Recent Advances in Copper-Based Organic Complexes and Nanoparticles for Tumor Theranostics
Abstract
1. Introduction
2. Copper Intake, Distribution, and Efflux in Normal and Tumor Cells
3. Copper Regulation in Cancer
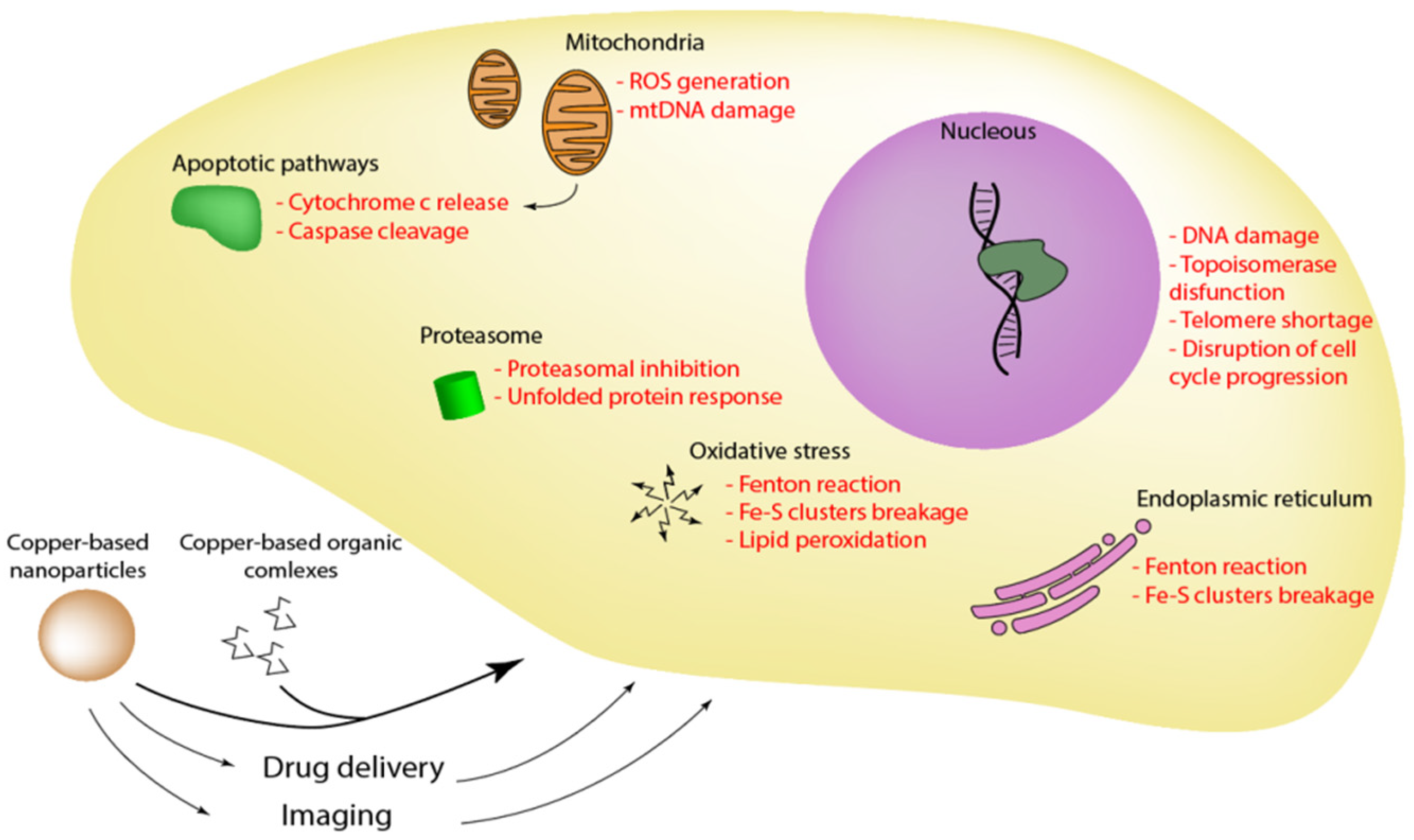
4. Therapeutic Effects of Copper-Based Compounds and Nanocarriers
5. Copper Nanoparticles for Cancer Imaging and Drug Delivery
6. Clinical Application of Copper-Based Nanoparticles in Oncology
7. The Combination of Nanoparticles with Other Treatment Modalities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef] [PubMed]
- Skopp, A.; Boyd, S.D.; Ullrich, M.S.; Liu, L.; Winkler, D.D. Copper–zinc superoxide dismutase (Sod1) activation terminates interaction between its copper chaperone (Ccs) and the cytosolic metal-binding domain of the copper importer Ctr1. Biometals 2019, 32, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Nývltová, E.; Dietz, J.V.; Seravalli, J.; Khalimonchuk, O.; Barrientos, A. Coordination of metal center biogenesis in human cytochrome c oxidase. Nat. Commun. 2022, 13, 3615. [Google Scholar] [CrossRef] [PubMed]
- Postma, G.C.; Nicastro, C.N.; Valdez, L.B.; Mikusic, I.A.R.; Grecco, A.; Minatel, L. Decrease lysyl oxidase activity in hearts of copper-deficient bovines. J. Trace Elem. Med. Biol. 2021, 65, 126715. [Google Scholar] [CrossRef]
- Grasso, M.; Bond, G.J.; Kim, Y.J.; Boyd, S.; Dzebo, M.M.; Valenzuela, S.; Tsang, T.; Schibrowsky, N.A.; Alwan, K.B.; Blackburn, N.J.; et al. The copper chaperone CCS facilitates copper binding to MEK1/2 to promote kinase activation. JBC 2021, 297, 101314. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, L.; Cotruvo, J.A., Jr.; Chan, J.; Kaluarachchi, H.; Muchenditsi, A.; Pendyala, V.S.; Jia, S.; Aron, A.T.; Ackerman, C.M.; Vander Wal, M.N.; et al. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 2016, 12, 586–592. [Google Scholar] [CrossRef]
- Guo, H.; Li, K.; Wang, W.; Wang, C.; Shen, Y. Effects of copper on hemocyte apoptosis, ROS production, and gene expression in white shrimp Litopenaeus vannamei. Biol. Trace Elem. Res. 2017, 179, 318–326. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflug Arch. Eur. J. Phy. 2020, 472, 1415–1429. [Google Scholar] [CrossRef]
- Lee, J.; Petris, M.J.; Thiele, D.J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002, 277, 4380–4387. [Google Scholar] [CrossRef]
- Ren, F.; Logeman B., L.; Zhang, X.; Liu, Y.; Thiele D., J.; Yuan, P. X-ray structures of the high-affinity copper transporter Ctr1. Nat. Commun. 2019, 10, 1386. [Google Scholar] [CrossRef]
- Chen, H.; Xu, C.; Yu, Q.; Zhong, C.; Peng, Y.; Chen, J.; Chen, G. Comprehensive landscape of STEAP family functions and prognostic prediction value in glioblastoma. J. Cell. Physiol. 2021, 236, 2988–3000. [Google Scholar] [CrossRef] [PubMed]
- Inesi, G. Molecular features of copper binding proteins involved in copper homeostasis. IUBMB Life 2017, 69, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Luchinat, E.; Barbieri, L.; Banci, L. A molecular chaperone activity of CCS restores the maturation of SOD1 fALS mutants. Sci. Rep. 2017, 7, 17433. [Google Scholar] [CrossRef]
- Banks, C.J.; Andersen, J.L. Mechanisms of SOD1 regulation by post-translational modifications. Redox Biol. 2019, 26, 101270. [Google Scholar] [CrossRef] [PubMed]
- Vanišová, M.; Burská, D.; Křížová, J.; Daňhelovská, T.; Dosoudilová, Ž.; Zeman, J.; Stibůrek, L.; Hansíková, H. Stable COX17 downregulation leads to alterations in mitochondrial ultrastructure, decreased copper con-tent and impaired cytochrome c oxidase biogenesis in HEK293 cells. Folia Biol. 2019, 65, 181–187. [Google Scholar]
- La Fontaine, S.; Mercer, J.F. Trafficking of the copper-ATPases, ATP7A and ATP7B: Role in copper homeostasis. Arch. Biochem. Biophys. 2007, 463, 149–167. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, H.; Chen, C.; Ren, K.; Xu, Y.; Liu, X.; He, L. 2 Curcumin enhances cisplatin sensitivity of human NSCLC cell lines through influencing Cu-Sp1-CTR1 regulatory loop. Phytomedicine 2018, 48, 51–61. [Google Scholar] [CrossRef]
- Sinani, D.; Adle, D.J.; Kim, H.; Lee, J. Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J. Biol. Chem. 2007, 282, 26775–26785. [Google Scholar] [CrossRef]
- Sun, S.; Cai, J.; Yang, Q.; Zhao, S.; Wang, Z. The association between copper transporters and the prognosis of cancer patients undergoing chemotherapy: A meta-analysis of literatures and datasets. Oncotarget 2017, 8, 16036. [Google Scholar] [CrossRef]
- Ilyechova, E.Y.; Bonaldi, E.; Orlov, I.A.; Skomorokhova, E.A.; Puchkova, L.V.; Broggini, M. CRISP-R/Cas9 mediated deletion of copper transport genes CTR1 and DMT1 in NSCLC cell line H1299. Biological and pharmacological consequences. Cells 2019, 8, 322. [Google Scholar] [CrossRef]
- Akerfeldt, M.C.; Tran, C.M.N.; Shen, C.; Hambley, T.W.; New, E.J. Interactions of cisplatin and the copper transporter CTR1 in human colon cancer cells. J. Biol. Inorg. Chem. 2017, 22, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. PNAS 2002, 99, 14298–14302. [Google Scholar] [CrossRef] [PubMed]
- Safaei, R.; Maktabi, M.H.; Blair, B.G.; Larson, C.A.; Howell, S. Effects of the loss of Atox1 on the cellular pharmacology of cisplatin. J. Inorg. Biochem. 2009, 103, 333–341. [Google Scholar] [CrossRef]
- Bompiani, K.M.; Tsai, C.Y.; Achatz, F.P.; Liebig, J.K.; Howell, S.B. Copper transporters and chaperones CTR1, CTR2, ATOX1, and CCS as determinants of cisplatin sensitivity. Metallomics 2016, 8, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Blockhuys, S.; Brady, D.C.; Wittung-Stafshede, P. Evaluation of copper chaperone ATOX1 as prognostic biomarker in breast cancer. Breast Cancer 2020, 27, 505–509. [Google Scholar] [CrossRef]
- Blockhuys, S.; Wittung-Stafshede, P. Copper chaperone Atox1 plays role in breast cancer cell migration. Biochem. Biophys. Res. Commun. 2017, 483, 301–304. [Google Scholar] [CrossRef]
- Itoh, S.; Kim, H.W.; Nakagawa, O.; Ozumi, K.; Lessner, S.M.; Aoki, H.; Akram, K.; McKinney, R.D.; Ushio-Fukai, M.; Fukai, T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell prolifera-tion. J. Biol. Chem. 2008, 283, 9157–9167. [Google Scholar] [CrossRef]
- Beaino, W.; Guo, Y.; Chang, A.J.; Anderson, C.J. Roles of Atox1 and p53 in the trafficking of copper-64 to tumor cell nuclei: Implications for cancer ther-apy. J. Biol. Inorg. Chem. 2014, 19, 427–438. [Google Scholar] [CrossRef]
- Singh, R.P.; Jeyaraju, D.V.; Voisin, V.; Xu, C.; Barghout, S.H.; Khan, D.H.; Hurren, R.; Gronda, M.; Wang, X.; Jitkova, Y.; et al. Targeting the Mitochondrial Metallochaperone Cox17 Reduces DNA Methylation and Promotes AML Differentiation through a Copper Dependent Mechanism. Blood 2018, 132, 1339. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, Q.; Wang, Z.; Xi, Z.; Xu, D.; Liu, Y. Cisplatin binds to human copper chaperone Cox17: The mechanistic implication of drug delivery to mitochondria. Chem. Commun. 2014, 50, 2667–2669. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.; Wu, H.; Xi, Z.; Liu, Y. Glutathione selectively modulates the binding of platinum drugs to human copper chaperone Cox17. Biochem. J. 2015, 472, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, F.; Fiorillo, M.; Lisanti, M.P. Mitochondrial markers predict recurrence, metastasis and tamoxifen-resistance in breast cancer patients: Early detection of treatment failure with companion diagnostics. Oncotarget 2017, 8, 68730–68745. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.P.; Sun, H.F.; Fu, W.Y.; Li, L.D.; Zhao, Y.; Chen, M.T.; Jin, W. High expression of COX5B is associated with poor prognosis in breast cancer. Future Oncol. 2017, 13, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Bikas, A.; Jensen, K.; Patel, A.; Costello, J.; Reynolds, S.M.; Mendonca-Torres, M.C.; Thakur, S.; Klubo-Gwiezdzinska, J.; Ylli, D.; Wartofsky, L.; et al. Cytochrome C Oxidase Subunit 4 (COX4): A Potential Therapeutic Target for the Treatment of Medullary Thyroid Cancer. Cancers 2020, 12, 2548. [Google Scholar] [CrossRef]
- Yi, J.F.; Li, Y.-M.; Liu, T.; He, W.-T.; Li, X.; Zhou, W.-C.; Kang, S.-L.; Zeng, X.-T.; Zhang, J.-Q. Mn-SOD and CuZn-SOD polymorphisms and interactions with risk factors in gastric cancer. World J. Gastroenterol. 2010, 16, 4738–4746. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Eryilmaz, R.; Demir, H.; Aykan, S.; Demir, C. Determination of oxidative stress levels and some antioxidant en-zyme activities in prostate cancer. Aging Male 2019, 22, 198–206. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Xu, J.; Hu, S.; Zhan, N.; Wang, H.; Gao, C.; Li, J.; Xu, X. SOD1 Promotes Cell Proliferation and Metastasis in Non-small Cell Lung Cancer via an miR-409-3p/SOD1/SETDB1 Epigenetic Regulatory Feedforward Loop. Front. Cell Dev. Biol. 2020, 8, 213. [Google Scholar] [CrossRef]
- Li, Y.; Liang, R.; Zhang, X.; Wang, J.; Shan, C.; Liu, S.; Li, L.; Zhang, S. Copper Chaperone for Superoxide Dismutase Promotes Breast Cancer Cell Proliferation and Migration via ROS-Mediated MAPK/ERK Signaling. Front. Pharmacol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Wang, J.; Luo, C.; Shan, C.; You, Q.; Lu, J.; Elf, S.; Zhou, Y.; Wen, Y.; Vinkenborg, J.L.; Fan, J.; et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat. Chem. 2015, 7, 968–979. [Google Scholar] [CrossRef]
- Wen, C.; Shan, C.L.; Sun, W.J.; Wan, Y.; Lin, R.; Chen, B.; Dai, H.-T.; Tang, K.-Y.; Xiang, X.-H.; Yang, J.-Y.; et al. 2021; preprint. [CrossRef]
- Samimi, G.; Varki, N.M.; Wilczynski, S.; Safaei, R.; Alberts, D.S.; Howell, S.B. Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin. Cancer Res. 2003, 96, 5853–5859. [Google Scholar]
- Chisholm, C.L.; Wang, H.; Wong, A.H.; Vazquez-Ortiz, G.; Chen, W.; Xu, X.; Deng, C.X. Ammonium tetrathiomolybdate treatment tar-gets the copper transporter ATP7A and enhances sensitivity of breast cancer to cisplatin. Oncotarget 2016, 7, 84439. [Google Scholar] [CrossRef] [PubMed]
- Seiko, I.; Frank, M.; Karen, S.; Douglas, H. Enhancing Tumor-Specific Uptake of the Anticancer Drug Cisplatin with a Copper Chelator. Cancer Cell 2010, 17, 574–583. [Google Scholar] [CrossRef]
- Mangala, L.S.; Zuzel, V.; Schmandt, R.; Leshane, E.S.; Halder, J.B.; Armaiz-Pena, G.N.; Spannuth, W.A.; Tanaka, T.; Shahzad, M.M.K.; Lin, Y.G.; et al. Therapeutic Targeting of ATP7B in Ovarian Carcinoma. Clin. Cancer Res. 2009, 15, 3770–3780. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Maruša, H.; Borut, K.; Katarina, Č. The contribution of copper efflux transporters ATP7A and ATP7B to chemo-resistance and personalized medicine in ovarian cancer. Biomed. Pharmacother. 2020, 129, 110401. [Google Scholar] [CrossRef]
- Yu, Z.; Cao, W.; Ren, Y.; Zhang, Q.; Liu, J. ATPase copper transporter A, negatively regulated by miR-148a-3p, contributes to cisplatin resistance in breast cancer cells. Clin. Transl. Med. 2020, 10, 57–73. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Cell Physiol. 2002, 282, 9157–9167. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, W.; Kang, Y.J. Copper affects the binding of HIF-1alpha to the critical motifs of its target genes. Metallomics 2019, 11, 429–438. [Google Scholar] [CrossRef]
- Wee, N.K.; Weinstein, D.C.; Fraser, S.T.; Assinder, S.J. The mammalian copper transporters CTR1 and CTR2 and their roles in development and disease. Int. J. Biochem. Cell Biol. 2013, 45, 960–963. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Luo, J.; Chen, X.; Ma, K.; He, H.; Li, W.; Cui, J. Serum Copper Level and the Copper-to-Zinc Ratio Could Be Useful in the Prediction of Lung Cancer and Its Prognosis: A Case-Control Study in Northeast China. Nutr. Cancer 2021, 73, 1908–1915. [Google Scholar] [CrossRef]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper is required for oncogenic BRAF signal-ling and tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Shin, M.J.; Choi, Y.J.; Kwon, H.J.; Lee, S.H.; Lee, S.; Lee, S.; Park, J.; Han, K.H.; Eum, W.S.; et al. Tat-ATOX1 inhibits inflammatory responses via regulation of MAPK and NF-kappaB pathways. BMB Rep. 2018, 51, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Finley, J.C.; Ali, S.S.; Patel, H.H.; Howell, S.B. Copper influx transporter 1 is required for FGF, PDGF and EGF-induced MAPK signaling. Biochem. Pharmacol. 2012, 84, 1007–1013. [Google Scholar] [CrossRef]
- Li, Y.; Fu, S.Y.; Wang, L.H.; Wang, F.Y.; Wang, N.N.; Cao, Q.; Wang, Y.-T.; Yangab, J.-Y.; Wu, C.-F. Copper improves the anti-angiogenic activity of disulfiram through the EGFR/Src/VEGF pathway in gliomas. Cancer Lett. 2015, 369, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef]
- Gutfilen, B.; Souza, S.A.; Valentini, G. Copper-64: A real theranostic agent. Drug Des. Devel. Ther. 2018, 12, 3235–3245. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, H.; Liu, H.; Wang, S.; Li, J.; Zhou, Q.; Chen, X.; Ren, X.; Jing, Y.; Deng, Y.; et al. Rational Design of Multifunctional CuS Nanoparticle-PEG Composite Soft Hydrogel-Coated 3D Hard Polycaprolactone Scaffolds for Efficient Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2202470. [Google Scholar] [CrossRef]
- Xue, X.; Liu, H.; Wang, S.; Hu, Y.; Huang, B.; Li, M.; Chen, X.; Ren, X.; Jing, Y.; Deng, Y.; et al. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/anti-inflammatory therapy of osteoarthritis. Compos. B Eng. 2022, 237, 109855. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S.; Ge, G.; Guo, Z.; Zhao, M.; Liu, Z. Boosting Chemodynamic Therapy by Tumor-Targeting and Cellular Redox Homeostasis-Disrupting Nanoparticles. ACS Appl. Mater. 2022, 14, 44098–44110. [Google Scholar] [CrossRef]
- Fanizza, E.; Mastrogiacomo, R.; Pugliese, O.; Guglielmelli, A.; De Sio, L.; Castaldo, R.; Scavo, M.P.; Giancaspro, M.; Rizzi, F.; Gentile, G.; et al. NIR-Absorbing Mesoporous Silica-Coated Copper Sulphide Nanostructures for Light-to-Thermal Energy Conversion. Nanomaterials 2022, 12, 2545. [Google Scholar] [CrossRef]
- Avila-Rodriguez, M.A.; Rios, C.; Carrasco-Hernandez, J.; Manrique-Arias, J.C.; Martinez-Hernandez, R.; Garcia-Perez, F.O.; Martinez-Rodriguez, E.; Romero-Piña, M.E.; Diaz-Ruiz, A. Biodistribution and radiation dosimetry of [(64)Cu]copper dichloride: First-in-human study in healthy volunteers. EJNMMI Res. 2017, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Kell, A.; Simard, B.; Xiang, B.; Lin, H.Y.; Tian, G. The cell labeling efficacy, cytotoxicity and relaxivity of cop-per-activated MRI/PET imaging contrast agents. Biomaterials 2011, 32, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sultan, D.; Detering, L.; Cho, S.; Sun, G.; Pierce, R.; Wooley, K.L.; Liu, Y. Copper-64-alloyed gold nanoparticles for cancer imaging: Im-proved radiolabel stability and diagnostic accuracy. Angew. Chem. Int. Ed. Engl. 2014, 53, 156–159. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjug. Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Konarikova, K.; Perdikaris, G.A.; Gbelcova, H.; Andrezalova, L.; Sveda, M.; Ruml, T.; Laubertová, L.; Žitňanová, I. Effect of Schiff base Cu(II) complexes on signaling pathways in HT-29 cells. Mol. Med. Rep. 2016, 14, 4436–4444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Langer, V.; Gyepesová, D.; Scholtzová, E.; Mach, P.; Kohútová, M.; Valent, A.; Smrčok, L.U. Crystal and electronic structure of aqua (N-salicylidene-methylester-L-glutamato) Cu (II) monohydrate. Z. Kristallogr. Cryst. Mater. 2004, 219, 112–116. [Google Scholar] [CrossRef]
- Nakao, Y.; Sakurai, K.I.; Nakahara, A. Copper (II) chelates of Schiff bases derived from salicylaldehyde and various α-amino acids. Bull. Chem. Soc. Jpn. 1967, 40, 1536–1538. [Google Scholar] [CrossRef]
- Krätsmár-Šmogrovič, J.; Pavelčík, F.; Soldánová, J.; Sivy, J.; Seressová, V.; Žemlička, M. The Crystal and Molecular Structure and Properties of Diaqua [N-salicylidene–(S)–(+)–glutamato] copper (II) Monohydrate. Z. Nat. B 1991, 46, 1323–1327. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, D.; Xu, J.; Fang, J. Redox-Dependent Copper Carrier Promotes Cellular Copper Uptake and Oxidative Stress-Mediated Apoptosis of Cancer Cells. ACS Appl. Mater. Interfaces 2018, 10, 33010–33021. [Google Scholar] [CrossRef]
- Kunos, C.A.; Andrews, S.J.; Moore, K.N.; Chon, H.S.; Ivy, S.P. Randomized Phase II Trial of Triapine-Cisplatin-Radiotherapy for Locally Advanced Stage Uterine Cervix or Vaginal Cancers. Front. Oncol. 2019, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Vutey, V.; Castelli, S.; D’Annessa, I.; Samia, L.B.; Souza-Fagundes, E.M.; Beraldo, H.; Desideria, A. Human topoisomerase IB is a target of a thiosemicarbazone copper(II) complex. Arch. Biochem. Biophys. 2016, 606, 34–40. [Google Scholar] [CrossRef]
- Kaur, P.; Johnson, A.; Northcote-Smith, J.; Lu, C.; Suntharalingam, K. Immunogenic Cell Death of Breast Cancer Stem Cells Induced by an Endoplasmic Reticulum-Targeting Copper(II) Complex. Chembiochem 2020, 21, 3618–3624. [Google Scholar] [CrossRef]
- Tardito, S.; Isella, C.; Medico, E.; Marchio, L.; Bevilacqua, E.; Hatzoglou, M.; Bussolati, O.; Franchi-Gazzola, R. The thioxotriazole copper(II) complex A0 induces endoplasmic reticulum stress and paraptotic death in human cancer cells. J. Biol. Chem. 2009, 284, 24306–24319. [Google Scholar] [CrossRef] [PubMed]
- Passeri, G.; Northcote-Smith, J.; Suntharalingam, K. Delivery of an immunogenic cell death-inducing copper complex to cancer stem cells using polymeric nanoparticles. RSC Adv. 2022, 12, 5290–5299. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Yague, E.; Wang, L.Y.; Dai, L.L.; Yang, Z.B.; Zhi, S.; Zhang, N.; Zhao, X.-M.; Hu, Y.-H. Novel Copper Complexes That Inhibit the Proteasome and Trig-ger Apoptosis in Triple-Negative Breast Cancer Cells. ACS Med. Chem. Lett. 2019, 10, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Fenk, K.D.; Huang, D.; Sen, S.; Cowan, J.A. Rapid Telomere Reduction in Cancer Cells Induced by G-Quadruplex-Targeting Copper Complexes. J. Med. Chem. 2019, 62, 5040–5048. [Google Scholar] [CrossRef] [PubMed]
- Deka, B.; Sarkar, T.; Banerjee, S.; Kumar, A.; Mukherjee, S.; Deka, S.; Saikia, K.K.; Hussain, A. Novel mitochondria targeted copper(ii) complexes of ferrocenyl terpyridine and anticancer active 8-hydroxyquinolines showing remarkable cytotoxicity, DNA and protein binding affinity. Dalton Trans. 2017, 46, 396–409. [Google Scholar] [CrossRef]
- Weissleder, R. Molecular imaging in cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef]
- Ku, G.; Chen, J.; Vittal, J.J. Copper sulfide nanoparticles as a new class of photoacoustic contrast agent for deep tissue imaging at 1064 nm. ACS Nano. 2012, 6, 7489–7496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Siphanto, R.; Thumma, K.K.; Kolkman, R.G.M.; van Leeuwen, T.G.; de Mul, F.F.M.; van Neck, J.W.; van Adrichem, L.N.A.; Steenbergen, W. Serial noninvasive photoacoustic imaging of neovascularization in tumor angiogenesis. Opt. Express 2005, 13, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, L.V. Photoacoustic tomography: Fundamentals, advances and prospects. Contrast Media Mol. Imaging 2011, 6, 332–345. [Google Scholar] [CrossRef]
- Zha, Z.; Deng, Z.; Li, Y.; Li, C.; Wang, J.; Wang, S.; Quc, E.; Dai, Z. Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale 2013, 5, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Yang, Z.; Liu, Y.; Xu, Y.; Gu, B.; Chen, J.; Su, P.; Tong, L.; Wang, L. Two-photon semiconducting polymer nanoparticles as a new platform for imaging of intracellular pH variation. Biosens. Bioelectron. 2019, 126, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Ungureanu, C.; van Leeuwen, T.G. Gold nanorods as molecular contrast agents in photoacoustic imaging: The promises and the caveats. Contrast Media Mol. Imaging 2011, 6, 389–400. [Google Scholar] [CrossRef]
- Cherukula, K.; Manickavasagam Lekshmi, K.; Uthaman, S.; Cho, K.; Cho, C.S.; Park, I.K. Multifunctional inorganic nanoparticles: Recent progress in thermal therapy and imaging. Nanomaterials 2016, 6, 76. [Google Scholar] [CrossRef]
- Zhou, M.; Ku, G.; Pageon, L.; Li, C. Theranostic probe for simultaneous in vivo photoacoustic imaging and confined photothermolysis by pulsed laser at 1064 nm in 4T1 breast cancer model. Nanoscale 2014, 6, 15228–15235. [Google Scholar] [CrossRef]
- Ding, K.; Zeng, J.; Jing, L.; Qiao, R.; Liu, C.; Jiao, M.; Libc, Z.; Gao, M. Aqueous synthesis of PEGylated copper sulfide nanoparticles for photoacoustic imaging of tumors. Nanoscale 2015, 7, 11075–11081. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zhao, J.; He, M.; Huang, Y.; Zhao, S. A tumor microenvironment–induced absorption red-shifted polymer nanoparticle for simultaneously activated photoacoustic imaging and photothermal therapy. Sci. Adv. 2021, 7, eabe3588. [Google Scholar] [CrossRef] [PubMed]
- Bindra, A.K.; Wang, D.; Zheng, Z.; Jana, D.; Zhou, W.; Yan, S.; Wuac, H.; Zheng, Y.; Zhao, Y. Self-assembled semiconducting polymer based hybrid nanoagents for synergistic tumor treatment. Biomaterials 2021, 279, 121188. [Google Scholar] [CrossRef] [PubMed]
- Phelps, M.E. Positron emission tomography provides molecular imaging of biological processes. PNAS 2000, 97, 9226–9233. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.; Huang, M.; Lu, W.; Song, S.; Melancon, M.P.; Tian, M.; Liang, D.; Li, C. A chelator-free multifunctional [64Cu] CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J. Am. Chem. Soc. 2010, 132, 15351–15358. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, S.; Esposito, T.V.; Neufeld-Peters, J.; Bergamo, M.; Yang, H.; Saatchi, K.; Schaffer, P.; Häfeli, U.O.; Ruiz-Molina, D.; Rodríguez-Rodríguez, C. Hybrid Metal–Phenol Nanoparticles with Polydopamine-like Coating for PET/SPECT/CT Imaging. ACS Appl. Mater. Interfaces 2021, 13, 10705–10718. [Google Scholar] [CrossRef]
- Zhang, H.; Song, F.; Dong, C.; Yu, L.; Chang, C.; Chen, Y. Co-delivery of nanoparticle and molecular drug by hollow mesoporous organosilica for tumor-activated and photothermal-augmented chemotherapy of breast cancer. J. Nanobiotechnology 2021, 19, 290. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Y.; Du, S.; Wu, Y.; Shen, P.; Yan, T.; Li, X.; Song, Y.; Zha, Z.; Han, X. Controlled CRISPR-Cas9 Ribonucleoprotein Delivery for Sensitized Photothermal Therapy. Small 2021, 17, 2101155. [Google Scholar] [CrossRef]
- Druzhkova, I.N.; Shirmanova, M.V.; Kuznetsova, D.S.; Lukina, M.M.; Zagaynova, E.V. Modern Approaches to Testing Drug Sensitivity of Patients’ Tumors (Review). Sovrem Tekhnologii Med. 2021, 12, 91–102. [Google Scholar] [CrossRef]
- Ganesan, K.; Wang, Y.; Gao, F.; Liu, Q.; Zhang, C.; Li, P.; Zhang, L.; Chen, J. Targeting Engineered Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2021, 13, 1829. [Google Scholar] [CrossRef]
- Es-Haghi, A.; Taghavizadeh, Y.M.; Sharifalhoseini, M.; Baghani, M.; Yousefi, E.; Rahdar, A.; Baino, F. Application of Response Sur-face Methodology for Optimizing the Therapeutic Activity of ZnO Nanoparticles Biosynthesized from Aspergillus niger. Biomimetics 2021, 6, 34. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Pourbagheri-Sigaroodi, A.; Kaveh, V.; Sheikholeslami, S.A.; Salari, S.; Bashash, D. The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: Recent advances and opportunities. Crit. Rev. Oncol. Hematol. 2021, 157, 103160. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Heo, Y.J.; Han, D.K. New opportunities for nanoparticles in cancer immunotherapy. Biomater. Res. 2018, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Copper ferrite nanoparticle-induced cytotoxicity and oxidative stress in human breast cancer MCF-7 cells. Colloids Surf. B. Biointerfaces 2016, 142, 46–54. [Google Scholar] [CrossRef]
- Rajagopal, G.; Nivetha, A.; Sundar, M.; Panneerselvam, T.; Murugesan, S.; Parasuraman, P.; Kumar, S.; Ilangoa, S.; Kunjiappanh, S. Mixed phytochemicals medi-ated synthesis of copper nanoparticles for anticancer and larvicidal applications. Heliyon 2021, 7, e7360. [Google Scholar] [CrossRef] [PubMed]
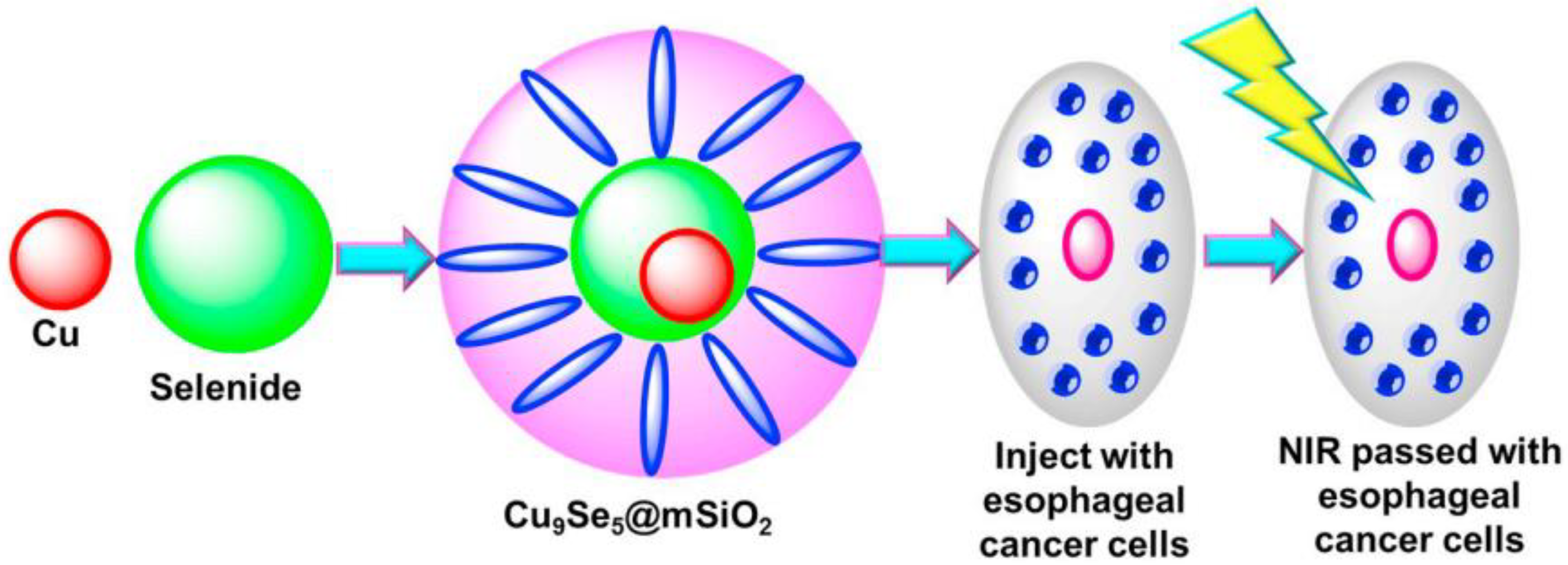
- Wang, S.; Liu, J.; Qiu, S.; Yu, J. Facile fabrication of Cu9-S5 loaded core-shell nanoparticles for near infrared radiation medi-ated tumor therapeutic strategy in human esophageal squamous carcinoma cells nursing care of esophageal cancer pa-tients. J. Photochem. Photobiol. B. 2019, 199, 111583. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, K.; Liang, J.; Gao, F.; Li, J.; Guan, F. Hyaluronic acid/polyethyleneimine nanoparticles loaded with copper ion and disulfiram for esophageal cancer. Carbohydr. Polym. 2021, 261, 117846. [Google Scholar] [CrossRef]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Cur-rent status and perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
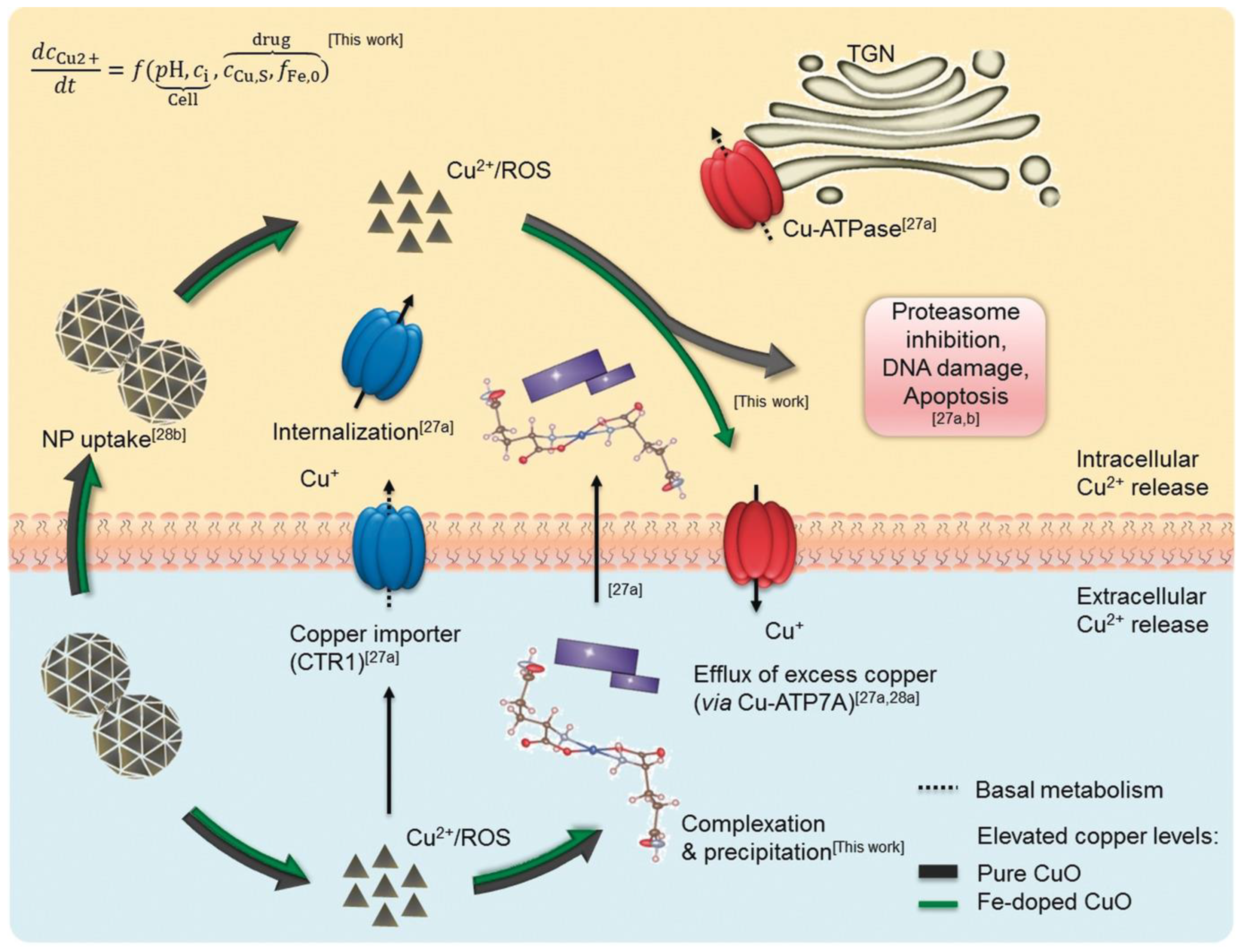
- Naatz, H.; Manshian, B.B.; Rios, L.C.; Tsikourkitoudi, V.; Deligiannakis, Y.; Birkenstock, J.; Pokhrel, S.; Mädler, L. Model-Based Nanoengineered Pharmacokinetics of Iron-Doped Copper Oxide for Nanomedical Applications. Angew. Chem. Int. Ed. Engl. 2020, 59, 1828–1836. [Google Scholar] [CrossRef]
- Kalaiarasi, A.; Sankar, R.; Anusha, C.; Saravanan, K.; Aarthy, K.; Karthic, S.; Ravikumar, V. Copper oxide nanoparticles induce anticancer activity in A549 lung cancer cells by inhibition of histone deacetylase. Biotechnol. Lett. 2018, 40, 249–256. [Google Scholar] [CrossRef]
- Giri, R.K.; Chaki, S.; Khimani, A.J.; Vaidya, Y.H.; Thakor, P.; Thakkar, A.B.; Pandya, S.J.; Deshpande, M.P. Biocompatible CuInS2 Nanoparticles as Poten-tial Antimicrobial, Antioxidant, and Cytotoxic Agents. ACS Omega 2021, 6, 26533–26544. [Google Scholar] [CrossRef] [PubMed]
- Li, W.B.; Stangl, S.; Klapproth, A.; Shevtsov, M.; Hernandez, A.; Kimm, M.A.; Schuemann, J.; Qiu, R.; Michalke, B.; Bernal, M.A.; et al. Application of High-Z Gold Nanoparticles in Targeted Cancer Radiotherapy-Pharmacokinetic Modeling, Monte Carlo Simulation and Radiobiological Effect Model-ing. Cancers 2021, 13, 5370. [Google Scholar] [CrossRef] [PubMed]
- Klapproth, A.P.; Shevtsov, M.; Stangl, S.; Li, W.B.; Multhoff, G. A New Pharmacokinetic Model Describing the Biodistribution of Intravenously and Intratumorally Administered Superparamagnetic Iron Oxide Nanoparticles (SPIONs) in a GL261 Xenograft Glioblastoma Model. Int. J. Nanomed. 2020, 15, 4677–4689. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Cheng, Y.; Qi, F.; Wu, Y.; Han, X.; Yan, J.; Zhang, H. Biodegradable Copper-Based Nanoparticles Augmented Chemody-namic Therapy through Deep Penetration and Suppressing Antioxidant Activity in Tumors. Adv. Healthc. Mater. 2021, 10, e2100412. [Google Scholar] [CrossRef]
- Koh, J.Y.; Lee, S.J. Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol. Brain 2020, 13, 116. [Google Scholar] [CrossRef]
- Lelievre, P.; Sancey, L.; Coll, J.L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef]
- Camats, M.; Pla, D.; Gomez, M. Copper nanocatalysts applied in coupling reactions: A mechanistic insight. Nanoscale 2021, 13, 18817–18838. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Zamani, A.; Abtahi, F.S. Preparation of Cu nanoparticles fixed on cellulosic walnut shell material and in-vestigation of its antibacterial, antioxidant and anticancer effects. Heliyon 2020, 6, e3528. [Google Scholar] [CrossRef]
- Naikoo, G.; Al-Mashali, F.; Arshad, F.; Al-Maashani, N.; Hassan, I.U.; Al-Baraami, Z.; Faruck, L.H.; Qurashi, A.; Ahmed, W.; Asiri, A.M. An Overview of Copper Nanoparti-cles: Synthesis, Characterisation and Anticancer Activity. Curr. Pharm. Des. 2021, 27, 4416–4432. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparti-cles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Da, S.D.; De Luca, A.; Squitti, R.; Rongioletti, M.; Rossi, L.; Machado, C.; Cerchiaro, G. Copper in tumors and the use of copper-based compounds in cancer treatment. J. Inorg. Biochem. 2022, 226, 111634. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Ebrahim, N.M.; Gaber, M.H. In-vitro evaluation of copper/copper oxide nanoparticles cytotoxicity and geno-toxicity in normal and cancer lung cell lines. J. Trace. Elem. Med. Biol. 2020, 60, 126481. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.R.; Kanchi, S.; Naidoo, E.B. In-vitro evaluation of copper nanoparticles cytotoxicity on prostate cancer cell lines and their antioxidant, sensing and catalytic activity: One-pot green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Feng, W.; Xu, W.; Yu, L.; Xiang, H.; Chen, Y.; Zhou, J. The Coppery Age: Copper (Cu)-Involved Nanotheranostics. Adv. Sci. 2020, 21, 2001549. [Google Scholar] [CrossRef]
- Li, W.; Zamani, R.; Rivera Gil, P.; Pelaz, B.; Ibáñez, M.; Cadavid, D.; Shavel, A.; Alvarez-Puebla, R.A.; Parak, W.J.; Arbiol, J.; et al. CuTe Nanocrystals: Shape and Size Control, Plasmonic Properties, and Use as SERS Probes and Photothermal Agents. J. Am. Chem. Soc. 2013, 135, 7098–7101. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic Cu9S5 Nanocrystals: A Photothermal Agent with a 25.7% Heat Conversion Effi-ciency for Photothermal Ablation of Cancer Cells in Vivo. ACS Nano. 2011, 5, 9761–9771. [Google Scholar] [CrossRef]
- Tian, Q.; Tang, M.; Sun, Y.; Zou, R.J.; Chen, Z.G.; Zhu, M.F.; Yang, S.P.; Wang, J.L.; Wang, J.H.; Hu, J.Q. Hydrophilic Flower-Like CuS Superstructures as an Efficient 980 nm Laser-Driven Photother-mal Agent for Ablation of Cancer Cells. Adv. Mater. 2011, 23, 3542–3547. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Fu, F.; Xu, K.; Zou, R.; Wang, Q.; Zhang, B.; Chena, Z.; Hu, J. Facile synthesis of biocompatible cysteine-coated CuS nanoparticles with high photothermal conver-sion efficiency for cancer therapy. Dalton Trans. 2014, 43, 11709–11715. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Wang, X.; Yang, D.; Yang, C.; Lou, Y.; Chen, J.; He, N. Near-infrared light-induced dissociation of zeolitic imidazole framework-8 (ZIF-8) with encapsulated CuS nanoparticles and their application as a therapeutic nanoplatform. Chem. Comm. 2016, 52, 12210–12213. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Q.; Zhang, L.; Zhang, H.; Han, Y.; Sun, Q.; Cheng, Z.; Qin, H.; Doub, S.; Li, Z. Vacancy engineering of Cu2−xSe nanoparticles with tunable LSPR and magnetism for dual-modal imaging guided photothermal therapy of cancer. Nanoscale 2018, 10, 3130–3143. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Ji, G.; Liu, Y.; Xu, X.; Lei, P.; Du, K.; Song, S.; Feng, J.; Zhang, H. Multifunctional Cu–Ag2S nanoparticles with high photothermal conversion efficiency for photoa-coustic imaging-guided photothermal therapy in vivo. Nanoscale 2018, 10, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Xu, M.; Zhang, W.; Yang, Z.; Huang, L.; Liu, J.; Zhang, Y.; Gu, L.; Yu, Y.; Hao, W.; et al. alStructurally Well-Defined Au@Cu2−xS Core–Shell Nanocrystals for Improved Cancer Treatment Based on Enhanced Photothermal Efficiency. Adv. Mater. 2016, 28, 3094–3101. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liow, C.H.; Zhang, M.; Huang, R.; Li, C.; Shen, H.; Liu, M.; Zou, Y.; Gao, N.; Zhang, Z.; et al. Surface Plasmon Resonance Enhanced Light Absorption and Photothermal Therapy in the Second Near-Infrared Window. J. Am. Chem. Soc. 2014, 136, 15684–15693. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Chen, C.; Ma, M.; Zeng, J.; Li, S.; Xia, Y.; Gao, M. Monodisperse Dual Plasmonic Au@Cu2–xE (E= S, Se) Core@Shell Supraparticles: Aqueous Fab-rication, Multimodal Imaging, and Tumor Therapy at in Vivo Level. ACS Nano. 2017, 11, 8273–8281. [Google Scholar] [CrossRef]
- Chen, W.; Qin, M.; Chen, X.; Wang, Q.; Zhang, Z.; Sun, X. Combining photothermal therapy and immunotherapy against melanoma by polydopamine-coated Al2O3nanoparticles. Theranostics 2018, 8, 2229–2241. [Google Scholar] [CrossRef]
- Wu, Z.-C.; Li, W.-P.; Luo, C.-H.; Su, C.H.; Yeh, C.S. Rattle-Type Fe3O4@CuS Developed to Conduct Magnetically Guided Photoinduced Hyper-thermia at First and Second NIR Biological Windows. Adv. Funct. Mater. 2015, 25, 6527–6537. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in Cancer Biology and Therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- An, L.; Wang, X.; Rui, X.; Lin, J.; Yang, H.; Tian, Q.; Tao, C.; Yang, S. The In Situ Sulfidation of Cu2O by Endogenous H2S for Colon Cancer Theranostics. Angew. Chem. Int. Ed 2018, 57, 15782–15786. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; An, L.; Lin, J.; Tian, Q.; Yang, S. Surface Plasmon Resonance–Enhanced Photoacoustic Imaging and Photothermal Therapy of Endog-enous H2S-Triggered Au@Cu2O. Small 2019, 15, 1903473. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Q.; Guo, Z.; Li, M.; Yang, X.; Wan, G.; Chen, H.; Zhang, Q.; Wang, Y. An Intelligent Biomimetic Nanoplatform for Holistic Treatment of Metastatic Tri-ple-Negative Breast Cancer via Photothermal Ablation and Immune Remodeling. ACS Nano. 2020, 14, 15161–15181. [Google Scholar] [CrossRef] [PubMed]



| Copper-Based Compound | Mechanism of Action | |
|---|---|---|
| Diagnostic tool | 64-CuCl2 [64] | Contrast agent in PET/MRI scanning |
| Combination of SPIONs and Cu(II) [62] | ||
| Gold-copper alloyed NPs [63] | ||
| Therapeutic agent | Schiff base copper (II) complexes [66] | Activation of extrinsic or intrinsic apoptotic pathways |
| Copper-based nanoparticles [96,103] | Copper ions release, oxidative stress, DNA damage | |
| Thiosemicarbazones copper (II) complex [72] | Topoisomerase inhibition | |
| Polypyridyl-Schiff-base copper complex [74] | Targets endoplasmic reticulum leading to immunogenic cell death | |
| G-quadruplex-targeting copper complex [77] | Rapid reduction of telomeres in cancer cells | |
| Ferrocenyl terpyridine copper complexes [78] | Targets mitochondria, causes mtDNA damage | |
| Combined approach | Copper chalcogenides [126] | Photothermal ablation and NIR-triggered chemotherapy |
| Alloyed CuAg or CuAu NPs [132,133] | ||
| PEG-[64Cu]CuS NPs [94] | Combined radiotherapy and hyperthermia against metastatic tumor cells | |
| Copper-doped iron NPs [109,131] | Magnetic guidance and copper release with subsequent oxidative stress |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsymbal, S.; Li, G.; Agadzhanian, N.; Sun, Y.; Zhang, J.; Dukhinova, M.; Fedorov, V.; Shevtsov, M. Recent Advances in Copper-Based Organic Complexes and Nanoparticles for Tumor Theranostics. Molecules 2022, 27, 7066. https://doi.org/10.3390/molecules27207066
Tsymbal S, Li G, Agadzhanian N, Sun Y, Zhang J, Dukhinova M, Fedorov V, Shevtsov M. Recent Advances in Copper-Based Organic Complexes and Nanoparticles for Tumor Theranostics. Molecules. 2022; 27(20):7066. https://doi.org/10.3390/molecules27207066
Chicago/Turabian StyleTsymbal, Sergey, Ge Li, Nikol Agadzhanian, Yuhao Sun, Jiazhennan Zhang, Marina Dukhinova, Viacheslav Fedorov, and Maxim Shevtsov. 2022. "Recent Advances in Copper-Based Organic Complexes and Nanoparticles for Tumor Theranostics" Molecules 27, no. 20: 7066. https://doi.org/10.3390/molecules27207066
APA StyleTsymbal, S., Li, G., Agadzhanian, N., Sun, Y., Zhang, J., Dukhinova, M., Fedorov, V., & Shevtsov, M. (2022). Recent Advances in Copper-Based Organic Complexes and Nanoparticles for Tumor Theranostics. Molecules, 27(20), 7066. https://doi.org/10.3390/molecules27207066