The Inhibition of α-Glucosidase, α-Amylase and Protein Glycation by Phenolic Extracts of Cotoneaster bullatus, Cotoneaster zabelii, and Cotoneaster integerrimus Leaves and Fruits: Focus on Anti-Hyperglycemic Activity and Kinetic Parameters
Abstract
1. Introduction
2. Results
2.1. Polyphenolic Profiling of Fruit and Leaf Extracts
2.2. Effect of Leaf and Fruit Extracts on the α-Glucosidase and α-Amylase Inhibition
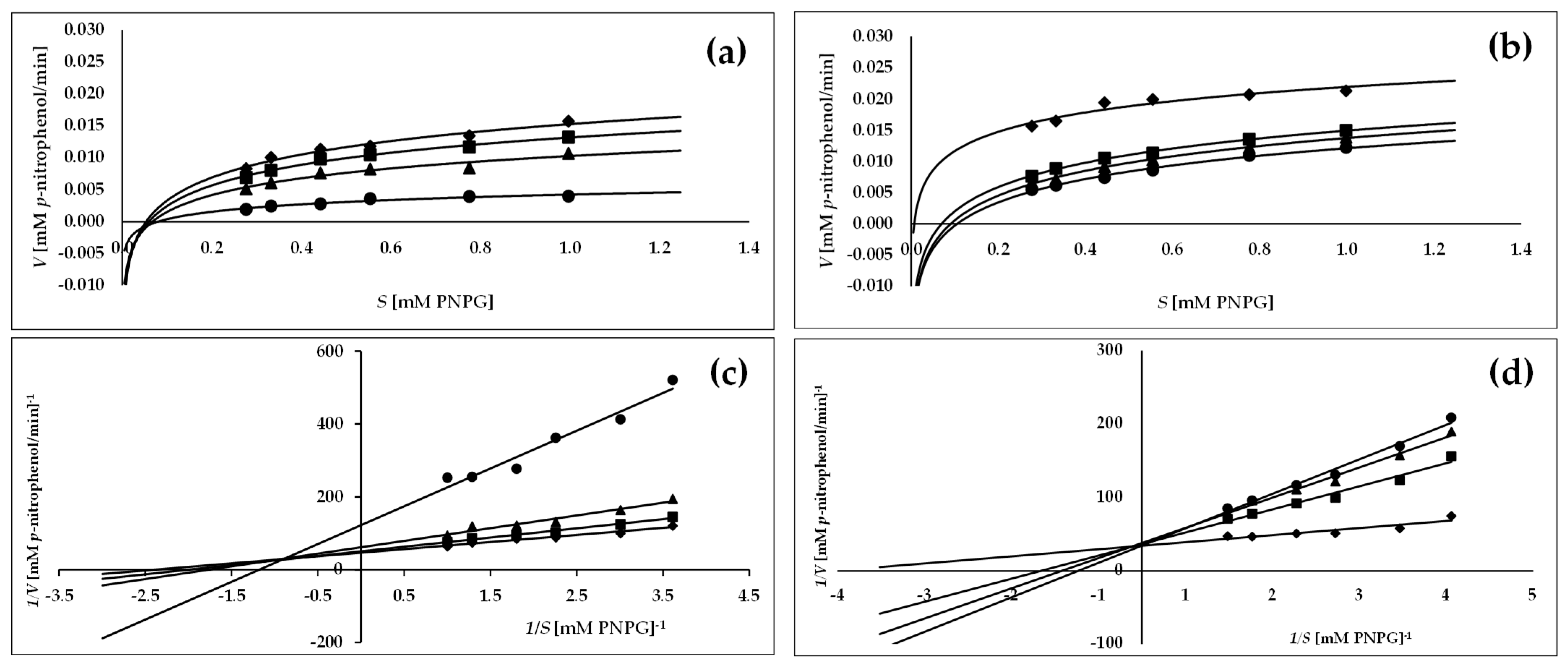
2.3. Mode of α-Glucosidase and α-Amylase Inhibition by Leaf Extract of C. bullatus
2.4. Effect of Leaf and Fruit Extracts on NonEnzymatic Protein Glycation
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Plant Material and Sample Preparation
4.3. Quantitative Phytochemical Profiling of Cotoneaster Leaf and Fruit Phenolics
4.4. α-Glucosidase Inhibitory Assay
4.5. α-Amylase Inhibitory Assay
4.6. Kinetic Parameters of α-Glucosidase and α-Amylase Inhibition
4.7. Protein Glycation Inhibitory Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 diabetes-global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Goh, S.Y.; Cooper, M.E. The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant extracts for type 2 diabetes: From traditional medicine to modern drug discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef]
- Willcox, M.L.; Elugbaju, C.; Al-Anbaki, M.; Lown, M.; Graz, B. Effectiveness of medicinal plants for glycaemic control in type 2 diabetes: An overview of meta-analyses of clinical trials. Front. Pharmacol. 2021, 26, 777561. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytother. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. 2019, 13, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols rich diets and risk of type 2 diabetes. Nutrients 2021, 13, 1445. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A. An Overview of the Genus Cotoneaster (Rosaceae): Phytochemistry, Biological Activity, and Toxicology. Antioxidants 2020, 9, 1002. [Google Scholar] [CrossRef]
- Holzer, V.M.D.; Lower-Nedza, A.D.; Nandintsetseg, M.; Batkhuu, J.; Brantner, A.H. Antioxidant constituents of Cotoneaster melanocarpus Lodd. Antioxidants 2013, 2, 265–272. [Google Scholar] [CrossRef]
- Les, F.; López, V.; Caprioli, G.; Iannarelli, R.; Fiorini, D.; Innocenti, M.; Bellumori, M.; Maggi, F. Chemical constituents, radical scavenging activity and enzyme inhibitory capacity of fruits from Cotoneaster pannosus Franch. Food Funct. 2017, 8, 1775–1784. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef]
- Uysal, A.; Zengin, G.; Mollica, A.; Gunes, E.; Locatelli, M.; Yilmaz, T.; Aktumsek, A. Chemical and biological insights on Cotoneaster integerrimus: A new (−)-epicatechin source for food and medicinal applications. Phytomedicine 2016, 15, 979–988. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Sokkar, N.M.; El-Gindi, O.; Ali, Z.Y.; Alfishawy, I.A. Phytoconstituents investigation, anti-diabetic and anti-dyslipidemic activities of Cotoneaster horizontalis Decne cultivated in Egypt. Life Sci. J. 2012, 9, 394–403. [Google Scholar]
- Kicel, A.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Marchelak, A.; Sopinska, M.; Ciszewski, P.; Nowak, P.; Olszewska, M.A. Polyphenol-rich extracts from Cotoneaster leaves inhibit pro-inflammatory enzymes and protect human plasma components against oxidative stress in vitro. Molecules 2018, 23, 2472. [Google Scholar] [CrossRef]
- Kicel, A.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Rutkowska, M.; Wajs-Bonikowska, A.; Granica, S.; Nowak, P.; Olszewska, M.A. Multifunctional phytocompounds in Cotoneaster fruits: Phytochemical profiling, cellular safety, anti-inflammatory and antioxidant effects in chemical and human plasma models in vitro. Oxid. Med. Cell. Longev. 2018, 2018, 3482521. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A.; Owczarek, A.; Gralak, P.; Ciszewski, P.; Olszewska, M.A. Polyphenolic profile, antioxidant activity, and pro-inflammatory enzymes inhibition of leaves, flowers, bark and fruits of Cotoneaster integerrimus: A comparative study. Phytochem. Lett. 2019, 30, 349–355. [Google Scholar] [CrossRef]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. The role of oxidative stress and inflammation in CVD aging. Biomed. Res. Int. 2014, 2014, 615312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.-M.G.; Hux, N.P.; Philips, S.J.; Towns, M.H. Michaelis–Menten graphs, Lineweaver–Burk plots, and reaction schemes: Investigating introductory biochemistry students’ conceptions of representations in enzyme kinetics. J. Chem. Educ. 2019, 96, 1833–1845. [Google Scholar] [CrossRef]
- Aledo, J.C. Enzyme kinetic parameters estimation: A tricky task? Biochem. Mol. Biol. Educ. 2021, 49, 633–638. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Jiphimai, P.; Prutanopajai, P.; Chanathong, B.; Sapwarobol, S.; Ariyapitipan, T. Evaluation of alpha-glucosidase, alpha-amylase and protein glycation inhibitory activities of edible plants. Int. J. Food Sci. Nutr. 2010, 61, 295–305. [Google Scholar] [CrossRef]
- Elya, B.; Basah, K.; Mun’im, A.; Yuliastuti, W.; Bangun, A.; Septiana, E.K. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. J. Biomed. Biotechnol. 2012, 2012, 281078. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef]
- Al Kazaz, M.; Desseaux, V.; Marchis-Mouren, G.; Prodanov, E.; Santimone, M. The mechanism of porcine pancreatic alpha-amylase. Inhibition of maltopentaose hydrolysis by acarbose, maltose and maltotriose. Eur. J. Biochem. 1998, 252, 100–107. [Google Scholar] [CrossRef]
- Yoon, S.H.; Robyt, J.F. Study of the inhibition of four alpha amylases by acarbose and its 4IV-α-maltohexaosyl and 4IV-α-maltododecaosyl analogues. Carbohydr. Res. 2003, 338, 1969–1980. [Google Scholar] [CrossRef]
- Ferey-Roux, G.; Perrier, J.; Forest, E.; Marchis-Mouren, G.; Puigserver, A.; Santimone, M. The human pancreatic α-amylase isoforms: Isolation, structural studies and kinetics of inhibition by acarbose. Biochim. Biophys. Acta 1998, 1388, 10–20. [Google Scholar] [CrossRef]
- Bischoff, H. The mechanism of alpha-glucosidase inhibition in the management of diabetes. Clin. Investig. Med. 1995, 18, 303–311. [Google Scholar]
- Yan, J.; Zhang, G.; Pan, J.; Wang, Y. α-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014, 64, 213–223. [Google Scholar] [CrossRef]
- Chougale, A.D.; Ghadyale, V.A.; Panaskar, S.N.; Arvindekar, A.U. Alpha glucosidase inhibition by stem extract of Tinospora cordifolia. J. Enzym. Inhib. Med. Chem. 2009, 24, 998–1001. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Dong, J. Inhibitory properties of aqueous ethanol extracts of propolis on alpha-glucosidase. Evid. Based Complement. Alternat. Med. 2015, 2015, 587383. [Google Scholar] [CrossRef]
- Ghadyale, V.; Takalikar, S.; Haldavnekar, V.; Arvindekar, A. Effective Control of Postprandial Glucose Level through Inhibition of Intestinal Alpha Glucosidase by Cymbopogon martinii (Roxb.). Evid. Based Complement. Alternat. Med. 2012, 2012, 372909. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of alpha-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef]
- Wu, X.; Hu, M.; Hu, X.; Ding, H.; Gong, D.; Zhang, G. Inhibitory mechanism of epicatechin gallate on α-amylase and α-glucosidase and its combinational effect with acarbose or epigallocatechin gallate. J. Mol. Liq. 2019, 290, 111202. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Inhibition kinetics of catechin and ferulic acid on polyphenoloxidase from cephalothorax of Pacific white shrimp (Litopenaeus vannamei). Food Chem. 2012, 131, 569–573. [Google Scholar] [CrossRef]
- Sylvain, G.; Patrice, P.; Jean-Marc, B.; Véronique, C. Inhibition of β-glucosidase (Amygdalae dulces) by (+)-catechin oxidation products and procyanidin dimers. Biosci. Biotechnol. Biochem. 1996, 60, 1131–1135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadowska-Bartosz, I.; Bartosz, G. Prevention of protein glycation by natural compounds. Molecules 2015, 20, 3309–3334. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeong, I.H.; Kim, C.S.; Lee, Y.M.; Kim, J.M.; Kim, J.S. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch. Pharm. Res. 2011, 34, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Yoo, N.H.; Kim, N.H.; Lee, Y.M.; Kim, C.S.; Kim, J.; Kim, J.S. 3,5-Di-O-caffeoyl-epi-quinic acid from the leaves and stems of Erigeron annuus inhibits protein glycation, aldose reductase, and cataractogenesis. Biol. Pharm. Bull. 2010, 33, 329–333. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bastos, D.H.M.; Schulze, J.; Souza, M.F.F. Caffeic and chlorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins. Fitoterapia 2009, 80, 339–344. [Google Scholar] [CrossRef]
- Bains, Y.; Gugliucci, A. Ilex paraguariensis and its main component chlorogenic acid inhibit fructose formation of advanced glycation endproducts with amino acids at conditions compatible with those in the digestive system. Fitoterapia 2017, 117, 6–10. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, X. Structures required of polyphenols for inhibiting advanced glycation end products formation. Curr. Drug Metab. 2013, 14, 414–431. [Google Scholar] [CrossRef]
- Kicel, A.; Owczarek, A.; Kapusta, P.; Kolodziejczyk-Czepas, J.; Olszewska, M.A. Contribution of Individual Polyphenols to Antioxidant Activity of Cotoneaster bullatus and Cotoneaster zabelii Leaves—Structural Relationships, Synergy Effects and Application for Quality Control. Antioxidants 2020, 9, 69. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Michel, P. Antioxidant activity of inflorescences, leaves and fruits of three Sorbus species in relation to their polyphenolic composition. Nat. Prod. Res. 2009, 23, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Jeong, Y.-K.; Wang, M.-H.; Lee, W.-Y.; Rhee, H.-I. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef] [PubMed]
- McCue, P.P.; Shetty, K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac. J. Clin. Nutr. 2004, 13, 101–106. [Google Scholar] [PubMed]
- Rutkowska, M.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Zakrzewska, A.; Magiera, A.; Olszewska, M.A. Novel insight into biological activity and phytochemical composition of Sorbus aucuparia L. fruits: Fractionated extracts as inhibitors of protein glycation and oxidative/nitrative damage of human plasma components. Food Res. Int. 2021, 147, 110526. [Google Scholar] [CrossRef] [PubMed]
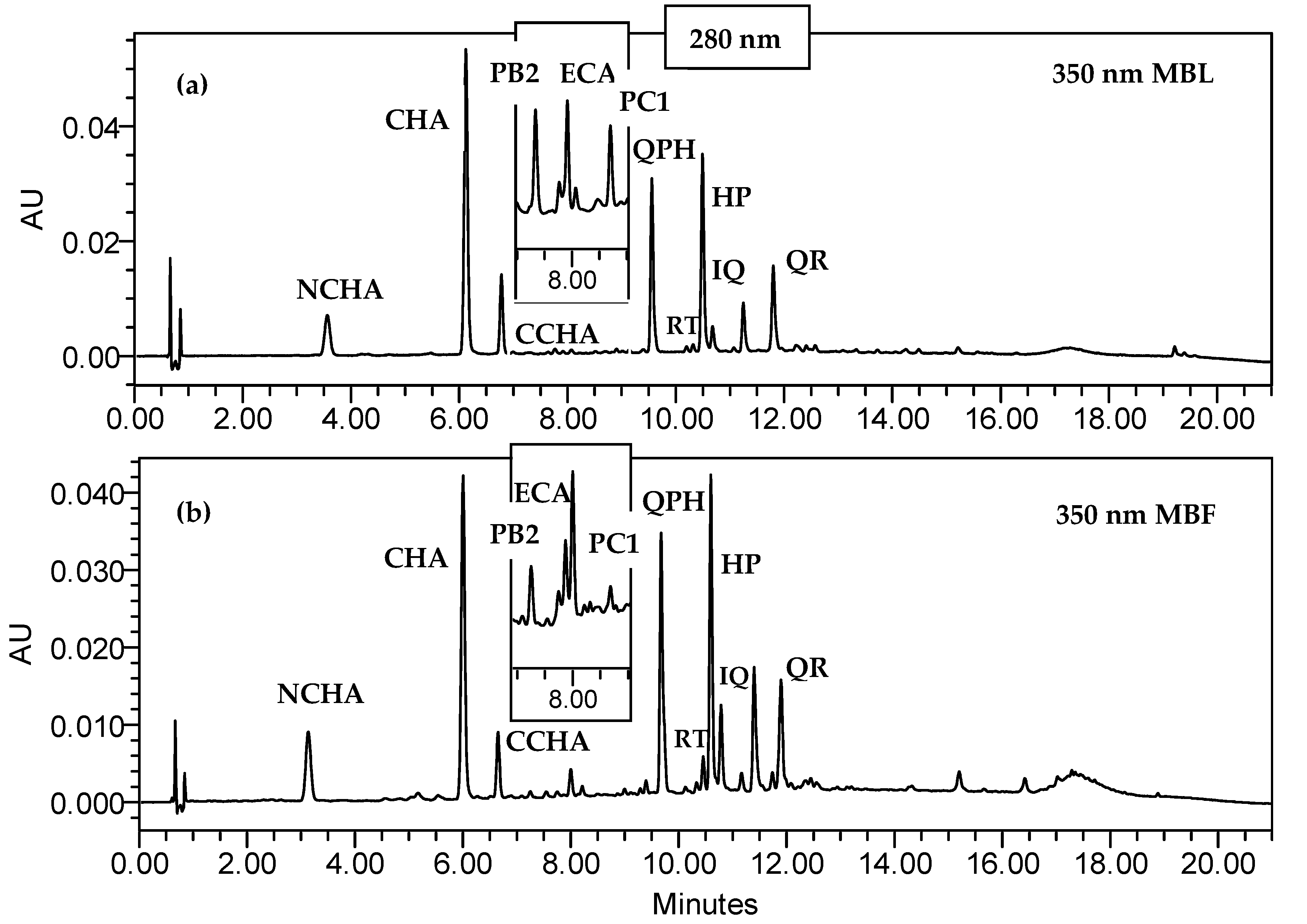

| MBL | MZL | MIL | MBF | MZF | MIF | |
|---|---|---|---|---|---|---|
| Individual analyte | mg/g dw | |||||
| NCHA | 3.71 ± 0.10 C | 1.99 ± 0.01 A | 2.66 ± 0.06 B | 0.79 ± 0.00 E | 0.09 ± 0.00 A | 0.70 ± 0.00 C |
| CHA | 17.07 ± 0.88 H | 9.24 ± 0.11 F | 61.53 ± 0.80 F | 2.36 ± 0.00 K | 2.17 ± 0.07 C | 1.52 ± 0.03 E |
| CCHA | 3.64 ± 0.15 C | 2.70 ± 0.03 B | 2.78 ± 0.01 B | 0.42 ± 0.01 C | 0.26 ± 0.02 A,B | 0.29 ± 0.01 A |
| PB2 | 18.45 ± 1.00 I | 12.09 ± 0.15 G | n.d. | 1.42 ± 0.02 I | 4.93 ± 0.08 E | 2.86 ± 0.16 F |
| ECA | 12.62 ± 0.65 F | 8.98 ± 0.10 E | n.d. | 1.28 ± 0.01 H | 6.99 ± 0.12 F | 2.94 ± 0.12 F |
| PC1 | 15.04 ± 0.76 G | 8.95 ± 0.06 E | n.d. | 1.10 ± 0.03 F | 4.22 ± 0.20 D | 3.13 ± 0.04 G |
| CAD | n.d. | 32.05 ± 0.25 H | n.d. | n.d. | n.d. | n.d. |
| QPH | 7.75 ± 0.32 E | n.d. | n.d. | 1.59 ± 0.02 J | n.d. | n.d. |
| RT | 0.27 ± 0.01 A | 3.35 ± 0.01 C | 4.33 ± 0.07 C | 0.18 ± 0.00 A | 0.29 ± 0.01 B | 0.98 ± 0.02 D |
| HP | 5.86 ± 0.15 D | 1.73 ± 0.03 A | 39.17 ± 0.43 E | 1.16 ± 0.00 G | 0.33 ± 0.01 B | 1.48 ± 0.02 E |
| IQ | 0.83 ± 0.05 B | 2.38 ± 0.03 B | 20.38 ± 0.22 D | 0.35 ± 0.00 B | 0.27 ± 0.01 A,B | 0.47 ± 0.02 B |
| QR | 3.42 ± 0.18 C | 4.36 ± 0.05 D | 1.72 ± 0.03 A | 0.62 ± 0.02 D | 0.21 ± 0.01 A,B | 0.22 ± 0.00 A |
| Phenolic fraction | mg/g dw | |||||
| TPC (GAE) | 316.27 ± 3.94 | 337.63 ± 2.33 | 200.30 ± 2.90 | 62.13 ± 3.15 | 77.86 ± 2.07 | 81.26 ± 0.77 |
| TPA (CYE) | 135.32 ± 1.88 | 147.91 ± 0.50 | 86.64 ± 2.42 | 20.42 ± 1.16 | 34.11 ± 0.44 | 35.68 ± 0.87 |
| TPH | 100.42 ± 4.92 | 103.10 ± 1.00 | 134.89 ± 1.65 | 12.18 ± 0.05 | 22.27 ± 0.11 | 15.47 ± 0.42 |
| TCA | 24.42 ± 1.02 | 43.97 ± 0.44 | 66.97 ± 0.85 | 3.57 ± 0.01 | 2.52 ± 0.05 | 2.52 ± 0.03 |
| TFL | 19.62 ± 0.85 | 14.94 ± 0.10 | 67.91 ± 0.80 | 4.01 ± 0.01 | 1.31 ± 0.03 | 3.76 ± 0.07 |
| TLPA | 56.38 ± 2.86 | 44.19 ± 0.45 | n.d. | 3.79 ± 0.04 | 17.50 ± 0.24 | 8.93 ± 0.32 |
| Analyte | α-Glucosidase | α-Amylase | Protein Glycation |
|---|---|---|---|
| Phenolic Extracts | |||
| µg/mL | µg/mL | µg/mL | |
| MBL | 8.56 ± 0.32 A | 41.82 ± 1.78 A | 32.62 ± 3.71 C |
| MZL | 9.53 ± 0.33 A | 33.03 ± 1.63 A | 36.53 ± 3.64 C |
| MIL | 53.92 ± 3.48 B | 1511.41 ± 50.23 E | 36.28 ± 2.35 C |
| MBF | 80.12 ± 3.91 C | 941.13 ± 49.98 C | 166.62 ± 7.71 G |
| MZF | 57.73 ± 2.86 B | 1082.52 ± 43.33 D | 118.94 ± 8.82 F |
| MIF | 48.89 ± 4.81 B | 976.20 ± 6.65 C | 106.36 ± 6.47 E |
| Pure Compounds/Standards | |||
| µg/mL | µg/mL | µg/mL | |
| PB2 | 416.37 ± 11.23 H | >200 | 2.20 ± 0.01 A |
| ECA | 231.66 ± 8.09 E | >200 | 8.79 ± 0.13 A, B |
| CAD | 43.70 ± 2.18 B | >400 | 17.26 ± 1.09 B |
| QPH | 311.80 ± 1.97 G | >400 | 15.78 ± 1.14 A, B |
| HP | 283.48 ± 3.47 F | >400 | 14.34 ± 1.15 A, B |
| AR | 169.52 ± 9.45 D | 5.78 ± 0.27 A | n.d. |
| AG | n.d. | n.d. | 71.09 ± 4.20 D |
| Inhibitor | Concentration of Inhibitor (µg/mL) | Vmax (mM/min) | Km (mM) | Vmax/Km | Type Inhibition | ||
|---|---|---|---|---|---|---|---|
| α-glucosidase | |||||||
| MBL | no inhibitor | Vmaxo | 0.024 ± 0.001 D | Kmo | 0.454 ± 0.020 A | 0.048 D | Mixed |
| 5.0 | Vmax1 | 0.020 ± 0.00 1C | Km1 | 0.533 ± 0.010 A,B | 0.038 C | ||
| 6.0 | Vmax2 | 0.016 ± 0.001 B | Km2 | 0.592 ± 0.030 C | 0.027 B | ||
| 9.0 | Vmax3 | 0.008 ± 0.001 A | Km3 | 0.780 ± 0.040 D | 0.012 A | ||
| AR | no inhibitor | Vmaxo | 0.029 ± 0.001A | Kmo | 0.280 ± 0.015 A | 0.136 C | Competitive |
| 110.0 | Vmax1 | 0.028 ± 0.001A | Km1 | 0.867 ± 0.010 B | 0.032 B | ||
| 160.0 | Vmax2 | 0.027 ± 0.001A | Km2 | 1.086 ± 0.010 C | 0.025 A,B | ||
| 215.0 | Vmax3 | 0.029 ± 0.001A | Km3 | 1.370 ± 0.020 D | 0.021 A | ||
| α-amylase | |||||||
| MBL | no inhibitor | Vmaxo | 0.465 ± 0.009 C | Kmo | 0.860 ± 0.050 A | 0.541 D | Mixed |
| 20.0 | Vmax1 | 0.333 ± 0.011 B | Km1 | 1.249 ± 0.045 B | 0.267 C | ||
| 40.0 | Vmax2 | 0.276 ± 0.015 A,B | Km2 | 1.360 ± 0.030 C | 0.203 B | ||
| 60.0 | Vmax3 | 0.226 ± 0.001 A | Km3 | 1.515 ± 0.025 D | 0.149 A | ||
| AR | no inhibitor | Vmaxo | 0.382 ± 0.020 C | Kmo | 0.873 ± 0.009 A | 0.438 D | Mixed |
| 3.0 | Vmax1 | 0.321 ± 0.009 C | Km1 | 0.906 ± 0.009 B | 0.354 C | ||
| 8.0 | Vmax2 | 0.241 ± 0.015 B | Km2 | 0.986 ± 0.009 C | 0.326 B | ||
| 10.0 | Vmax3 | 0.189 ± 0.009 A | Km3 | 1.274 ± 0.009 D | 0.148 A | ||
| r (p) for: | TPC (mg GAE/g) | TPA (mg CYE/g) | TPH (mg/g) | TCA (mg/g) | TFL (mg/g) | TLPA (mg/g) |
|---|---|---|---|---|---|---|
| α-glucosidase (µg/mL) | −0.9119 (0.011) * | −0.9216 (0.009) * | −0.5819 (0.226) | −0.3844 (0.452) | −0.0648 (0.903) | −0.8957 (0.016) * |
| α-amylase (µg/mL) | −0.7301 (0.039) * | −0.7205 (0.048) * | −0.1924 (0.715) | 0.0055 (0.993) | 0.3719 (0.468) | −0.9274 (0.008) * |
| protein glycation (µg/mL) | −0.8854 (0.019) * | −0.8988 (0.015) * | −0.9121 (0.011) * | −0.7899 (0.042) * | −0.6140 (0.195) | −0.5612 (0.240) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicel, A.; Magiera, A.; Skrzywanek, M.; Malczuk, M.; Olszewska, M.A. The Inhibition of α-Glucosidase, α-Amylase and Protein Glycation by Phenolic Extracts of Cotoneaster bullatus, Cotoneaster zabelii, and Cotoneaster integerrimus Leaves and Fruits: Focus on Anti-Hyperglycemic Activity and Kinetic Parameters. Molecules 2022, 27, 7081. https://doi.org/10.3390/molecules27207081
Kicel A, Magiera A, Skrzywanek M, Malczuk M, Olszewska MA. The Inhibition of α-Glucosidase, α-Amylase and Protein Glycation by Phenolic Extracts of Cotoneaster bullatus, Cotoneaster zabelii, and Cotoneaster integerrimus Leaves and Fruits: Focus on Anti-Hyperglycemic Activity and Kinetic Parameters. Molecules. 2022; 27(20):7081. https://doi.org/10.3390/molecules27207081
Chicago/Turabian StyleKicel, Agnieszka, Anna Magiera, Marta Skrzywanek, Mariola Malczuk, and Monika Anna Olszewska. 2022. "The Inhibition of α-Glucosidase, α-Amylase and Protein Glycation by Phenolic Extracts of Cotoneaster bullatus, Cotoneaster zabelii, and Cotoneaster integerrimus Leaves and Fruits: Focus on Anti-Hyperglycemic Activity and Kinetic Parameters" Molecules 27, no. 20: 7081. https://doi.org/10.3390/molecules27207081
APA StyleKicel, A., Magiera, A., Skrzywanek, M., Malczuk, M., & Olszewska, M. A. (2022). The Inhibition of α-Glucosidase, α-Amylase and Protein Glycation by Phenolic Extracts of Cotoneaster bullatus, Cotoneaster zabelii, and Cotoneaster integerrimus Leaves and Fruits: Focus on Anti-Hyperglycemic Activity and Kinetic Parameters. Molecules, 27(20), 7081. https://doi.org/10.3390/molecules27207081