When Protein Syn-G. Chem. Biol. 2003, 10, 161–168. [Google Scholar] [CrossRef]
- Cassia-Santos, M.R.; Souza, A.G.; Soledade, L.E.B.; Varela, J.A.; Longo, E. Thermal and Structural Investigation of (Sn1-xTix)O2 Obtained by the Polymeric Precursor Method. J. Therm. Anal. Calorim. 2005, 79, 415–420. [Google Scholar] [CrossRef]
- López Morales, F.; Zayas, T.; Contreras, O.E.; Salgado, L. Effect of Sn Precursor on the Synthesis of SnO2 and Sb-Doped SnO2 Particles via Polymeric Precursor Method. Front. Mater. Sci. 2013, 7, 387–395. [Google Scholar] [CrossRef]
- Rodrigues, E.C.P.E.; Olivi, P. Preparation and Characterization of Sb-Doped SnO2 Films with Controlled Stoichiometry from Polymeric Precursors. J. Phys. Chem. Solids 2003, 64, 1105–1112. [Google Scholar] [CrossRef]
- Carreo, N.L.V.; Maciel, A.P.; Leite, E.R.; Lisboa-Filho, P.N.; Longo, E.; Valentini, A.; Probst, L.F.D.; Paiva-Santos, C.O.; Schreiner, W.H. The Influence of Cation Segregation on the Methanol Decomposition on Nanostructured SnO2. Sens. Actuators B Chem. 2002, 86, 185–192. [Google Scholar] [CrossRef]
- Leite, E.R.; Cerri, J.A.; Longo, E.; Varela, J.A.; Paskocima, C.A. Sintering of Ultrafine Undoped SnO2 Powder. J. Eur. Ceram. Soc. 2001, 21, 669–675. [Google Scholar] [CrossRef]
- Castro, R.H.R.; Hidalgo, P.; Muccillo, R.; Gouvêa, D. Microstructure and Structure of NiO–SnO2 and Fe2O3–SnO2 Systems. Appl. Surf. Sci. 2003, 214, 172–177. [Google Scholar] [CrossRef]
- Maciel, A.P.; Lisboa-Filho, P.N.; Leite, E.R.; Paiva-Santos, C.O.; Schreiner, W.H.; Maniette, Y.; Longo, E. Microstructural and Morphological Analysis of Pure and Ce-Doped Tin Dioxide Nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 707–713. [Google Scholar] [CrossRef]
- Aragón, F.H.; Coaquira, J.A.H.; Hidalgo, P.; Brito, S.L.M.; Gouvêa, D.; Castro, R.H.R. Structural and Magnetic Properties of Pure and Nickel Doped SnO2 Nanoparticles. J. Phys. Condens. Mater. 2010, 22, 496003. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.H.R.; Pereira, G.J.; Gouvêa, D. Surface Modification of SnO2 Nanoparticles Containing Mg or Fe: Effects on Sintering. Appl. Surf. Sci. 2007, 253, 4581–4585. [Google Scholar] [CrossRef]
- Yuasa, M.; Masaki, T.; Kida, T.; Shimanoe, K.; Yamazoe, N. Nano-Sized PdO Loaded SnO2 Nanoparticles by Reverse Micelle Method for Highly Sensitive CO Gas Sensor. Sens. Actuators B Chem. 2009, 136, 99–104. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Wang, W.H.; Bao, M. Expeditious synthesis of SnO2 nanoparticles through controlled hydrolysis and condensation of Tin alkoxide in reverse microemulsion. Ceram. Int. 2017, 43, 4702–4705. [Google Scholar] [CrossRef]
- Zamand, N.; Nakhaei Pour, A.; Housaindokht, M.R.; Izadyar, M. Size-Controlled Synthesis of SnO2 Nanoparticles Using Reverse Microemulsion Method. Solid State Sci. 2014, 33, 6–11. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y. Ultrasensitive and Low Detection Limit of Acetone Gas Sensor Based on ZnO/SnO2 thick Films. RSC Adv. 2020, 10, 35958–35965. [Google Scholar] [CrossRef]
- Salimian Rizi, V.; Sharifianjazi, F.; Jafarikhorami, H.; Parvin, N.; Saei Fard, L.; Irani, M.; Esmaeilkhanian, A. Sol-Gel Derived SnO2/Ag2O Ceramic Nanocomposite for H2 Gas Sensing Applications. Mater. Res. Express 2019, 6, 1150g2. [Google Scholar] [CrossRef]
- Marzec, A.; Radecka, M.; Maziarz, W.; Kusior, A.; Pedzich, Z. Structural, Optical and Electrical Properties of Nanocrystalline TiO2, SnO2 and Their Composites Obtained by the Sol-Gel Method. J. Eur. Ceram. Soc. 2016, 36, 2981–2989. [Google Scholar] [CrossRef]
- Yin, X.T.; Tao, L. Fabrication and Gas Sensing Properties of Au-Loaded SnO2 Composite Nanoparticles for Low Concentration Hydrogen. J. Alloys Compd. 2017, 727, 254–259. [Google Scholar] [CrossRef]
- Yin, X.T.; Wu, S.S.; Dastan, D.; Nie, S.; Liu, Y.; Li, Z.G.; Zhou, Y.W.; Li, J.; Faik, A.; Shan, K.; et al. Sensing Selectivity of SnO2-Mn3O4 Nanocomposite Sensors for the Detection of H2 and CO Gases. Surf. Interfaces 2021, 25, 101190. [Google Scholar] [CrossRef]
- Joshi, G.; Rajput, J.K.; Purohit, L.P. SnO2–Co3O4 Pores Composites for CO2 Gas Sensing at Low Operating Temperature. Microporous Mesoporous Mater. 2021, 326, 2–9. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Wang, Z.; Tsukimoto, S.; Saito, M.; Ikuhara, Y. Selective Detection of Formaldehyde Gas Using a Cd-Doped TiO2-SnO2 Sensor. Sensors 2009, 9, 9029–9038. [Google Scholar] [CrossRef]
- Chesler, P.; Hornoiu, C.; Mihaiu, S.; Vladut, C.; Moreno, J.M.C.; Anastasescu, M.; Moldovan, C.; Brasoveanu, C.; Firtat, B.; Muscalu, G.; et al. Nanostructured SnO2-ZnO Composite Gas Sensors for Selective Detection of Carbon Monoxide. Beilstein J. Nanotechnol. 2016, 7, 2045–2056. [Google Scholar] [CrossRef]
- Wen, Z.; Tian-mo, L. Gas-Sensing Properties of SnO2-TiO2-Based Sensor for Volatile Organic Compound Gas and Its Sensing Mechanism. Phys. B Condens. Mater. 2010, 405, 1345–1348. [Google Scholar] [CrossRef]
- Jian, K.S.; Chang, C.J.; Wu, J.J.; Chang, Y.C.; Tsay, C.Y.; Chen, J.H.; Horng, T.L.; Lee, G.J.; Karuppasamy, L.; Anandan, S.; et al. High Response CO Sensor Based on a Polyaniline/SnO2 Nanocomposite. Polymers 2019, 11, 184. [Google Scholar] [CrossRef]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef]
- Maiti, A.; Rodriguez, J.A.; Law, M.; Kung, P.; McKinney, J.R.; Yang, P. SnO2 Nanoribbons as NO2 Sensors: Insights from First Principles Calculations. Nano Lett. 2003, 3, 1025–1028. [Google Scholar] [CrossRef]
- Hwang, I.S.; Choi, J.K.; Kim, S.J.; Dong, K.Y.; Kwon, J.H.; Ju, B.K.; Lee, J.H. Enhanced H2S Sensing Characteristics of SnO2 Nanowires Functionalized with CuO. Sens. Actuators B Chem. 2009, 142, 105–110. [Google Scholar] [CrossRef]
- Yuan, K.P.; Zhu, L.Y.; Yang, J.H.; Hang, C.Z.; Tao, J.J.; Ma, H.P.; Jiang, A.Q.; Zhang, D.W.; Lu, H.L. Precise Preparation of WO3@SnO2 Core Shell Nanosheets for Efficient NH3 Gas Sensing. J. Colloid Interface Sci. 2020, 568, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Koziej, D.; Weimar, U. Metal Oxide-Based Gas Sensor Research: How To? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Yamazoe, N. New Approaches for Improving Semiconductor Gas Sensors. Sens. Actuators B. Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Comini, E. Metal Oxide Nano-Crystals for Gas Sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef]
- Murata, N.; Suzuki, T.; Lin, Y.; Nitani, H.; Niwa, Y.; Wada, T.; Uo, M.; Asakura, K. Structure of Atomically Dispersed Pt in a SnO2 Thin Film under Reaction Conditions: Origin of Its High Performance in Micro Electromechanical System Gas Sensor Catalysis. ACS Appl. Mater. Interfaces 2022, 14, 39507–39514. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. A Low Temperature Operated NO2 Gas Sensor Based on TeO2/SnO2 p-n Heterointerface. Sens. Actuators B Chem. 2013, 176, 875–883. [Google Scholar] [CrossRef]
- Haridas, D.; Sreenivas, K.; Gupta, V. Improved Response Characteristics of SnO2 Thin Film Loaded with Nanoscale Catalysts for LPG Detection. Sens. Actuators B Chem. 2008, 133, 270–275. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen Ionosorption and Spectroscopic Evidence for Adsorbed Oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef]
- Bonu, V.; Das, A.; Prasad, A.K.; Krishna, N.G.; Dhara, S.; Tyagi, A.K. Influence of in-plane and bridging oxygen vacancies of SnO2 nanostructures on CH4 sensing at low operating temperatures. Appl. Phys. Lett. 2014, 105, 243102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).