Viscoelastic Properties of Water-Absorbed Poly(methyl methacrylate) Doped with Lithium Salts with Various Anions
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Procedure
3.1. Materials and Sample Preparation
3.2. Characterization
3.3. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tominaga, Y.; Izumi, Y.; Kwak, G.H.; Asai, S.; Sumita, M. Effect of supercritical carbon dioxide processing on ionic association and conduction in a crystalline poly (ethylene oxide)-LiCF3SO3 complex. Macromolecules 2003, 36, 8766–8772. [Google Scholar] [CrossRef]
- Tominaga, Y.; Asai, S.; Sumita, M. Relation between ionic conductivity and solubility of CO2 in pressurized solid polymer electrolytes. Macromolecules 2007, 40, 3348–3354. [Google Scholar] [CrossRef]
- Agnihotry, S.A.; Pradeep, P.; Sekhon, S.S. PMMA based gel electrolyte for EC smart windows. Electrochim. Acta 1999, 44, 3121–3126. [Google Scholar] [CrossRef]
- TianKhoon, L.; Ataollahi, N.; Hassan, N.H.; Ahmad, A. Studies of porous solid polymeric electrolytes based on poly (vinylidene fluoride) and poly (methyl methacrylate) grafted natural rubber for applications in electrochemical devices. J. Solid State Electrochem. 2016, 20, 203–213. [Google Scholar] [CrossRef]
- Liang, B.; Tang, S.; Jiang, Q.; Chen, C.; Chen, X.; Li, S.; Yan, X. Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-Al2O3. Electrochim. Acta 2015, 169, 334–341. [Google Scholar] [CrossRef]
- Ito, A.; Phulkerd, P.; Ayerdurai, V.; Soga, M.; Courtoux, A.; Miyagawa, A.; Yamaguchi, M. Enhancement of the glass transition temperature of poly (methyl methacrylate) by salt. Polym. J. 2018, 50, 857–863. [Google Scholar] [CrossRef]
- Ito, A.; Nitta, K.-h. Additive effects of lithium salts with various anionic species in poly (methyl methacrylate). Molecules 2021, 26, 4096. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Nitta, K.-h. Rheological and mechanical properties of poly (methyl methacrylate) associated with lithium salts. Nihon Reoroji Gakkaishi 2022, 50, 87–93. [Google Scholar] [CrossRef]
- Ito, A.; Maeno, R.; Yamaguchi, M. Control of optical and mechanical properties of poly (methyl methacrylate) by introducing lithium salt. Opt. Mater. 2018, 83, 152–156. [Google Scholar] [CrossRef]
- Ito, A.; Shin, A.; Nitta, K.-h. Rheological and mechanical properties of poly (methyl methacrylate) doped with lithium salts. Polym. J. 2022, 54, 41–46. [Google Scholar] [CrossRef]
- Ishiyama, C.; Higo, Y. Effects of humidity on Young’s modulus in poly (methyl methacrylate). J. Polym. Sci. Part B Polym. Phys. 2002, 40, 460–465. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 1595, 350, 6260. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Borodin, O.; Sun, W.; Fun, X.; Yang, C.; Wang, F.; Gao, T.; Ma, Z.; Schroeder, M.; Cresce, A.; et al. Advanced High-Voltage Aqueous Lithium-Ion Battery Enabled by “Water-inBisalt” Electrolyte. Angew. Chem. 2016, 128, 7252–7257. [Google Scholar] [CrossRef]
- Jaumaux, P.; Yang, X.; Zhang, B.; Safaei, J.; Tang, X.; Zhou, D.; Wang, C.; Wang, G. Localized Water-In-Salt Electrolyte for Aqueous Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 19965–19973. [Google Scholar] [CrossRef] [PubMed]
- Ionita, D.; Cristea, M.; Banabic, D. Viscoelastic behavior of PMMA in relation to deformation mode. J. Therm. Anal. Calorim. 2015, 120, 1775–1783. [Google Scholar] [CrossRef]
- Ceccorulli, G.; Pizzoli, M. Effect of water on the relaxation spectrum of poly(methylmethacrylate). Polym. Bull. 2001, 47, 283–289. [Google Scholar] [CrossRef]
- Shen, J.; Chen, C.C.; Sauer, J.A. Effects of sorbed water on properties of low and high molecular weight PMMA: 1. Deformation and fracture behaviour. Polymer 1985, 26, 511–518. [Google Scholar] [CrossRef]
- Starkweather, H.W. Noncooperative relaxations. Macromolecules 1988, 21, 1798–1802. [Google Scholar] [CrossRef]
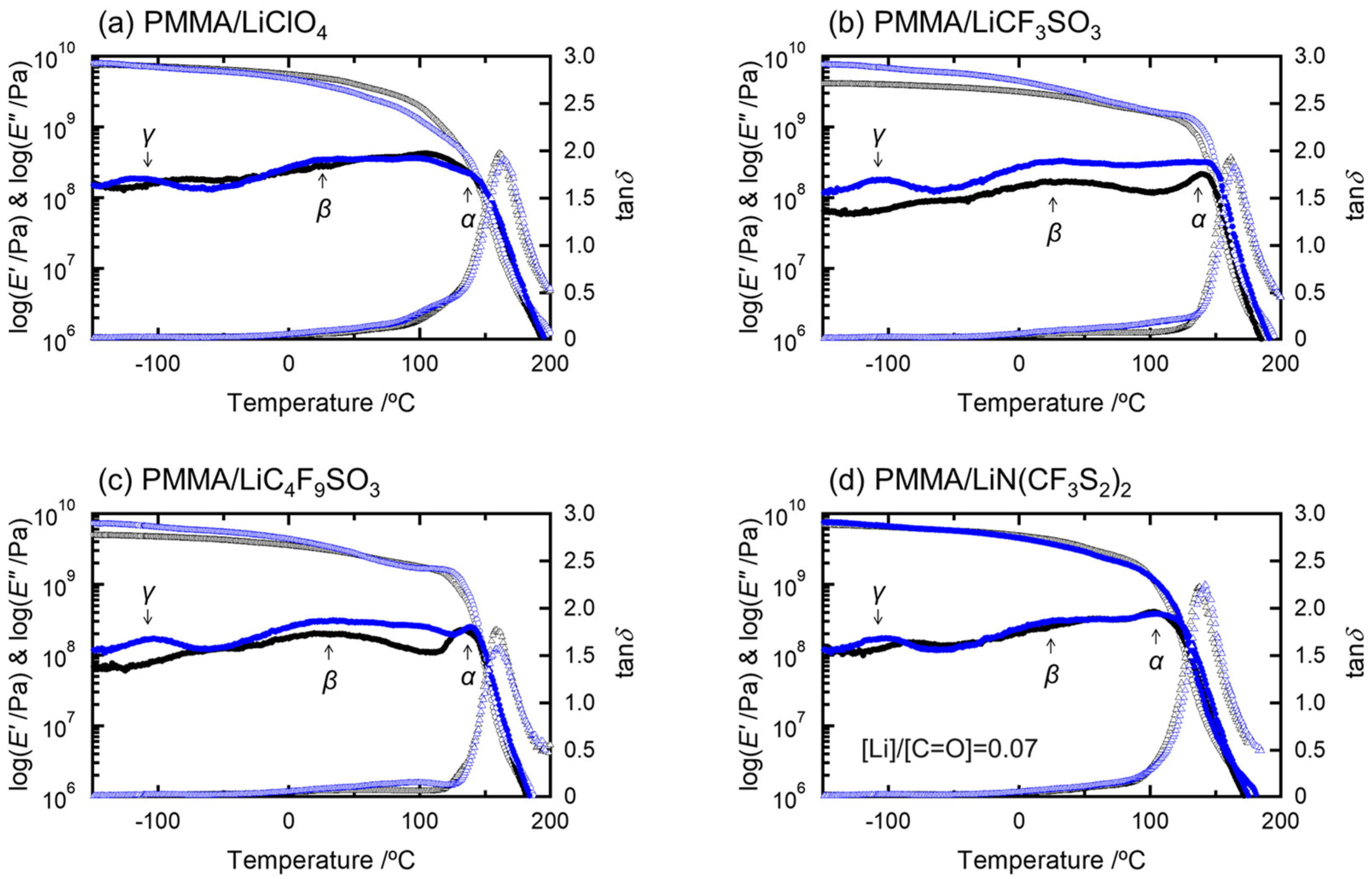
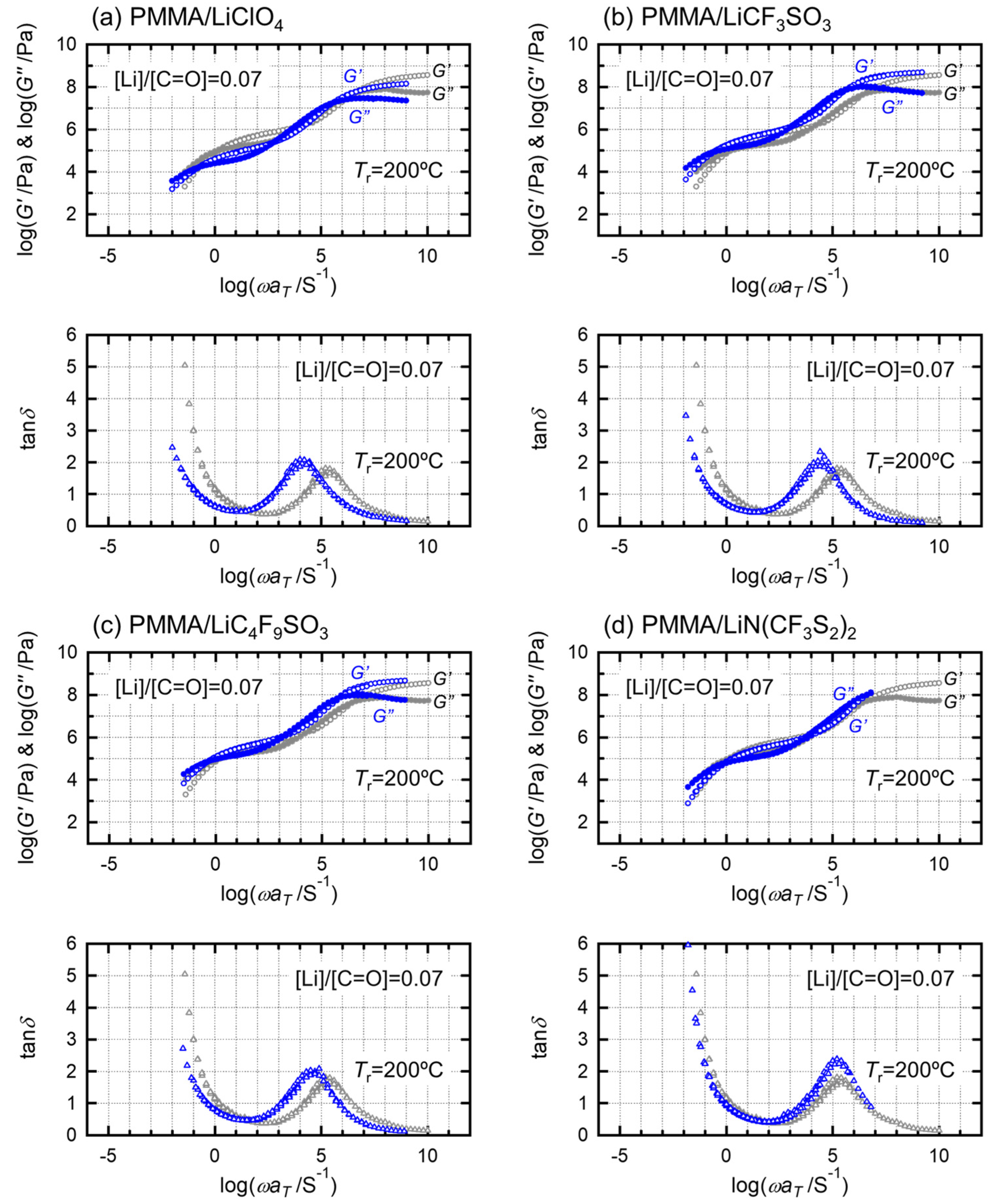

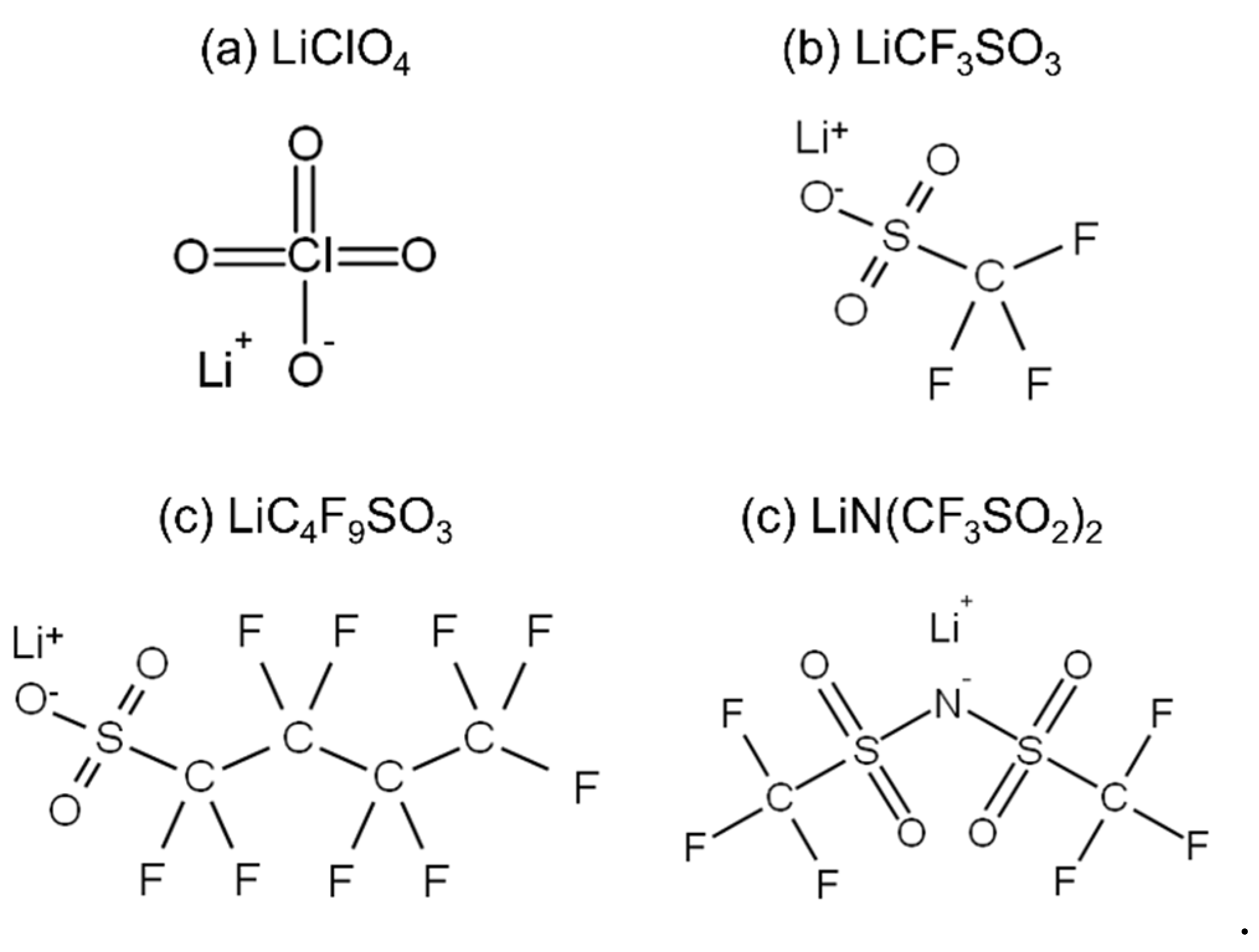
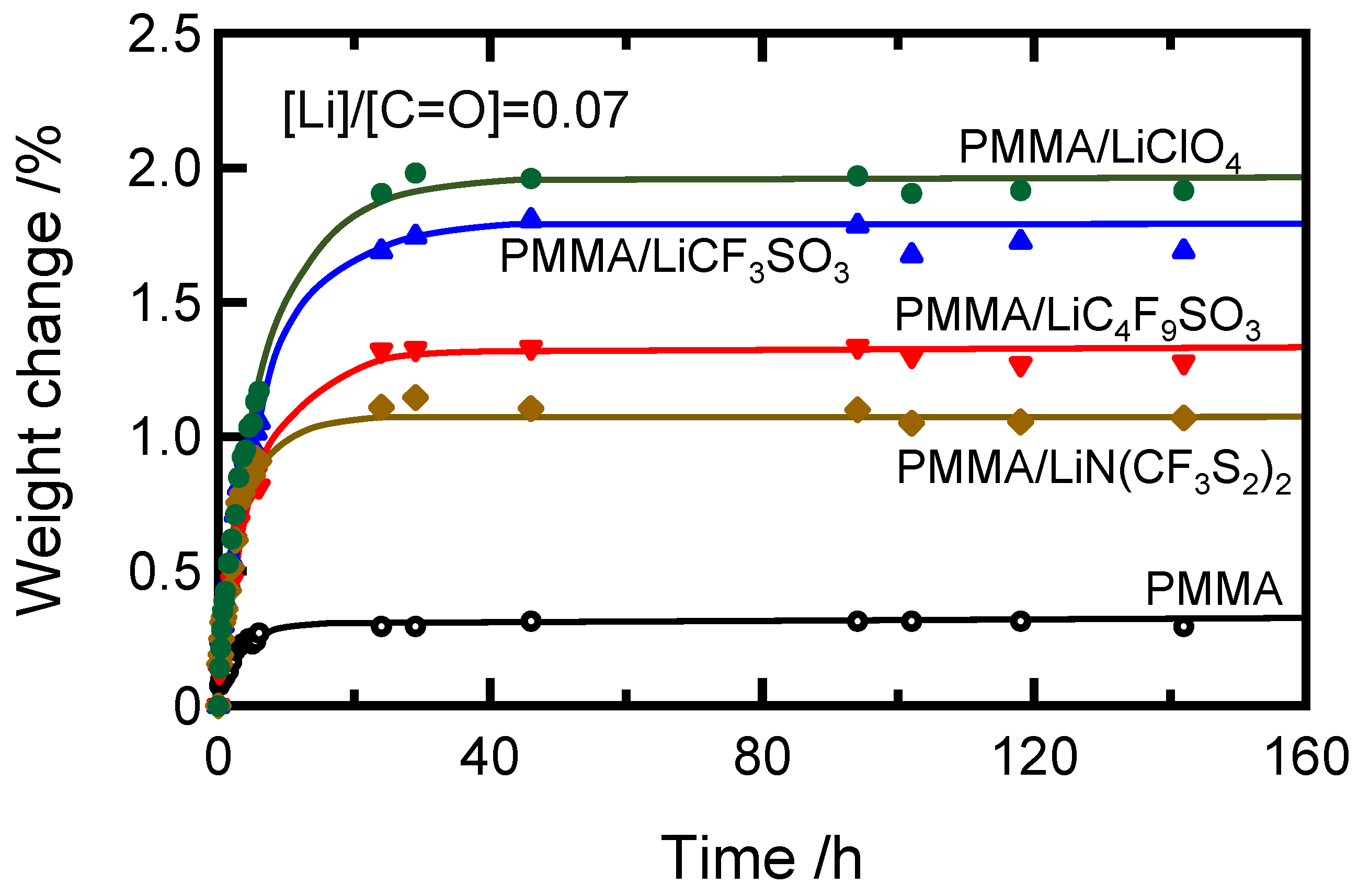
| Sample Code | Condition | Weight Percent of Salt/wt.% | Molar Ratio of Salt/mol mol−1 | Absorbed Water Content/wt.% |
|---|---|---|---|---|
| PMMA | Dried | 0 | 0 | 0 |
| Water absorbed | 0.31 | |||
| PMMA/LiClO4 | Dried | 7 | 0.07 | 0 |
| Water absorbed | 0.19 | |||
| PMMALiCF3SO3 | Dried | 10 | 0.07 | 0 |
| Water absorbed | 1.7 | |||
| PMMA/LiC4F9SO3 | Dried | 18 | 0.07 | 0 |
| Water absorbed | 1.3 | |||
| PMMA/LiN(CF3SO2)2 | Dried | 17 | 0.07 | 0 |
| Water absorbed | 1.1 |
| Sample Code | <τG>/<τG0> | <νe>/<νe0> | <τF>/<τF0> |
|---|---|---|---|
| PMMA | 1 | 1 | 1 |
| PMMA/LiClO4 | 8.9 | 0.14 | 9.8 |
| PMMALiCF3SO3 | 6.3 | 0.83 | 4.9 |
| PMMA/LiC4F9SO3 | 3.2 | 0.81 | 3.1 |
| PMMA/LiN(CF3SO2)2 | 0.63 | 0.72 | 1.7 |
| Sample Code | Condition | Toughness/MJm−3 |
|---|---|---|
| PMMA | Dried | 7.4 |
| Water absorbed | 8.3 | |
| PMMA/LiClO4 | Dried | 4.2 |
| Water absorbed | 4.7 | |
| PMMALiCF3SO3 | Dried | 3.7 |
| Water absorbed | 5.5 | |
| PMMA/LiC4F9SO3 | Dried | 3.8 |
| Water absorbed | 2.2 | |
| PMMA/LiN(CF3SO2)2 | Dried | 4.9 |
| Water absorbed | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, A.; Shin, A.; Nitta, K.-h. Viscoelastic Properties of Water-Absorbed Poly(methyl methacrylate) Doped with Lithium Salts with Various Anions. Molecules 2022, 27, 7114. https://doi.org/10.3390/molecules27207114
Ito A, Shin A, Nitta K-h. Viscoelastic Properties of Water-Absorbed Poly(methyl methacrylate) Doped with Lithium Salts with Various Anions. Molecules. 2022; 27(20):7114. https://doi.org/10.3390/molecules27207114
Chicago/Turabian StyleIto, Asae, Arisa Shin, and Koh-hei Nitta. 2022. "Viscoelastic Properties of Water-Absorbed Poly(methyl methacrylate) Doped with Lithium Salts with Various Anions" Molecules 27, no. 20: 7114. https://doi.org/10.3390/molecules27207114
APA StyleIto, A., Shin, A., & Nitta, K.-h. (2022). Viscoelastic Properties of Water-Absorbed Poly(methyl methacrylate) Doped with Lithium Salts with Various Anions. Molecules, 27(20), 7114. https://doi.org/10.3390/molecules27207114







