Photoinduced Processes in Rhenium(I) Terpyridine Complexes Bearing Remote Amine Groups: New Insights from Transient Absorption Spectroscopy
Abstract
:1. Introduction
2. Results
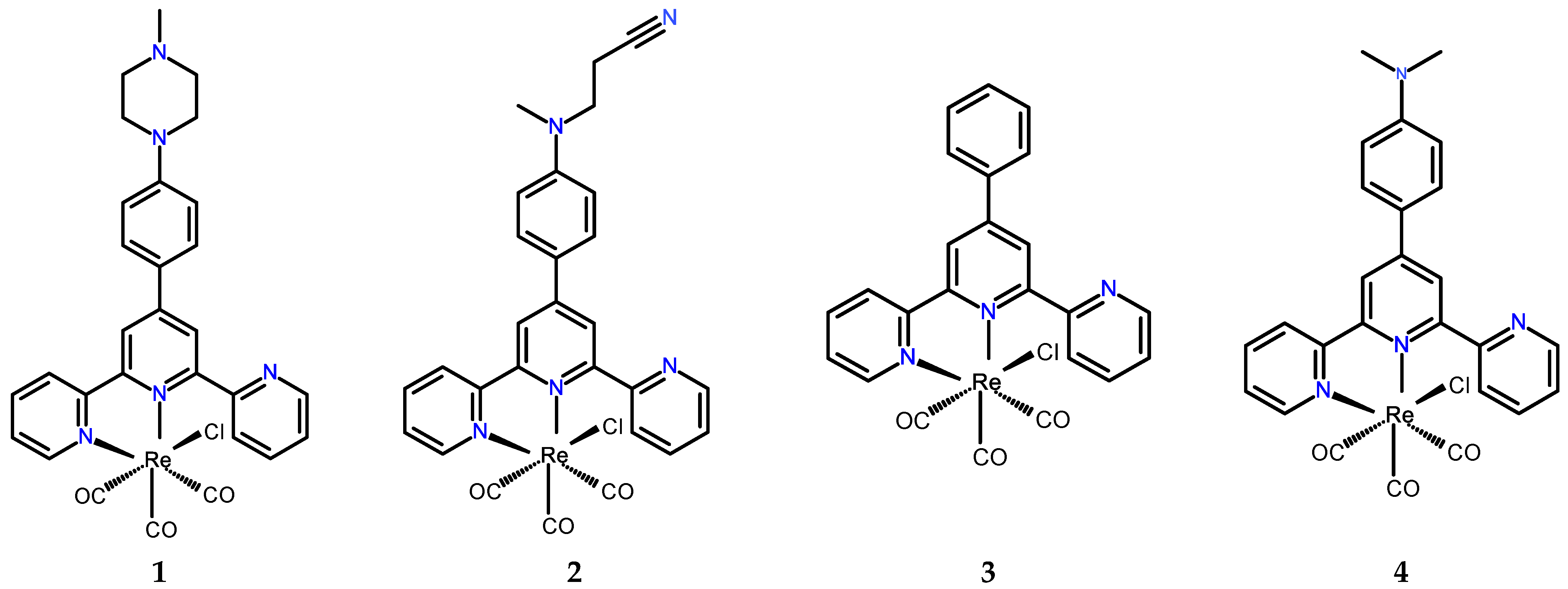
2.1. Synthesis and Molecular Structures
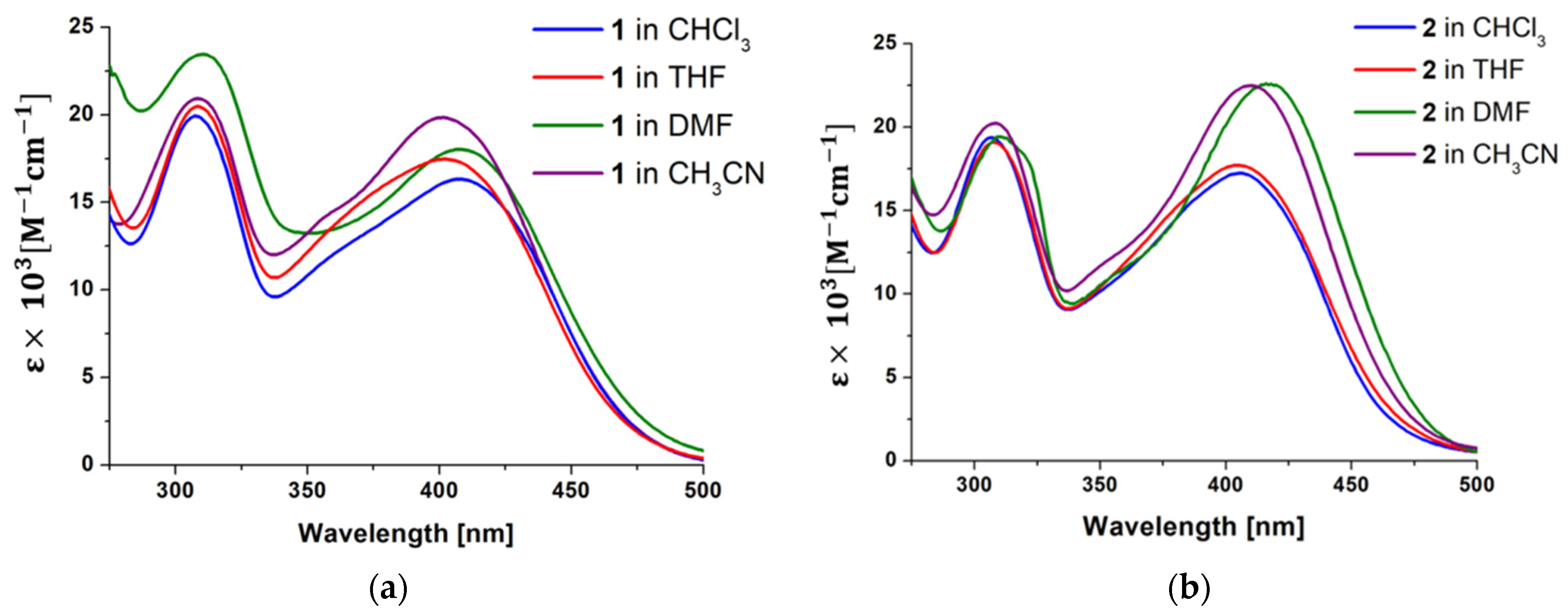
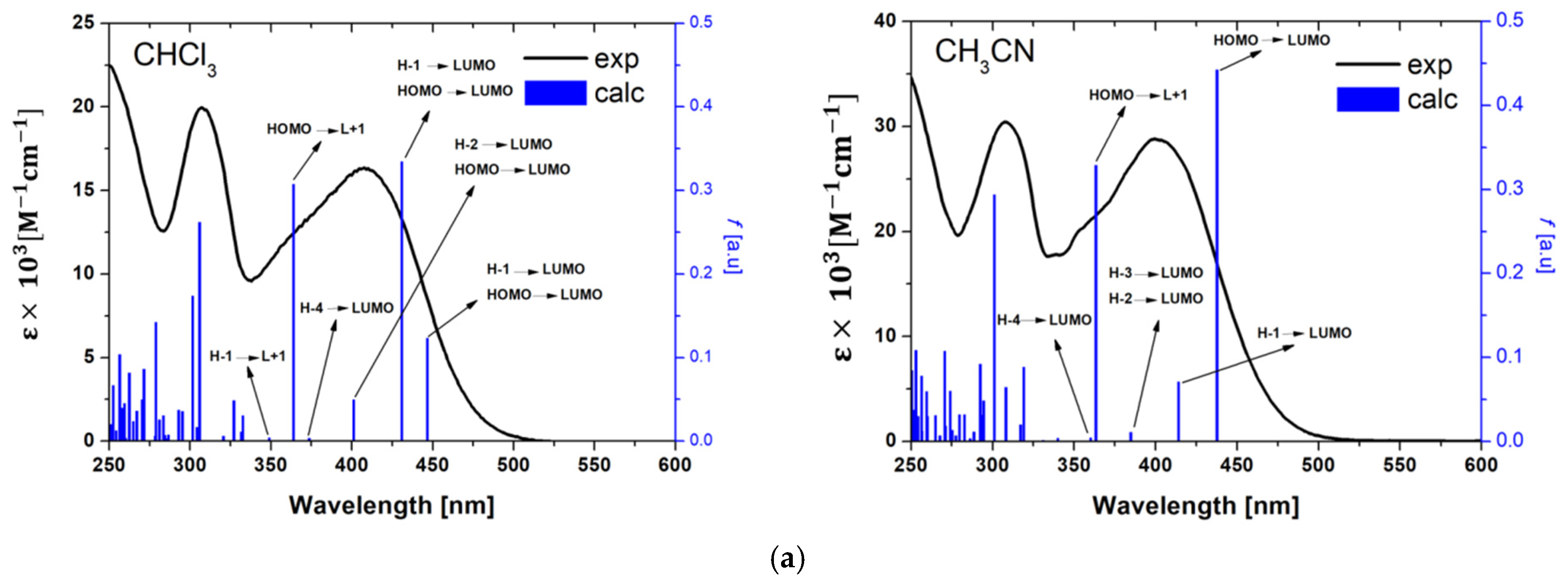
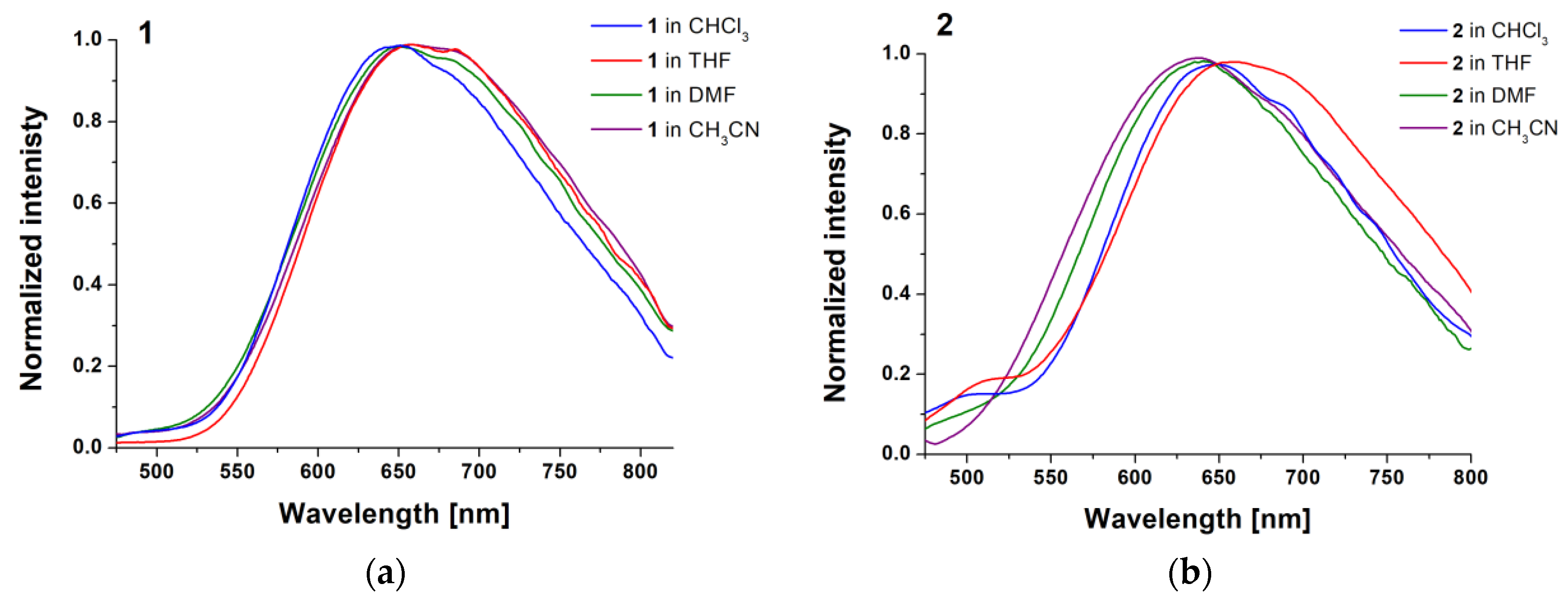
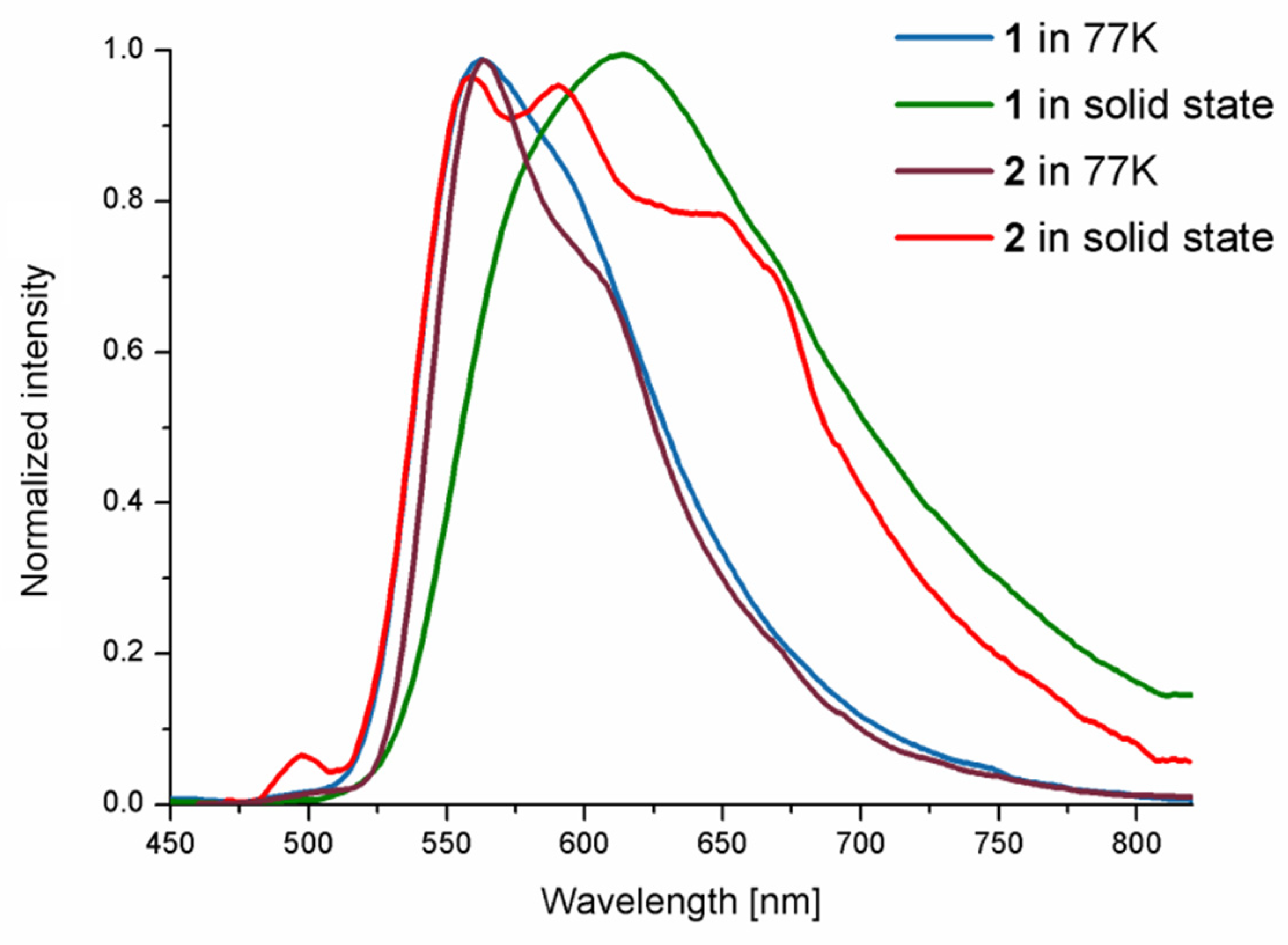
2.2. Photophysical Behavior—Experimental and Theoretical Insights
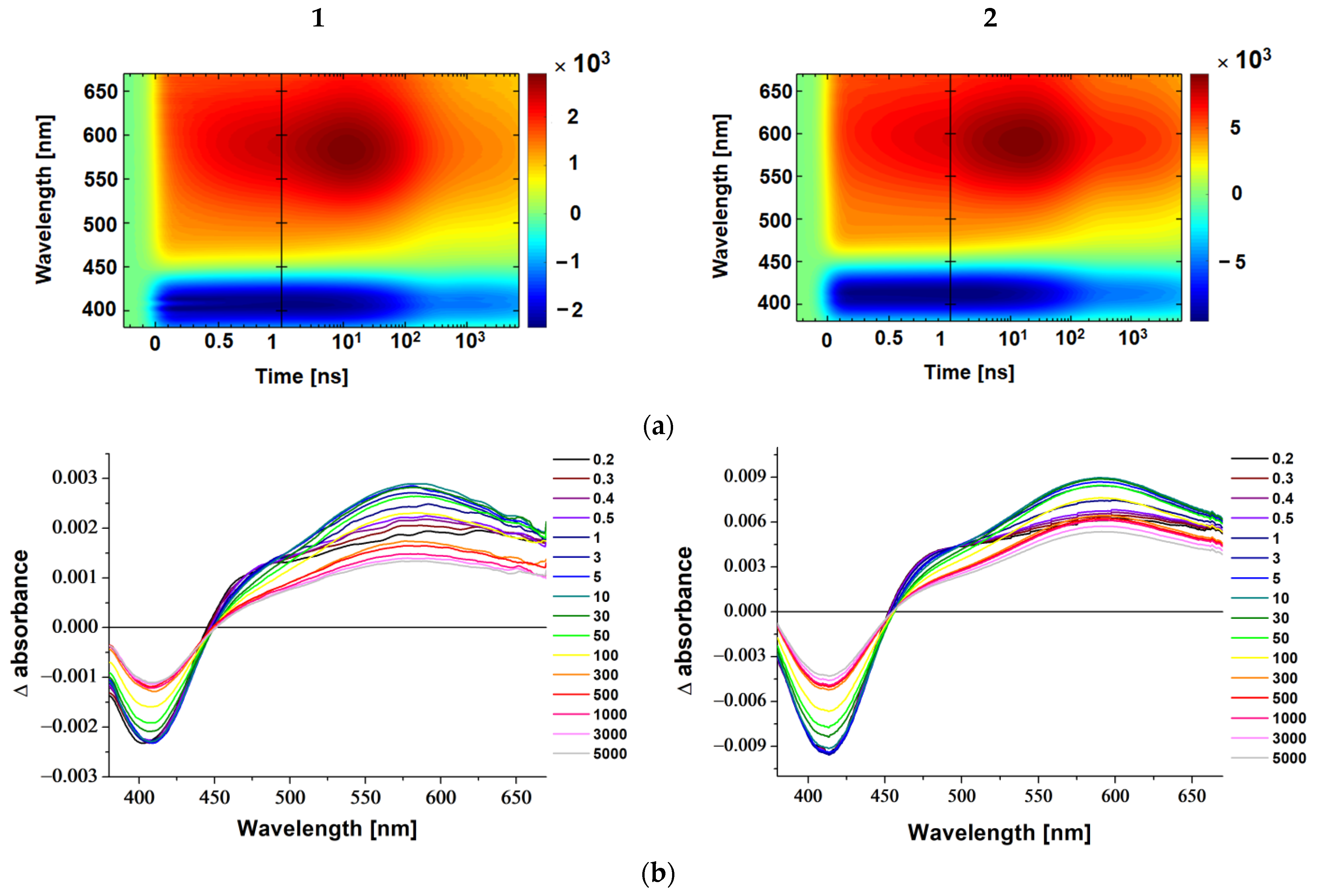
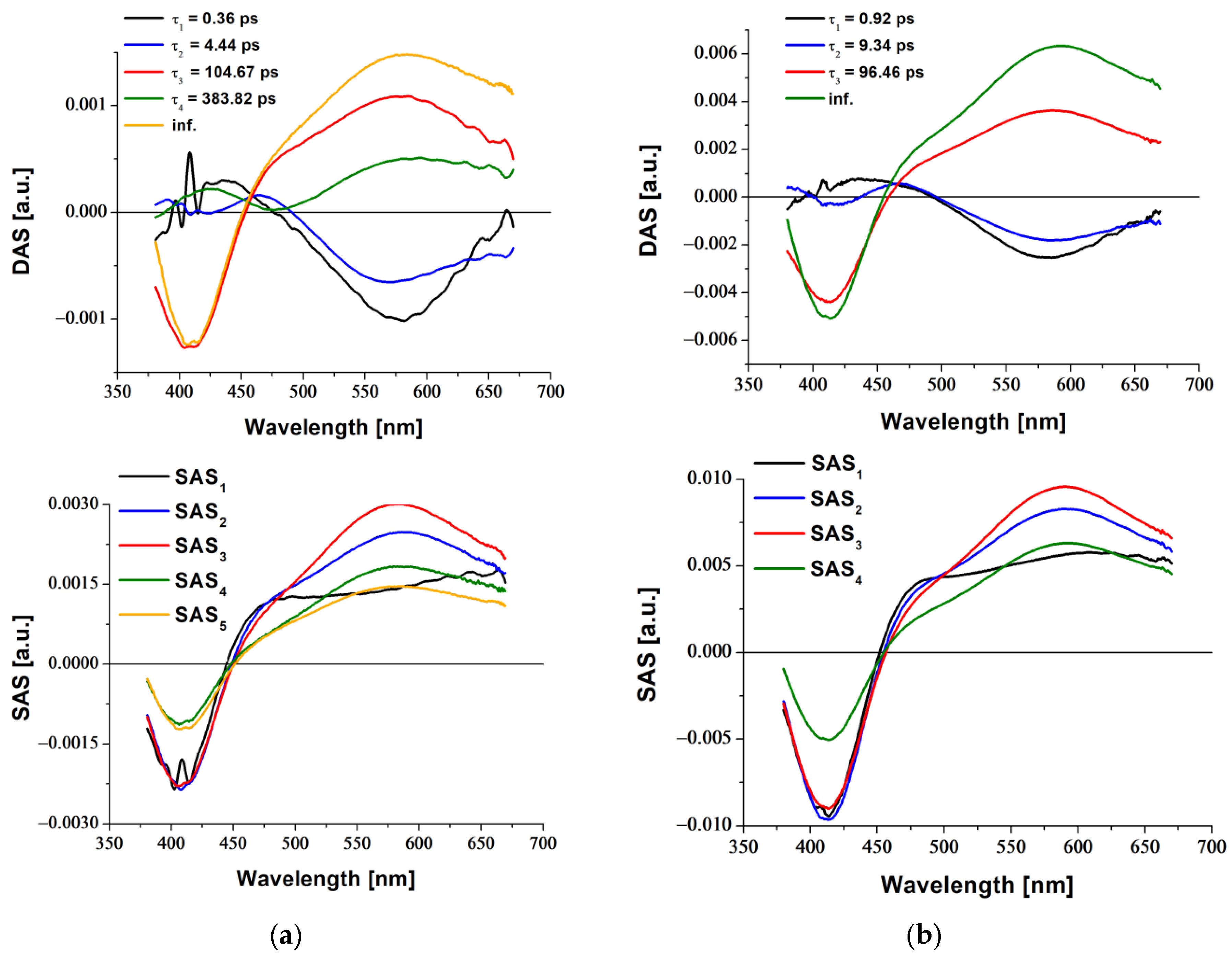
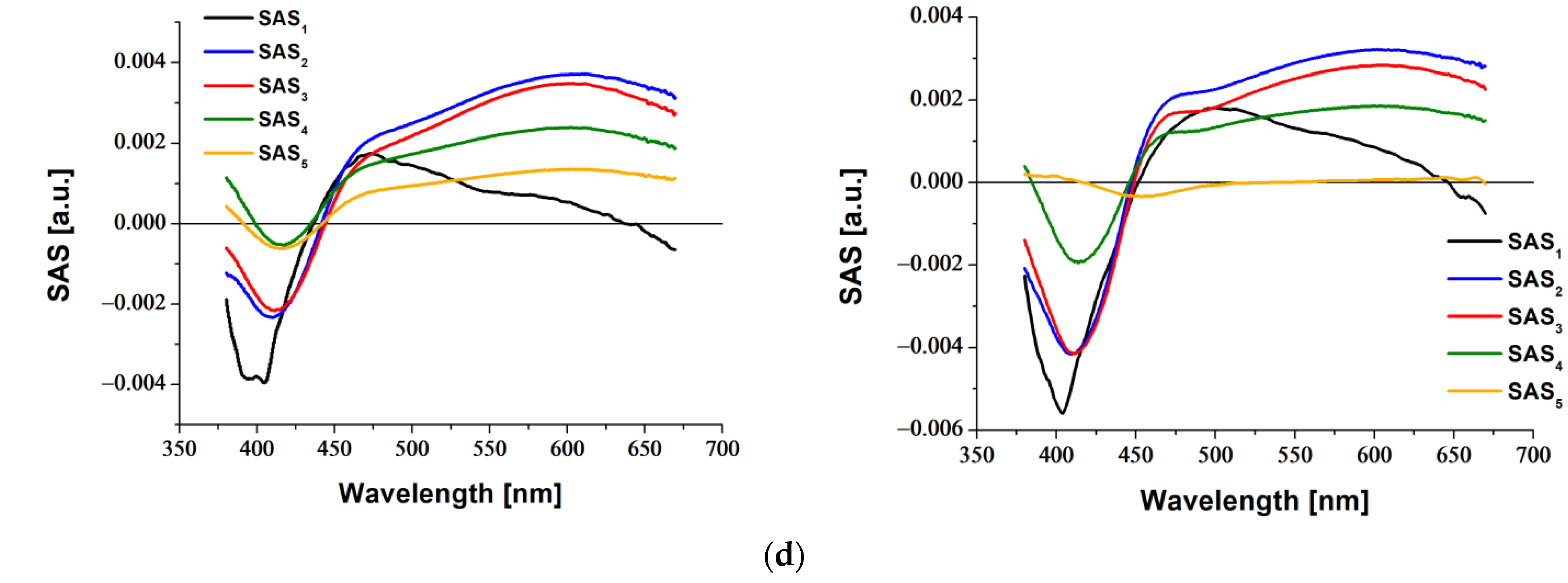
2.3. Transient Absorption Spectroscopy
3. Materials and Methods
3.1. Materials and General Information
3.2. Preparation of [ReCl(CO)3(N-Methyl-piperazinyl-C6H4-terpy-κ2N)] (1) and [ReCl(CO)3((CH3)(CH2CH2CN)N-C6H4-Terpy-κ2N)] (2)
3.3. X-ray Crystallography
3.4. Absorption and Emission Spectra
3.5. Femtosecond Transient Absorption
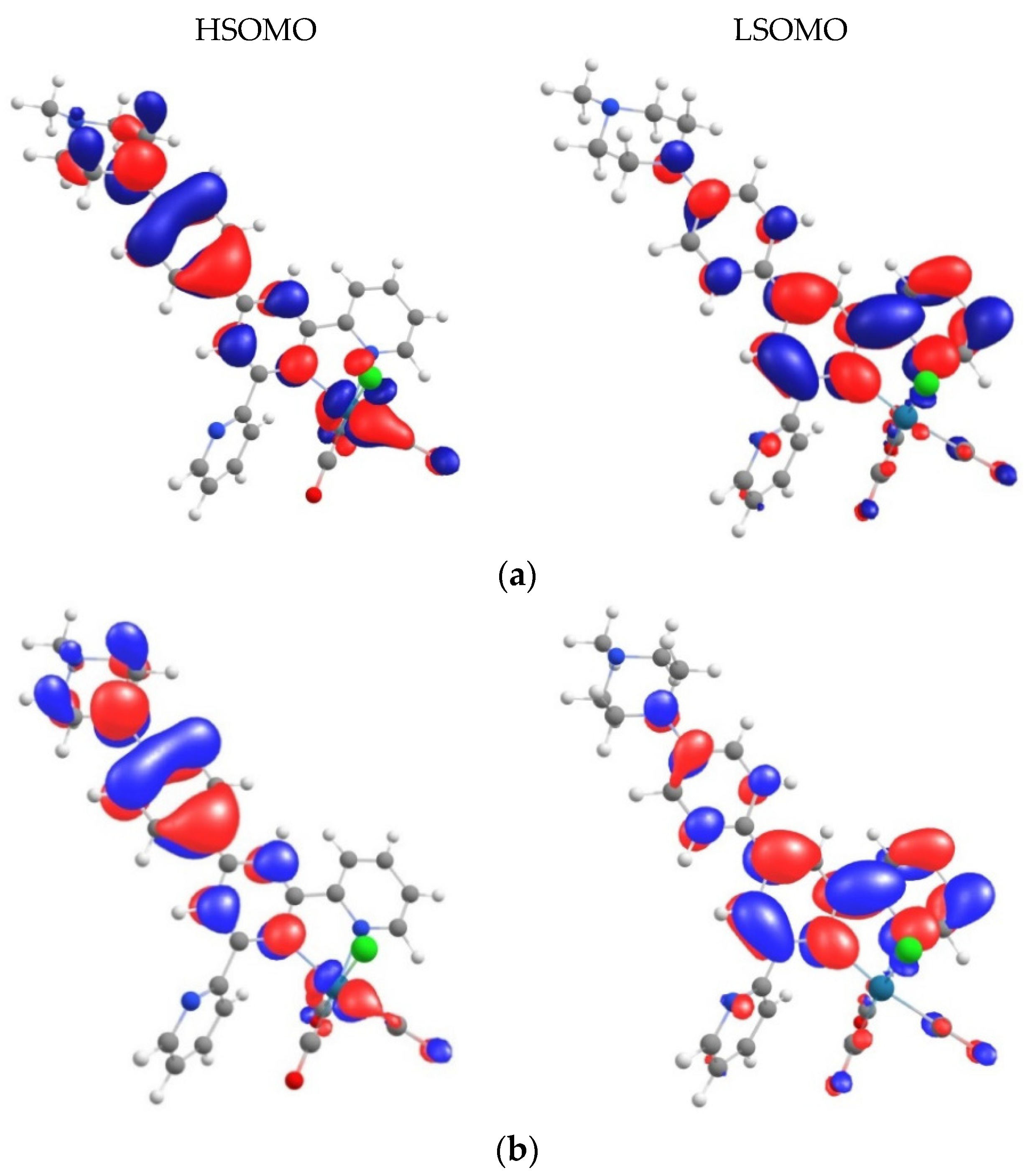
3.6. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wrighton, M.; Morse, D.L. Nature of the Lowest Excited State in Tricarbonylchloro-1,10-Phenanthrolinerhenium(I) and Related Complexes. J. Am. Chem. Soc. 1974, 96, 998–1003. [Google Scholar] [CrossRef]
- Stufkens, D.J.; Vlček, A. Ligand-Dependent Excited State Behaviour of Re(I) and Ru(II) Carbonyl–Diimine Complexes. Coord. Chem. Rev. 1998, 177, 127–179. [Google Scholar] [CrossRef]
- Striplin, D.R.; Crosby, G.A. Photophysical Investigations of Rhenium(I)Cl(CO)3(Phenanthroline) Complexes. Coord. Chem. Rev. 2001, 211, 163–175. [Google Scholar] [CrossRef]
- Coleman, A.; Brennan, C.; Vos, J.G.; Pryce, M.T. Photophysical Properties and Applications of Re(I) and Re(I)–Ru(II) Carbonyl Polypyridyl Complexes. Coord. Chem. Rev. 2008, 252, 2585–2595. [Google Scholar] [CrossRef]
- Kumar, A.; Sun, S.-S.; Lees, A.J. Photophysics and Photochemistry of Organometallic Rhenium Diimine Complexes. In Photophysics of Organometallics; Lees, A.J., Ed.; Topics in Organometallic Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; pp. 37–71. ISBN 978-3-642-04729-9. [Google Scholar]
- Vlček, A. Ultrafast Excited-State Processes in Re(I) Carbonyl-Diimine Complexes: From Excitation to Photochemistry. In Photophysics of Organometallics; Lees, A.J., Ed.; Topics in Organometallic Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; pp. 115–158. ISBN 978-3-642-04729-9. [Google Scholar]
- Takeda, H.; Koike, K.; Morimoto, T.; Inumaru, H.; Ishitani, O. Photochemistry and Photocatalysis of Rhenium(I) Diimine Complexes. In Advances in Inorganic Chemistry; van Eldik, R., Stochel, G., Eds.; Inorganic Photochemistry; Academic Press: Cambridge, MA, USA, 2011; Volume 63, pp. 137–186. [Google Scholar]
- Baková, R.; Chergui, M.; Daniel, C.; Vlček, A.; Záliš, S. Relativistic Effects in Spectroscopy and Photophysics of Heavy-Metal Complexes Illustrated by Spin–Orbit Calculations of [Re(Imidazole)(CO)3(Phen)]+. Coord. Chem. Rev. 2011, 255, 975–989. [Google Scholar] [CrossRef]
- Ko, C.-C.; Cheung, A.W.-Y.; Lo, L.T.-L.; Siu, J.W.-K.; Ng, C.-O.; Yiu, S.-M. Syntheses and Photophysical Studies of New Classes of Luminescent Isocyano Rhenium(I) Diimine Complexes. Coord. Chem. Rev. 2012, 256, 1546–1555. [Google Scholar] [CrossRef]
- Bertrand, H.C.; Clède, S.; Guillot, R.; Lambert, F.; Policar, C. Luminescence Modulations of Rhenium Tricarbonyl Complexes Induced by Structural Variations. Inorg. Chem. 2014, 53, 6204–6223. [Google Scholar] [CrossRef]
- Woźna, A.; Kapturkiewicz, A. The Luminescence Properties of the Heteroleptic [Re(CO)3(N∩N)Cl] and [Re(CO)3(N∩N)(CH3CN)]+ Complexes in View of the Combined Marcus–Jortner and Mulliken–Hush Formalism. Phys. Chem. Chem. Phys. 2015, 17, 30468–30480. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.-W. Luminescent Rhenium(I) and Iridium(III) Polypyridine Complexes as Biological Probes, Imaging Reagents, and Photocytotoxic Agents. Acc. Chem. Res. 2015, 48, 2985–2995. [Google Scholar] [CrossRef]
- Li, X.; Zhao, G.-W.; Hu, Y.-X.; Zhao, J.-H.; Dong, Y.; Zhou, L.; Lv, Y.-L.; Chi, H.-J.; Su, Z. Rational Design and Characterization of Novel Phosphorescent Rhenium(I) Complexes for Extremely High-Efficiency Organic Light-Emitting Diodes. J. Mater. Chem. C 2017, 5, 7629–7636. [Google Scholar] [CrossRef]
- Hostachy, S.; Policar, C.; Delsuc, N. Re(I) Carbonyl Complexes: Multimodal Platforms for Inorganic Chemical Biology. Coord. Chem. Rev. 2017, 351, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Yip, A.M.-H.; Lo, K.K.-W. Luminescent Rhenium(I), Ruthenium(II), and Iridium(III) Polypyridine Complexes Containing a Poly(Ethylene Glycol) Pendant or Bioorthogonal Reaction Group as Biological Probes and Photocytotoxic Agents. Coord. Chem. Rev. 2018, 361, 138–163. [Google Scholar] [CrossRef]
- Barnsley, J.E.; Shillito, G.E.; Larsen, C.B.; van der Salm, H.; Horvath, R.; Sun, X.Z.; Wu, X.; George, M.W.; Lucas, N.T.; Gordon, K.C. Generation of Microsecond Charge-Separated Excited States in Rhenium(I) Diimine Complexes: Driving Force Is the Dominant Factor in Controlling Lifetime. Inorg. Chem. 2019, 58, 9785–9795. [Google Scholar] [CrossRef] [PubMed]
- Maroń, A.M.; Szlapa-Kula, A.; Matussek, M.; Kruszynski, R.; Siwy, M.; Janeczek, H.; Grzelak, J.; Maćkowski, S.; Schab-Balcerzak, E.; Machura, B. Photoluminescence Enhancement of Re(I) Carbonyl Complexes Bearing D–A and D–π–A Ligands. Dalton Trans. 2020, 49, 4441–4453. [Google Scholar] [CrossRef]
- Shillito, G.E.; Preston, D.; Traber, P.; Steinmetzer, J.; McAdam, C.J.; Crowley, J.D.; Wagner, P.; Kupfer, S.; Gordon, K.C. Excited-State Switching Frustrates the Tuning of Properties in Triphenylamine-Donor-Ligand Rhenium(I) and Platinum(II) Complexes. Inorg. Chem. 2020, 59, 6736–6746. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Xu, S.; Hanatani, Y.; Tsuda, S.; Umeda, R. Rhenium Complex-Catalyzed Deoxygenation and Silylation of Alcohols with Hydrosilane. Tetrahedron Lett. 2022, 99, 153839. [Google Scholar] [CrossRef]
- Valdés, E.; Cepeda-Plaza, M.; Günther, G.; Vega, A.; Palacios, R.; Gómez, M.L.; Pizarro, N. An Amine Linker Group Modulates Luminescent Properties in a Rhenium(I) Tricarbonyl Complex. How Can It Be Applied for Ratiometric Oxygen Sensing? Dye. Pigment. 2020, 172, 107787. [Google Scholar] [CrossRef]
- Portenkirchner, E.; Oppelt, K.; Egbe, D.A.M.; Knör, G.; Sariçiftçi, N.S. Electro- and Photo-Chemistry of Rhenium and Rhodium Complexes for Carbon Dioxide and Proton Reduction: A Mini Review. Nanomater. Energy 2013, 2, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Nichols, A.W.; Machan, C.W. Secondary-Sphere Effects in Molecular Electrocatalytic CO2 Reduction. Front. Chem. 2019, 7, 397. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, K.; Sinha Roy, S.; Amatya, E.; Sleeper, E.A.; Le Magueres, P.; Jurss, J.W. Enhanced Electrochemical CO2 Reduction by a Series of Molecular Rhenium Catalysts Decorated with Second-Sphere Hydrogen-Bond Donors. Inorg. Chem. 2020, 59, 6087–6099. [Google Scholar] [CrossRef]
- Li, F.; Zhang, M.; Feng, J.; Cheng, G.; Wu, Z.; Ma, Y.; Liu, S.; Sheng, J.; Lee, S.T. Red Electrophosphorescence Devices Based on Rhenium Complexes. Appl. Phys. Lett. 2003, 83, 365–367. [Google Scholar] [CrossRef]
- Lundin, N.J.; Blackman, A.G.; Gordon, K.C.; Officer, D.L. Synthesis and Characterization of a Multicomponent Rhenium(I) Complex for Application as an OLED Dopant. Angew. Chem. Int. Ed. 2006, 45, 2582–2584. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Li, W.; Chu, B.; Han, L.; Zhu, J.; Su, Z.; Bi, D.; Wang, D.; Yang, D.; et al. Very High-Efficiency Organic Light-Emitting Diodes Based on Cyclometallated Rhenium (I) Complex. Appl. Phys. Lett. 2008, 92, 083302. [Google Scholar] [CrossRef] [Green Version]
- Klemens, T.; Świtlicka-Olszewska, A.; Machura, B.; Grucela, M.; Janeczek, H.; Schab-Balcerzak, E.; Szlapa, A.; Kula, S.; Krompiec, S.; Smolarek, K.; et al. Synthesis, Photophysical Properties and Application in Organic Light Emitting Devices of Rhenium(I) Carbonyls Incorporating Functionalized 2,2′:6′,2′′-Terpyridines. RSC Adv. 2016, 6, 56335–56352. [Google Scholar] [CrossRef]
- Klemens, T.; Świtlicka-Olszewska, A.; Machura, B.; Grucela, M.; Schab-Balcerzak, E.; Smolarek, K.; Mackowski, S.; Szlapa, A.; Kula, S.; Krompiec, S.; et al. Rhenium(I) Terpyridine Complexes–Synthesis, Photophysical Properties and Application in Organic Light Emitting Devices. Dalton Trans. 2016, 45, 1746–1762. [Google Scholar] [CrossRef]
- Zhao, G.-W.; Zhao, J.-H.; Hu, Y.-X.; Zhang, D.-Y.; Li, X. Recent Advances of Neutral Rhenium(I) Tricarbonyl Complexes for Application in Organic Light-Emitting Diodes. Synth. Met. 2016, 212, 131–141. [Google Scholar] [CrossRef]
- Polo, A.S.; Itokazu, M.K.; Murakami Iha, N.Y. Metal Complex Sensitizers in Dye-Sensitized Solar Cells. Coord. Chem. Rev. 2004, 248, 1343–1361. [Google Scholar] [CrossRef]
- Si, Z.; Li, J.; Li, B.; Zhao, F.; Liu, S.; Li, W. Synthesis, Structural Characterization, and Electrophosphorescent Properties of Rhenium(I) Complexes Containing Carrier-Transporting Groups. Inorg. Chem. 2007, 46, 6155–6163. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Jia, J.; Wu, H.-S. Theoretical Studies of COOH Group Effect on the Performance of Rhenium (I) Tricarbonyl Complexes with Bispyridine Sulfur-Rich Core Ligand as Dyes in DSSC. Chem. Acc. 2012, 131, 1266. [Google Scholar] [CrossRef]
- Komreddy, V.; Ensz, K.; Nguyen, H.; Paul Rillema, D. Synthesis and Characterization of Rhenium(I) 4,4′-Dicarboxy-2,2′-Bipyridine Tricarbonyl Complexes for Solar Energy Conversion. Inorg. Chim. Acta 2020, 511, 119815. [Google Scholar] [CrossRef]
- Bauer, E.B.; Haase, A.A.; Reich, R.M.; Crans, D.C.; Kühn, F.E. Organometallic and Coordination Rhenium Compounds and Their Potential in Cancer Therapy. Coord. Chem. Rev. 2019, 393, 79–117. [Google Scholar] [CrossRef]
- Konkankit, C.C.; Marker, S.C.; Knopf, K.M.; Wilson, J.J. Anticancer Activity of Complexes of the Third Row Transition Metals, Rhenium, Osmium, and Iridium. Dalton Trans. 2018, 47, 9934–9974. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Pavic, A.; Voutier, N.; Vojnovic, S.; Crochet, A.; Nikodinovic-Runic, J.; Zobi, F. Identification of Novel Potent and Non-Toxic Anticancer, Anti-Angiogenic and Antimetastatic Rhenium Complexes against Colorectal Carcinoma. Eur. J. Med. Chem. 2020, 204, 112583. [Google Scholar] [CrossRef]
- Wang, F.-X.; Liang, J.-H.; Zhang, H.; Wang, Z.-H.; Wan, Q.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. Mitochondria-Accumulating Rhenium(I) Tricarbonyl Complexes Induce Cell Death via Irreversible Oxidative Stress and Glutathione Metabolism Disturbance. ACS Appl. Mater. Interfaces 2019, 11, 13123–13133. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.-Y.; Tan, C.-P.; Rao, L.-S.; Zhang, H.; Zheng, Y.; Hao, L.; Ji, L.-N.; Mao, Z.-W. Recoding the Cancer Epigenome by Intervening in Metabolism and Iron Homeostasis with Mitochondria-Targeted Rhenium(I) Complexes. Angew. Chem. Int. Ed. 2020, 59, 18755–18762. [Google Scholar] [CrossRef]
- Konkankit, C.C.; Vaughn, B.A.; Huang, Z.; Boros, E.; Wilson, J.J. Systematically Altering the Lipophilicity of Rhenium(I) Tricarbonyl Anticancer Agents to Tune the Rate at Which They Induce Cell Death. Dalton Trans. 2020, 49, 16062–16066. [Google Scholar] [CrossRef]
- He, S.-F.; Pan, N.-L.; Chen, B.-B.; Liao, J.-X.; Huang, M.; Qiu, H.-J.; Jiang, D.-C.; Wang, J.-J.; Chen, J.-X.; Sun, J. Mitochondria-Targeted Re(I) Complexes Bearing Guanidinium as Ligands and Their Anticancer Activity. J. Biol. Inorg. Chem. 2020, 25, 1107–1116. [Google Scholar] [CrossRef]
- Marker, S.C.; King, A.P.; Swanda, R.V.; Vaughn, B.; Boros, E.; Qian, S.-B.; Wilson, J.J. Exploring Ovarian Cancer Cell Resistance to Rhenium Anticancer Complexes. Angew. Chem. Int. Ed. 2020, 59, 13391–13400. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, L.; Wei, F.; Li, L.; Zhao, S.; Gong, P.; Cai, L.; Wong, K.M.-C. Versatile Strategy To Generate a Rhodamine Triplet State as Mitochondria-Targeting Visible-Light Photosensitizers for Efficient Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 8797–8806. [Google Scholar] [CrossRef]
- Liew, H.S.; Mai, C.-W.; Zulkefeli, M.; Madheswaran, T.; Kiew, L.V.; Delsuc, N.; Low, M.L. Recent Emergence of Rhenium(I) Tricarbonyl Complexes as Photosensitisers for Cancer Therapy. Molecules 2020, 25, 4176. [Google Scholar] [CrossRef]
- Karges, J.; Kalaj, M.; Gembicky, M.; Cohen, S.M. ReI Tricarbonyl Complexes as Coordinate Covalent Inhibitors for the SARS-CoV-2 Main Cysteine Protease. Angew. Chem. Int. Ed. 2021, 60, 10716–10723. [Google Scholar] [CrossRef] [PubMed]
- Vlček, A.; Busby, M. Ultrafast Ligand-to-Ligand Electron and Energy Transfer in the Complexes Fac-[ReI(L)(CO)3(Bpy)]N+. Coord. Chem. Rev. 2006, 250, 1755–1762. [Google Scholar] [CrossRef]
- Vlček, A.; Záliš, S. Modeling of Charge-Transfer Transitions and Excited States in D6 Transition Metal Complexes by DFT Techniques. Coord. Chem. Rev. 2007, 251, 258–287. [Google Scholar] [CrossRef]
- Yue, Y.; Grusenmeyer, T.; Ma, Z.; Zhang, P.; Pham, T.T.; Mague, J.T.; Donahue, J.P.; Schmehl, R.H.; Beratan, D.N.; Rubtsov, I.V. Evaluating the Extent of Intramolecular Charge Transfer in the Excited States of Rhenium(I) Donor–Acceptor Complexes with Time-Resolved Vibrational Spectroscopy. J. Phys. Chem. B 2013, 117, 15903–15916. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-K.; Wei, X.-G.; Yiu, S.-M.; Ko, C.-C.; Lau, K.-C. Strongly Phosphorescent Neutral Rhenium(I) Isocyanoborato Complexes: Synthesis, Characterization, and Photophysical, Electrochemical, and Computational Studies. Chem. Eur. J. 2015, 21, 2603–2612. [Google Scholar] [CrossRef] [PubMed]
- Favale, J.M.; Danilov, E.O.; Yarnell, J.E.; Castellano, F.N. Photophysical Processes in Rhenium(I) Diiminetricarbonyl Arylisocyanides Featuring Three Interacting Triplet Excited States. Inorg. Chem. 2019, 58, 8750–8762. [Google Scholar] [CrossRef]
- Yarnell, J.E.; Deaton, J.C.; McCusker, C.E.; Castellano, F.N. Bidirectional “Ping-Pong” Energy Transfer and 3000-Fold Lifetime Enhancement in a Re(I) Charge Transfer Complex. Inorg. Chem. 2011, 50, 7820–7830. [Google Scholar] [CrossRef]
- Kisel, K.S.; Eskelinen, T.; Zafar, W.; Solomatina, A.I.; Hirva, P.; Grachova, E.V.; Tunik, S.P.; Koshevoy, I.O. Chromophore-Functionalized Phenanthro-Diimine Ligands and Their Re(I) Complexes. Inorg. Chem. 2018, 57, 6349–6361. [Google Scholar] [CrossRef]
- Szlapa-Kula, A.; Małecka, M.; Maroń, A.M.; Janeczek, H.; Siwy, M.; Schab-Balcerzak, E.; Szalkowski, M.; Maćkowski, S.; Pedzinski, T.; Erfurt, K.; et al. In-Depth Studies of Ground- and Excited-State Properties of Re(I) Carbonyl Complexes Bearing 2,2′:6′,2″-Terpyridine and 2,6-Bis(Pyrazin-2-Yl)Pyridine Coupled with π-Conjugated Aryl Chromophores. Inorg. Chem. 2021, 60, 18726–18738. [Google Scholar] [CrossRef]
- Shillito, G.E.; Hall, T.B.J.; Preston, D.; Traber, P.; Wu, L.; Reynolds, K.E.A.; Horvath, R.; Sun, X.Z.; Lucas, N.T.; Crowley, J.D.; et al. Dramatic Alteration of 3ILCT Lifetimes Using Ancillary Ligands in [Re(L)(CO)3(Phen-TPA)]N+ Complexes: An Integrated Spectroscopic and Theoretical Study. J. Am. Chem. Soc. 2018, 140, 4534–4542. [Google Scholar] [CrossRef]
- Yarnell, J.E.; Wells, K.A.; Palmer, J.R.; Breaux, J.M.; Castellano, F.N. Excited-State Triplet Equilibria in a Series of Re(I)-Naphthalimide Bichromophores. J. Phys. Chem. B 2019, 123, 7611–7627. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Terán, R.; Sévery, L. Living Long and Prosperous: Productive Intraligand Charge-Transfer States from a Rhenium(I) Terpyridine Photosensitizer with Enhanced Light Absorption. Inorg. Chem. 2021, 60, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, H.; Gao, W.; Wu, Q.; Fan, X.; Mu, Y.; Ma, C. New Rhenium(I) Complexes with Substituted Diimine Ligands for Highly Efficient Phosphorescent Devices Fabricated by a Solution Process. J. Mater. Chem. 2012, 22, 3485–3492. [Google Scholar] [CrossRef]
- Yu, T.; Tsang, D.P.-K.; Au, V.K.-M.; Lam, W.H.; Chan, M.-Y.; Yam, V.W.-W. Deep Red to Near-Infrared Emitting Rhenium(I) Complexes: Synthesis, Characterization, Electrochemistry, Photophysics, and Electroluminescence Studies. Chem. Eur. J. 2013, 19, 13418–13427. [Google Scholar] [CrossRef]
- Cargill Thompson, A.M.W. The Synthesis of 2,2′:6′,2″-Terpyridine Ligands—Versatile Building Blocks for Supramolecular Chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Hofmeier, H.; Schubert, U.S. Recent Developments in the Supramolecular Chemistry of Terpyridine–Metal Complexes. Chem. Soc. Rev. 2004, 33, 373–399. [Google Scholar] [CrossRef]
- Wild, A.; Winter, A.; Schlütter, F.; Schubert, U.S. Advances in the Field of π-Conjugated 2,2′:6′,2″-Terpyridines. Chem. Soc. Rev. 2011, 40, 1459–1511. [Google Scholar] [CrossRef]
- Winter, A.; Hager, M.D.; Newkome, G.R.; Schubert, U.S. The Marriage of Terpyridines and Inorganic Nanoparticles: Synthetic Aspects, Characterization Techniques, and Potential Applications. Adv. Mater. 2011, 23, 5728–5748. [Google Scholar] [CrossRef]
- Czerwińska, K.; Golec, M.; Skonieczna, M.; Palion-Gazda, J.; Zygadło, D.; Szlapa-Kula, A.; Krompiec, S.; Machura, B.; Szurko, A. Cytotoxic Gold( Iii ) Complexes Incorporating a 2,2′:6′,2′′-Terpyridine Ligand Framework–the Impact of the Substituent in the 4′-Position of a Terpy Ring. Dalton Trans. 2017, 46, 3381–3392. [Google Scholar] [CrossRef]
- Toledo, D.; Brovelli, F.; Soto-Delgado, J.; Peña, O.; Pivan, J.-Y.; Moreno, Y. Influence of Structural Changes on Photophysical Properties of Terpyridine Derivates: Experimental Studies and Theoretical Calculations. J. Mol. Struct. 2018, 1153, 282–291. [Google Scholar] [CrossRef]
- Palion-Gazda, J.; Machura, B.; Klemens, T.; Szlapa-Kula, A.; Krompiec, S.; Siwy, M.; Janeczek, H.; Schab-Balcerzak, E.; Grzelak, J.; Maćkowski, S. Structure-Dependent and Environment-Responsive Optical Properties of the Trisheterocyclic Systems with Electron Donating Amino Groups. Dye. Pigment. 2019, 166, 283–300. [Google Scholar] [CrossRef]
- Malarz, K.; Zych, D.; Kuczak, M.; Musioł, R.; Mrozek-Wilczkiewicz, A. Anticancer Activity of 4′-Phenyl-2,2′:6′,2″-Terpyridines–behind the Metal Complexation. Eur. J. Med. Chem. 2020, 189, 112039. [Google Scholar] [CrossRef] [PubMed]
- Klemens, T.; Świtlicka, A.; Szlapa-Kula, A.; Łapok, Ł.; Obłoza, M.; Siwy, M.; Szalkowski, M.; Maćkowski, S.; Libera, M.; Schab-Balcerzak, E.; et al. Tuning Optical Properties of Re(I) Carbonyl Complexes by Modifying Push–Pull Ligands Structure. Organometallics 2019, 38, 4206–4223. [Google Scholar] [CrossRef]
- Choroba, K.; Maroń, A.; Świtlicka, A.; Szłapa-Kula, A.; Siwy, M.; Grzelak, J.; Maćkowski, S.; Pedzinski, T.; Schab-Balcerzak, E.; Machura, B. Carbazole Effect on Ground- and Excited-State Properties of Rhenium(I) Carbonyl Complexes with Extended Terpy-like Ligands. Dalton Trans. 2021, 50, 3943–3958. [Google Scholar] [CrossRef] [PubMed]
- Klemens, T.; Świtlicka, A.; Machura, B.; Kula, S.; Krompiec, S.; Łaba, K.; Korzec, M.; Siwy, M.; Janeczek, H.; Schab-Balcerzak, E.; et al. A Family of Solution Processable Ligands and Their Re(I) Complexes towards Light Emitting Applications. Dye. Pigment. 2019, 163, 86–101. [Google Scholar] [CrossRef]
- Laramée-Milette, B.; Zaccheroni, N.; Palomba, F.; Hanan, G.S. Visible and Near-IR Emissions from K2N- and K3N-Terpyridine Rhenium(I) Assemblies Obtained by an [N×1] Head-to-Tail Bonding Strategy. Chem. Eur. J. 2017, 23, 6370–6379. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Banu, A.; Coogan, M.P.; Edwards, P.G.; Hossain, G.; Malik, K.M.A. Functionalisation of Terpyridine Complexes Containing the Re(CO)3+ Moiety. Dalton Trans. 2010, 39, 6993–7003. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Q.-L.; Zhang, S.; Li, H.-Y.; Wang, C.-C.; Li, T.-Y.; Jing, Y.-M.; Huang, W.; Zheng, Y.-X.; Accorsi, G. Synthesis and Photoluminescence Properties of Rhenium(I) Complexes Based on 2,2′:6′,2′′-Terpyridine Derivatives with Hole-Transporting Units. Dalton Trans. 2013, 42, 2716–2723. [Google Scholar] [CrossRef]
- Klemens, T.; Świtlicka, A.; Szlapa-Kula, A.; Krompiec, S.; Lodowski, P.; Chrobok, A.; Godlewska, M.; Kotowicz, S.; Siwy, M.; Bednarczyk, K.; et al. Experimental and Computational Exploration of Photophysical and Electroluminescent Properties of Modified 2,2′:6′,2″-Terpyridine, 2,6-Di(Thiazol-2-Yl)Pyridine and 2,6-Di(Pyrazin-2-Yl)Pyridine Ligands and Their Re(I) Complexes. Appl. Organomet. Chem. 2018, 32, e4611. [Google Scholar] [CrossRef]
- Małecka, M.; Machura, B.; Świtlicka, A.; Kotowicz, S.; Szafraniec-Gorol, G.; Siwy, M.; Szalkowski, M.; Maćkowski, S.; Schab-Balcerzak, E. Towards Better Understanding of Photophysical Properties of Rhenium(I) Tricarbonyl Complexes with Terpy-like Ligands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118124. [Google Scholar] [CrossRef]
- Sutton, J.J.; Preston, D.; Traber, P.; Steinmetzer, J.; Wu, X.; Kayal, S.; Sun, X.-Z.; Crowley, J.D.; George, M.W.; Kupfer, S.; et al. Excited-State Switching in Rhenium(I) Bipyridyl Complexes with Donor–Donor and Donor–Acceptor Substituents. J. Am. Chem. Soc. 2021, 143, 9082–9093. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.-T.; Zhu, N.; Yam, V.W.-W. Synthesis and Characterization of Luminescent Rhenium(I) Tricarbonyl Diimine Complexes with a Triarylboron Moiety and the Study of Their Fluoride Ion-Binding Properties. Inorg. Chem. 2009, 48, 9664–9670. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.I.; Shaw, J.R.; Simon, J.A.; Thummel, R.P.; Schmehl, R.H. The Photophysical Behavior of D6 Complexes Having Nearly Isoenergetic MLCT and Ligand Localized Excited States. Coord. Chem. Rev. 1998, 171, 43–59. [Google Scholar] [CrossRef]
- Liu, B.; Monro, S.; Li, Z.; Jabed, M.A.; Ramirez, D.; Cameron, C.G.; Colón, K.; Roque, J.; Kilina, S.; Tian, J.; et al. New Class of Homoleptic and Heteroleptic Bis(Terpyridine) Iridium(III) Complexes with Strong Photodynamic Therapy Effects. ACS Appl. Bio Mater. 2019, 2, 2964–2977. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Y.; Xiao, X.; Chen, X.; Hu, M.; Geng, X.; Wang, Z.; Zhao, J. Recent Development of the Transition Metal Complexes Showing Strong Absorption of Visible Light and Long-Lived Triplet Excited State: From Molecular Structure Design to Photophysical Properties and Applications. Coord. Chem. Rev. 2020, 417, 213371. [Google Scholar] [CrossRef]
- Wells, K.A.; Yarnell, J.E.; Sheykhi, S.; Palmer, J.R.; Yonemoto, D.T.; Joyce, R.; Garakyaraghi, S.; Castellano, F.N. Accessing the Triplet Manifold of Naphthalene Benzimidazole–Phenanthroline in Rhenium(I) Bichromophores. Dalton Trans. 2021, 50, 13086–13095. [Google Scholar] [CrossRef]
- Gu, J.; Yan, Y.; Helbig, B.J.; Huang, Z.; Lian, T.; Schmehl, R.H. The Influence of Ligand Localized Excited States on the Photophysics of Second Row and Third Row Transition Metal Terpyridyl Complexes: Recent Examples and a Case Study. Coord. Chem. Rev. 2015, 282, 100–109. [Google Scholar] [CrossRef] [Green Version]
- van Wilderen, L.J.; Lincoln, C.N.; van Thor, J.J. Modelling Multi-Pulse Population Dynamics from Ultrafast Spectroscopy. PLoS ONE 2011, 6, e17373. [Google Scholar] [CrossRef] [Green Version]
- Slavov, C.; Hartmann, H.; Wachtveitl, J. Implementation and Evaluation of Data Analysis Strategies for Time-Resolved Optical Spectroscopy. Anal. Chem. 2015, 87, 2328–2336. [Google Scholar] [CrossRef]
- Choroba, K.; Kotowicz, S.; Maroń, A.; Świtlicka, A.; Szłapa-Kula, A.; Siwy, M.; Grzelak, J.; Sulowska, K.; Maćkowski, S.; Schab-Balcerzak, E.; et al. Ground- and Excited-State Properties of Re(I) Carbonyl Complexes–Effect of Triimine Ligand Core and Appended Heteroaromatic Groups. Dye. Pigment. 2021, 192, 109472. [Google Scholar] [CrossRef]
- CrysAlis PRO. 2011.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.a.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choroba, K.; Kula, S.; Maroń, A.; Machura, B.; Małecki, J.; Szłapa-Kula, A.; Siwy, M.; Grzelak, J.; Maćkowski, S.; Schab-Balcerzak, E. Aryl Substituted 2,6-Di(Thiazol-2-Yl)Pyridines –Excited-State Characterization and Potential for OLEDs. Dye. Pigment. 2019, 169, 89–104. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-Adjustedab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theoret. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Mennucci, B.; Tomasi, J. Ab Initio Study of Ionic Solutions by a Polarizable Continuum Dielectric Model. Chem. Phys. Lett. 1998, 286, 253–260. [Google Scholar] [CrossRef]




| Compound | λ/nm (104 ε/dm3·mol−1·cm−1) | |||
|---|---|---|---|---|
| CHCl3 | THF | CH3CN | DMF | |
| 1 | 405 (1.63) | 405 (1.75) | 402 (1.93) | 409 (1.66) |
| 2 | 405 (1.72) | 406 (1.78) | 405 (2.24) | 417 (2.26) |
| Compound | Medium | λex [nm] | λem [nm] | τav | Φ |
|---|---|---|---|---|---|
| 1 | CHCl3 | 405 | 645 | 8 ns | 0.009 |
| THF | 405 | 665 | 6 ns | 0.010 | |
| DMF | 409 | 650 | 248 ns | 0.001 | |
| CH3CN | 405 | 665 | 146 ns | <0.001 | |
| 77 K (EtOH-MeOH) | 420 | 560 | 178 μs | –– | |
| solid | 475 | 613 | 553 ns | 0.071 | |
| 2 | CHCl3 | 430 | 643 | 8 ns | 0.026 |
| THF | 445 | 665 | 6 ns | 0.027 | |
| DMF | 420 | 640 | 579 ns | 0.003 | |
| CH3CN | 415 | 640 | 79 ns | 0.003 | |
| 77 K (EtOH-MeOH) | 410 | 564 | 173 μs | – | |
| solid | 450 | 560,589,649 | 828 ns | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palion-Gazda, J.; Szłapa-Kula, A.; Penkala, M.; Erfurt, K.; Machura, B. Photoinduced Processes in Rhenium(I) Terpyridine Complexes Bearing Remote Amine Groups: New Insights from Transient Absorption Spectroscopy. Molecules 2022, 27, 7147. https://doi.org/10.3390/molecules27217147
Palion-Gazda J, Szłapa-Kula A, Penkala M, Erfurt K, Machura B. Photoinduced Processes in Rhenium(I) Terpyridine Complexes Bearing Remote Amine Groups: New Insights from Transient Absorption Spectroscopy. Molecules. 2022; 27(21):7147. https://doi.org/10.3390/molecules27217147
Chicago/Turabian StylePalion-Gazda, Joanna, Agata Szłapa-Kula, Mateusz Penkala, Karol Erfurt, and Barbara Machura. 2022. "Photoinduced Processes in Rhenium(I) Terpyridine Complexes Bearing Remote Amine Groups: New Insights from Transient Absorption Spectroscopy" Molecules 27, no. 21: 7147. https://doi.org/10.3390/molecules27217147






