Abstract
A different type of MnO2-induced oxidative cyclization of dihydrotriazines has been developed. These dihydrotriazines are considered as a “formal” Schiff’s base. This method provided easy access to naphthofuro-fused triazine via the C-C/C-O oxidative coupling reaction. The reaction sequence comprised the nucleophilic addition of 2-naphthol or phenol to 1,2,4-triazine, followed by oxidative cyclization. The scope and limitations of this novel coupling reaction have been investigated. Further application of the synthesized compound has been demonstrated by synthesizing carbazole-substituted benzofuro-fused triazines. The scalability of the reaction was demonstrated at a 40 mmol load. The mechanistic study strongly suggests that this reaction proceeds through the formation of an O-coordinated manganese complex.
1. Introduction
In organic synthesis, C−H functionalization in the presence of transition metal catalysts has become one of the fundamental methods, and has had a massive impact on synthetic organic chemistry, medicinal chemistry, and material science [1,2,3,4,5,6,7,8]. In this context, cross dehydrogenative coupling (CDC) reactions have gained much interest in the last decade [9,10,11,12,13,14,15] among all types of C-H functionalization/activation reactions. This type of coupling reaction allows the construction of a C-C bond or C-X bond directly from C-H-containing substrates in the presence of an oxidant via the formal removal of a H2 molecule. In addition, these methods avoid the prefunctionalization of starting materials, which makes the synthetic routes straightforward and more efficient. For CDC reactions, various transition metals such as Pd, Cu, Ag, Rh, and Ru have been extensively studied due to their high efficiency. However, the exploration of manganese catalysis in CDC reactions is in high demand due to its low price, ready availability, sustainability, nontoxicity, and environmentally friendly properties [16]. Simple manganese salts were sensibly employed in the CDC reaction due to their ability to undergo the reaction in a radical way.
Benzofuro-fused N-heterocycles are considered as common structural motifs in biologically active compounds, drug candidates and fluorescence materials (Figure 1). For example, benzofuro [2,3-b]pyridine, in particular Elbfluorene I, and its derivatives are important cyclin-dependent kinase inhibitors [17,18,19,20], the benzofuro [3,2-d]pyrimidine derivative Amuvatinib II is a multitarget tyrosine kinase inhibitor [21,22,23,24], and benzofuro[2,3-b]pyrazine III was designed as a deep-blue fluorescent emitter [25].
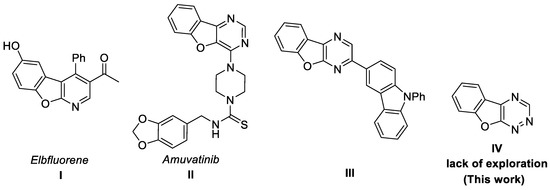
Figure 1.
Representative benzofuro-fused N-heterocycles.
Considering the importance of this moiety, several methods have been developed for developing benzofuro-fused N-heterocycles such as pyridine, pyrimidine and pyrazine derivatives. The first synthetic approach comprises the annulation of the heterocyclic ring to a benzofuran core (Scheme 1a) [26,27,28,29,30,31,32,33,34]. Alternatively, other approaches include the intramolecular cyclization (C-C bond formation) of arylhetaryl ethers [35,36,37,38,39,40] (Scheme 1b) or the intramolecular cyclization (C-O bond formation) of 2-hetaryl-substituted phenol derivatives [41,42,43,44,45,46,47] (Scheme 1c), as well as the intermolecular tandem C-C/C-O cross-coupling reaction of prefunctionalized substrate [48,49,50] to form a furan ring fused between the benzene and mono/diazine ring. However, these approaches usually require multistep synthesis, harsh reaction conditions, and the use of transition metal catalysts or special reagents and conditions.
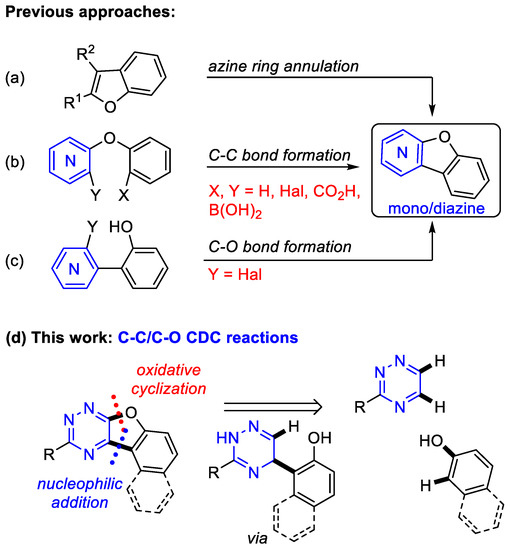
Scheme 1.
Approaches to Benzofuro-fused N-heterocycles. (a–c): Known approaches; (d) Approach of this work.
On the other hand, it is worth mentioning that information about the synthetic and applied data of benzofuro-fused triazines is lacking in the literature. To date, only a few studies have investigated the synthesis of benzofurotriazine derivatives. In 1988, Eid et al. reported the synthesis of naphthofuro[2,3-e][1,2,4]triazine in a 33% overall yield via the annulation of the triazine core to naphthofuran-1,2-dione [51]. The reaction was carried out in four steps. Later, Seitz and Richter reported that the intramolecular [4+2]-cycloaddition of 2-(tetrazinyloxy)benzonitrile led to the formation of benzofuro[3,2-e][1,2,4]triazine derivatives [52]. Neunhoeffer et al. synthesized benzofuro[2,3-e][1,2,4]triazine at 26% yield using the tandem SNH-SNipso reaction of resorcinol and 1,2,4-triazine with a good leaving group [53]. Unfortunately, these methods represent the only examples of benzofurotriazine derivatives, and provide poor yields of the desired products. At the same time, 1,2,4-triazines represent readily accessible and cheap building blocks for the construction of pyridine [54,55,56,57,58,59,60,61,62], pyrimidine [63,64] or pyrazine [59,65,66] cores via the sequence of Diels-Alder/retro-Diels-Alder reactions.
In a continuation of our research on CDC reactions in triazines [54,67] and diazines [68], herein, we are pleased to report an unusual synthesis of benzofuro-fused 1,2,4-triazines via the sequence of C-C/C-O CDC reactions of 1,2,4-triazines with 2-naphthols or phenols (Scheme 1d). The reaction proceeded through the formation of 1,4-dihydrotriazine, followed by oxidative cyclization.
2. Results and Discussion
Based on retrosynthetic analysis of benzofurotriazine (Scheme 1d), we assume that 5,6-unsubstituted triazine and α-unsubstituted phenol are the best building blocks for the construction of the desired molecule through a sequence of C-C/C-O cross-coupling reactions. Earlier, our [67,69,70] and other [70,71] research groups demonstrated that 5,6-unsubstituted 1,2,4-triazines may be used in a two-step CDC reaction with various aromatic C-nucleophiles via the formation of 1,4-dihydrotriazine derivatives as intermediates, followed by aromatization to bi(het)aryl products. As mentioned above, the prefunctionalized azine is required for C-O cross-coupling reaction [53]. On the other hand, it is well known that the phenolic Schiff’s bases readily undergoes intramolecular oxidative cyclization in the presence of various oxidizing agents, in particular, hypervalent iodine compounds [72,73,74,75], lead(IV) acetate [76,77,78], or manganese salts such as Mn(OAc)3.2H2O [78,79] and MnO2 [80]. We hypothesized that 1,4-dihydrotriazines containing 2-hydroxyaryl moiety can be considered as a “formal” phenolic Schiff’s base (Scheme 2).
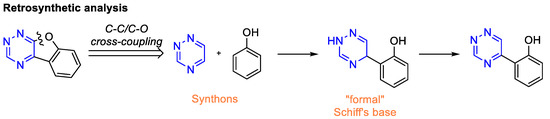
Scheme 2.
Retrosynthetic analyses of benzofurotriazine scaffold.
Based on this hypothesis, we have focused our attention on the oxidative cyclization of dihydrotriazines easily obtainable from triazine and naphthol. For example, the reaction of readily available 3-methylthio-1,2,4-triazine 1a and naphthol 2a yields dihydrotriazine 3aa (Scheme 3), which was used for the initial screening of the optimal conditions. Using standard oxidizing agent, such as phenyliodonine(III) diacetate, phenyliodonine(III) bis(trifluoroacetate) or Pb(OAc)4, for the oxidative cyclization of the phenolic Schiff’s base, only a complex mixture was isolated from the reaction. Surprisingly, when using MnO2 for the oxidation of the “formal” Schiff’s base 3aa, the desired oxidative coupling product naphthofurotriazine 4aa was formed in one step. At the same time, the side product 5aa was also observed in the reaction (Scheme 3). After comprehensive screening (Please see Supporting Information for details, Section S6), we found that the vigorous stirring (1500 rpm) of 3aa in CHCl3 at 50 °C in the presence of 3 equiv. of γ-MnO2 [81] provided the naphthofurotriazine 4aa in an almost quantitative yield after 3 h (Table 1, entry 1). Besides γ-MnO2, other manganese salts such as Mn(OAc)3.2H2O, Mn(OAc)2.4H2O and MnCl2, Mn(acac)2 were not so effective for this reaction, or provided 4aa in very poor yields (Table 1, entries 3-6), except MnO2 impregnated with nitric acid [82], which afforded 4aa in a good yield (Table 1, entry 2). One may assume that Mn(OAc)3 has low oxidative potential in organic media [83] compared to MnO2. Other alternative oxidants such as Ag2O, DTBP and DDQ led to low yields (Table 1, entries 7-9), and p-chloranil exclusively provided compound 5aa in a high yield (Table 1, entry 10). The use of other solvents such as 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), EtOH, DCE or benzene clearly gave worse results (Table 1, entries 11-14). By increasing the temperature, the yield of 4aa was decreased (Table 1, entry 15). In contrast, lower conversion was observed at room temperature (Table 1, entry 16). Carrying out the reaction in the presence of a decreased amount of MnO2 (Table 1, entry 17) had negative effects on the efficiency of the reaction. In contrast, using 5 equiv. of MnO2 increased the yield of the side product 5aa (Table 1, entry 18). At the same time, all attempts were unsuccessful to cyclize 5aa to 4aa.
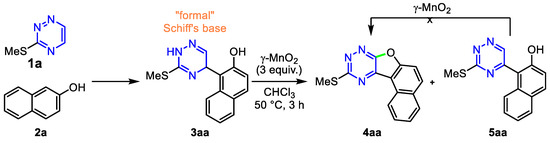
Scheme 3.
Synthesis and oxidation of dihydrotriazine 3aa.

Table 1.
Optimization of the reaction conditions 1.
In order to study the applicability of the proposed oxidative coupling reaction, we synthesized a series of starting dihydrotriazines 3. It was observed that our earlier proposed method [54] of the nucleophilic addition of 5,7-dimethoxycoumarins to 1,2,4-triazines with some modifications allowed us to prepare a series of compounds 3 using a variety of 3-S-substituted 1,2,4-triazines 1 and 2-naphthols 2 (Scheme 4). In all cases, the reaction proceeded with high regioselectivity to give compounds 3 in good to high yields. When methoxy- or hydroxy-substituted 2-naphthols 2b-e were involved in the reaction with 1,2,4-triazine, the best yields were achieved in the presence of BF3.OEt2 under refluxed conditions in methanol.
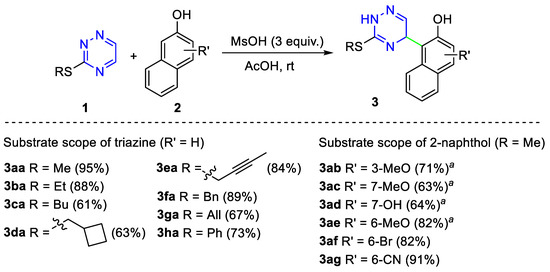
Scheme 4.
Substrate scope for the synthesis of compounds 3. Reaction conditions: 1 (1 mmol), 2 (1 mmol), MsOH (3 mmol) in AcOH (4 mL). a 1 (1 mmol), 2 (1 mmol), BF3.OEt2 (8 mmol) in methanol 4 mL under reflux.
With the optimized reaction conditions and a set of dihydrotriazines 3 in hand, we then examined the applicability and scope of this MnO2-induced oxidative cyclization reaction of dihydrotriazines 3. At first, the scope of the reaction was studied with respect to different S-substituents in the dihydrotriazine core, and the results are summarized in Scheme 5. The naphthofuro-fused triazine 4aa was isolated in a 95% yield under optimal reaction conditions after recrystallization from MeCN. Other 3-alkylthio-substituted triazine derivatives 3ba-3da and 3fa also underwent oxidative cyclization, producing only the desired cyclic product 4 in good to high yields. Moreover, 3-(but-2-yn-1-yl)- and 3-allylthio derivatives 3ea and 3ga smoothly transformed to 4ea and 4ga in 70% and 78% yields, respectively. However, in the case of phenylthio-substituted derivative 3ha, a 5:1 mixture of 4ha and 5ha was isolated. Next, an investigation of this coupling reaction on 3-methylthiotriazine adducts 3ab-3ag showed that the naphthyl ring substituted with various functional groups at different positions afforded the corresponding products with good to excellent yields. For example, bromo-, hydroxy-, methoxy- and cyano-substituted adducts 3ab-3ag underwent oxidative cyclization with high regioselectivity to give only naphthofuro[3,2-e]triazine derivatives 4 in up to 91% yields (Scheme 5).
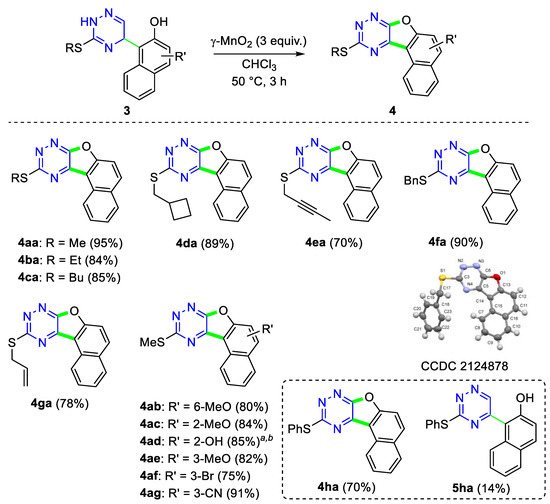
Scheme 5.
Substrate scope for the synthesis of benzofuro-fused 1,2,4-triazines 4. Reaction conditions: 3 (0.2 mmol), γ-MnO2 (0.6 mmol) in CHCl3 (3 mL), 50 °C, 3 h. a Two-step yield; b CHCl3:EtOH (v/v = 4:1, 3 mL).
Encouraged by these results, we then investigated the oxidative cyclization reaction of triazine not bearing S-substituents (Scheme 6). In particular, 3-phenyl and 3-(4-methoxyphenyl) (PMP) derivatives 3ia and 3ja prepared under standard conditions (MsOH, AcOH) underwent MnO2-induced oxidative cyclization to afford cyclic products 4ia and 4ja, respectively, as minor products with up to 28% yield. In contrast, 3-methyltriazine 1k smoothly reacted with 2-naphthol 2a in AcOH without the addition of MsOH, leading to the corresponding adduct 3la, which was oxidized in the presence of MnO2 to generate the desired 4la as a major product in a 48% overall yield. Similar to triazine 1k, 3-benzyltriazine 1l was also involved in the same cascade reaction to give the mixture of 4ka and 5ka in a ratio of 1:1. In addition, we were pleased to find that the oxidative cyclization of 3ma bearing the N-morpholinyl group in a triazine core produced the respective oxidative product 4ma in a 75% yield. Actually, the adduct 3ma was synthesized in situ by the interaction between triazine 1m and 2-naphthol 2a in the presence of BF3.OEt2 under reflux in methanol.
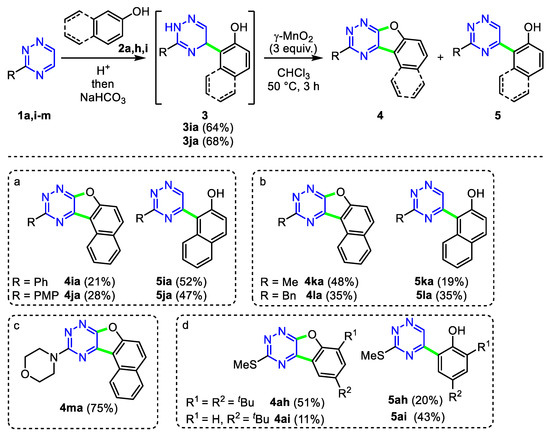
Scheme 6.
Addition/oxidative cyclization sequence of triazine with naphthol and phenol. Reaction conditions: 1 (1 mmol), 2 (1 mmol), (a) MsOH (3 mmol), AcOH (5 mL), rt; (b) AcOH (5 mL), rt; (c) BF3.OEt2 (3 mmol), MeOH (5 mL), reflux; (d) TFA (4 mL), rt.
Further, we explored the reactivity of p-substituted phenols in these sequence reactions. Unfortunately, all attempts to prepare the starting materials (3) under standard conditions (MsOH, AcOH, rt) failed, and only starting materials 1 and 2 were isolated from the reactions. However, we found that the use of trifluoroacetic acid (TFA) as the activator and medium at room temperature could allow the formation of unstable compounds 3ah and 3ai by the nucleophilic addition of phenol to the triazine core. These two compounds (3ah and 3ai) underwent the oxidative cyclization reaction, giving benzofuro[3,2-e]triazine 4 in lower to moderate yields. At the same time, biaryl by-products 5ah and 5ai were also isolated.
For further assessing the synthetic utility of the method, we performed the addition and coupling reaction sequence again at the gram scale. Thus, under slightly optimized conditions, we synthesized compound 4ad from triazine 1a and naphthol 2d at 40 mmol loading in an 85% yield via two steps (Scheme 4).
The thiomethyl group is a versatile moiety for coupling reactions. In triazines, the thiomethyl group may be easily substituted with aryl boronic acids [84,85] or trialkyl(aryl)stannanes [86] using Liebeskind–Srogl coupling [87]. To demonstrate the synthetic potential of benzofuro-annulated triazines, we performed the substitution of the thiomethyl group with an aryl substituent. The reaction of triazine 4aa with 4-carbazolyl-phenylboronic acid 6 provided the corresponding coupling product 7 in a 73% yield (Scheme 7a).
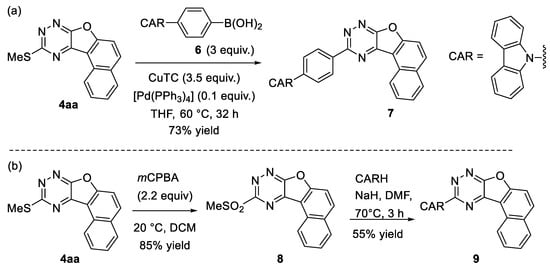
Scheme 7.
Further application of the synthesized compound. (a) Suzuki coupling; (b) Replacement of good leaving group.
Furthermore, a thiomethyl group can easily be oxidized with mCPBA to afford the methylsulfonyl group, which can be substituted with various nucleophiles [88,89,90]. Treatment of the synthesized compound 4aa with mCPBA gave the corresponding sulfonyl derivative 8 at an 85% yield. After that, we successfully synthesized carbazole-substituted naphthofuro-fused 1,2,4-triazine 9 via the subsequent replacement of the sulfonyl group in 8 with carbazole in the presence of sodium hydride (Scheme 7b). It is worth mentioning that these types of carbazole-substituted triazine derivatives have potential uses in biological fields [91,92] and OLED applications [93].
To gain some mechanistic insights into this oxidative cyclization, we first carried out several control experiments. When (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) or butylated hydroxytoluene (BHT) was added to the oxidative cyclization of 3aa under the standard reaction conditions (Scheme 8a), the desired product 4aa was obtained in a yield up 81%, suggesting that radicals may not be involved in the catalytic cycle, in contrast to the earlier published cyclization of the Schiff’s base in the presence of Mn salt [78,79]. The slight decrease in yield is probably due to the deactivation of manganese oxide under the reducing action of TEMPO and BHT. In addition, a high yield of 4aa was achieved, even when performing the reaction under a N2 atmosphere, demonstrating that aerobic oxygen is not the oxidizing agent in this transformation (Scheme 8a).
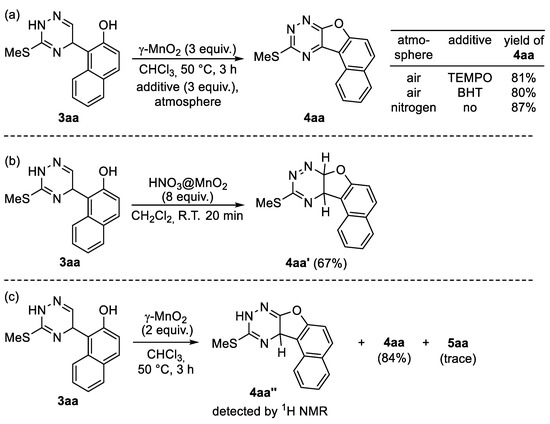
Scheme 8.
Control experiments for mechanistic investigation. (a) Oxidation in an inert and oxygen atmosphere; (b) Using MnO2 impregnated with HNO3; (c) Isolation of intermediates.
Subsequently, in order to get some information about possible reaction intermediates, we carried out the oxidative cyclization of 3aa under various conditions. After several trials, we managed to isolate one of the possible intermediates, 4aa′, in the presence of MnO2 impregnated with nitric acid [82] in CH2Cl2 at room temperature (Scheme 8b). The structure of the intermediate 4aa′ was supported by NMR and HRMS data. The 1H NMR spectrum comprises two dihydrotriazine proton doublets at 5.69 and 5.66 ppm with an SSCC (spin–spin coupling constant) of 10.8 Hz. Another intermediate 4aa′′ was detected by 1H NMR analysis (Please see Supporting Information for details, Section S6) in the crystallized reaction mixture when the reaction was carried out in the presence of a twofold excess of MnO2 (Scheme 8c). We ascribed the structure of dihydrotriazine to this compound since a single proton resonance at the sp3 carbon is observed in the 1H NMR spectrum.
After summarizing these preliminary mechanistic studies, a plausible reaction mechanism of the oxidative cyclization has been postulated (Scheme 9). The reaction may proceed through two different pathways: path a and path b. In path a, the reaction starts with the formation of an O-coordinated complex A, which agrees well with the oxidation of alcohol to aldehyde in the presence of MnO2 [94]. Then, complex A undergoes intramolecular nucleophilic addition to generate an intermediate 4aa′ with the elimination of Mn(II) species detected by an EPR experiment (Please see Supporting Information for details, Section S6). Then, the quick tautomerization of 4aa′ leads to the intermediate 4aa′′, which is aromatized with the second equivalent of MnO2, as well as with 1,4-dihydropyridine [95,96,97,98] or 1,4-dihydrotriazine [71,99], to give the final product 4aa. On the other hand, if we consider path b, at the first step, MnO2 may coordinate with the nitrogen atom of the triazine core, leading to N-coordinated complex B, which is also aromatized with the formation of biaryl product 5aa. Thus, the formation of the final product depends on the position of the initial coordination of the manganese dioxide, through which the reaction can proceed through the regular aromatization of dihydrotriazine (path A) or through the path of oxidative cyclization (path A).
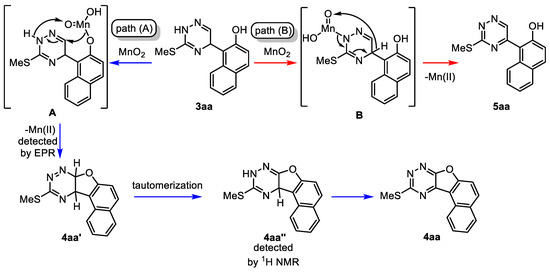
Scheme 9.
Proposed pathways of oxidation of dihydrotriazines. (A) Oxidative cyclization; (B) Regular aromatization.
In order to rationalize the regioselectivity of pathways of the products’ formation (4 vs. 5) we have performed a series of DFT calculations of the electron density of HOMO and HOMO-1 in the compounds 3aa, 3fa, 3ha and 3ia (Figure 2). The results show that the electron density on the oxygen atom of the hydroxyl group is comparable with the one on the nitrogen of the triazine core in compounds 3aa and 3ha. However, the larger energy gap between HOMO and HOMO-1 of 3aa compared with the energy gap in 3ha increased the regioselectivity of the formation of O-coordinated manganese ester. In the case of compound 3ia, the localization of the orbitals on the triazine N2 nitrogen (HOMO-1) was higher than those on the phenol oxygen (HOMO). So, the reaction proceeds partially via the aromatization of dihydrotriazine, rather than through oxidative cyclization. As follows from Figure 2, the important role of the alkylthio group is that it reduces the electron density at the nitrogen atom of dihydrotriazine, which leads to a reaction at the phenolic oxygen atom. Therefore, these results suggest that MnO2 may coordinate with either oxygen or nitrogen atoms, depending on the delocalization of the electron density of HOMO and HOMO-1 on the corresponding oxygen or nitrogen atom, and the energy gap between these orbitals.
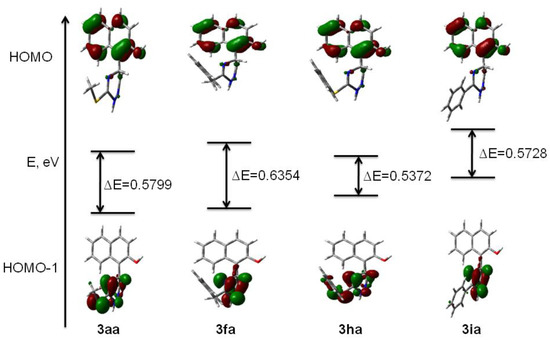
Figure 2.
Localizations of electron density on nitrogen and oxygen atoms for compounds 3aa, 3fa, 3ha, and 3ia.
3. Materials and Methods
General Information: All commercially available chemicals were used without further purifications. 1H NMR (400 MHz) and 13C NMR (101 MHz) spectra were registered on a Bruker DRX-400 Avance spectrometer with DMSO-d6 or CDCl3 as the solvent at ambient temperature. Chemical shifts are reported in ppm, and coupling constants are given in Hz. Data for 1H NMR are recorded as follows: chemical shift (ppm), multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; quin, quintet; sex, sextet; m, multiplet; br s, broad signal), integration and coupling constant (Hz). High-resolution mass spectra were recorded on an Agilent UHPLC/MS Accurate-Mass Q-TOF 1290/6545. EPR spectra were obtained using a Bruker Elexsys E500 CW-EPR spectrometer (modulation amplitude was set as 0.3 mT). The simulation of the EPR spectra was performed using the package EasySpin 5.2 software [100]. Molecular geometry optimization and the calculation of energies of molecules were carried out in the gas phase using the B3LYP DFT functional [101] with a 6-311 + G (d, p) basis set [102] according to [103] in Gaussian09 [104]. The plots of electron densities of molecular orbitals were obtained using the GaussView 6.0 software [105]. X-ray analysis for compound 5fa was executed on an Xcalibur 3 diffractometer (MoKα radiation, graphite monochromator, 295(2) K, φ- and ω-scanning with a step of 1°). Thin-layer chromatography (TLC) was performed on a silica gel-coated glass slide (Merck, Silica gel G for TLC). Column chromatography was carried out on silica gel (60 Å, 0.035−0.070 mm). Images of 1H and 13C NMR spectra are provided on pages S26–S81 of the Supplementary Materials.
3.1. Synthesis of S-substituted 3-thio-1,2,4-triazines 1a-f
S-substituted 3-thio-1,2,4-triazines 1a-f were prepared from the corresponding salt of S-substituted isothiosemicarbazide (2 mmol) and glyoxal solution according to the following procedure [106].
A solution of 40% glyoxal (8 mmol, 1160 mg) and NaHCO3 (5 mmol, 420 mg) in ice water (40 mL) was added to a solution of S-substituted isothiosemicarbazide hydrogen iodide (2 mmol) dissolved in ice water (40 mL). The reaction mixture was stirred for 15 min; during that time, the evolution of gas (CO2) was observed. The reaction mixture was left in the fridge overnight and the aqueous solution was extracted with chloroform. The combined organic layer was washed with 10% oxalic acid, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to obtain oil or a solid triazine compound.
3.1.1. Synthesis of 3-(phenylthio)-1,2,4-triazine 1h
Compound 1h was prepared via the oxidation of 1a with mCPBA using a modified procedure [107] followed by the treatment of compound 1a′ with thiophenol.
mCPBA (11.6 g, 77%, 52 mmol) and anhydrous Na2SO4 (4.0 g) were successively added to DCM (60 mL); the mixture was stirred for 15 min and then filtered and the filter cake was washed with 10 mL of DCM to obtain a clear dichloromethane solution of mCPBA. A dichloromethane solution of 3-methylthio-1,2,4-triazine 1a (3.0 g, 23.6 mmol) was added to this dichloromethane solution of mCPBA at −10 °C with stirring. The reaction mixture was allowed to heat to ambient temperature and then stirred for an additional 3 h. Dichloromethane was evaporated under reduced pressure to obtain a dry mixture of 3-(methylsulfonyl)-1,2,4-triazine 1a′ and m-chlorobenzoic acid. The mixture was dissolved in pyridine (40 mL) and thiophenol (5.3 mL, 5.72 g, 52 mmol) was added after. After 24 h the mixture was evaporated in vacuo, and the residue was treated with a mixture of dichloromethane and aqueous NaHCO3. The organic layer was evaporated, yielding pure compound 1h.
3.1.2. Synthesis of 3-phenyl- and 3-(4-methoxyphenyl)-1,2,4-triazines 1i and 1j
Compounds 1i and 1j were prepared according to the published procedure [108]. The spectroscopic data for compound 1i are in agreement with the literature [109].
3.1.3. Synthesis of 3-methyl- and 3-benzyl-1,2,4-triazine 1k and 1l
Compounds 1k and 1l were prepared according to the known procedure [110]. The spectroscopic data of compounds 1k are in agreement with the published data [110].
3.1.4. Synthesis of 4-(1,2,4-triazin-3-yl)morpholine 1m
Compound 1m was prepared according to the published procedure [111].
3.2. General Procedure for the Synthesis of Dihydrotriazines 3
3.2.1. Method A
To a stirred solution of triazine 1a-j (1 mmol, 1 equiv.) and 2-naphthol 2a,f,g (1 mmol, 1 equiv.) in acetic acid (4 mL), we added a methanesulfonic acid (195 μL, 3 mmol, 3 equiv.). The resulting mixture was stirred at room temperature for 1-5 h. The progress of the reaction was monitored using TLC. After the completion of the reaction, the reaction mixture was diluted with water (20 mL), neutralized with aq. NaHCO3 solution and extracted with AcOEt (3 × 10 mL). The combined organic phase was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel chromatography or recrystallization from the corresponding solvent to afford product 3.
3.2.2. Method B
To a stirred solution of triazine 1a (1 mmol, 1 equiv.) and 2-naphthol 2b-e (1 mmol, 1 equiv.) in methanol (4 mL) we added BF3.OEt2 (985 μL, 8 mmol, 8 equiv.) and the resulting mixture was refluxed for 5 h. After cooling the methanol was evaporated under reduced pressure, and the residue was dissolved in AcOEt (10 mL) and washed with 5% aq. NaHCO3 solution (50 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The crude product was recrystallized from MeCN to obtain the product 3ab-3ae.
3.3. General Procedure for the Synthesis of Naphthofuro-Fused Triazines 4
To a stirred solution of 3 (0.2 mmol, 1 equiv.) in CHCl3 (3 mL), MnO2 (52 mg, 0.6 mmol, 3 equiv.) was added in one portion. The resulting mixture was stirred at 50 °C for 3 h. The completion of the reaction was monitored by TLC. The reaction mixture was then cooled to room temperature; the MnO2 was filtered and the filter cake was washed with CHCl3 (3 × 10 mL). The combined organic phase was concentrated under reduced pressure. The residue was purified by chromatography on silica gel or recrystallization to afford the pure product 4.
3.3.1. 10-(Methylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4aa
Pale yellow needles after recrystallization from MeCN. Yield 51 mg, 95%; m.p. 185–187 °C. 1H NMR (CDCl3): 8.78–8.66 (m, 1H, H-1), 8.20–8.06 (m, 1H, H-5), 7.97–7.86 (m, 1H, H-4), 7.76–7.52 (m, 3H, H-2, H-3, H-6), 2.79 (s, 3H, SCH3); 13C NMR (CDCl3): 169.8, 160.0, 158.6, 143.7, 137.0, 130.5, 129.6, 129.2, 128.7, 126.7, 124.7, 112.7, 112.6, 14.8. Anal. Calcd. For C14H9N3OS: C, 62.91; H, 3.39; N, 15.72%; found: C, 62.96; H, 3.47; N, 15.79%.
3.3.2. 10-(Ethylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ba
Pale yellow needles after recrystallization from MeCN. Yield 47 mg, 84%; m.p. 141–143 °C. 1H NMR (CDCl3): 8.97–8.88 (m, 1H, H-1), 8.28–8.21 (m, 1H, H-5), 8.06–7.99 (m, 1H, H-4), 7.87–7.75 (m, 2H, H-2, H-6), 7.70–7.62 (m, 1H, H-3), 3.44 (q, 2H, J = 7.3 Hz, SCH2), 1.56 (t, 3H, J = 7.3 Hz, CH3); 13C NMR (CDCl3): 169.6, 160.1, 158.7, 143.9, 137.0, 130.6, 129.6, 129.3, 128.9, 126.8, 124.8, 112.9, 112.7, 26.1, 14.4. Anal. Calcd. For C15H11N3OS: C, 64.04; H, 3.94; N, 14.94%; found: C, 64.12; H, 3.99; N, 14.85%.
3.3.3. 10-(Butylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ca
Pale yellow needles after recrystallization from MeCN. Yield 53 mg, 85%; m.p. 121–123 °C. 1H NMR (CDCl3): 8.83–8.75 (m, 1H, H-1), 8.20–8.12 (m, 1H, H-5), 7.99–7.92 (m, 1H, H-4), 7.79–7.55 (m, 3H, H-2, H-3, H-6), 3.45–3.33 (m, 2H, SCH2), 1.96–1.83 (m, 2H, SCH2CH2), 1.68–1.53 (m, 2H, SCH2CH2CH2), 1.08–0.97 (m, 3H, CH3); 13C NMR (CDCl3): 169.8, 160.1, 158.7, 143.9, 137.1, 130.6, 129.6, 129.4, 128.9, 126.8, 124.9, 113.0, 112.8, 31.4, 31.3, 22.3, 13.9. Anal. Calcd. For C17H15N3OS: C, 66.00; H, 4.89; N, 13.58%; found: C, 66.09; H, 4.82; N, 13.50%.
3.3.4. 10-((Cyclobutylmethyl)thio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4da
Yellow needles after recrystallization from MeCN. Yield 57 mg, 89%; m.p. 154–156 °C. 1H NMR (CDCl3): 8.81–8.76 (m, 1H, H-1), 8.17 (d, 1H, J = 9.0 Hz, H-5), 7.98–7.93 (m, 1H, H-4), 7.78–7.72 (m, 1H, H-2), 7.70 (d, 1H, J = 9.0 Hz, H-6), 7.63–7.57 (m, 1H, H-3), 3.53–3.44 (m, 2H, SCH2), 2.90–2.80 (m, 1H, CH-1′), 2.29–2.18 (m, 2H, CH2-3′), 1.98–1.83 (m, 4H, CH2-2′, CH2-4′); 13C NMR (CDCl3): 169.7, 160.0, 158.7, 143.8, 137.0, 130.6, 129.6, 129.3, 128.8, 126.7, 124.8, 112.8, 112.7, 37.9, 34.7, 28.0, 18.2. Anal. Calcd. For C18H15N3OS: C, 67.27; H, 4.70; N, 13.07%; found: C, 67.35; H, 4.78; N, 13.12%.
3.3.5. 10-(But-2-yn-1-ylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ea
Brown powder after purification by chromatography on silica gel using n-hexane-ethyl acetate (10:1). Yield 45 mg, 70%; m.p. 163–165 °C. 1H NMR (CDCl3): 8.88–8.83 (m, 1H, H-1), 8.22 (d, 1H, J = 9.0 Hz, H-5), 8.02–7.97 (m, 1H, H-4), 7.82–7.77 (m, 1H, H-2), 7.74 (d, 1H, J = 9.0 Hz, H-6), 7.67–7.61 (m, 1H, H-3), 4.15 (q, 2H, J = 2.5 Hz, SCH2), 1.84 (t, 3H, J = 2.5 Hz, CH3); 13C NMR (CDCl3): 168.4, 160.1, 158.8, 143.9, 137.3, 130.6, 129.8, 129.8, 128.9, 126.9, 124.9, 112.9, 112.7, 79.5, 73.7, 21.0, 3.9. Anal. Calcd. For C17H11N3OS: C, 66.87; H, 3.63; N, 13.76%; found: C, 66.80; H, 3.60; N, 13.86%.
3.3.6. 10-(Benzylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4fa
Yellow needles after recrystallization from MeCN. Yield 63 mg, 90%; m.p. 161–163 °C. 1H NMR (CDCl3): 8.82–8.73 (m, 1H, H-1), 8.17 (d, 1H, J = 9.1 Hz, H-5), 7.97–7.93 (m, 1H, H-4), 7.78–7.72 (m, 1H, H-3), 7.70 (d, 1H, J = 9.1 Hz, H-6), 7.26–7.56 (m, 3H, H-2, Ph), 7.38–7.32 (m, 2H, Ph), 7.30–7.25 (m, 1H, Ph), 4.66 (m, 1H, SCH2); 13C NMR (CDCl3): 169.0, 160.2, 158.8, 143.9, 137.2, 137.1, 130.6, 129.7, 129.4, 129.4, 128.9, 128.7, 127.6, 126.8, 124.9, 112.9, 112.7, 36.1. Anal. Calcd. For C20H13N3OS: C, 69.95; H, 3.82; N, 12.24%; found: C, 69.85; H, 3.76; N, 12.20%.
3.3.7. 10-(Allylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ga
Pale yellow powder after recrystallization from MeCN. Yield 46 mg, 78%; m.p. 126–128 °C. 1H NMR (CDCl3): 8.83–8.74 (m, 1H, H-1), 8.17 (d, 1H, J = 9.1 Hz, H-5), 7.99–7.93 (m, 1H, H-4), 7.78–7.72 (m, 1H, H-3), 7.70 (d, 1H, J = 9.1 Hz, H-6), 7.64–7.57 (m, 1H, H-2), 6.13 (ddt, 1H, 3J = 6.9 Hz, 3J(cis) = 10.0 Hz, 3J(trans) = 17.0 Hz, CH-2′), 5.47 (dd, 1H, 3J(trans) = 16.9 Hz, J = 1.2 Hz, CH-3a′), 5.22 (d, 1H, 3J(cis) = 10.0 Hz, CH-3b′), 4.06 (d, 2H, 3J = 6.9 Hz, SCH2); 13C NMR (CDCl3): 168.9, 160.1, 158.7, 143.8, 137.1, 133.1, 130.6, 129.7, 129.3, 128.8, 126.8, 124.8, 118.7, 112.9, 112.7, 34.5. Anal. Calcd. For C16H9N3OS: C, 65.97; H, 3.11; N, 14.42%; found: C, 65.90; H, 3.18; N, 14.50%.
A mixture of 4ha and 5ha was separated by silica gel chromatography using n-hexane-ethyl acetate (17:1) to isolate 4ha and n-hexane-ethyl acetate (10:1) to give 5ha.
3.3.8. 10-(Phenylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ha
Yellow powder. Yield 46 mg, 70%; m.p. 196–198 °C. 1H NMR (CDCl3): 8.58–8.53 (m, 1H, H-1), 8.22–8.17 (m, 1H, H-5), 8.00–7.95 (m, 1H, H-4), 7.83–7.77 (m, 2H, Ph), 7.75–7.67 (m, 2H, H-3, H-6), 7.64–7.58 (m, 1H, H-2), 7.56–7.50 (m, 3H, Ph); 13C NMR (CDCl3): 170.0, 160.3, 158.8, 144.0, 137.2, 135.8, 130.6, 129.7, 129.6, 129.5, 129.3, 129.2, 128.8, 126.8, 124.8, 113.0, 112.7. Anal. Calcd. For C19H11N3OS: C, 69.29; H, 3.37; N, 12.76%; found: C, 69.20; H, 3.45; N, 12.70%.
3.3.9. 1-(3-(Phenylthio)-1,2,4-triazin-5-yl)naphthalen-2-ol 5ha
Yellow powder. Yield 9 mg, 14%; m.p. 149–151 °C. 1H NMR (CDCl3): 11.01 (s, 1H, OH), 9.51 (s, 1H, H-6′), 8.09–8.03 (m, 1H, H-8), 7.84 (d, 1H, J = 9.0 Hz, H-4), 7.82–7.76 (m, 1H, H-5), 7.75–7.67 (m, 2H, Ph), 7.59–7.51 (m, 4H, Ph, H-7), 7.44–7.37 (m, 1H, H-6), 7.07 (d, 1H, J = 9.0 Hz, H-3); 13C NMR (CDCl3): 172.3, 160.8, 155.7, 146.2, 136.2, 136.0, 130.8 (2C), 130.3, 129.5, 129.2, 128.7, 126.9, 124.7, 123.2, 119.7, 108.9. Anal. Calcd. For C19H13N3OS: C, 68.86; H, 3.95; N, 12.68%; found: C, 68.77; H, 3.90; N, 12.60%.
A mixture of 4ia and 5ia was separated by chromatography using n-hexane-ethyl acetate (15:1) to give 4ia and n-hexane-ethyl acetate (8:1) to give 5ia.
3.3.10. 10-Phenylnaphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ia
Pale yellow solid. Yield 12 mg, 21%, m.p. 189–191 °C. Rf = 0.54 (ethyl acetate:hexane, 1:1). 1H NMR (CDCl3): 9.09–8.99 (m, 1H, H-1), 8.75–8.64 (m, 2H, Ph), 8.24–8.14 (m, 1H, H-5), 8.03–7.94 (m, 1H, H-4), 7.86–7.73 (m, 2H, H-2, H-6), 7.66–7.52 (m, 4H, H-3, Ph); 13C NMR (CDCl3): 161.5, 160.8, 158.5, 143.9, 136.7, 135.7, 131.2, 130.7, 129.6, 129.3, 129.1, 129.0, 128.5, 126.7, 124.9, 113.7, 112.8. Anal. Calcd. For C19H11N3O: C, 76.76; H, 3.73; N, 14.13%; found: C, 76.83; H, 3.70; N, 14.19%.
3.3.11. 1-(3-Phenyl-1,2,4-triazin-5-yl)naphthalen-2-ol 5ia
Yellow solid. Yield 31 mg, 52%, m.p. 204–206 °C. Rf = 0.39 (ethyl acetate:hexane, 1:1). 1H NMR (DMSO-d6): 12.45–12.16 (br s, 1H, OH), 9.77 (s, 1H, H-6′), 8.56–8.47 (m, 2H, Ph), 8.21–8.11 (m, 1H, H-8), 7.95 (d, 1H, J = 9.0 Hz, H-4), 7.90–7.83 (m, 1H, H-5), 7.67–7.55 (m, 4H, H-6, Ph), 7.49–7.42 (m, 1H, H-7), 7.28 (d, 1H, J = 9.0 Hz, H-3); 13C NMR (DMSO-d6): 161.3, 160.3, 155.9, 148.3, 135.8, 134.2, 132.4, 131.0, 129.6, 129.3 (2C), 128.7, 128.4, 124.7, 123.1, 119.6, 109.4. Anal. Calcd. For C19H13N3O: C, 76.24; H, 4.38; N, 14.04%; found: C, 76.32; H, 4.45; N, 14.12%.
A mixture of 4ja and 5ja was separated by silica gel chromatography using n-hexane-ethyl acetate (17:1) to isolate 4ja and n-hexane-ethyl acetate (7:1) to give 5ja.
3.3.12. 10-(4-Methoxyphenyl)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ja
Pale yellow solid. Yield 18 mg, 28%; m.p. 205–207 °C. Rf = 0.53 (ethyl acetate:hexane, 1:1). 1H NMR (CDCl3): 9.05–8.99 (m, 1H, H-1), 8,64 (d, 2H, J = 8.8 Hz, Ph), 8.19 (d, 1H, J = 9.0 Hz, H-5), 8.02–7.94 (m, 1H, H-4), 7.85–7.78 (m, 1H, H-3), 7.76 (d, 1H, J = 9.0 Hz, H-6), 7.66–7.58 (m, 1H, H-2), 7.08 (d, 2H, J = 8.8 Hz, Ph), 3.93 (s, 3H, OCH3); 13C NMR (CDCl3): 162.3, 161.4, 160.6, 158.4, 143.9, 136.5, 130.7, 130.1, 129.5, 129.3, 129.1, 128.3, 126.7, 124.9, 114.3, 113.7, 112.8, 55.6. Anal. Calcd. For C20H13N3O2: C, 73.38; H, 4.00; N, 12.84%; found: C, 73.30; H, 3.92; N, 12.94%.
3.3.13. 1-(3-(4-Methoxyphenyl)-1,2,4-triazin-5-yl)naphthalen-2-ol 5ja
Yellow solid. Yield 31 mg, 47%; m.p. 178–180 °C. Rf = 0.41 (ethyl acetate:hexane, 1:1). 1H NMR (DMSO-d6): 12.33–12.08 (br s, 1H, OH), 9.59 (s, 1H, H-6′), 8.38 (d, 2H, J = 8.7 Hz, Ph), 8.09–8.01 (m, 1H, H-8), 7.87–7.81 (m, 1H, H-4), 7.80–7.72 (m, 1H, H-5), 7.54–7.45 (m, 1H, H-7 or H-6), 7.39–7.30 (m, 1H, H-6 or H-7), 7.22–7.13 (m, 1H, H-3), 7.03–6.95 (d, 2H, J = 8.7 Hz, Ph), 3.83 (s, 3H, OCH3); 13C NMR (DMSO-d6): 162.5, 156.8, 154.1, 151.0, 135.1, 132.6, 132.2, 131.6, 129.1, 128.4, 128.0, 127.8, 127.6, 123.4, 123.1, 118.1, 113.9, 55.7. Anal. Calcd. For C20H15N3O2: C, 72.94; H, 4.59; N, 12.76%; found: C, 72.83; H, 4.65; N, 12.70%.
3.3.14. 6-Methoxy-10-(methylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ab
Yellow powder after recrystallization from MeCN. Yield 48 mg, 80%; m.p. 183–185 °C. 1H NMR (CDCl3): 8.55–8.51 (m, 1H, H-4 or H-1), 7.71–7.66 (m, 1H, H-1 or H-4), 7.55–7.50 (m, 1H, H-3 or H-2), 7.79–7.50 (m, 1H, H-2 or H-3), 7.32 (s, 1H, H-5), 4.13 (s, 3H, OCH3), 2.78 (s, 3H, SCH3); 13C NMR (CDCl3): 169.9, 159.9, 149.9, 145.0, 143.3, 131.2, 127.6, 126.9, 126.8, 124.3, 123.6, 114.1, 113.3, 56.5, 14.8. Anal. Calcd. For C15H11N3O2S: C, 60.59; H, 3.73; N, 14.13%; found: C, 60.67; H, 3.65; N, 14.04%.
3.3.15. 2-Methoxy-10-(methylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ac
Yellow powder after recrystallization from MeCN. Yield 50 mg, 84%; m.p. 219–221 °C. 1H NMR (CDCl3): 8.22–8.19 (m, 1H, H-1), 8.14–8.10 (m, 1H, H-4), 7.88 (d, 1H, J = 8.9 Hz, H-4), 7.57 (d, 1H, J = 8.9 Hz, H-3), 7.27–7.22 (m, 1H, H-3), 4.06 (s, 3H, OCH3), 2.81 (s, 3H, SCH3); 13C NMR (CDCl3): 169.6, 161.0, 160.2, 159.4, 144.1, 136.8, 131.0, 125.7, 118.8, 112.0, 109.8, 104.2, 96.3, 55.8, 14.8. Anal. Calcd. For C15H11N3O2S: C, 60.59; H, 3.73; N, 14.13%; found: C, 60.50; H, 3.66; N, 14.10%.
3.3.16. 10-(Methylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazin-2-ol 4ad
According to the general procedure, in the mixture of CHCl3:EtOH (4:1) as solvent, 4ad was obtained as yellow powder after recrystallization from EtOH. Yield 38 mg, 68%; m.p. 282–284 °C. 1H NMR (DMSO-d6): 10.61 (s, 1H, OH), 8.30 (d, 1H, J = 8.9 Hz, H-6), 8.03–7.97 (m, 2H, H-1, H-4), 7.70 (d, 1H, J = 8.9 Hz, H-4), 7.57 (d, 1H, J = 8.9 Hz, H-3), 7.19–7.14 (m, 1H, H-3), 2.76 (s, 3H, SCH3); 13C NMR (DMSO-d6): 168.0, 159.9, 159.1, 159.1, 144.0, 137.4, 131.6, 130.2, 124.4, 118.4, 110.5, 108.9, 106.5, 14.1. Anal. Calcd. For C14H9N3O2S: C, 59.35; H, 3.20; N, 14.83%; found: C, 59.25; H, 3.15; N, 14.75%.
3.3.17. 3-Methoxy-10-(methylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ae
Yellow powder after recrystallization from MeCN. Yield 49 mg, 82%; m.p. 214–216 °C. 1H NMR (CDCl3): 8.83 (d, 1H, J = 8.9 Hz, H-1), 8.13 (d, 1H, J = 9.0 Hz, H-5), 7.74 (d, 1H, J = 9.0 Hz, H-6), 7.47 (dd, 1H, J = 8.9 Hz, J = 2.5 Hz, H-2), 7.33 (d, 1H, J = 2.5 Hz, H-4), 3.98 (s, 3H, OCH3), 2.83 (s, 3H, SCH3); 13C NMR (CDCl3): 169.7, 160.2, 158.3, 157.6, 143.9, 135.9, 132.2, 126.3, 123.8, 121.7, 113.1 (2C), 108.2, 55.6, 14.9. Anal. Calcd. For C15H11N3O2S: C, 60.59; H, 3.73; N, 14.13%; found: C, 60.65; H, 3.82; N, 14.10%.
3.3.18. 3-Bromo-10-(methylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4af
Yellow powder after recrystallization from toluene. Yield 53 mg, 75%; m.p. 244–246 °C. 1H NMR (CDCl3): 8.86 (d, 1H, J = 8.7 Hz, H-1), 8.23 (d, 1H, J = 1.8 Hz, H-4), 8.19 (d, 1H, J = 9.1 Hz, H-5), 7.92 (dd, 1H, J = 8.7 Hz, J = 1.8 Hz, H-2), 7.85 (d, 1H, J = 9.1 Hz, H-6), 2.85 (s, 3H, SCH3); 13C NMR (CDCl3): 170.2 160.3, 158.7, 143.6, 136.0, 133.0, 132.0, 131.5, 127.5, 126.6, 120.8, 114.1, 113.2, 14.9. Anal. Calcd. For C14H8BrN3OS: C, 48.57; H, 2.33; N, 12.14%; found: C, 48.50; H, 2.26; N, 12.06%.
3.3.19. 10-(Methylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine-3-carbonitrile 4ag
Yellow solid after recrystallization from MeCN. Yield 54 mg, 91%; m.p. 283–285 °C. 1H NMR (CDCl3): 9.09 (d, 1H, J = 8.5 Hz, H-1), 8.44 (d, 1H, J = 1.5 Hz, H-4) 8.33 (d, 1H, J = 9.1 Hz, H-5), 7.99 (dd, 1H, J = 8.5 Hz, J = 1.5 Hz, H-2), 7.96 (d, 1H, J = 9.1 Hz, H-6), 2.85 (s, 3H, SCH3); 13C NMR (CDCl3): 170.7, 160.4, 159.8, 143.3, 136.8, 134.9, 130.9, 130.6, 129.9, 126.3, 118.6, 115.1, 113.4, 110.7, 14.9. Anal. Calcd. For C15H8N4OS: C, 61.63; H, 2.76; N, 19.17%; found: C, 61.72; H, 2.86; N, 19.22%.
3.3.20. Synthesis of 10-alkyl naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazines 4ka and 4la
To a stirred solution of corresponding triazine 1k or 1l (1 mmol, 1 equiv.) in acetic acid (4 mL), 2-naphthol 2a (144 mg, 1 mmol, 1 equiv.) was added. Then the mixture was stirred at room temperature for 5 h, concentrated under reduced pressure, dissolved in CHCl3 (10 mL) and washed with saturated aq. NaHCO3 solution (10 mL). The organic layer was dried over anhydrous Na2SO4 and filtered. To the organic phase, MnO2 (261 mg, 3.0 mmol, 3 equiv.) was added in one portion and the mixture was stirred at 50 °C for 3 h. The reaction mixture was then cooled to room temperature. MnO2 was filtered and washed with CHCl3 (3 × 10 mL). The combined organic phase was concentrated under reduced pressure to give a mixture of 4 and 5, which was separated by chromatography on silica gel using a mixture of n-hexane-ethyl acetate as the eluent.
A mixture of 4ka and 5ka was separated by chromatography on silica gel using n-hexane-ethyl acetate (25:1) to isolate 4ka and n-hexane-ethyl acetate (8:1) to give 5ka.
3.3.21. 10-Methylnaphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ka
Yellow solid. Yield 113 mg, 48%; m.p. 190–192 °C. Rf = 0.46 (ethyl acetate:hexane, 1:1). 1H NMR (CDCl3): 8.98–8.92 (m, 1H, H-1), 8.24–8.17 (m, 1H, H-5), 8.04–7.98 (m, 1H, H-4), 7.84–7.72 (m, 2H, H-2, H-6), 7.68–7.59 (m, 1H, H-3), 3.08 (s, 3H, CH3); 13C NMR (CDCl3): 164.4, 160.6, 158.4, 144.0, 136.7, 130.7, 129.5, 129.3, 129.0, 126.7, 124.9, 113.4, 112.8, 23.9. Anal. Calcd. For C14H9N3O: C, 71.48; H, 3.86; N, 17.86%; found: C, 71.39; H, 3.93; N, 17.92%.
3.3.22. 1-(3-Methyl-1,2,4-triazin-5-yl)naphthalen-2-ol 5ka
Pale yellow solid. Yield 45 mg, 19%; m.p. 168–170 °C. Rf = 0.25 (ethyl acetate:hexane 1:1). 1H NMR (DMSO-d6): 10.46 (s, 1H, OH), 9.44 (s, 1H, H-6′), 8.01–7.96 (m, 1H, H-4), 7.92–7.85 (m, 1H, H-8 or H-5), 7.73–7.67 (m, 1H, H-5 or H-8), 7.45–7.29 (m, 3H, H-3, H-6, H-7), 2.85 (s, 3H, CH3); 13C NMR (DMSO-d6): 166.2, 156.4, 153.8, 150.2, 132.3, 132.1, 128.3, 127.9, 127.4, 123.3, 123.2, 118.1, 113.8, 23.6. Anal. Calcd. For C14H11N3O: C, 70.87; H, 4.67; N, 17.71%; found: C, 70.77; H, 4.72; N, 17.80%.
A mixture of 4la and 5la was separated by chromatography on silica gel using n-hexane-ethyl acetate (17:1) to give 4la, and n-hexane-ethyl acetate (10:1) to give 5la.
3.3.23. 10-Benzylnaphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4la
Pale yellow solid. Yield 109 mg, 35%; m.p. 226–228 °C. Rf = 0.57 (ethyl acetate:hexane, 1:1). 1H NMR (DMSO-d6): 8.89–8.82 (m, 1H, H-1), 8.49 (d, 1H, J = 9.0 Hz H-5), 8.25–8.20 (m, 1H, H-4), 8.06 (d, 1H, J = 9.0 Hz H-6), 7.93–7.87 (m, 1H, H-3 or H-2), 7.74–7.69 (m, 1H, H-2 or H-3), 7.49–7.44 (m, 2H, Ph), 7.37–7.32 (m, 2H, Ph), 7.27–7.22 (m, 1H, Ph), 4.62 (s, 2H, CH2); 13C NMR (DMSO-d6): 165.0, 160.2, 158.0, 143.9, 138.1, 137.0, 130.2, 129.5 (2C), 129.0 (2C), 128.3 (2C), 128.1, 126.4 (2C), 123.7, 112.9, 112.5, 42.7. Anal. Calcd. For C20H13N3O: C, 77.16; H, 4.21; N, 13.50%; found: C, 77.25; H, 4.30; N, 13.57%.
3.3.24. 1-(3-Methyl-1,2,4-triazin-5-yl)naphthalen-2-ol 5la
Pale yellow solid. Yield 110 mg, 35%; m.p. 155–157 °C. Rf = 0.34 (ethyl acetate:hexane, 1:1). 1H NMR (DMSO-d6): 10.55 (s, 1H, OH), 9.49 (s, 1H, H-6′), 8.01–7.94 (m, 1H, H-4), 7.90–7.84 (m, 1H, H-8 or H-5), 7.63–7.55 (m, 1H, H-5 or H-8), 7.43–7.22 (m, 8H, H-3, H-6, H-7, Ph), 4.47 (s, 2H, CH2); 13C NMR (DMSO-d6): 167.9, 156.7, 154.0, 150.6, 137.7, 132.6, 132.0, 129.2, 128.5, 128.3, 128.0, 127.3, 126.6, 123.4, 123.1, 118.0, 113.5, 43.0. Anal. Calcd. For C20H15N3O: C, 76.66; H, 4.83; N, 13.41%; found: C, 76.75; H, 4.74; N, 13.48%.
3.3.25. 10-Morpholinonaphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4ma
To a stirred solution of 4-(1,2,4-triazin-3-yl)morpholine 1m (1 mmol, 1 equiv.) and 2-naphthol 2a (1 mmol, 1 equiv.) in methanol (4 mL) BF3.OEt2 (370 μL, 3 mmol, 3 equiv.) was added dropwise, and the resulting mixture was refluxed for 3 h. After cooling to room temperature the methanol was evaporated under reduced pressure, and the residue was dissolved in CHCl3 (10 mL) and washed with aq. NaHCO3. Then, the organic layer was dried over Na2SO4 and filtered. To the resulting solution MnO2 (261 mg, 3 mmol, 3 equiv.) was added in one portion and the mixture was stirred at 50 °C for 3 h. The reaction mixture was cooled to room temperature. MnO2 was filtered and washed with CHCl3 (3 × 10 mL). The combined organic phase was concentrated under reduced pressure, and the residue was crystallized from MeCN to afford pure 4ma. Yellow powder. Yield 225 mg, 75%; m.p. 230–232 °C. 1H NMR (CDCl3): 8.87–8.82 (m, 1H, H-1), 8.19 (d, 1H, J = 9.1 Hz, H-5), 8.02–7.96 (m, 1H, H-4), 7.80–7.73 (m, 1H, H-3), 7.70 (d, 1H, J = 9.1 Hz, H-6), 7.65–7.58 (m, 1H, H-2), 4.07–4.00 (m, 4H, morpholine), 3.95–3.88 (m, 4H, morpholine); 13C NMR (CDCl3): 161.1, 158.9, 157.7, 144.2, 136.0, 130.5, 129.3, 129.3, 129.2, 126.3, 124.6, 113.5, 113.0, 67.0, 45.1. Anal. Calcd. For C17H14N4O2: C, 66.66; H, 4.61; N, 18.29%; found: C, 66.75; H, 4.54; N, 18.36%.
3.3.26. Synthesis of Benzofuro-Fused Triazines 4ah and 4ai
To a solution of triazine 1a (127 mg, 1 mmol) in TFA (4 mL), a corresponding phenol 2h or 2i (1 mmol) was added, and the resulting mixture was stirred at room temperature for 24 h. The completion of the reaction was monitored by TLC. Then, the reaction mixture was concentrated under reduced pressure. The residue was dissolved in CHCl3 (10 mL) and washed with 5% aq. NaHCO3. The organic layer was dried over Na2SO4 and filtered. MnO2 (52 mg, 0.6 mmol, 3 equiv.) was added to the resulting solution in one portion and the mixture was stirred at 50 °C for 3 h, cooled to room temperature, and MnO2 was filtered and the filter cake washed with CHCl3 (3 × 10 mL). The combined organic phase was concentrated under reduced pressure. The residue was purified by silica gel chromatography to afford the pure product, using n-hexane-ethyl acetate (80:1) to afford 4ah or 4ai and n-hexane-ethyl acetate (40:1) to give 5ah or 5ai.
3.3.27. 6,8-Di-tert-butyl-3-(methylthio)benzofuro[3,2-e][1,2,4]triazine 4ah
Yellow powder. Yield 167 mg, 51%; m.p. 105–107 °C. Rf = 0.67 (ethyl acetate:hexane, 1:1). 1H NMR (CDCl3): 8.08 (d, 1H, J = 1.8 Hz H-5), 7.78 (d, 1H, J = 1.8 Hz, H-7), 2.79 (s, 3H, SCH3), 1.57 (s, 9H, C(CH3)3), 1.41 (s, 9H, C(CH3)3); 13C NMR (CDCl3): 169.1, 160.7, 155.8, 148.6, 144.2, 136.1, 130.3, 118.8, 118.0, 35.4, 35.0, 31.7, 29.8, 14.8. Anal. Calcd. For C18H23N3OS: C, 65.62; H, 7.04; N, 12.75%; found: C, 65.72; H, 7.10; N, 12.82%.
3.3.28. 2,4-Di-tert-butyl-6-(3-(methylthio)-1,2,4-triazin-5-yl)phenol 5ah
Pale yellow powder. Yield 67 mg, 20%; m.p. 113–115 °C. Rf = 0.64 (ethyl acetate:hexane, 1:1). 1H NMR (CDCl3): 12.73 (s, 1H, OH), 9.50 (s, 1H, H-6′), 7.69 (d, 1H, J = 2.3 Hz, H-5), 7.57 (d, 1H, J = 2.3 Hz, H-3), 2.75 (s, 3H, SCH3), 1.46 (s, 9H, C(CH3)3), 1.35 (s, 9H, C(CH3)3); 13C NMR (CDCl3): 170.1, 160.0, 156.0, 141.8, 141.6, 139.0, 130.9, 121.2, 113.0, 35.5, 34.6, 31.5, 29.5, 14.1. Anal. Calcd. For C18H25N3OS: C, 65.22; H, 7.60; N, 12.68%; found: C, 65.31; H, 7.51; N, 12.74%.
3.3.29. 6-(tert-Butyl)-3-(methylthio)benzofuro[3,2-e][1,2,4]triazine 4ai
Yellow powder. Yield 30 mg, 11%; m.p. 136–138 °C. Rf = 0.64 (ethyl acetate:hexane, 1:1). 1H NMR (CDCl3): 8.23 (d, 1H, J = 1.8 Hz, H-5), 7.88 (dd, 1H, J = 1.8 Hz, J = 8.9 Hz, H-7), 7.61 (d, 1H, J = 8.9 Hz, H-8), 2.78 (s, 3H, SCH3), 1.42 (s, 9H, C(CH3)3); 13C NMR (CDCl3): 169.3, 161.0, 157.2, 149.0, 144.1, 133.5, 120.6, 118.5, 112.8, 35.3, 31.6, 14.8. Anal. Calcd. For C14H15N3OS: C, 61.52; H, 5.53; N, 15.37%; found: C, 61.59; H, 5.44; N, 15.30%.
3.3.30. 4-(tert-Butyl)-2-(3-(methylthio)-1,2,4-triazin-5-yl)phenol 5ai
Yellow powder. Yield 120 mg, 43%; m.p. 123–125 °C. Rf = 0.59 (ethyl acetate:hexane, 1:1). 1H NMR (CDCl3): 11.97 (s, 1H, OH), 9.49 (s, 1H, H-6′), 7.80 (d, 1H, J = 2.0 Hz, H-3), 7.53 (dd, 1H, J = 2.0 Hz, J = 8.8 Hz, H-5), 6.99 (d, 1H, J = 8.8 Hz, H-8), 2.71 (s, 3H, SCH3), 1.34 (s, 9H, C(CH3)3); 13C NMR (CDCl3): 170.6, 160.3, 155.3, 142.9, 141.2, 133.5, 123.2, 119.1, 113.2, 34.4, 31.4, 14.0. Anal. Calcd. For C14H17N3OS: C, 61.06; H, 6.22; N, 15.26%; found: C, 61.13; H, 6.29; N, 15.16%.
3.3.31. 40 mmol Scaled Synthesis of 3ad
To a stirred solution of triazine 1a (5.10 g, 40 mmol, 1 equiv.) and 2,7-dihydroxynaphthalene 2d (6.40 g, 40 mmol, 1 equiv.) in methanol (40 mL), BF3.OEt2 (40 mL, 320 mmol, 8 equiv.) was added dropwise and the resulting mixture was refluxed for 8 h. After cooling the methanol was evaporated under reduced pressure, and then the residue was treated with AcOEt (30 mL) and stirred for 15 min. The precipitate formed was filtered and washed with AcOEt (10 mL). The precipitate was suspended in AcOEt and the resulting mixture was washed with aq. NaHCO3 solution. The organic layer was dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure to give 3ad. The 3ad was dissolved in a mixture of CHCl3:EtOH (4:1, 300 mL). To the resulting solution, MnO2 (10.44 g, 120 mmol, 3 equiv.) was added in one portion. The resulting mixture was stirred at 50 °C for 6 h. The completion of the reaction was monitored by TLC. The reaction mixture was then cooled to room temperature, and the MnO2 was filtered and washed with CHCl3 (3 × 50 mL). The combined organic phase was concentrated under reduced pressure. The residue was recrystallized in EtOH to give pure 4ad (9.62 g, 85% in two steps).
3.4. Further Modifications of Compound 4aa
3.4.1. 10-(4-(Carbazol-9-yl)phenyl)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 7
To a solution of 10-(methylthio)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 4aa (100 mg, 1 equiv.) in dry THF (5 mL), we added CuTC (249 mg, 3.5 equiv), Pd[PPh3]4 (43 mg, 10 mol%) and (4-(9H-carbazol-9-yl)phenyl)boronic acid (322 mg, 3 equiv.). Then, the reaction mixture was stirred at reflux for 32 h. The progress of the reaction was monitored by TLC. After completion, the solvent was evaporated under reduced pressure and the residue was purified by flash chromatography using n-hexane-ethyl acetate (10:1→5:1) to give a pure product 7 as yellow powder. Yield 126 mg, 73%; m.p. 280–282 °C. 1H NMR (CDCl3): 9.22–9.18 (m, 1H, H-1), 9.01–8.97 (m, 2H, H-2′′), 8.34–8.29 (m, 1H, H-5), 8.20–8.16 (m, 2H, H-1′), 8.11–8.08 (m, 1H, H-4), 7.94–7.82 (m 4H, H-2, H-6, H-3′′), 7.74–7.70 (m, 1H, H-3), 7.60-7.57 (m, 2H, H-4′), 7.48–7.43 (m, 2H, H-3′ or H-2′), 7.36-7.31 (m, 2H, H-2′ or H-3′); 13C NMR (CDCl3): 161.1, 161.0, 158.8, 144.3, 140.7, 140.5, 137.1, 134.5, 130.9, 130.2, 129.8, 129.6, 129.2, 127.2, 126.9, 126.3, 125.1, 123.8, 120.6, 120.5, 113.8, 112.9, 110.1. Anal. Calcd. For C31H18N4O: C, 80.50; H, 3.92; N, 12.11%; found: C, 80.31; H, 4.07; N, 11.96%.
3.4.2. 10-(Methylsulfonyl)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 8
mCPBA (427 mg, ≤77%, 2.2 equiv.) was dissolved in dry DCM (5 mL), Na2SO4 (2.0 g) was added to the resulting solution and the mixture was stirred for 10 min. Na2SO4 was filtered and washed with DCM (3 × 5 mL). The obtained solution of mCPBA was added dropwise to a solution of 4aa (133 mg, 0.5 mmol) in DCM (4 mL) at 0 °C. Then the reaction mixture was stirred at room temperature for 12 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was quenched with an aqueous solution of NaHCO3, washed with water and dried over Na2SO4, and the solvent was evaporated under reduced pressure. The residue was purified by flash chromatography using n-hexane-chloroform (2:1) as eluent to give pure 8 as a yellow powder. Yield 127 mg, 85%; m.p. 248–251 °C. 1H NMR (CDCl3): 9.07–9.04 (m, 1H, H-1), 8.43–8.39 (m, 1H, H-5), 8.11–8.08 (m, 1H, H-4), 7.93–7.88 (m, 2H, H-2, H-6), 7.76–7.72 (m, 1H, H-3), 7.65–7.58 (m, 1H, H-2), 3.66 (s, 2H, SO2CH3); 13C NMR (CDCl3): 163.8, 161.6, 160.4, 145.3, 139.5, 131.0, 130.7, 129.7, 128.7, 127.8, 125.5, 113.0, 112.6, 40.6. Anal. Calcd. For C14H9N3O3S: C, 56.18; H, 3.03; N, 14.04%; found: C, 56.05; H, 3.20; N, 13.96%.
3.4.3. 10-(Carbazol-9-yl)naphtho[1′,2′:4,5]furo[3,2-e][1,2,4]triazine 9
To a solution of carbazole (106 mg, 1.9 equiv.) in dry DMF (3 mL), we added NaH (60% suspension in mineral oil, 19 mg, 1.4 equiv.) and the mixture was stirred for 10 min. Then methylsulfonyl derivative 8 (100 mg, 0.33 mmol) was added to the resulting solution and the mixture was heated at 70 °C for 12 h. After completion of the reaction, the mixture was diluted with water (15 mL), and the forming precipitate was filtered and washed with water and ethanol and purified by flash chromatography using n-hexane:chloroform (2:1) as the eluent to give pure 9 as a yellow powder. Yield 71 mg, 55%; m.p. 250–253 °C. 1H NMR (HMPA d-18): 9.02–8.98 (m, 1H, H-1), 8.95-8.90 (d, J = 9.1 Hz, 1H, H-5), 8.85–8.78 (m, 2H, H-1′), 8.58–8.54 (m, 1H, H-4), 8.51–8.46 (m, 2H, H-4′), 8.42 (d, J = 9.1 Hz, 1H, H-6), 8.15–8.09 (m, 1H, H-3 and H-2), 7.89–7.82 (m, 1H, H-2 and H-3), 7.68–7.61 (m, 2H, H-3′ or H-2′), 7.50–7.43 (m, 2H, H-2′ or H-3′); 13C NMR (HMPAd-18): 160.2, 159.8, 157.9, 144.5, 139.4, 139.3, 131.5, 130.9, 130.6, 128.9, 127.3, 127.1, 126.0, 124.0, 122.9, 121.0, 115.1, 113.8, 113.2. Anal. Calcd. For C25H14N4O: C, 77.71; H, 3.65; N, 14.50%; found: C, 77.90; H, 2.81; N, 14.29%.
4. Conclusions
In summary, we have developed an unusual MnO2-induced oxidative cyclization in adducts of phenols and triazines. This method provides easy two-step access to benzofuro-fused triazine via the nucleophilic addition of the 2-naphthol to 1,2,4-triazine, followed by oxidative cyclization. The scope and limitations of this novel reaction have been investigated. Further application of the synthesized compound has been demonstrated by synthesizing carbazole-substituted benzofuro-fused triazines. The mechanistic study has revealed that the process proceeds through the formation of an O-coordinated Mn complex. We believe that the present methodology will open a new door to synthesizing important building blocks of α-sulfonylamino ketones.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27207105/s1. Synthesis of the starting 1,2,4-triazines, 3, 4, 4aa derivatives; Preliminary mechanistic studies; DFT calculations; 1H and 13C NMR spectra for compounds 1,3–5,7–9, 4aa′.
Author Contributions
Conceptualization, I.A.K., S.S. and O.N.C.; methodology, R.F.F., A.D.S. and A.P.P., N.N.M., A.V.I. and P.N.M.; software, A.N.T.; validation, I.A.K. and S.S.; formal analysis, R.F.F., I.A.K. and S.S.; investigation, R.F.F., A.D.S., A.P.P., N.N.M., A.V.I. and P.N.M.; resources, R.F.F. and I.A.K.; data curation, R.F.F., I.A.K. and S.S.; writing—original draft preparation, R.F.F., I.A.K. and S.S.; writing—review and editing, S.S.; visualization, I.A.K. and S.S.; supervision, O.N.C.; project administration, R.F.F., I.A.K. and S.S.; funding acquisition, R.F.F. and I.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Scientific Foundation (Grant #21-13-00304, synthesis of compounds 4) and the Council on Grants of the President of the Russian Federation (Ref. #NSh- 1223.2022.1.3, synthesis of the starting compounds 1 and DFT studies).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the all compounds are available from the authors.
References
- Engle, K.M.; Mei, T.-S.; Wasa, M.; Yu, J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 2012, 45, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, N.; Hopkinson, M.N.; Wencel-Delord, J.; Glorius, F. Beyond Directing Groups: Transition-Metal-Catalyzed C-H Activation of Simple Arenes. Angew. Chem. Int. Ed. 2012, 51, 10236–10254. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, S.R.; Sanford, M.S. Controlling site selectivity in palladium-catalyzed C–H bond functionalization. Acc. Chem. Res. 2012, 45, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Direct C−H transformation via iron catalysis. Chem. Rev. 2011, 111, 1293–1314. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Yamaguchi, A.D.; Itami, K. C-H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew. Chem. Int. Ed. 2012, 51, 8960–9009. [Google Scholar] [CrossRef]
- Rit, R.K.; Yadav, M.R.; Ghosh, K.; Sahoo, A.K. Reusable directing groups [8-aminoquinoline, picolinamide, sulfoximine] in C(sp3)–H bond activation: Present and future. Tetrahedron 2015, 71, 4450–4459. [Google Scholar] [CrossRef]
- Bag, S.; Patra, T.; Modak, A.; Deb, A.; Maity, S.; Dutta, U.; Dey, A.; Kancherla, R.; Maji, A.; Hazra, A.; et al. Remote para-C–H Functionalization of Arenes by a D-Shaped Biphenyl Template-Based Assembly. J. Am. Chem. Soc. 2015, 137, 11888–11891. [Google Scholar] [CrossRef]
- Yoshikai, N. Cobalt-Catalyzed C-C Bond-Forming Reactions via Chelation-Assisted CH Activation. Yuki Gosei Kagaku Kyokaishi 2014, 72, 1198–1206. [Google Scholar] [CrossRef]
- Phillips, A.M.F.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. New Trends in Enantioselective Cross-Dehydrogenative Coupling. Catalysts 2020, 10, 529. [Google Scholar] [CrossRef]
- Bosque, I.; Chinchilla, R.; Gonzalez-Gomez, J.C.; Guijarro, D.; Alonso, F. Cross-dehydrogenative coupling involving benzylic and allylic C–H bonds. Org. Chem. Front. 2020, 7, 1717–1742. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Chen, T.; Han, L. Cross-Dehydrogenative Alkynylation: A Powerful Tool for the Synthesis of Internal Alkynes. ChemSusChem 2020, 13, 4776–4794. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K. Recent advances in oxidative C–C coupling reaction of amides with carbon nucleophiles. Tetrahedron Lett. 2017, 58, 4655–4662. [Google Scholar] [CrossRef]
- Li, C.-J. Cross-dehydrogenative coupling (CDC): Exploring C−C bond formations beyond functional group transformations. Acc. Chem. Res. 2009, 42, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Li, Z.; Li, C.-J. Cross-dehydrogenative coupling: A sustainable reaction for C–C bond formations. Green Chem. 2021, 23, 6789–6862. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Kang, H.; Li, J.; Li, C.-J. En route to intermolecular cross-dehydrogenative coupling reactions. J. Org. Chem. 2019, 84, 12705–12721. [Google Scholar] [CrossRef]
- Rohit, K.R.; Radhika, S.; Saranya, S.; Anilkumar, G. Manganese-Catalysed Dehydrogenative Coupling—An Overview. Adv. Synth. Catal. 2020, 362, 1602–1650. [Google Scholar] [CrossRef]
- Voigt, B.; Meijer, L.; Lozach, O.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Novel CDK inhibition profiles of structurally varied 1-aza-9-oxafluorenes. Bioorg. Med. Chem. Lett. 2005, 15, 823–825. [Google Scholar] [CrossRef]
- Tell, V.; Mahmoud, K.A.; Wichapong, K.; Schächtele, C.; Totzke, F.; Sippl, W.; Hilgeroth, A. Novel aspects in structure–activity relationships of profiled 1-aza-9-oxafluorenes as inhibitors of Alzheimer’s disease-relevant kinases cdk1, cdk5 and gsk3β. MedChemComm 2012, 3, 1413–1418. [Google Scholar] [CrossRef]
- Tell, V.; Holzer, M.; Herrmann, L.; Mahmoud, K.A.; Schächtele, C.; Totzke, F.; Hilgeroth, A. Multitargeted drug development: Discovery and profiling of dihydroxy substituted 1-aza-9-oxafluorenes as lead compounds targeting Alzheimer disease relevant kinases. Bioorg. Med. Chem. Lett. 2012, 22, 6914–6918. [Google Scholar] [CrossRef]
- Schade, N.; Koch, P.; Ansideri, F.; Krystof, V.; Holzer, M.; Hilgeroth, A. Evaluation of Novel Substituted Furopyridines as Inhibitors of Protein Kinases Related to Tau Pathology in Alzheimer’s Disease. Med. Chem. 2021, 17, 844–855. [Google Scholar] [CrossRef]
- Marciano, R.; David, H.B.; Akabayov, B.; Rotblat, B. The Amuvatinib Derivative, N-(2H-1,3-Benzodioxol-5-yl)-4-{thieno [3,2-d]pyrimidin-4-yl}piperazine-1-carboxamide, Inhibits Mitochondria and Kills Tumor Cells under Glucose Starvation. Int. J. Mol. Sci. 2020, 21, 1041. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.S.H.; Abdel Aziz, Y.M.; Elgawish, M.S.; Said, M.M.; Abouzid, K.A.M. Design, synthesis, biological evaluation and molecular modeling study of new thieno [2,3-d]pyrimidines with anti-proliferative activity on pancreatic cancer cell lines. Bioorg. Chem. 2020, 94, 103472. [Google Scholar] [CrossRef] [PubMed]
- Phillip, C.J.; Zaman, S.; Shentu, S.; Balakrishnan, K.; Zhang, J.; Baladandayuthapani, V.; Taverna, P.; Redkar, S.; Wang, M.; Stellrecht, C.M.; et al. Targeting MET kinase with the small-molecule inhibitor amuvatinib induces cytotoxicity in primary myeloma cells and cell lines. J. Hematol. Oncol. 2013, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.J. Targeting receptor tyrosine kinase MET in cancer: Small molecule inhibitors and clinical progress. J. Med. Chem. 2014, 57, 4427–4453. [Google Scholar] [CrossRef]
- Huang, C.-C.; Xue, M.-M.; Wu, F.-P.; Yuan, Y.; Liao, L.-S.; Fung, M.-K. Deep-blue and hybrid-white organic light emitting diodes based on a twisting carbazole-benzofuro [2,3-b] pyrazine fluorescent emitter. Molecules 2019, 24, 353. [Google Scholar] [CrossRef] [PubMed]
- Salman, G.A.; Nisa, R.U.; Iaroshenko, V.O.; Iqbal, J.; Ayub, K.; Langer, P. Pyrrole versus quinoline formation in the palladium catalyzed reaction of 2-alkynyl-3-bromothiophenes and 2-alkynyl-3-bromofurans with anilines. A combined experimental and computational study. Org. Biomol. Chem. 2012, 10, 9464–9473. [Google Scholar] [CrossRef] [PubMed]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-aryl-7-methoxybenzo[b]furo [3,2-d]pyrimidin-4-amines and their N-arylbenzo[b]thieno [3,2-d]pyrimidin-4-amine analogues as dual inhibitors of CLK1 and DYRK1A kinases. Eur. J. Med. Chem. 2013, 59, 283–295. [Google Scholar] [CrossRef]
- Rao, Y.; Li, Z.; Yin, G. Clean and efficient assembly of functionalized benzofuro [2,3-c] pyridines via metal-free one-pot domino reactions. Green Chem. 2014, 16, 2213–2218. [Google Scholar] [CrossRef]
- Brikci-Nigassa, N.M.; Bentabed-Ababsa, G.; Erb, W.; Chevallier, F.; Picot, L.; Vitek, L.; Fleury, A.; Thiéry, V.; Souab, M.; Robert, T.; et al. 2-Aminophenones, a common precursor to N-aryl isatins and acridines endowed with bioactivities. Tetrahedron 2018, 74, 1785–1801. [Google Scholar] [CrossRef]
- Yonekura, K.; Shinoda, M.; Yonekura, Y.; Tsuchimoto, T. Indium-Catalyzed Annulation of o-Acylanilines with Alkoxyheteroarenes: Synthesis of Heteroaryl[b]quinolines and Subsequent Transformation to Cryptolepine Derivatives. Molecules 2018, 23, 838. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Y.; Tang, J.; Zhang, L.; Liu, Q.; Li, Q.; Gao, G.; You, J. Ir-Catalyzed Cascade C–H Fusion of Aldoxime Ethers and Heteroarenes: Scope and Mechanisms. ACS Catal. 2020, 10, 203–209. [Google Scholar] [CrossRef]
- Bouarfa, S.; Bentabed-Ababsa, G.; Erb, W.; Picot, L.; Thiéry, V.; Roisnel, T.; Dorcet, V.; Mongin, F. Iodothiophenes and Related Compounds as Coupling Partners in Copper-Mediated N-Arylation of Anilines. Synthesis 2021, 53, 1271–1284. [Google Scholar] [CrossRef]
- Rong, B.; Xu, G.; Yan, H.; Zhang, S.; Wu, Q.; Zhu, N.; Fang, Z.; Duan, J.; Guo, K. Synthesis of benzofuro-and benzothieno [2,3-c]pyridines via copper-catalyzed [4+2] annulation of ketoxime acetates with acetoacetanilide. Org. Chem. Front. 2021, 8, 2939–2943. [Google Scholar] [CrossRef]
- Sadeghzadeh, P.; Pordel, M.; Davoodnia, A. Synthesis of 3H-[1]Benzofuro [2,3-b]imidazo [4,5-f]quinolines as New Fluorescent Heterocyclic Systems for Dye-Sensitized Solar Cells. Russ. J. Org. Chem. 2021, 57, 440–447. [Google Scholar] [CrossRef]
- Yue, W.S.; Li, J.J. A concise synthesis of all four possible benzo [4,5]furopyridines via palladium-mediated reactions. Org. Lett. 2002, 4, 2201–2203. [Google Scholar] [CrossRef]
- Sun, W.; Wang, M.; Zhang, Y.; Wang, L. Synthesis of Benzofuro [3,2-b]pyridines via Palladium-Catalyzed Dual C–H Activation of 3-Phenoxypyridine 1-Oxides. Org. Lett. 2015, 17, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, R.M.; Hickey, A.; Reen, F.J.; O’Gara, F.; McGlacken, G.P. Synthesis of Benzofuroquinolines via Phosphine-Free Direct Arylation of 4-Phenoxyquinolines in Air. Eur. J. Org. Chem. 2018, 2018, 6140–6149. [Google Scholar] [CrossRef]
- Shanahan, R.M.; Hickey, A.; Bateman, L.M.; Light, M.E.; McGlacken, G.P. One-Pot Cross-Coupling/C–H Functionalization Reactions: Quinoline as a Substrate and Ligand through N–Pd Interaction. J. Org. Chem. 2020, 85, 2585–2596. [Google Scholar] [CrossRef]
- Rathod, P.K.; Jonnalagadda, S.; Panaganti, L. A simple and efficient synthesis of benzofuroquinolines via the decarboxylative cross-coupling. Tetrahedron Lett. 2021, 66, 152808. [Google Scholar] [CrossRef]
- Nakamura, S.; Tohnai, N.; Nishii, Y.; Hinoue, T.; Miura, M. Effect of Substitution Pattern of tert-Butyl Groups in a Bisbenzofuropyrazine Core π-System on Optical Properties: Unique Mechanochromic Fluorescence Behavior. ChemPhotoChem 2019, 3, 46–53. [Google Scholar] [CrossRef]
- Singh, R.; Bhatia, H.; Prakash, P.; Debroye, E.; Dey, S.; Dehaen, W. Tandem Nenitzescu reaction/nucleophilic aromatic substitution to form novel pyrido fused indole frameworks. Eur. J. Org. Chem. 2021, 2021, 4865–4875. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, H.; He, Q.; Yang, C. Synthesis of multisubstituted 2-aminopyrroles/pyridines via chemoselective Michael addition/intramolecular cyclization reaction. Org. Lett. 2014, 16, 4186–4189. [Google Scholar] [CrossRef] [PubMed]
- Miliutina, M.; Janke, J.; Hassan, S.; Zaib, S.; Iqbal, J.; Lecka, J.; Sévigny, J.; Villinger, A.; Friedrich, A.; Lochbrunner, S.; et al. A domino reaction of 3-chlorochromones with aminoheterocycles. Synthesis of pyrazolopyridines and benzofuropyridines and their optical and ecto-5′-nucleotidase inhibitory effects. Org. Biomol. Chem. 2018, 16, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jing, C.; Hang, D.; Fan, H.; Duan, L.; Fang, S.; Yan, L. Synthesis, characterization, and photoelectric properties of iridium(III) complexes containing an N hetero-dibenzofuran C^N ligand. RSC Adv. 2021, 11, 11004–11010. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fitzgerald, A.E.; Mani, N.S. Facile assembly of fused benzo [4,5]furo heterocycles. J. Org. Chem. 2008, 73, 2951–2954. [Google Scholar] [CrossRef] [PubMed]
- Cramp, S.; Dyke, H.J.; Higgs, C.; Clark, D.E.; Gill, M.; Savy, P.; Jennings, N.; Price, S.; Lockey, P.M.; Norman, D.; et al. Identification and hit-to-lead exploration of a novel series of histamine H4 receptor inverse agonists. Bioorg. Med. Chem. Lett. 2010, 20, 2516–2519. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Steglich, W. Synthesis of some benzofuronaphthyridines and benzofuronaphthyridine derivatives. J. Heterocycl. Chem. 1993, 30, 909–912. [Google Scholar] [CrossRef]
- Xiao, X.; Lai, M.; Song, Z.; Geng, M.; Ding, J.; Xie, H.; Zhang, A. Design, synthesis and pharmacological evaluation of bicyclic and tetracyclic pyridopyrimidinone analogues as new KRASG12C inhibitors. Eur. J. Med. Chem. 2021, 213, 113082. [Google Scholar] [CrossRef]
- Ondachi, P.W.; Comins, D.L. Synthesis of Fused-Ring Nicotine Derivatives from (S)-Nicotine. J. Org. Chem. 2010, 75, 1706–1716. [Google Scholar] [CrossRef]
- Kumar, K.S.; Adepu, R.; Kapavarapu, R.; Rambabu, D.; Krishna, G.R.; Reddy, C.M.; Priya, K.K.; Parsa, K.V.L.; Pal, M. AlCl3 induced C-arylation/cyclization in a single pot: A new route to benzofuran fused N-heterocycles of pharmacological interest. Tetrahedron Lett. 2012, 53, 1134–1138. [Google Scholar] [CrossRef]
- Eid, M.M.; Kadry, A.M.; Hassan, R.A. Synthesis and reactions of some 6-(2-hydroxy-1-naphthyl)-1,2,4-triazines. J. Heterocycl. Chem. 1988, 25, 1117–1118. [Google Scholar] [CrossRef]
- Seitz, G.; Richter, J. Donorsubstituierte Benzonitrile als Seitenkettendienophile bei der intramolekularen [4+2]-Cycloaddition mit inversem Elektronenbedarf. Chem. Ber. 1989, 122, 2177–2181. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Rusinov, G.L.; Beresnev, D.G.; Charushin, V.N.; Neunhoeffer, H. A simple one pot synthesis of condensed 1,2,4-triazines by using the tandem aN-SNipso and SNH-SNipsa reactions. J. Heterocycl. Chem. 2001, 38, 901–907. [Google Scholar] [CrossRef]
- Fatykhov, R.F.; Savchuk, M.I.; Starnovskaya, E.S.; Bobkina, M.V.; Kopchuk, D.S.; Nosova, E.V.; Zyryanov, G.V.; Khalymbadzha, I.A.; Chupakhin, O.N.; Charushin, V.N.; et al. Nucleophilic substitution of hydrogen–the Boger reaction sequence as an approach towards 8-(pyridin-2-yl) coumarins. Mendeleev Commun. 2019, 29, 299–300. [Google Scholar] [CrossRef]
- Savchuk, M.I.; Kopchuk, D.S.; Taniya, O.S.; Nikonov, I.L.; Egorov, I.N.; Santra, S.; Zyryanov, G.V.; Chupakhin, O.N.; Charushin, V.N. 5-Aryl-6-arylthio-2, 2′-bipyridine and 6-Arylthio-2, 5-diarylpyridine Fluorophores: Pot, Atom, Step Economic (PASE) Synthesis and Photophysical Studies. J. Fluoresc. 2021, 31, 1099–1111. [Google Scholar] [CrossRef]
- Raw, S.A.; Taylor, R.J.K. Highly substituted pyridines via tethered imine–enamine (TIE) methodology. Chem. Commun. 2004, 508–509. [Google Scholar] [CrossRef]
- Papadopoulou, M.V.; Taylor, E.C. Intramolecular Diels-Alder reactions of 1,2,4-triazines. Synthesis of 3-alkylpyridines via Raney nickel desulfurization of thieno [2,3-b] pyridines. Tetrahedron 2021, 89, 132158. [Google Scholar] [CrossRef]
- Moseev, T.D.; Nikiforov, E.A.; Varaksin, M.V.; Starnovskaya, E.S.; Savchuk, M.I.; Nikonov, I.L.; Kopchuk, D.S.; Zyryanov, G.V.; Chupakhin, O.N.; Charushin, V.N. Novel Pentafluorophenyl-and Alkoxyphenyl-Appended 2, 2′-Bipyridine Push–Pull Fluorophores: A Convenient Synthesis and Photophysical Studies. Synthesis 2021, 53, 3597–3607. [Google Scholar] [CrossRef]
- Lipińska, T.; Branowska, D.; Rykowski, A. 1,2,4-triazines in organic synthesis. 8. Intramolecular diels-alder reaction of 5-acyl-1, 2, 4-triazineoxime ethers. New route of synthesis of alkylhetarylketones. Chem. Heterocycl. Compd. 1999, 35, 334–342. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Kopchuk, D.S.; Kim, G.A.; Shevyrin, V.A.; Santra, S.; Rahman, M.; Taniya, O.S.; Zyryanov, G.V.; Rusinov, V.L.; Chupakhin, O.N. Water-soluble luminescent lanthanide complexes based on C6-DTTA-appended 5-aryl-2, 2′-bipyridines. Polyhedron 2020, 181, 114473. [Google Scholar] [CrossRef]
- Fernández, S.Y.; Raw, S.A.; Taylor, R.J.K. Improved methodologies for the preparation of highly substituted pyridines. J. Org. Chem. 2005, 70, 10086–10095. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ren, N.; Ma, X.; Nie, J.; Zhang, F.-G.; Ma, J.-A. Silver-Catalyzed [3 + 3] Dipolar Cycloaddition of Trifluorodiazoethane and Glycine Imines: Access to Highly Functionalized Trifluoromethyl-Substituted Triazines and Pyridines. ACS Catal. 2019, 9, 4600–4608. [Google Scholar] [CrossRef]
- Yamanaka, H.; Sagi, M.; Wada, K.; Konno, S. Studies on as-triazine derivatives. XV, Intramolecular reverse-electron demand Diels-Alter reaction of 1,2,4-triazine derivatives. Heterocycles 1990, 30, 1009–1021. [Google Scholar] [CrossRef]
- Taylor, E.C.; Pont, J.L. Intramolecular Diels-Alder reactions of 1,2,4-triazines. Synthesis of condensed pyrimidines. J. Org. Chem. 1987, 52, 4287–4292. [Google Scholar] [CrossRef]
- Taylor, E.C.; Pont, J.L.; Warner, J.C. Heterodienophilic intramolecular Diels-Alder reactions of 1,2,4-triazines: Synthesis of novel polycyclic condensed pyrazines and lumazines. Tetrahedron 1987, 43, 5159–5168. [Google Scholar] [CrossRef]
- Taylor, E.C.; French, L.G. Intramolecular Diels-Alder reactions of 1,2,4-triazines. Routes to condensed pyrazines via cycloaddition of nitrile dienophiles. J. Org. Chem. 1989, 54, 1245–1249. [Google Scholar] [CrossRef]
- Khalymbadzha, I.A.; Chupakhin, O.N.; Fatykhov, R.F.; Charushin, V.N.; Schepochkin, A.V.; Kartsev, V.G. Transition-metal-free cross-dehydrogenative coupling of triazines with 5,7-dihydroxycoumarins. Synlett 2016, 27, 2606–2610. [Google Scholar] [CrossRef]
- Khalymbadzha, I.A.; Fatykhov, R.F.; Chupakhin, O.N.; Charushin, V.N.; Tseitler, T.A.; Sharapov, A.D.; Inytina, A.K.; Kartsev, V.G. Transition-Metal-Free C–C Coupling of 5, 7-Dihydroxybenzopyrones with Quinoxalones and Pteridinones. Synthesis 2018, 50, 2423–2431. [Google Scholar] [CrossRef]
- Utepova, I.A.; Nemytov, A.I.; Ishkhanian, V.A.; Chupakhin, O.N.; Charushin, V.N. Metal-free C–H/C–H coupling of 1,3-diazines and 1,2,4-triazines with 2-naphthols facilitated by Brønsted acids. Tetrahedron 2020, 76, 131391. [Google Scholar] [CrossRef]
- Fatykhov, R.; Khalymbadzha, I.; Chupakhin, O. Cross-Dehydrogenative Coupling Reactions between Phenols and Hetarenes: Modern Trends in Cross-Coupling Chemistry of Phenols. Adv. Synth. Catal. 2022, 364, 1052–1068. [Google Scholar] [CrossRef]
- Alphonse, F.-A.; Suzenet, F.; Keromnes, A.; Lebret, B.; Guillaumet, G. A general approach to selective functionalization of 1, 2, 4-triazines using organometallics in palladium-catalyzed cross-coupling and addition reactions. Synthesis 2004, 2004, 2893–2899. [Google Scholar] [CrossRef]
- Costa, S.P.G.; Oliveira, E.; Lodeiro, C.; Raposo, M.M.M. Heteroaromatic alanine derivatives bearing (oligo) thiophene units: Synthesis and photophysical properties. Tetrahedron Lett. 2008, 49, 5258–5261. [Google Scholar] [CrossRef][Green Version]
- Verma, V.; Singh, K.; Kumar, D.; Klapötke, T.M.; Stierstorfer, J.; Narasimhan, B.; Qazi, A.K.; Hamid, A.; Jaglan, S. Synthesis, antimicrobial and cytotoxicity study of 1,3-disubstituted-1H-naphtho [1,2-e][1,3]oxazines. Eur. J. Med. Chem. 2012, 56, 195–202. [Google Scholar] [CrossRef]
- Koleda, O.; Broese, T.; Noetzel, J.; Roemelt, M.; Suna, E.; Francke, R. Synthesis of benzoxazoles using electrochemically generated hypervalent iodine. J. Org. Chem. 2017, 82, 11669–11681. [Google Scholar] [CrossRef]
- Babbs, A.; Berg, A.; Chatzopoulou, M.; Davies, K.E.; Davies, S.G.; Edwards, B.; Elsey, D.J.; Emer, E.; Guiraud, S.; Harriman, S.; et al. 2-Arylbenzo[d]oxazole Phosphinate Esters as Second-Generation Modulators of Utrophin for the Treatment of Duchenne Muscular Dystrophy. J. Med. Chem. 2020, 63, 7880–7891. [Google Scholar] [CrossRef]
- Ravinaik, B.; Ramachandran, D.; Rao, M.V.B. Synthesis and anticancer evaluation of amide derivatives of 1, 3, 4-oxadiazole linked with benzoxazole. Russ. J. Gen. Chem. 2019, 89, 1003–1008. [Google Scholar] [CrossRef]
- Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. Heterocyclic amino acids as fluorescent reporters for transition metals: Synthesis and evaluation of novel furyl-benzoxazol-5-yl-l-alanines. N. J. Chem. 2018, 42, 3483–3492. [Google Scholar] [CrossRef]
- Varma, R.S.; Kumar, D. Manganese triacetate oxidation of phenolic schiffs bases: Synthesis of 2-arylbenzoxazoles. J. Heterocycl. Chem. 1998, 35, 1539–1540. [Google Scholar] [CrossRef]
- Ozokan, K.G.; Gumus, M.K.; Kaban, S. Synthesis of hetaryl-substituted benzoxazoles via oxidative cyclization of phenolic schiff’s bases. J. Heterocycl. Chem. 2008, 45, 1831–1834. [Google Scholar] [CrossRef]
- Bougrin, K.; Loupy, A.; Soufiaoui, M. Trois nouvelles voies de synthèse des dérivés 1,3-azoliques sous micro-ondes. Tetrahedron 1998, 54, 8055–8064. [Google Scholar] [CrossRef]
- Vereshchagin, L.I.; Gainulina, S.R.; Podskrebysheva, S.A.; Gaivoroskii, L.A.; Okhapkina, L.L.; Vorob’eva, V.G.; Latyshev, V.P. Reactivity of Manganese Oxides in the Oxidation of Alcohols. J. Org. Chem. USSR 1972, 8, 1143–1147. [Google Scholar]
- Cassis, R.; Valderrama, J.A. Studies on Quinones. XI. Synthesis of Quinones from Hydroquinones by Using Manganese Dioxide and Acid-Impregnated Manganese Dioxide. Synth. Commun. 1983, 13, 347–356. [Google Scholar] [CrossRef]
- Yamaguchi, K.S.; Sawyer, D.T. The Redox Chemistry of Manganese(III) and -(IV) Complexes. Israel J. Chem. 1985, 25, 164–176. [Google Scholar] [CrossRef]
- Moir, M.; Lane, S.; Montgomery, A.P.; Hibbs, D.; Connor, M.; Kassiou, M. The discovery of a potent and selective pyrazolo-[2,3-e]-[1,2,4]-triazine cannabinoid type 2 receptor agonist. Eur. J. Med. Chem. 2021, 210, 113087. [Google Scholar] [CrossRef]
- Zhu, Z.; Glinkerman, C.M.; Boger, D.L. Selective N1/N4 1,4-Cycloaddition of 1,2,4,5-Tetrazines Enabled by Solvent Hydrogen Bonding. J. Am. Chem. Soc. 2020, 142, 20778–20787. [Google Scholar] [CrossRef] [PubMed]
- Branowska, D.; Olender, E.; Świętochowska, M.; Karczmarzyk, Z.; Wysocki, W.; Cichosz, I.; Woźna, A.; Urbańczyk-Lipkowska, Z.; Kalicki, P.; Gil, M. Synthesis and optical properties of some 3,4-(ethylenedioxythiophen-2-yl)-1,2,4-triazine derivatives. Tetrahedron 2017, 73, 411–417. [Google Scholar] [CrossRef]
- Fatykhov, R.F.; Chupakhin, O.N.; Rusinov, V.L.; Khalymbadzha, I.A. Copper catalysis for triazines. In Copper in N-Heterocyclic Chemistry; Srivastava, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 161–220. [Google Scholar] [CrossRef]
- Finlay, M.R.V.; Anderton, M.; Bailey, A.; Boyd, S.; Brookfield, J.; Cairnduff, C.; Charles, M.; Cheasty, A.; Critchlow, S.E.; Culshaw, J.; et al. Discovery of a Thiadiazole–Pyridazine-Based Allosteric Glutaminase 1 Inhibitor Series That Demonstrates Oral Bioavailability and Activity in Tumor Xenograft Models. J. Med. Chem. 2019, 62, 14, 6540–6560. [Google Scholar] [CrossRef]
- Sahin, Z.; Biltekin, S.N.; Ozansoy, M.; Hemiş, B.; Ozansoy, M.B.; Yurttaş, L.; Berk, B.; Demirayak, Ş. Synthesis and in vitro antitumor activities of novel thioamide substituted piperazinyl-1,2,4-triazines. J. Heterocycl. Chem. 2022, 59, 1333–1340. [Google Scholar] [CrossRef]
- Bielawska, A.; Bielawski, K.; Czarnomysy, R.; Gornowicz, A.; Mojzych, M.; Szymanowska, A. The anticancer action of a novel 1,2,4-triazine sulfonamide derivative in colon cancer cells. Molecules 2021, 26, 2045. [Google Scholar] [CrossRef]
- Bashir, M.; Bano, A.; Ijaz, A.S.; Chaudhary, B.A. Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review. Molecules 2015, 20, 13496–13517. [Google Scholar] [CrossRef]
- Majid, A.; Ashid, M.; Nasir, H.; Joshi, A. A Convenient Synthesis and Reactions of some Substituted 1,2,4-Triazine, and Their Derivatives with Carbazole, Sulfonamide and Trityl Chloride Moiety of Biological Interest. Eur. J. Mol. Clin. Med. 2020, 7, 994–1002. [Google Scholar]
- Zassowski, P.; Ledwon, P.; Kurowska, A.; Herman, A.P.; Lapkowski, M.; Cherpak, V.; Hotra, Z.; Turyk, P.; Ivaniuk, K.; Stakhira, P.; et al. 1,3,5-Triazine and carbazole derivatives for OLED applications. Dyes Pigment. 2018, 149, 804–811. [Google Scholar] [CrossRef]
- Fatiadi, A.J. Active manganese dioxide oxidation in organic chemistry-part I. Synthesis 1976, 1976, 65–104. [Google Scholar] [CrossRef]
- Miri, R.; Firuzi, O.; Peymani, P.; Zamani, M.; Mehdipour, A.R.; Heydari, Z.; Farahani, M.M.; Shafiee, A. Synthesis, Cytotoxicity, and QSAR Study of New Aza-cyclopenta[b]fluorene-1,9-dione Derivatives. Chem. Biol. Drug Des. 2012, 79, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bagley, M.C.; Lubinu, M.C. Microwave-assisted oxidative aromatization of Hantzsch 1, 4-dihydropyridines using manganese dioxide. Synthesis 2006, 2006, 1283–1288. [Google Scholar] [CrossRef]
- Sengoku, T.; Murata, Y.; Suzuki, C.; Takahashi, M.; Yoda, H. Synthesis of new chiral lactam-fused pyridine derivatives. RSC Adv. 2015, 5, 73562–73565. [Google Scholar] [CrossRef]
- Quinonero, O.; Jean, M.; Vanthuyne, N.; Roussel, C.; Bonne, D.; Constantieux, T.; Bressy, C.; Bugaut, X.; Rodriguez, J. Combining organocatalysis with central-to-axial chirality conversion: Atroposelective hantzsch-type synthesis of 4-arylpyridines. Angew. Chem. Int. Ed. 2016, 55, 1401–1405. [Google Scholar] [CrossRef]
- Shabunina, O.V.; Starnovskaya, E.S.; Shaitz, Y.K.; Kopchuk, D.S.; Sadieva, L.K.; Kim, G.A.; Taniya, O.S.; Nikonov, I.L.; Santra, S.; Zyryanov, G.V.; et al. Asymmetrically substituted 5,5′′-diaryl-2,2′:6′,2′′-terpyridines as efficient fluorescence “turn-on” probes for Zn2+ in food/cosmetic samples and human urine. J. Photochem. Photobiol. Chem. 2021, 408, 113101. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView; Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2019. [Google Scholar]
- Ban, K.; Duffy, S.; Khakham, Y.; Avery, V.M.; Hughes, A.; Montagnat, O.; Katneni, K.; Ryan, E.; Baell, J.B. 3-Alkylthio-1,2,4-triazine dimers with potent antimalarial activity. Bioorg. Med. Chem. Lett. 2010, 20, 6024–6029. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.C.; Macor, J.E.; Pont, J.L. Intramolecular diels-alder reactions of 1, 2, 4-triazines.: A general synthesis of furo [2,3-b]pyridines, 2,3-dihydropyrano [2,3-b]pyridines, and pyrrolo [2,3-b]pyridines. Tetrahedron 1987, 43, 5145–5158. [Google Scholar] [CrossRef]
- Von Neunhoeffer, H.; Hennig, H.; Frühauf, H.-W.; Mutterer, M. Zur synthese von 1,2,4-triazinen. Tetrahedron Lett. 1969, 10, 3147–3150. [Google Scholar] [CrossRef]
- Shkurko, O.P.; Gogin, L.L.; Baram, S.G.; Mamaev, V.P. Electronic spectra of asym-triazinyl groups. Chem. Heterocycl. Compd. 1987, 23, 216–221. [Google Scholar] [CrossRef]
- Courcot, B.; Tran, D.N.; Fraisse, B.; Bonhomme, F.; Marsura, A.; Ghermani, N.E. Electronic Properties of 3,3′-Dimethyl-5,5′-bis(1,2,4-triazine): Towards Design of Supramolecular Arrangements of N-Heterocyclic CuI Complexes. Chem. Eur. J. 2007, 13, 3414–3423. [Google Scholar] [CrossRef]
- Alekseev, S.G.; Charushin, V.N.; Chupakhin, O.N.; Shorshnev, S.V.; Chernyshev, A.I.; Klyuev, N.A. Reactions of azinium cations. 6. N(1)-alkyl-1,2,4-triazinium salts. Reactions with indoles—The first case of the double addition of nucleophiles to a triazine ring. Chem. Heterocycl. Compd. 1986, 22, 1242–1249. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).