Chemopreventive Effect on Human Colon Adenocarcinoma Cells of Styrylquinolines: Synthesis, Cytotoxicity, Proapoptotic Effect and Molecular Docking Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Remarks
2.1.2. General Procedure for the Synthesis of Styrylquinolines
Styrylquinolines 3a–e
Obtention of Compounds 4a and 4b
2.2. Biological Activity Assays
2.2.1. Cell Lines and Culture Conditions
2.2.2. Cytotoxic Activity
2.2.3. Antiproliferative Activity
2.2.4. Reactive Oxygen Species (ROS) Levels
2.2.5. Assessment of Apoptosis
2.2.6. Determination of Inflammatory Cytokines and Apoptotic Proteins
2.2.7. Statistical Analysis
2.3. Computational Methods
3. Results
3.1. Chemistry
3.2. Effect of Styrylquinolines on Cell Viability of Malignant and Nonmalignant Cells
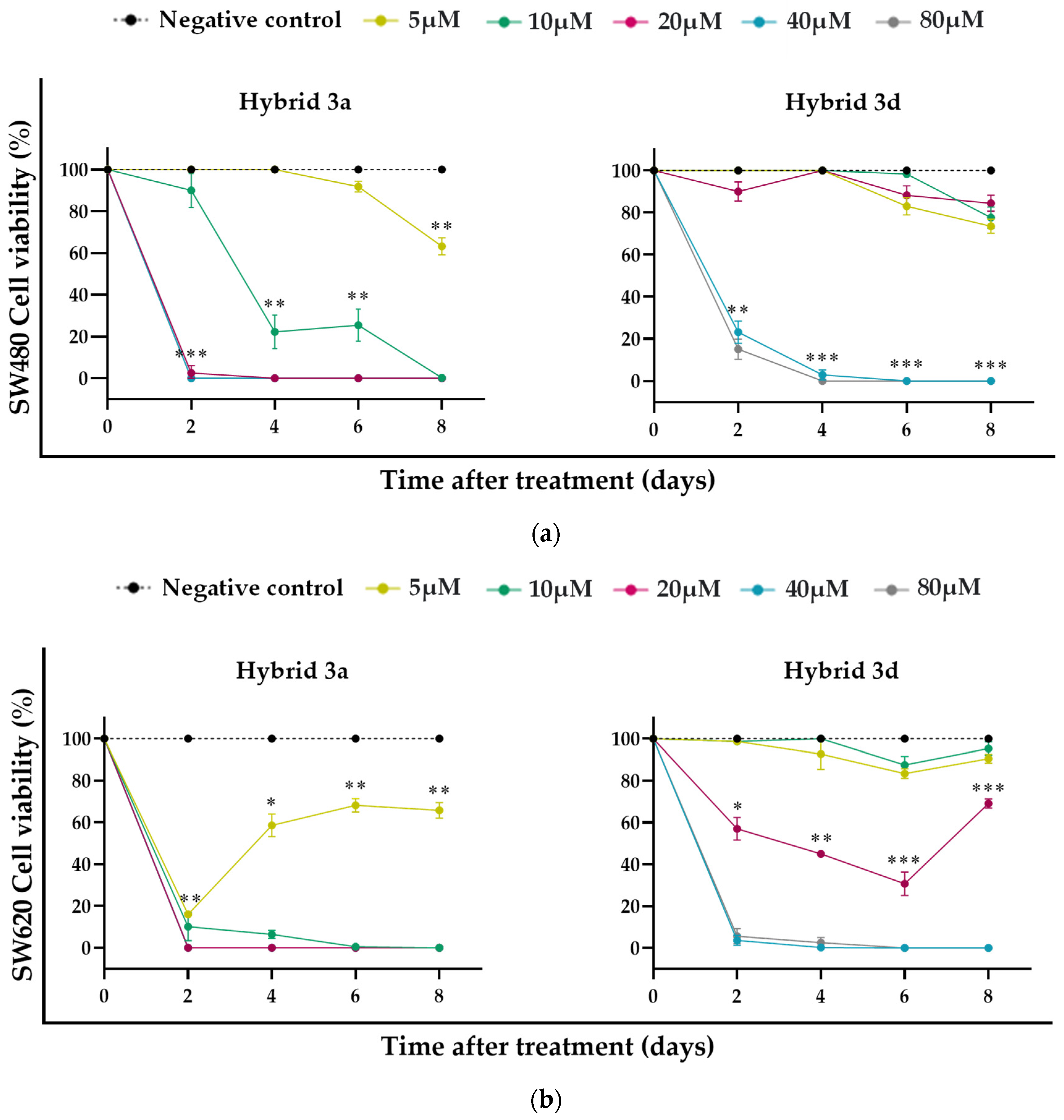
3.3. Antiproliferative Effect of Styrylquinolines
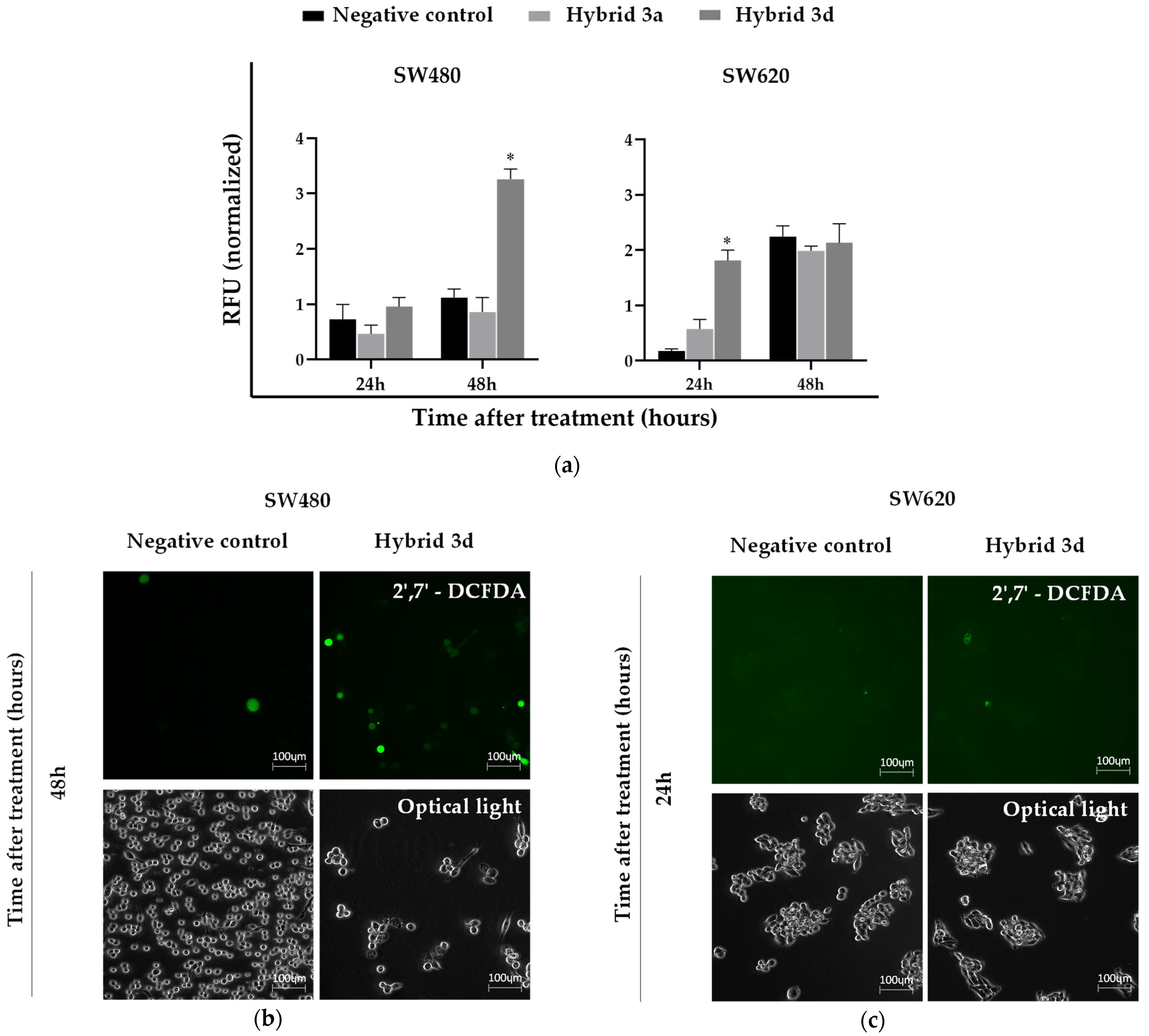
3.4. ROS Production Induced by Styrylquinolines
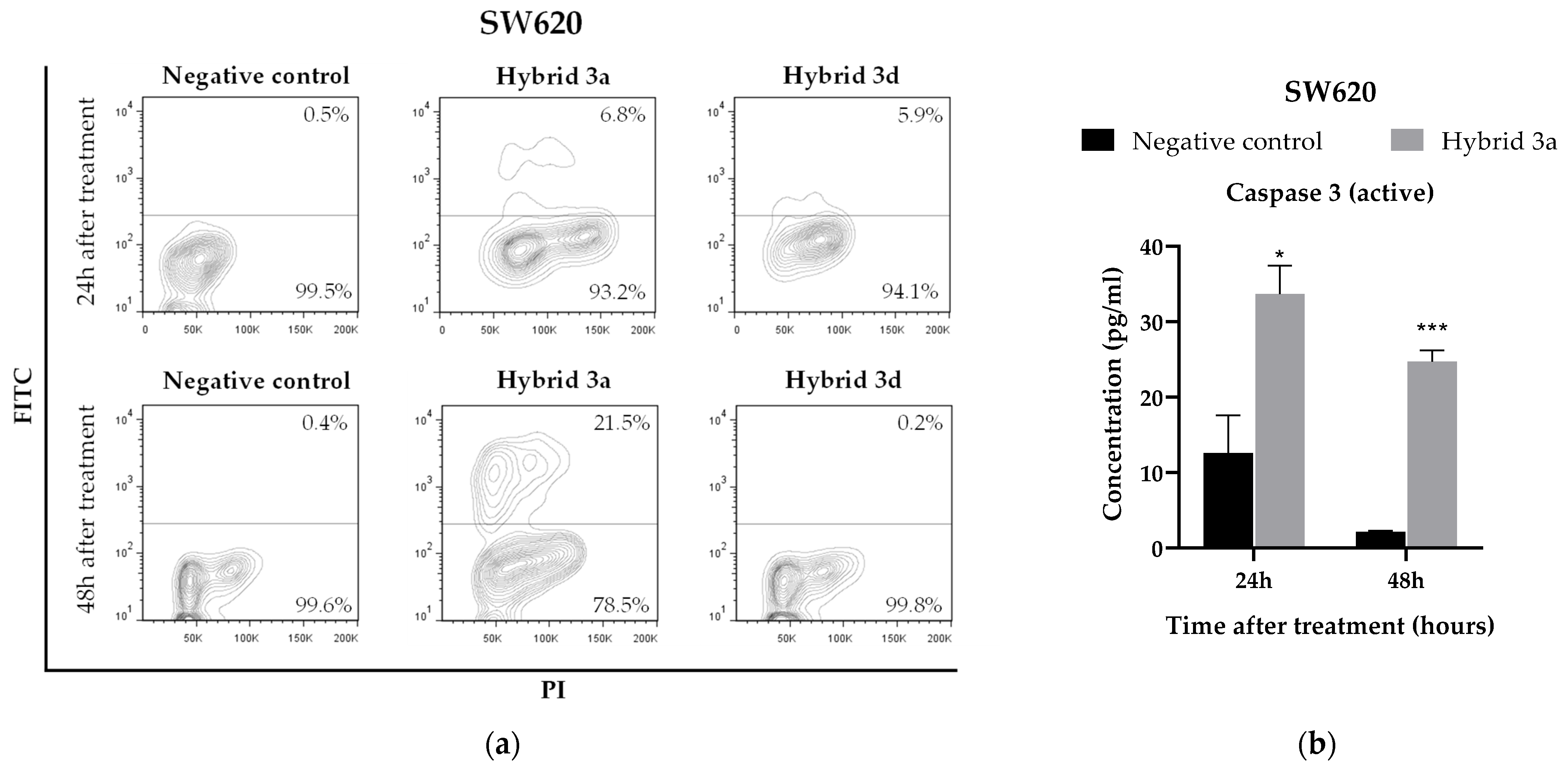
3.5. Apoptosis Induction by Styrylquinolines
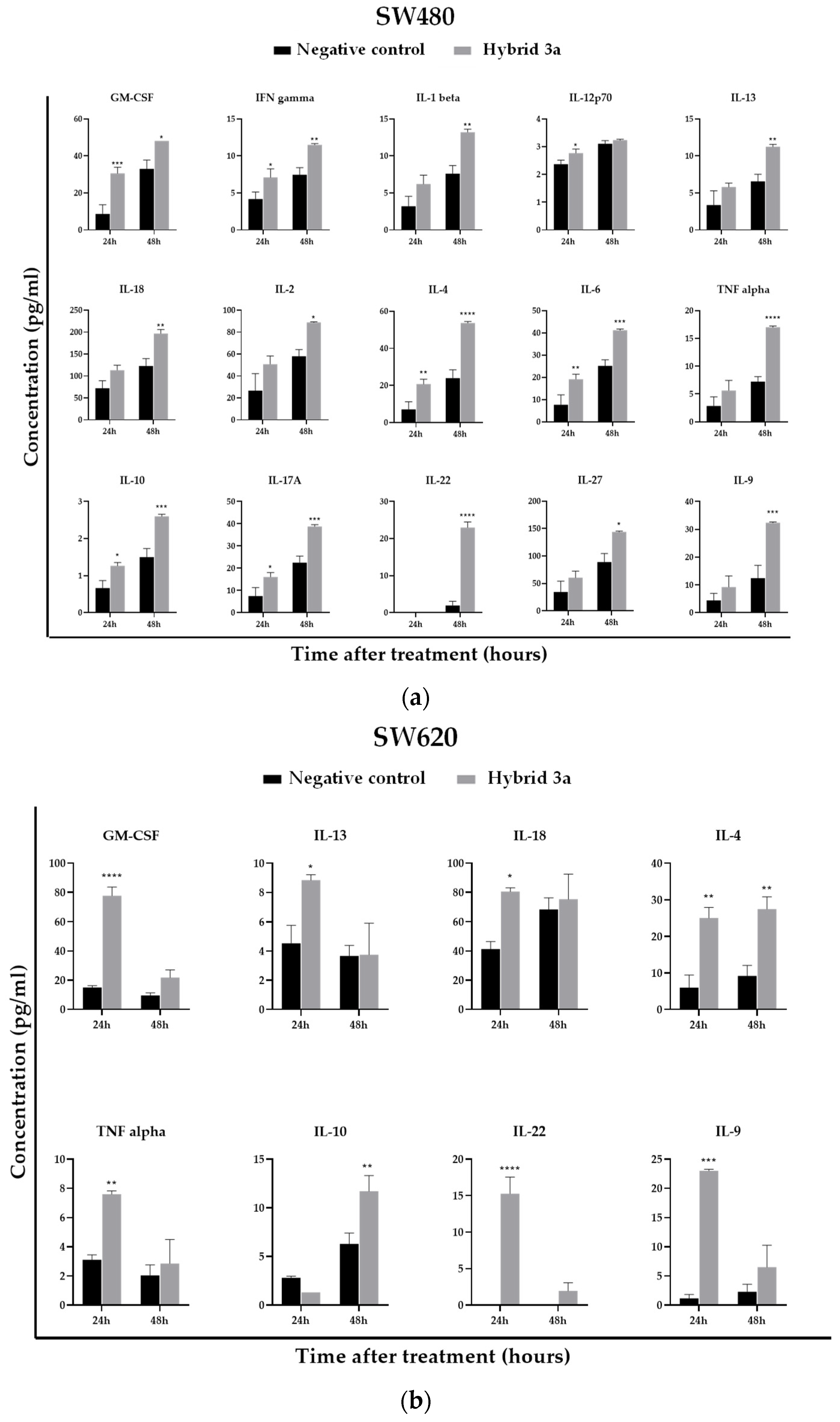
3.6. Effect of Styrylquinolines on the Production of Immunological Markers Associated with the Inflammatory Response
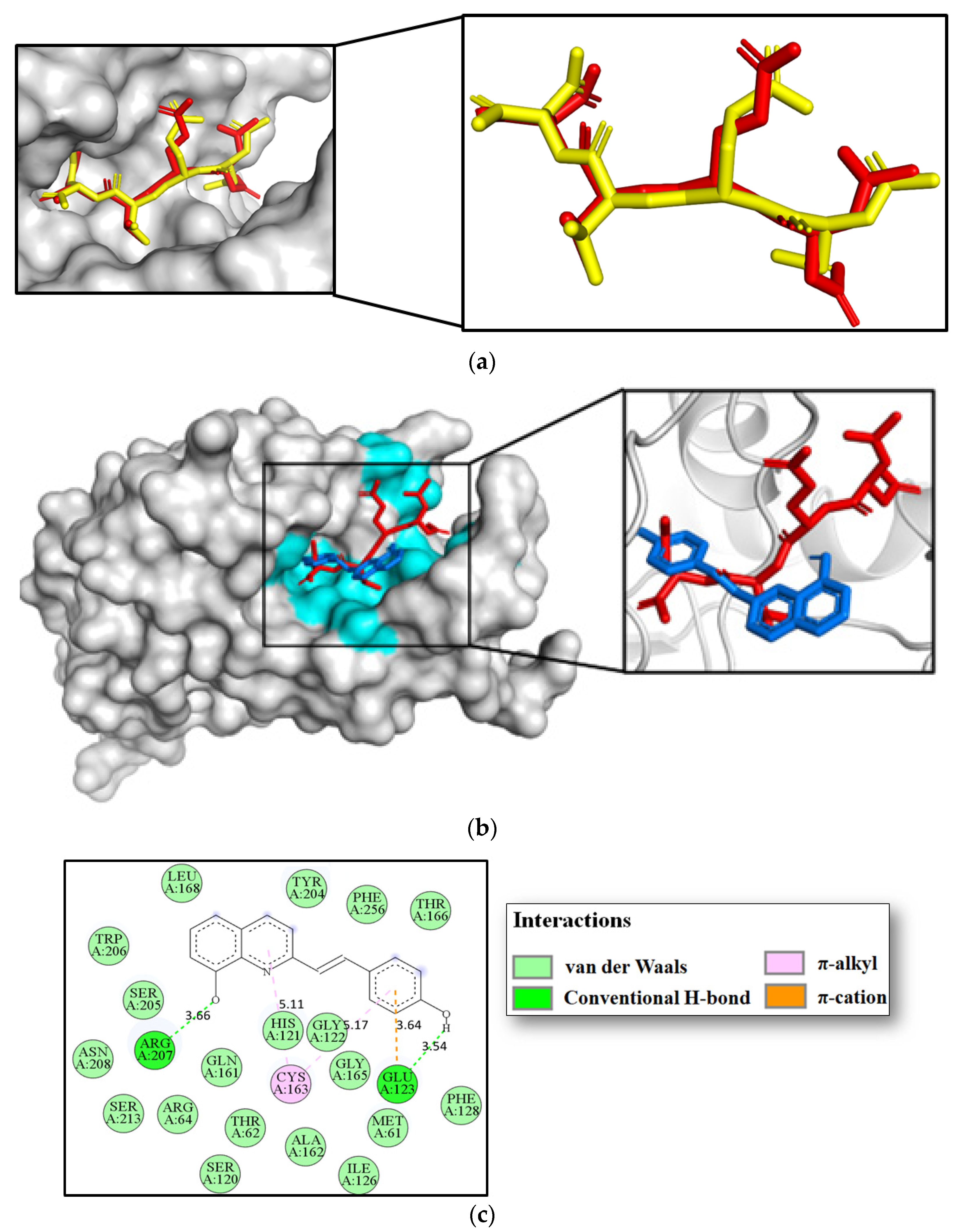
3.7. Molecular Docking Studies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Organización Mundial de la Salud Cáncer. 2021. pp. 1–7. Available online: https://www.who.int/es/news-room/fact-sheets/detail/cancer (accessed on 20 September 2022).
- The Global Cancer Observatory (GLOBOCAN) Colorectal Cancer. 2021. p. 1. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 20 September 2022).
- American Cancer Society Treating Colorectal Cancer. 2020. pp. 1–52. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/treating/by-stage-colon.html (accessed on 20 September 2022).
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32231332 (accessed on 20 September 2022). [CrossRef] [PubMed]
- Herrmann, J. Vascular toxic effects of cancer therapies. Nat. Rev. Cardiol. 2020, 17, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef]
- Kazemirad, H.; Kazerani, H.R. Cardioprotective effects of resveratrol following myocardial ischemia and reperfusion. Mol. Biol. Rep. 2020, 47, 5843–5850. [Google Scholar] [CrossRef]
- Miguel, C.A.; Noya-Riobó, M.V.; Mazzone, G.L.; Villar, M.J.; Coronel, M.F. Antioxidant, anti-inflammatory and neuroprotective actions of resveratrol after experimental nervous system insults. Special focus on the molecular mechanisms involved. Neurochem. Int. 2021, 150, 105188. [Google Scholar] [CrossRef]
- Yang, M.-D.; Sun, Y.; Zhou, W.-J.; Xie, X.-Z.; Zhou, Q.-M.; Lu, Y.-Y.; Su, S.-B. Resveratrol Enhances Inhibition Effects of Cisplatin on Cell Migration and Invasion and Tumor Growth in Breast Cancer MDA-MB-231 Cell Models In Vivo and In Vitro. Molecules 2021, 26, 2204. [Google Scholar] [CrossRef]
- Moutabian, H.; Majdaeen, M.; Ghahramani-Asl, R.; Yadollahi, M.; Gharepapagh, E.; Ataei, G.; Falahatpour, Z.; Bagheri, H.; Farhood, B. A systematic review of the therapeutic effects of resveratrol in combination with 5-fluorouracil during colorectal cancer treatment: With a special focus on the oxidant, apoptotic, and anti-inflammatory activities. Cancer Cell Int. 2022, 22, 142. [Google Scholar] [CrossRef]
- He, L.; Fan, F.; Hou, X.; Gao, C.; Meng, L.; Meng, S.; Huang, S.; Wu, H. Resveratrol suppresses pulmonary tumor metastasis by inhibiting platelet-mediated angiogenic responses. J. Surg. Res. 2017, 217, 113–122. [Google Scholar] [CrossRef]
- Fu, Y.; Ye, Y.; Zhu, G.; Xu, Y.; Sun, J.; Wu, H.; Feng, F.; Wen, Z.; Jiang, S.; Li, Y.; et al. Resveratrol induces human colorectal cancer cell apoptosis by activating the mitochondrial pathway via increasing reactive oxygen species. Mol. Med. Rep. 2020, 23, 170. [Google Scholar] [CrossRef]
- Kumar, S.; Chang, Y.-C.; Lai, K.-H.; Hwang, T.-L. Resveratrol, a Molecule with Anti-Inflammatory and Anti-Cancer Activities: Natural Product to Chemical Synthesis. Curr. Med. Chem. 2021, 28, 3773–3786. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 1923, 8-Hydroxyquinoline. 2021. p. 54. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8-Hydroxyquinoline (accessed on 20 September 2022).
- Balthazar, J.D.; Soosaimanickam, M.P.; Emmanuel, C.; Krishnaraj, T.; Sheikh, A.; Alghafis, S.F.; Ibrahim, H.-I.M. 8-Hydroxyquinoline a natural chelating agent from Streptomyces spp. inhibits A549 lung cancer cell lines via BCL2/STAT3 regulating pathways. World J. Microbiol. Biotechnol. 2022, 38, 182. [Google Scholar] [CrossRef] [PubMed]
- Joaquim, A.R.; Boff, R.T.; Adam, F.C.; Lima-Morales, D.; Cesare, M.A.; Kaminski, T.F.; Teixeira, M.L.; Fuentefria, A.M.; Andrade, S.F.; Martins, A.F. Antibacterial and synergistic activity of a new 8-hydroxyquinoline derivative against methicillin-resistant Staphylococcus aureus. Future Microbiol. 2022, 17, 425–436. [Google Scholar] [CrossRef]
- Reginatto, P.; Joaquim, A.R.; Rocha, D.A.; Berlitz, S.J.; Külkamp-Guerreiro, I.C.; De Andrade, S.F.; Fuentefria, A.M. 8-hydroxyquinoline and quinazoline derivatives as potential new alternatives to combat Candida spp. Biofilm. Lett. Appl. Microbiol. 2022, 74, 395–404. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Fang, H.; Hou, X. Design, synthesis and preliminary bioactivity evaluations of 8-hydroxyquinoline derivatives as matrix metalloproteinase (MMP) inhibitors. Eur. J. Med. Chem. 2019, 181, 111563. [Google Scholar] [CrossRef]
- Wang, L.; Deng, K.; Gong, L.; Zhou, L.; Sayed, S.; Li, H.; Sun, Q.; Su, Z.; Wang, Z.; Liu, S.; et al. Chlorquinaldol targets the β-catenin and T-cell factor 4 complex and exerts anti-colorectal cancer activity. Pharmacol. Res. 2020, 159, 104955. [Google Scholar] [CrossRef]
- Chan, L.-P.; Tseng, Y.-P.; Ding, H.-Y.; Pan, S.-M.; Chiang, F.-Y.; Wang, L.-F.; Chou, T.-H.; Lien, P.-J.; Liu, C.; Kuo, P.-L.; et al. Tris(8-Hydroxyquinoline)iron induces apoptotic cell death via oxidative stress and by activating death receptor signaling pathway in human head and neck carcinoma cells. Phytomedicine 2019, 63, 153005. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [Green Version]
- Nepovimova, E.; Kuca, K. Multi-Target-Directed Ligands in Alzheimer’s Disease Therapy. In Neurodegenerative Diseases—Molecular Mechanisms and Current Therapeutic Approaches; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/books/neurodegenerative-diseases-molecular-mechanisms-and-current-therapeutic-approaches/multi-target-directed-ligands-in-alzheimer-s-disease-therapy (accessed on 20 September 2022).
- Szemik-Hojniak, A.; Deperasińska, I.; Nizhnik, Y.P. Photophysical behavior of a potential drug candidate, trans-[2-(4-methoxystyryl)]quinoline-1-oxide tuned by environment effects. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2017, 187, 198–206. [Google Scholar] [CrossRef]
- Espinosa, R.; Robledo, S.; Guzmán, C.; Arbeláez, N.; Yepes, L.; Santafé, G.; Sáez, A. Synthesis and evaluation of the in vitro and in vivo antitrypanosomal activity of 2-styrylquinolines. Heliyon 2021, 7, e07024. [Google Scholar] [CrossRef]
- Mrozek-Wilczkiewicz, A.; Spaczynska, E.; Malarz, K.; Cieslik, W.; Rams-Baron, M.; Kryštof, V.; Musiol, R. Design, Synthesis and In Vitro Activity of Anticancer Styrylquinolines. The p53 Independent Mechanism of Action. PLoS ONE 2015, 10, e0142678. [Google Scholar] [CrossRef]
- Mirzaei, S.; Eisvand, F.; Hadizadeh, F.; Mosaffa, F.; Ghasemi, A.; Ghodsi, R. Design, synthesis and biological evaluation of novel 5,6,7-trimethoxy-N-aryl-2-styrylquinolin-4-amines as potential anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem. 2020, 98, 103711. [Google Scholar] [CrossRef] [PubMed]
- Mrozek-Wilczkiewicz, A.; Kuczak, M.; Malarz, K.; Cieślik, W.; Spaczyńska, E.; Musiol, R. The synthesis and anticancer activity of 2-styrylquinoline derivatives. A p53 independent mechanism of action. Eur. J. Med. Chem. 2019, 177, 338–349. [Google Scholar] [CrossRef]
- Herrera-R, A.; Castrillón, W.; Otero, E.; Ruiz, E.; Carda, M.; Agut, R.; Naranjo, T.; Moreno, G.; Maldonado, M.E.; Cardona-G, W. Synthesis and antiproliferative activity of 3- and 7-styrylcoumarins. Med. Chem. Res. 2018, 27, 1893–1905. [Google Scholar] [CrossRef]
- Puerta, J.D.; Usme-Ciro, J.A.; Gallego-Gómez, J.C. Implementación de un control interno en la detección molecular de las principales especies de micoplasmas contaminantes de cultivos celulares. Salud Uninorte 2013, 29, 160–173. [Google Scholar]
- Hernández, C.; Moreno, G.; Herrera-R, A.; Cardona-G, W. New Hybrids Based on Curcumin and Resveratrol: Synthesis, Cytotoxicity and Antiproliferative Activity against Colorectal Cancer Cells. Molecules 2021, 26, 2661. [Google Scholar] [CrossRef]
- Kim, H.; Xue, X. Detection of Total Reactive Oxygen Species in Adherent Cells by 2’,7’-Dichlorodihydrofluorescein Diacetate Staining. J. Vis. Exp. 2020, e60682. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Podeszwa, B.; Niedbala, H.; Polanski, J.; Musiol, R.; Tabak, D.; Finster, J.; Serafin, K.; Milczarek, M.; Wietrzyk, J.; Boryczka, S.; et al. Investigating the antiproliferative activity of quinoline-5,8-diones and styrylquinolinecarboxylic acids on tumor cell lines. Bioorg. Med. Chem. Lett. 2007, 17, 6138–6141. [Google Scholar] [CrossRef]
- Cieslik, W.; Musiol, R.; Nycz, J.E.; Jampilek, J.; Vejsova, M.; Wolff, M.; Machura, B.; Polanski, J. Contribution to investigation of antimicrobial activity of styrylquinolines. Bioorg. Med. Chem. 2012, 20, 6960–6968. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.-S.; Chen, W.; Wang, C.; Tzeng, C.-C.; Chen, Y.-L. Synthesis and antiproliferative evaluations of certain 2-phenylvinylquinoline (2-styrylquinoline) and 2-furanylvinylquinoline derivatives. Bioorg. Med. Chem. 2010, 18, 124–133. [Google Scholar] [CrossRef] [PubMed]
- PHILLIPS, J.P.; BREESE, R.; BARRALL, E.M. Styryl Derivatives of 8-Quinolinol. J. Org. Chem. 1959, 24, 1104–1106. [Google Scholar] [CrossRef]
- Ouali, M.; Laboulais, C.; Leh, H.; Gill, D.; Desmaële, D.; Mekouar, K.; Zouhiri, F.; D’Angelo, J.; Auclair, C.; Mouscadet, J.F.; et al. Modeling of the inhibition of retroviral integrases by styrylquinoline derivatives. J. Med. Chem. 2000, 43, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Otero, E.; García, E.; Palacios, G.; Yepes, L.M.; Carda, M.; Agut, R.; Vélez, I.D.; Cardona, W.I.; Robledo, S.M. Triclosan-caffeic acid hybrids: Synthesis, leishmanicidal, trypanocidal and cytotoxic activities. Eur. J. Med. Chem. 2017, 141, 73–83. [Google Scholar] [CrossRef]
- Otero, E.; Robledo, S.M.; Díaz, S.; Carda, M.; Muñoz, D.; Paños, J.; Vélez, I.D.; Cardona, W. Synthesis and leishmanicidal activity of cinnamic acid esters: Structure–activity relationship. Med. Chem. Res. 2014, 23, 1378–1386. [Google Scholar] [CrossRef] [Green Version]
- Maciag, J.J.; Mackenzie, S.H.; Tucker, M.B.; Schipper, J.L.; Swartz, P.; Clark, A.C. Tunable allosteric library of caspase-3 identifies coupling between conserved water molecules and conformational selection. Proc. Natl. Acad. Sci. USA 2016, 113, E6080–E6088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Han, J.; Ma, Y.; Zhang, J.; Zhang, Z.; Wang, G. Demethylzeylasteral inhibits cell proliferation and enhances cell chemosensitivity to 5-fluorouracil in Colorectal Cancer cells. J. Cancer 2020, 11, 6059–6069. [Google Scholar] [CrossRef] [PubMed]
- Arul, M.; Roslani, A.C.; Cheah, S.H. Heterogeneity in cancer cells: Variation in drug response in different primary and secondary colorectal cancer cell lines in vitro. Vitr. Cell. Dev. Biol.-Anim. 2017, 53, 435–447. [Google Scholar] [CrossRef]
- Yan, W.; Yang, W.; Liu, Z.; Wu, G. Characterization of microRNA expression in primary human colon adenocarcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). Onco. Targets. Ther. 2018, 11, 4701–4709. [Google Scholar] [CrossRef] [Green Version]
- Ge, T.; Zhang, Y. Tanshinone IIA reverses oxaliplatin resistance in colorectal cancer through microRNA-30b-5p/AVEN axis. Open Med. 2022, 17, 1228–1240. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, L.; Meng, J.; Dai, M. Circular RNA circ_0068464 combined with microRNA-383 regulates Wnt/β-catenin pathway to promote the progression of colorectal cancer. Bioengineered 2022, 13, 5113–5125. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, X.; Ke, H.; Pan, X.; Ai, J.; Xie, R.; Lan, G.; Hu, Y.; Wu, Y. microRNA-16-5p suppresses cell proliferation and angiogenesis in colorectal cancer by negatively regulating forkhead box K1 to block the PI3K/Akt/mTOR pathway. Eur. J. Histochem. 2022, 66. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Li, C.; Fan, W.; Zhang, M.; Li, X.; Chen, W.; Li, W.; Liang, R.; Li, Z.; Zhu, X. Brilliant glycans and glycosylation: Seq and ye shall find. Int. J. Biol. Macromol. 2021, 189, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Dong, X.; Wang, Y.; Qiu, L.; Peng, F.; Luo, Z. β3GnT8 regulates oxaliplatin resistance by altering integrin�β1 glycosylation in colon cancer cells. Oncol. Rep. 2018, 39, 2006–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, T.; Wen, T.; Ge, Y.; Liu, J.; Yang, L.; Jiang, Y.; Dong, X.; Liu, H.; Yao, J.; An, G. Disruption of Core 1-mediated O-glycosylation oppositely regulates CD44 expression in human colon cancer cells and tumor-derived exosomes. Biochem. Biophys. Res. Commun. 2020, 521, 514–520. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ginckels, P.; Holvoet, P. Oxidative Stress and Inflammation in Cardiovascular Diseases and Cancer: Role of Non-coding RNAs. Yale J. Biol. Med. 2022, 95, 129–152. [Google Scholar]
- Mihanfar, A.; Yousefi, B.; Ghazizadeh Darband, S.; Sadighparvar, S.; Kaviani, M.; Majidinia, M. Melatonin increases 5-flurouracil-mediated apoptosis of colorectal cancer cells through enhancing oxidative stress and downregulating survivin and XIAP. Bioimpacts 2021, 11, 253–261. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, X.; Yu, T.; Huang, H.; Yang, C.; Zhang, L.; Yang, S.; Luo, X.; Luo, J. Lycorine inhibits cell proliferation, migration and invasion, and primarily exerts in vitro cytostatic effects in human colorectal cancer via activating the ROS/p38 and AKT signaling pathways. Oncol. Rep. 2021, 45, 19. [Google Scholar] [CrossRef]
- Malarz, K.; Mrozek-Wilczkiewicz, A.; Serda, M.; Rejmund, M.; Polanski, J.; Musiol, R. The role of oxidative stress in activity of anticancer thiosemicarbazones. Oncotarget 2018, 9, 17689–17710. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Fan, Z.; Hu, S.; Lu, C.; Xiang, Y.; Liao, S. Ferroptosis: A New Strategy for Cancer Therapy. Front. Oncol. 2022, 12, 830561. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, R.; Li, H.; Yu, H.; Ren, K.; Jia, S.; Li, Y.; Wang, Q. Insight into the Double-Edged Role of Ferroptosis in Disease. Biomolecules 2021, 11, 1790. [Google Scholar] [CrossRef] [PubMed]
- Florean, C.; Song, S.; Dicato, M.; Diederich, M. Redox biology of regulated cell death in cancer: A focus on necroptosis and ferroptosis. Free Radic. Biol. Med. 2019, 134, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, Y.-J. Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells. Nutr. Res. Pract. 2021, 15, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; You, J.H.; Kim, M.-S.; Roh, J.-L. Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020, 37, 101697. [Google Scholar] [CrossRef]
- Roberts, J.Z.; Crawford, N.; Longley, D.B. The role of Ubiquitination in Apoptosis and Necroptosis. Cell Death Differ. 2022, 29, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, R.; Jain, S.; Vaidya, A. Caspase-3: A primary target for natural and synthetic compounds for cancer therapy. Chem. Biol. Drug Des. 2021, 98, 144–165. [Google Scholar] [CrossRef] [PubMed]
- Zhenqwen, A.; Flores-Borja, F.; Irshad, S.; Deng, J.; Ng, T. Pleiotropic Role and Bidirectional Immunomodulation of Innate Lymphoid Cells in Cancer. Front. Immunol. 2020, 10, 3111. [Google Scholar] [CrossRef] [Green Version]
- Choudhry, H.; Helmi, N.; Abdulaal, W.H.; Zeyadi, M.; Zamzami, M.A.; Wu, W.; Mahmoud, M.M.; Warsi, M.K.; Rasool, M.; Jamal, M.S. Prospects of IL-2 in Cancer Immunotherapy. Biomed Res. Int. 2018, 2018, 9056173. [Google Scholar] [CrossRef] [Green Version]
- Ding, M.; Fei, Y.; Zhu, J.; Ma, J.; Zhu, G.; Zhen, N.; Zhu, J.; Mao, S.; Sun, F.; Wang, F.; et al. IL-27 improves adoptive CD8 + T cells’ antitumor activity via enhancing cell survival and memory T cell differentiation. Cancer Sci. 2022, 113, 2258–2271. [Google Scholar] [CrossRef]
- Wang, J.; Sun, M.; Zhao, H.; Huang, Y.; Li, D.; Mao, D.; Zhang, Z.; Zhu, X.; Dong, X.; Zhao, X. IL-9 Exerts Antitumor Effects in Colon Cancer and Transforms the Tumor Microenvironment In Vivo. Technol. Cancer Res. Treat. 2019, 18, 1533033819857737. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Ma, X.; Ye, L.; Su, P.; Xiong, W.; Bi, E.; Wang, Q.; Xian, M.; Yang, M.; Qian, J.; et al. IL-9/STAT3/fatty acid oxidation–mediated lipid peroxidation contributes to Tc9 cell longevity and enhanced antitumor activity. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Droeser, R.A.; Lezzi, G. IL-22-mediates Cross-talk between Tumor Cells and Immune Cells Associated with Favorable Prognosis in Human Colorectal Cancer. J. Cell. Immunol. 2021, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Jin, G.; Fang, J.; Lu, Y. IL-4 together with IL-1β induces antitumor Th9 cell differentiation in the absence of TGF-β signaling. Nat. Commun. 2019, 10, 1376. [Google Scholar] [CrossRef] [Green Version]
- Loyon, R.; Jary, M.; Salomé, B.; Gomez-Cadena, A.; Galaine, J.; Kroemer, M.; Romero, P.; Trabanelli, S.; Adotévi, O.; Borg, C.; et al. Peripheral Innate Lymphoid Cells Are Increased in First Line Metastatic Colorectal Carcinoma Patients: A Negative Correlation With Th1 Immune Responses. Front. Immunol. 2019, 10, 2121. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Wang, Y.; Huang, C.-Y.; Zhou, Z.-Q.; Zhao, J.-J.; Zhang, X.-F.; Pan, Q.-Z.; Wu, J.-X.; Weng, D.-S.; Tang, Y.; et al. IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology 2018, 7, e1373234. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Smith, A.S.; Bowers, J.S.; Wyatt, M.M.; Nelson, M.H.; Rangel Rivera, G.O.; Horton, J.D.; Krieg, C.; Armeson, K.; et al. IL6 Fuels Durable Memory for Th17 Cell–Mediated Responses to Tumors. Cancer Res. 2020, 80, 3920–3932. [Google Scholar] [CrossRef] [PubMed]
- Chonov, D.C.; Ignatova, M.M.K.; Ananiev, J.R.; Gulubova, M.V. IL-6 Activities in the Tumour Microenvironment. Part 1. Open Access Maced. J. Med. Sci. 2019, 7, 2391–2398. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Li, N.; Pei, S.; Lian, Y.; Li, L.; Peng, Y.; Liu, Q.; Guo, J.; Wang, X.; Han, Y.; et al. Histone methyltransferase WHSC1 loss dampens MHC-I antigen presentation pathway to impair IFN-γ-stimulated anti-tumor immunity. J. Clin. Investig. 2022, 132, e153167. [Google Scholar] [CrossRef]
- Yan, W.-L.; Wu, C.-C.; Shen, K.-Y.; Liu, S.-J. Activation of GM-CSF and TLR2 signaling synergistically enhances antigen-specific antitumor immunity and modulates the tumor microenvironment. J. Immunother. Cancer 2021, 9, e002758. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Z.; Sun, P.; Song, G.; Wang, L.; Sun, Z.; Yuan, N.; Wang, Q.; Lun, L. Interleukin-18 Is a Prognostic Marker and Plays a Tumor Suppressive Role in Colon Cancer. Dis. Markers 2020, 2020, 6439614. [Google Scholar] [CrossRef]

| Compound | 24 h | 48 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HaCaT IC50 (µM) | SW480 IC50 (µM) | SI | SW620 IC50 (µM) | SI | HaCaT IC50 (µM) | SW480 IC50 (µM) | SI | SW620 IC50 (µM) | SI | |
| 3a | 231.9 ± 29.2 | 67.7 ± 2.8 | 3.4 | 60.5 ± 2.9 | 3.8 | 47.9 ± 3.3 | 26.5 ± 4.0 | 1.8 | 16.6 ± 2.2 | 2.9 |
| 3b | 57.2 ± 6.3 | 72.6 ± 11.0 | 0.8 | 49.1 ± 4.6 | 1.2 | 33.5 ± 4.9 | 39.0 ± 3.6 | 0.9 | 9.7 ± 2.0 | 3.4 |
| 3c | 110.5 ± 12.7 | 37.1 ± 3.8 | 3.0 | 62.0 ± 4.0 | 1.8 | 38.4 ± 6.2 | 38.4 ± 3.8 | 1.0 | 20.7 ± 3.1 | 1.9 |
| 3d | 163.9 ± 14.1 | 159.7 ± 16.6 | 1.0 | 54.6 ± 7.5 | 3.0 | 78.9 ± 2.3 | 42.5 ± 5.2 | 1.9 | 6.4 ± 1.1 | 12.3 |
| 4a | 65.1 ± 8.4 | 117.4 ± 5.3 | 0.6 | 51.0 ± 5.6 | 1.3 | 69.7 ± 3.0 | 63.2 ± 7.1 | 1.1 | 28.9 ± 1.2 | 2.4 |
| 4b | 109.3 ± 8.1 | 93.4 ± 16.6 | 1.2 | 58.8 ± 5.5 | 1.9 | 53.6 ± 2.2 | 43.2 ± 2.0 | 1.2 | 18.6 ± 2.0 | 2.9 |
| Resveratrol | 179.9 ± 7.2 | 217.1 ± 12.8 | 0.8 | 168.9 ± 3.9 | 1.1 | 98.2 ± 7.0 | 135.7 ± 9.1 | 0.7 | 125.2 ± 4.6 | 0.8 |
| 8-HQ | 87.1 ± 9.4 | 89.6 ± 11.8 | 1.0 | 55.3 ± 3.6 | 1.6 | 61.6 ± 4.8 | 38.8 ± 3.2 | 1.6 | 36.3 ± 4.8 | 1.7 |
| 5-FU | >2000 | >2000 | 2.0 | >2000 | 0.9 | 38.78 ± 7.2 | 748.3 ± 157.6 | 0.4 | 295.5 ± 39.7 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedoya-Betancur, V.; Correa, E.; Rendón, J.P.; Yepes-Pérez, A.F.; Cardona-Galeano, W.; Naranjo, T.W. Chemopreventive Effect on Human Colon Adenocarcinoma Cells of Styrylquinolines: Synthesis, Cytotoxicity, Proapoptotic Effect and Molecular Docking Analysis. Molecules 2022, 27, 7108. https://doi.org/10.3390/molecules27207108
Bedoya-Betancur V, Correa E, Rendón JP, Yepes-Pérez AF, Cardona-Galeano W, Naranjo TW. Chemopreventive Effect on Human Colon Adenocarcinoma Cells of Styrylquinolines: Synthesis, Cytotoxicity, Proapoptotic Effect and Molecular Docking Analysis. Molecules. 2022; 27(20):7108. https://doi.org/10.3390/molecules27207108
Chicago/Turabian StyleBedoya-Betancur, Vanesa, Elizabeth Correa, Juan Pablo Rendón, Andrés F. Yepes-Pérez, Wilson Cardona-Galeano, and Tonny W. Naranjo. 2022. "Chemopreventive Effect on Human Colon Adenocarcinoma Cells of Styrylquinolines: Synthesis, Cytotoxicity, Proapoptotic Effect and Molecular Docking Analysis" Molecules 27, no. 20: 7108. https://doi.org/10.3390/molecules27207108





