Electron Spin Resonance Dosimetry Studies of Irradiated Sulfite Salts
Abstract
1. Introduction
2. Results and Discussion
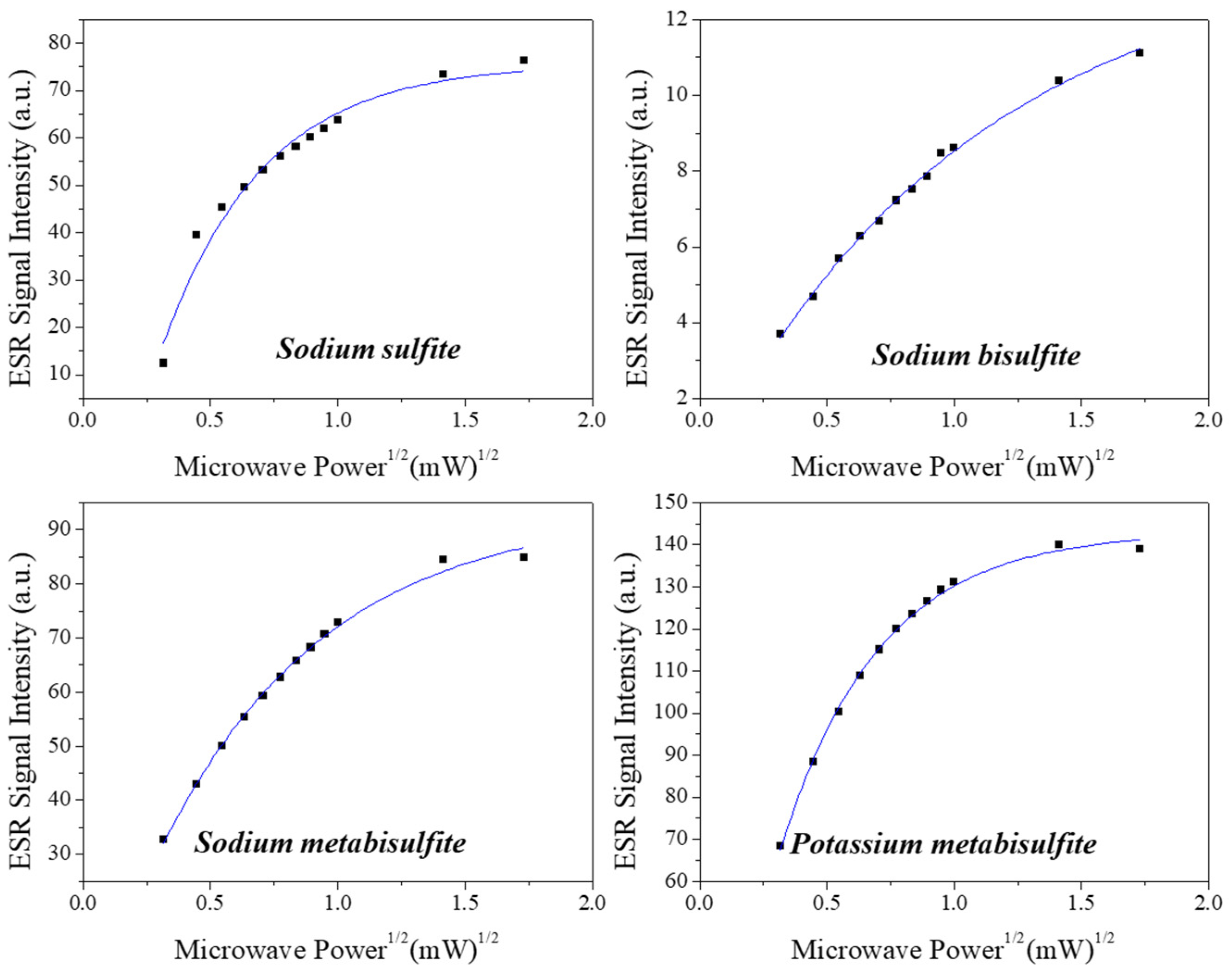
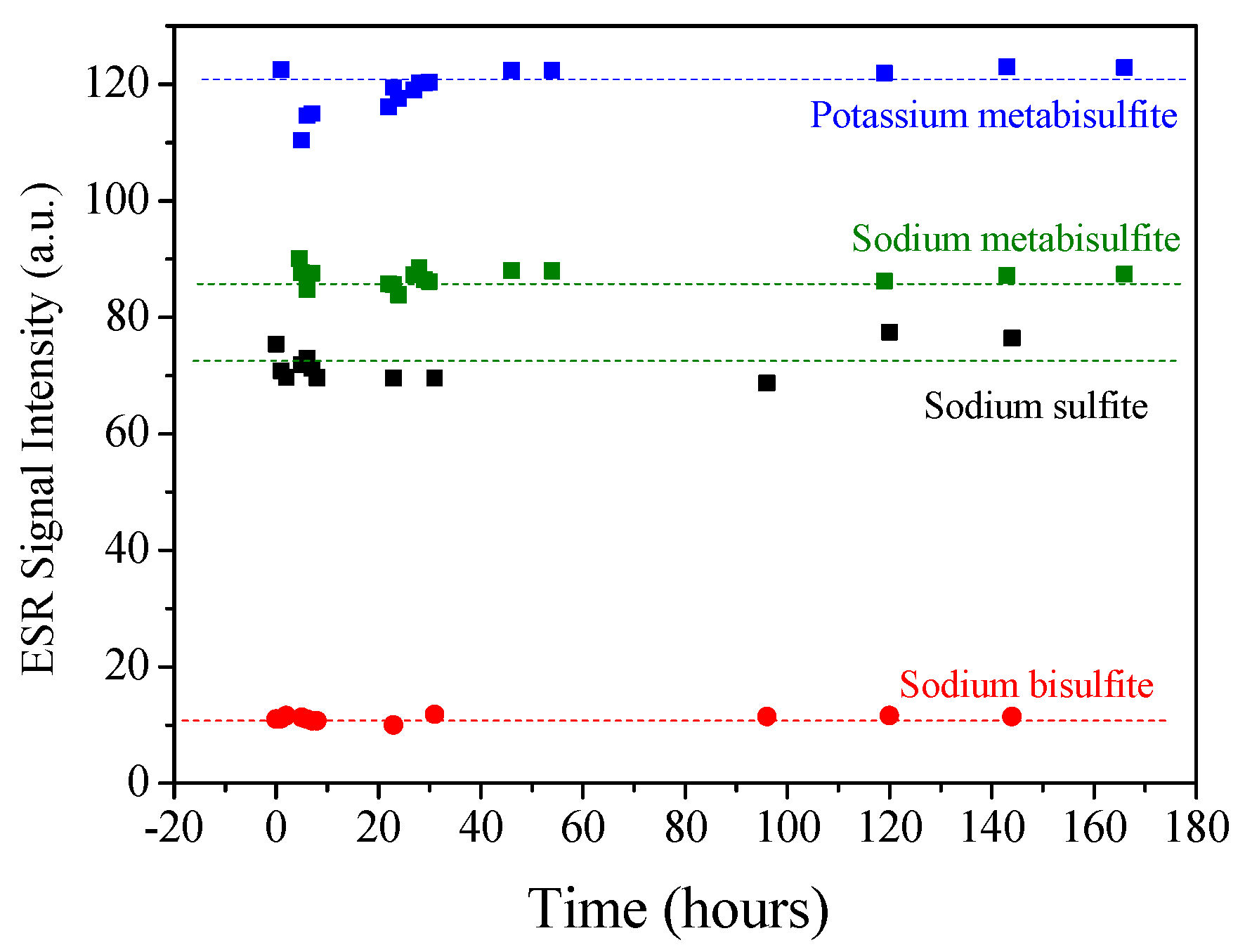
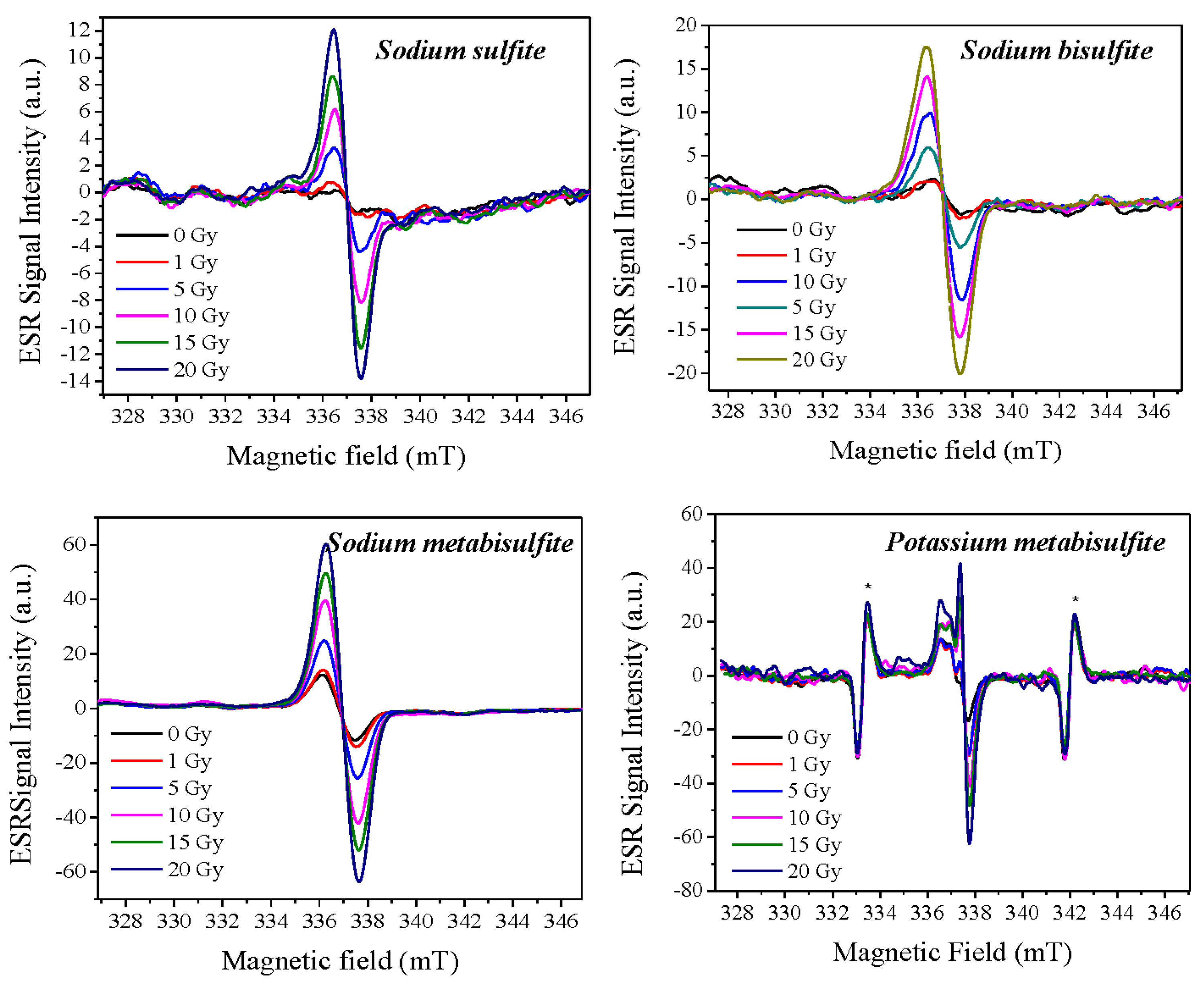
2.1. ESR Spectra Characterization
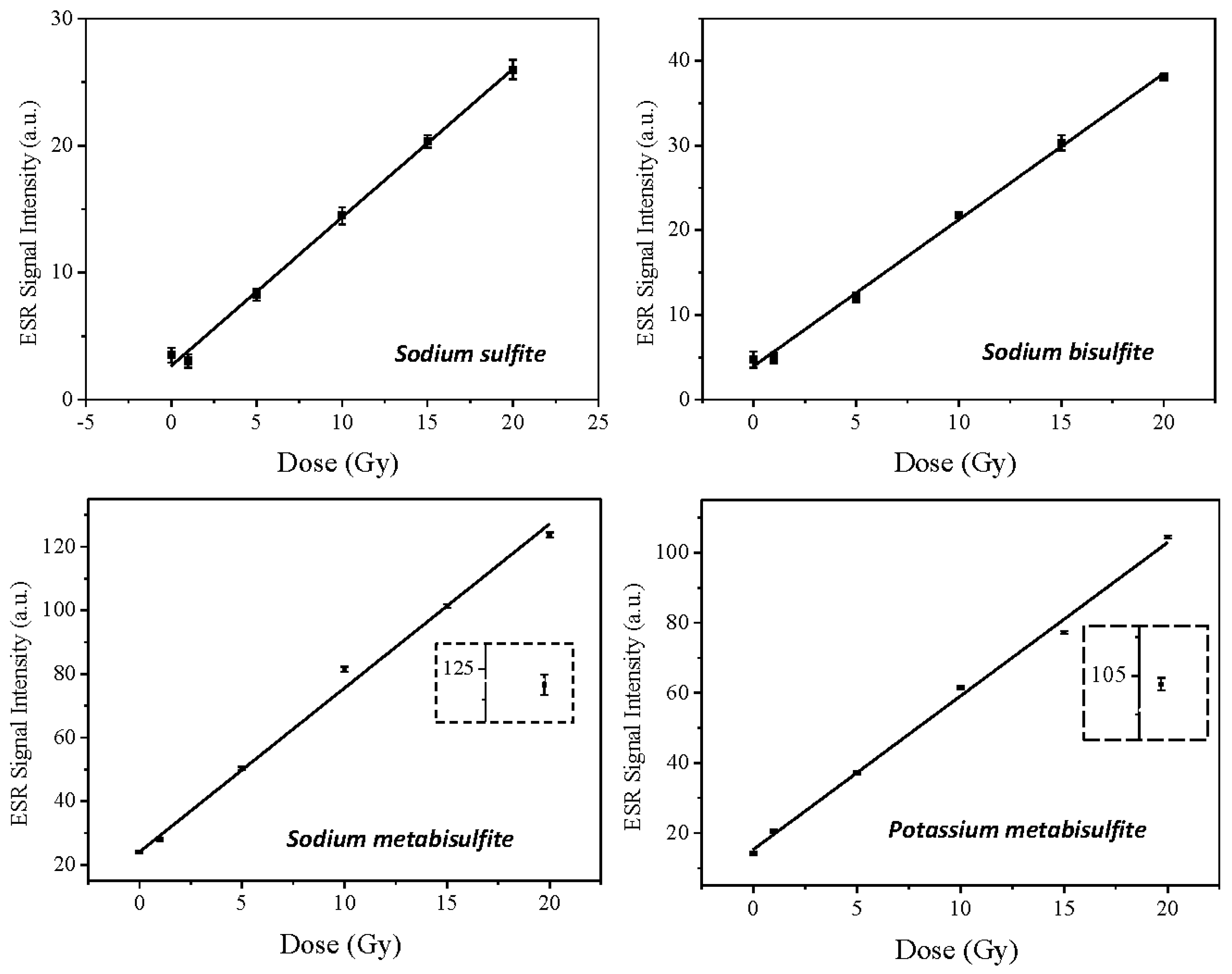
2.2. Dose-Response Curve
3. Materials and Methods
3.1. Irradiation
3.2. ESR Spectra Characterization
3.3. Dose-Response Curve
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gould, G.W.; Russell, N.J. Sulfite. In Food Preservatives; Russell, N.J., Gould, G.W., Eds.; Springer: Boston, MA, USA, 2003; pp. 85–101. ISBN 978-0-387-30042-9. [Google Scholar]
- Garcia-fuentes, A.R.; Wirtz, S.; Vos, E.; Verhagen, H. Short Review of Sulphites as Food Additives. Eur. J. Nutr. Food Saf. 2015, 5, 113–120. [Google Scholar] [CrossRef]
- Gancheva, V.; Sagstuen, E.; Yordanov, N.D. Study on the EPR/dosimetric properties of some substituted alanines. Radiat. Phys. Chem. 2006, 75, 329–335. [Google Scholar] [CrossRef]
- Ikeya, M. New Applications of Electron Paramagnetic Resonance: Dating, Dosimetry and Microscopy; World Scientific: Singapore, 1993; p. 500. [Google Scholar]
- Ikeya, M.; Fillho, O.B.; Mascarenhas, S. ESR dating of cave deposits from Akiyoshi-do Cave in Japan and Diabo Cavern in Brazil. J. Speleol. Soc. Jpn. 1984, 9, 58–67. [Google Scholar]
- Kinoshita, A.; Brunetti, A.; Avelar, W.E.P.; Mantelatto, F.L.M.; Simões, M.G.; Fransozo, A.; Baffa, O. ESR dating of a subfossil shell from Couve Island, Ubatuba, Brazil. Appl. Radiat. Isot. 2002, 57, 497–500. [Google Scholar] [CrossRef]
- Küçükuysal, C.; Engin, B.; Türkmenoğlu, A.G.; Aydaş, C. ESR dating of calcrete nodules from Bala, Ankara (Turkey): Preliminary results. Appl. Radiat. Isot. 2011, 69, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.-Y.; Toyoda, S.; Tomita, M.; Yamamoto, Y. Thermal stability and closure temperature of barite for electron spin resonance dating. Quat. Geochronol. 2022, 71, 101332. [Google Scholar] [CrossRef]
- Bogushevich, S.; Ugolev, I. Inorganic EPR dosimeter for medical radiology. Appl. Radiat. Isot. 2000, 52, 1217–1219. [Google Scholar] [CrossRef]
- Baran, M.P.; Bugay, O.A.; Kolesnik, S.P.; Maksimenko, V.M.; Teslenko, V.V.; Petrenko, T.L.; Desrosiers, M.F. Barium dithionate as an EPR dosemeter. Radiat. Prot. Dosim. 2006, 120, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Danilczuk, M.; Gustafsson, H.; Sastry, M.D.; Lund, E.; Lund, A. Ammonium dithionate—A new material for highly sensitive EPR dosimetry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 69, 18–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murthy, G.S.; Eager, R.L.; McCallum, K.J. Radiation Chemistry of Dithionates. Can. J. Chem. 1971, 49, 3733–3738. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, H.; Lund, A.; Hole, E.O.; Sagstuen, E. SO3− radicals for EPR dosimetry: X- and Q band EPR study and LET dependency of crystalline potassium dithionate. Radiat. Meas. 2013, 59, 123–128. [Google Scholar] [CrossRef]
- Chantry, G.W.; Horsfield, A.; Morton, J.R.; Rowlands, J.R.; Whiffen, D.H. The optical and electron resonance spectra of SO3−. Mol. Phys. 1962, 5, 233–239. [Google Scholar] [CrossRef]
- Gallo, S.; Iacoviello, G.; Bartolotta, A.; Dondi, D.; Panzeca, S.; Marrale, M. ESR dosimeter material properties of phenols compound exposed to radiotherapeutic electron beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2017, 407, 110–117. [Google Scholar] [CrossRef]
- Longo, A.; Basile, S.; Brai, M.; Marrale, M.; Tranchina, L. ESR response of watch glasses to proton beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2010, 268, 2712–2718. [Google Scholar] [CrossRef]


| Compound | g-Factor | LW (mT) |
|---|---|---|
| Sodium sulfite | 2.0085 2.0062 2.0062 | 0.6266 0.2427 |
| Sodium bisulfite | 2.0133 2.0089 2.0098 | 0.3168 0.6736 |
| 2.0047 2.0071 2.0098 | 0.0083 0.3740 | |
| Sodium metabisulfite | 2.0089 2.0045 2.0067 | 0.5546 0.2112 |
| Potassium metabisulfite | 2.0130 2.0093 2.0092 | 0.9715 0.2011 |
| 2.0052 2.0074 2.0078 | 0.2404 0.0961 |
| Parameter | Sodium Sulfite | Sodium Bisulfite | Sodium Metabisulfite | Potassium Metabisulfite |
|---|---|---|---|---|
| Center magnetic field | ~337 mT | |||
| Microwave frequency | 9 GHz | |||
| Resonant Cavity | Cylindrical resonator, mode TE011, Q factor 6000 | |||
| Modulation frequency | 100 kHz | |||
| Microwave power | 1 mW | |||
| Modulation amplitude | 1.0 mT | 1.4 mT | 1.4 mT | 0.1 mT |
| Sweep width | 10 mT | |||
| Time constant | 0.3 s | |||
| Gain | 10 × 100 | |||
| Sweep time | 1 min | |||
| Number of scans | 3 | |||
| Sample mass | ~80 mg | ~85 mg | ~60 mg | ~90 mg |
| Compound | Model | I0 | α | Adj. R-Square |
|---|---|---|---|---|
| Sodium sulfite | linear | 2.7 | 2.3 | 0.997 |
| Sodium bisulfite | linear | 4.0 | 2.3 | 0.997 |
| Sodium metabisulfite | linear | 24.1 | 4.7 | 0.997 |
| Potassium metabisulfite | linear | 15.3 | 3.5 | 0.995 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rech, A.B.; Kinoshita, A.; Donate, P.M.; Nascimento, O.R.; Baffa, O. Electron Spin Resonance Dosimetry Studies of Irradiated Sulfite Salts. Molecules 2022, 27, 7047. https://doi.org/10.3390/molecules27207047
Rech AB, Kinoshita A, Donate PM, Nascimento OR, Baffa O. Electron Spin Resonance Dosimetry Studies of Irradiated Sulfite Salts. Molecules. 2022; 27(20):7047. https://doi.org/10.3390/molecules27207047
Chicago/Turabian StyleRech, Amanda Burg, Angela Kinoshita, Paulo Marcos Donate, Otaciro Rangel Nascimento, and Oswaldo Baffa. 2022. "Electron Spin Resonance Dosimetry Studies of Irradiated Sulfite Salts" Molecules 27, no. 20: 7047. https://doi.org/10.3390/molecules27207047
APA StyleRech, A. B., Kinoshita, A., Donate, P. M., Nascimento, O. R., & Baffa, O. (2022). Electron Spin Resonance Dosimetry Studies of Irradiated Sulfite Salts. Molecules, 27(20), 7047. https://doi.org/10.3390/molecules27207047







