Anxiolytic-like Effect of Quercetin Possibly through GABA Receptor Interaction Pathway: In Vivo and In Silico Studies
Abstract
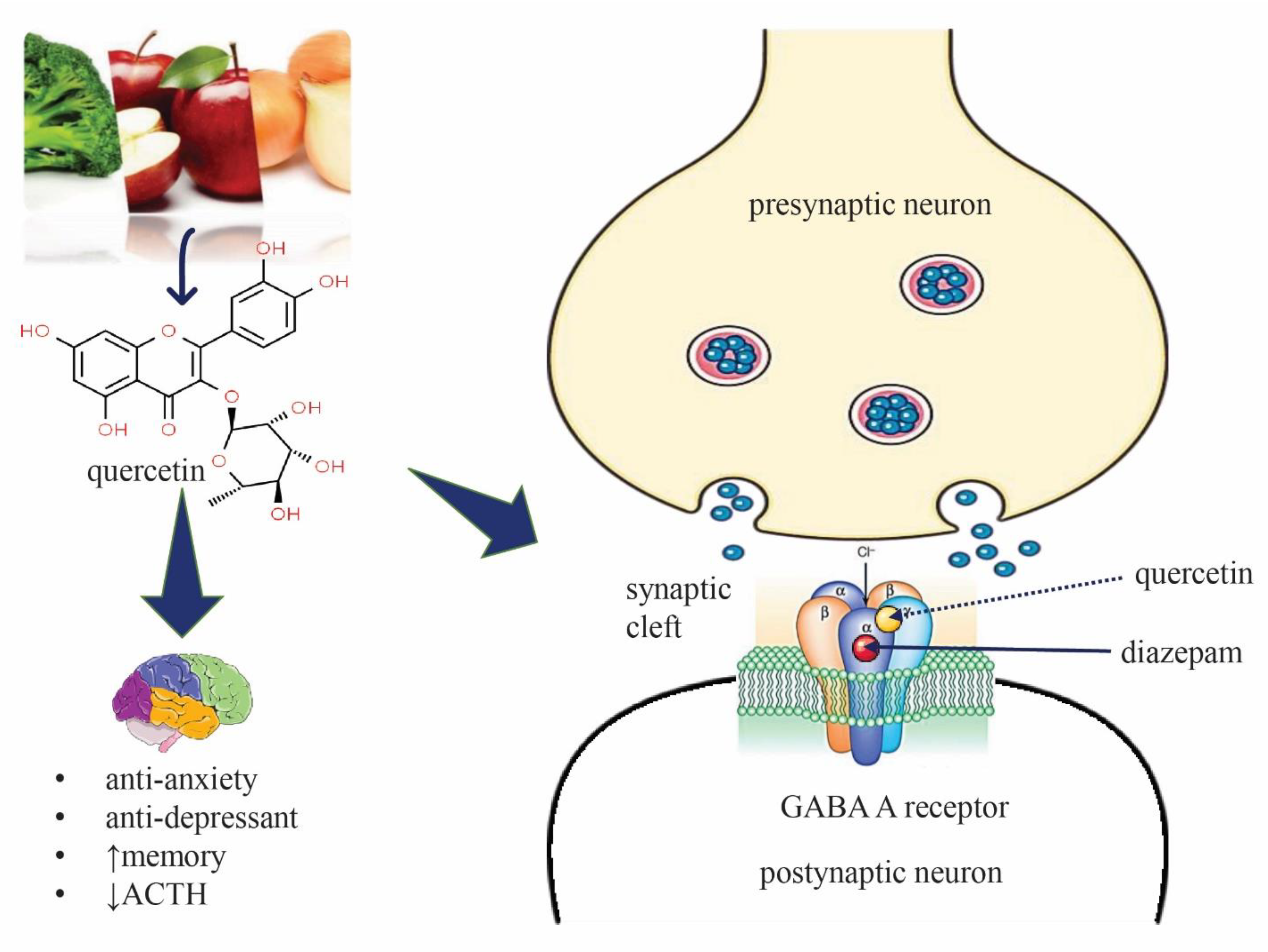
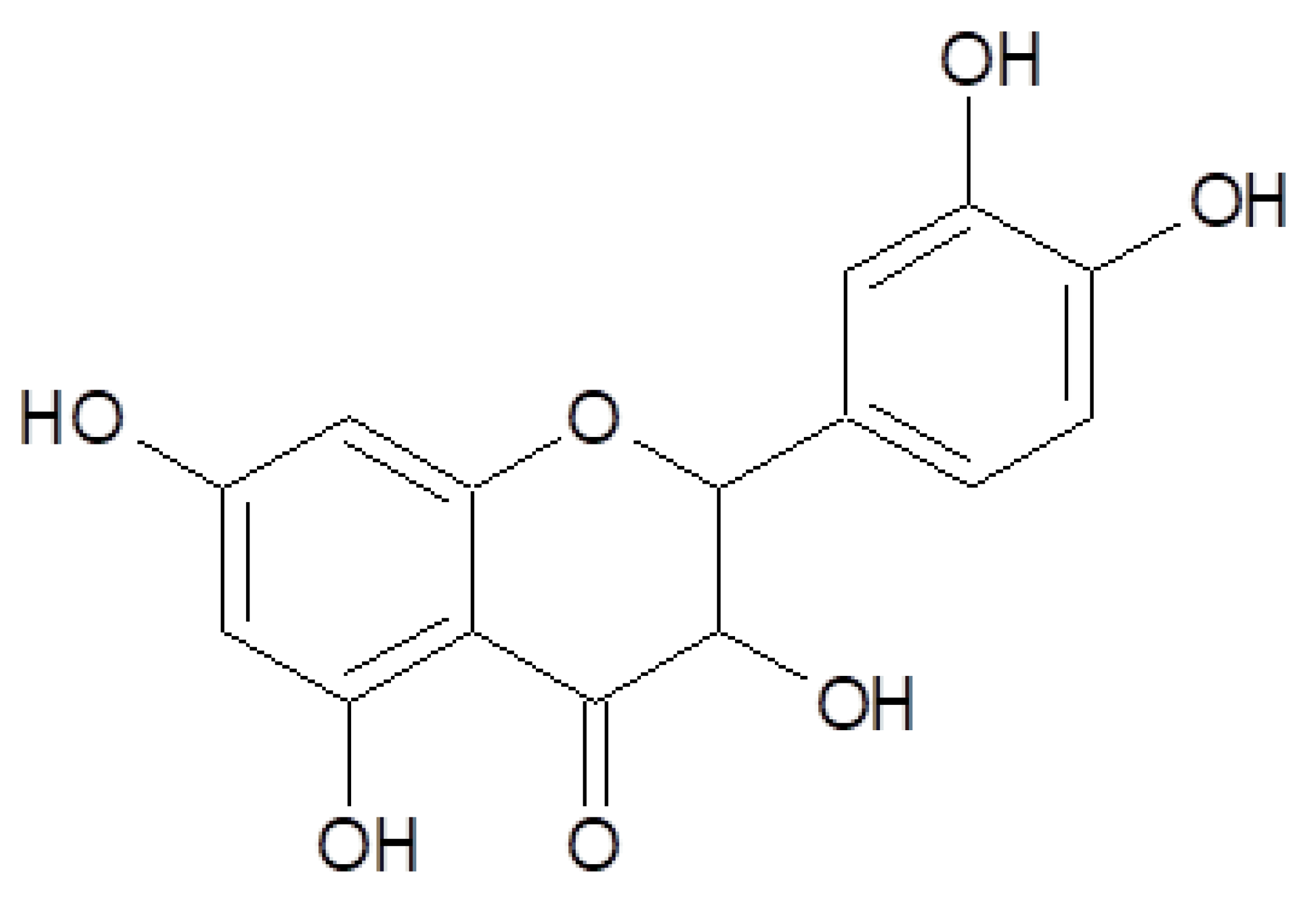
:1. Introduction
2. Results
2.1. Animal Study
2.1.1. Open-Field Test
2.1.2. Hole Cross Test
2.1.3. Swing Test
2.1.4. Light–Dark Test
2.2. In silico Study
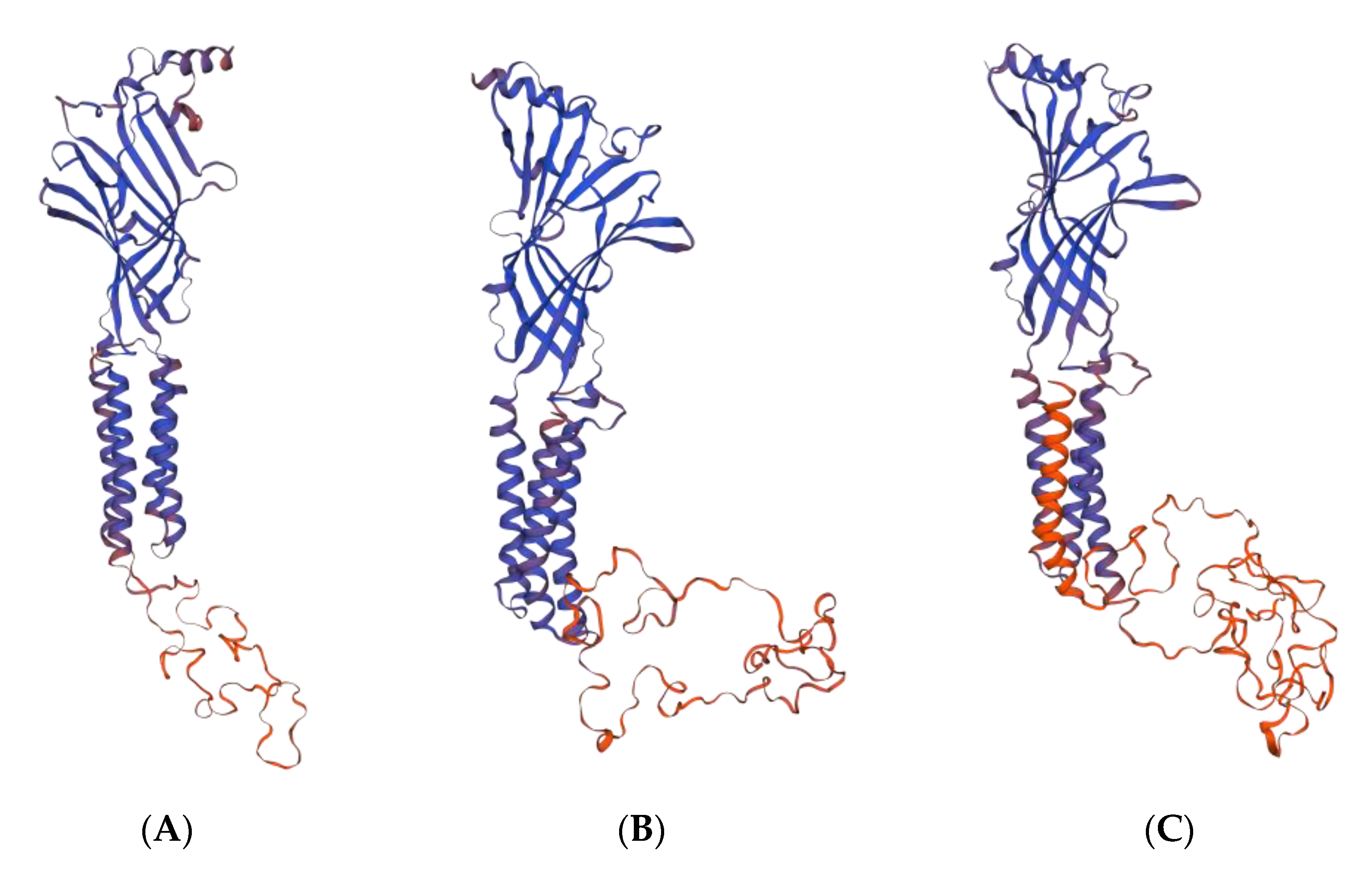
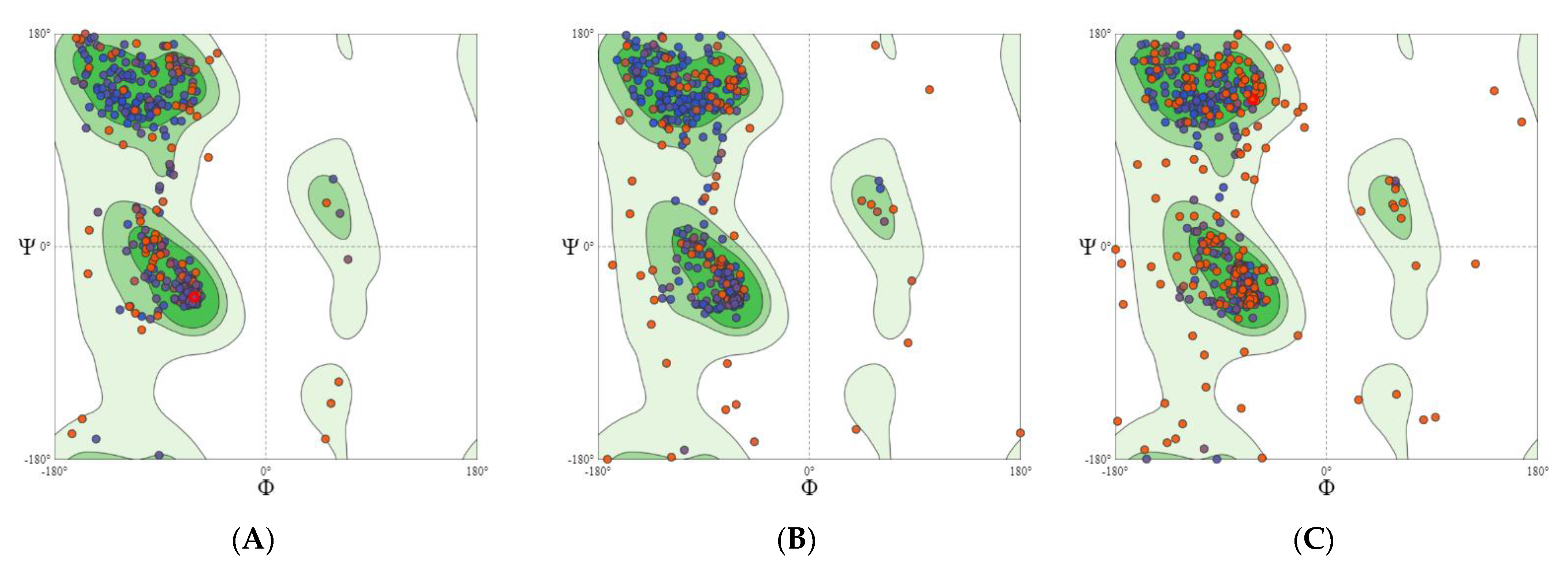
2.2.1. GABA Homology Model
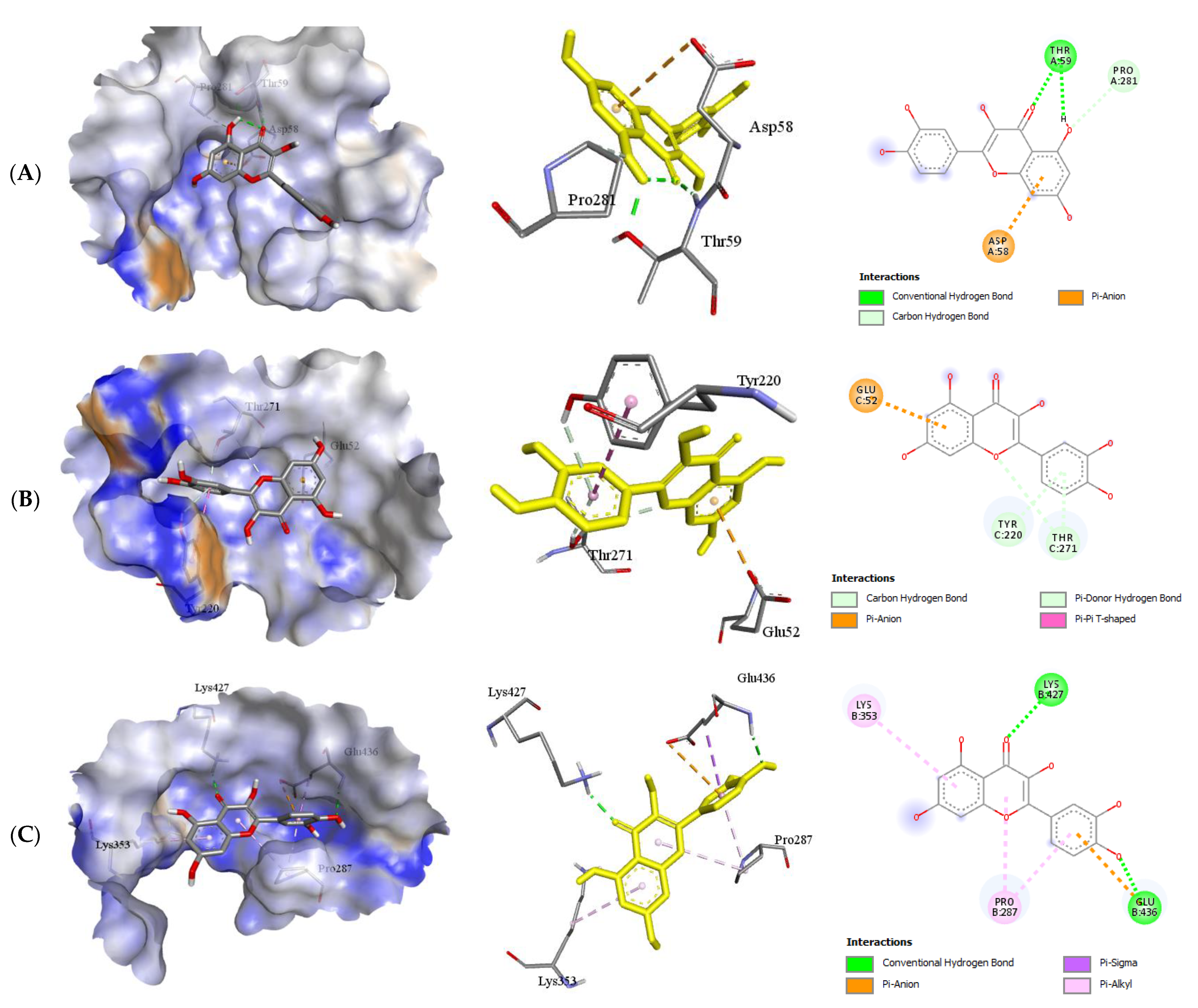
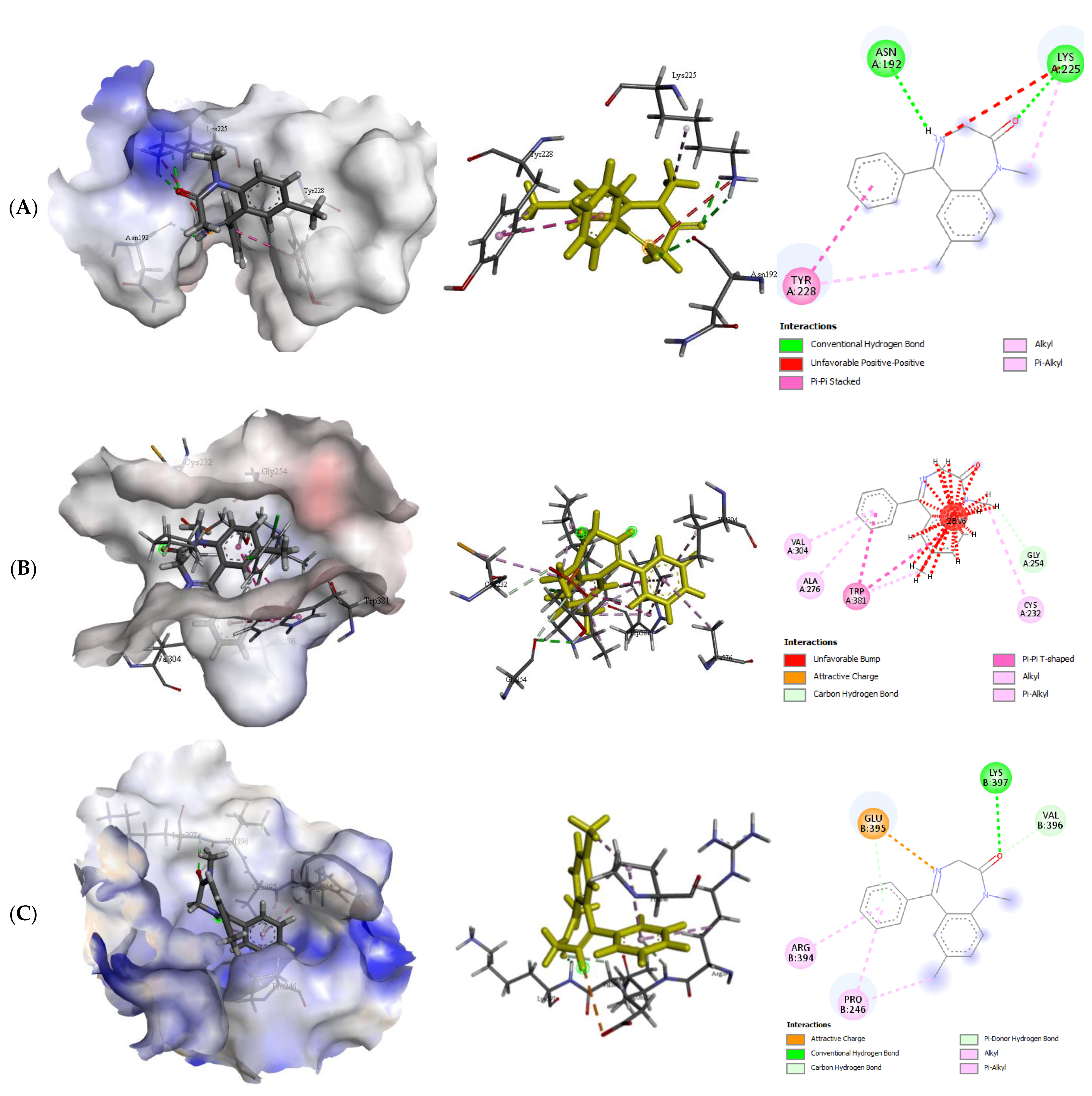
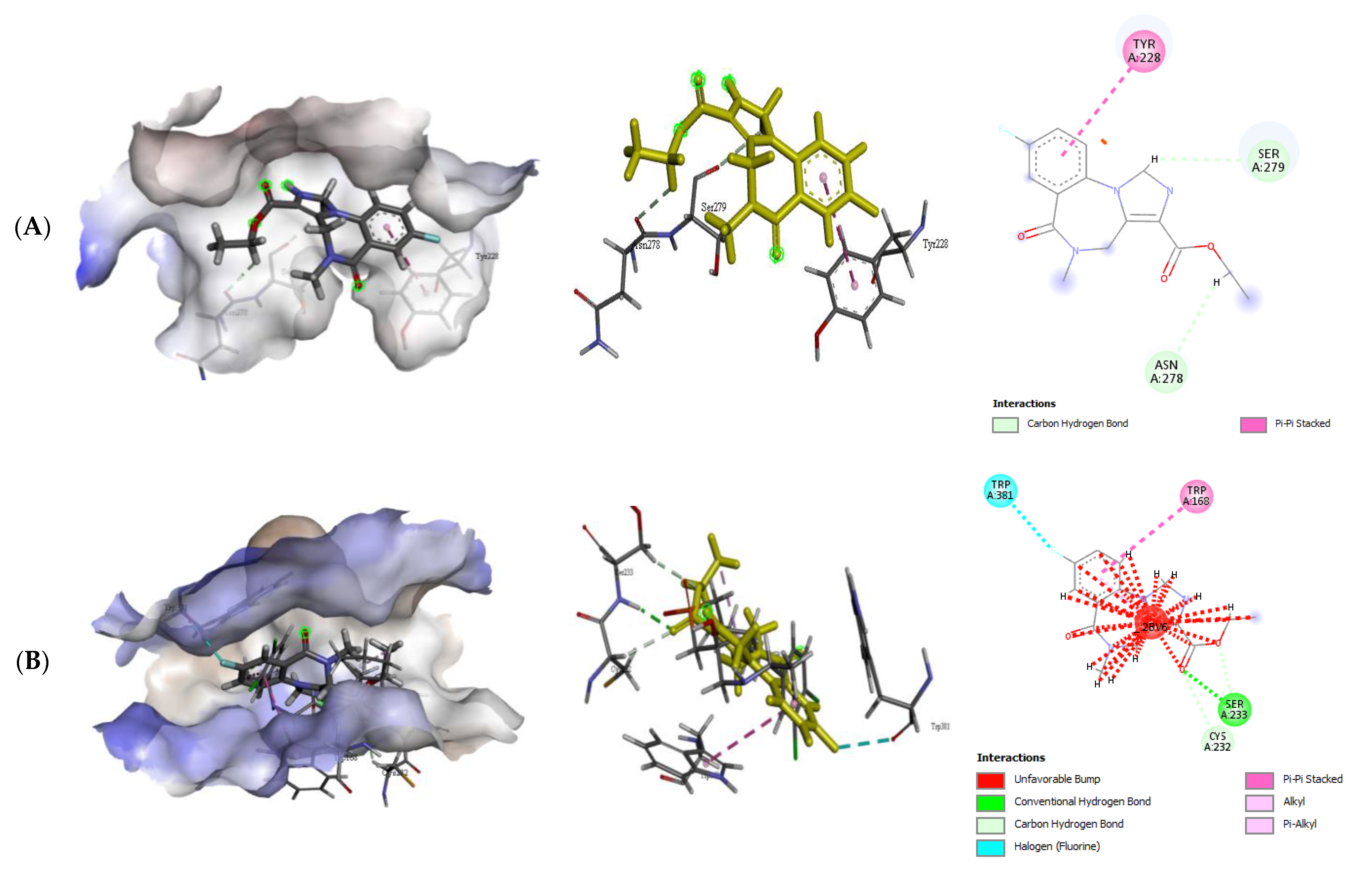
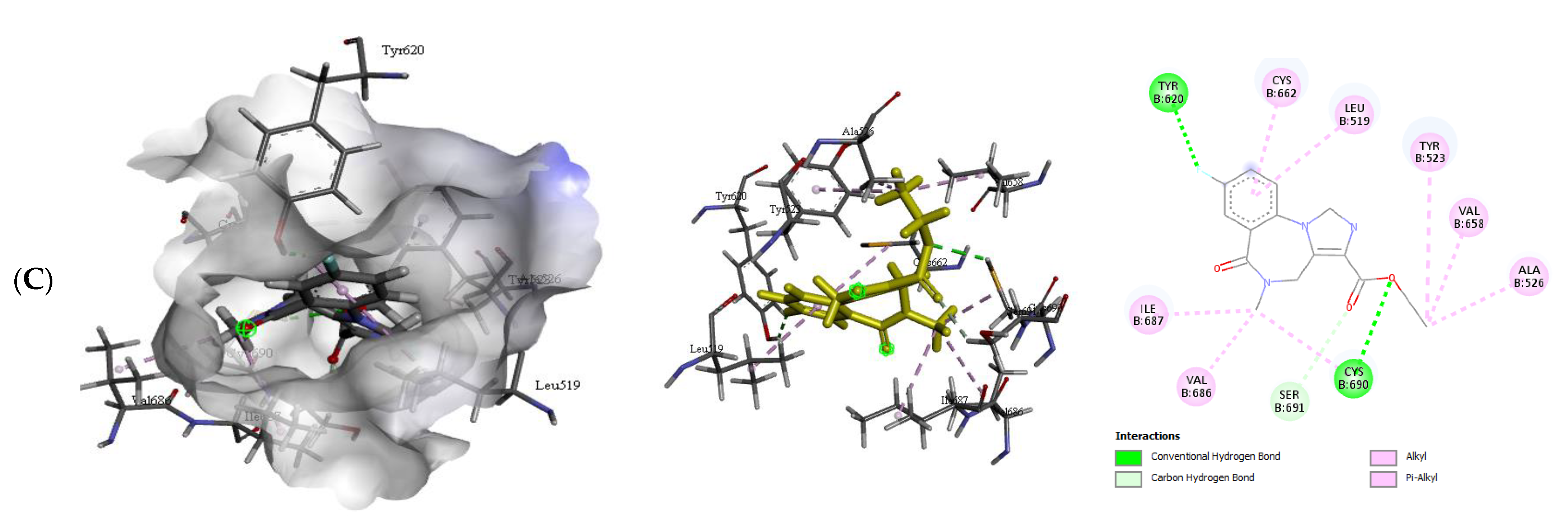
2.2.2. Quercetin (QUR), Diazepam (DZP), and Flumazenil (FLU) with GABA Receptor Interaction
2.2.3. MD Simulation Study
2.2.4. Binding Free Energy (MM-PBSA) Analysis
3. Discussion
4. Materials and Methods
4.1. In Vivo (Animal) Study
4.1.1. Chemicals and Reagents
4.1.2. Animal Model
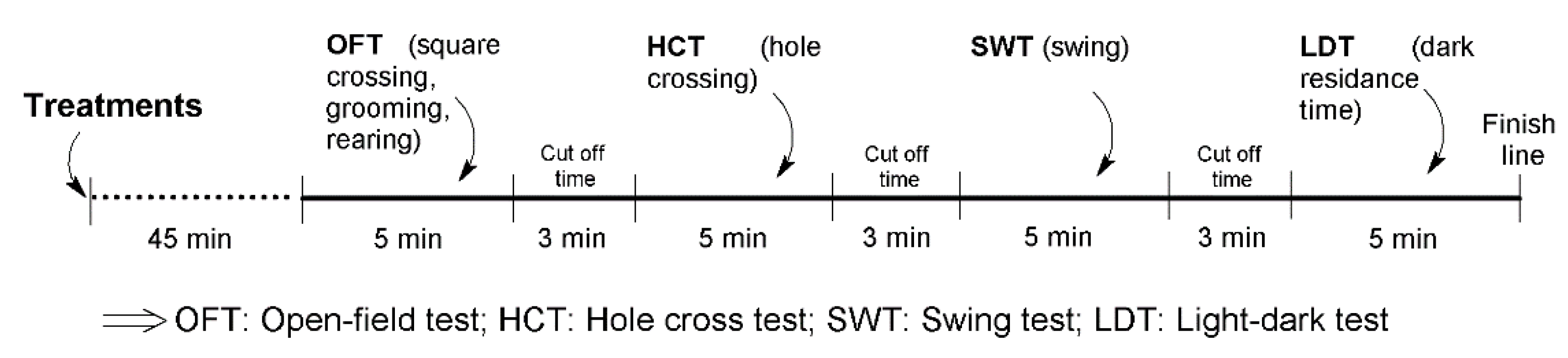
4.1.3. Study Design
4.1.4. Experimental Protocol
4.1.5. Open-Field Test
4.1.6. Hole Cross Test
4.1.7. Swing Test
4.1.8. Light–Dark Test
4.1.9. Statistical Analysis
4.2. Molecular Docking (In Silico) Study
4.2.1. Protein and Ligand Preparation
4.2.2. Docking Protocol
4.2.3. Molecular Dynamic (MD) Simulation and MM-PBSA Study
5. Overall Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Telles-Correia, D.; Saraiva, S.; Gonçalves, J. Mental Disorder—The Need for an Accurate Definition. Front. Psychiatry 2018, 9, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crocq, M.-A. A History of Anxiety: From Hippocrates to DSM. Dialogues Clin. Neurosci. 2022; in press. [Google Scholar] [CrossRef]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Marina Salgado Castillo, C.; Martorell, M. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Nussbaum, L.; Hogea, L.M.; Călina, D.; Andreescu, N.; Grădinaru, R.; Ștefănescu, R.; Puiu, M. Modern Treatment Approaches in Psychoses. Pharmacogenetic, Neuroimagistic and Clinical Implications. Farmacia 2017, 65, 75–81. [Google Scholar]
- Ströhle, A.; Gensichen, J.; Domschke, K. The Diagnosis and Treatment of Anxiety Disorders. Dtsch. Arztebl. Int. 2018, 115, 611. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Docea, A.O.; Calina, D.; Buga, A.M.; Zlatian, O.; Gutnikov, S.; Kostoff, R.N.; Aschner, M. Hormetic Neurobehavioral Effects of Low Dose Toxic Chemical Mixtures in Real-Life Risk Simulation (RLRS) in Rats. Food Chem. Toxicol. 2019, 125, 141–149. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Koek, W.; Mitchell, N.C.; Daws, L.C. Biphasic Effects of Selective Serotonin Reuptake Inhibitors on Anxiety: Rapid Reversal of Escitalopram’s Anxiogenic Effects in the Novelty-Induced Hypophagia (NIH) Test in Mice? Behav. Pharmacol. 2018, 29, 365. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Boyunegmez Tumer, T.; Catarina Moreira, A. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [Green Version]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Antioxidant Activity of Quercetin and Its Glucosides from Propolis: A Theoretical Study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G. Anticancer and Apoptosis-inducing Effects of Quercetin in Vitro and in Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosari-Nasab, M.; Shokouhi, G.; Ghorbanihaghjo, A.; Mesgari-Abbasi, M.; Salari, A.-A. Quercetin Mitigates Anxiety-like Behavior and Normalizes Hypothalamus–Pituitary–Adrenal Axis Function in a Mouse Model of Mild Traumatic Brain Injury. Behav. Pharmacol. 2019, 30, 282–289. [Google Scholar] [CrossRef]
- Merzoug, S.; Toumi, M.L.; Tahraoui, A. Quercetin Mitigates Adriamycin-Induced Anxiety-and Depression-like Behaviors, Immune Dysfunction, and Brain Oxidative Stress in Rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2014, 387, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Saleem, A.; Yasmin, F.; Shehzad, M.A. Quercetin Protects against Stress-Induced Anxiety-and Depression-Like Behavior and Improves Memory in Male Mice. Physiol. Res. 2018, 67, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Moghbelinejad, S.; Alizadeh, S.; Mohammadi, G.; Khodabandehloo, F.; Rashvand, Z.; Najafipour, R.; Nassiri-Asl, M. The Effects of Quercetin on the Gene Expression of the GABAA Receptor A5 Subunit Gene in a Mouse Model of Kainic Acid-Induced Seizure. J. Physiol. Sci. 2017, 67, 339–343. [Google Scholar] [CrossRef]
- Moghbelinejad, S.; Rashvand, Z.; Khodabandehloo, F.; Mohammadi, G.; Nassiri-Asl, M. Modulation of the Expression of the GABAA Receptor Β1 and Β3 Subunits by Pretreatment with Quercetin in the KA Model of Epilepsy in Mice: The Effect of Quercetin on GABAA Receptor Beta Subunits. J. Pharmacopunct. 2016, 19, 163. [Google Scholar]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Nascimento, I.J.; de Aquino, T.M.; da Silva Santos-Júnior, P.F.; de Araújo-Júnior, J.X.; da Silva-Júnior, E.F. Molecular Modeling Applied to Design of Cysteine Protease Inhibitors—A Powerful Tool for the Identification of Hit Compounds against Neglected Tropical Diseases. Front. Comput. Chem. 2020, 5, 63–110. [Google Scholar]
- Muhammed, M.T.; Aki-Yalcin, E. Homology Modeling in Drug Discovery: Overview, Current Applications, and Future Perspectives. Chem. Biol. Drug Des. 2019, 93, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Kanazawa, L.K.S.; Vecchia, D.D.; Wendler, E.M.; de AS Hocayen, P.; Beirao, P.S., Jr.; de Mélo, M.L.; dos Reis Lívero, F.A.; Corso, C.R.; Stipp, M.C.; Acco, A. Effects of Acute and Chronic Quercetin Administration on Methylphenidate-Induced Hyperlocomotion and Oxidative Stress. Life Sci. 2017, 171, 1–8. [Google Scholar] [CrossRef]
- He, Y.; Ouyang, J.; Hu, Z.; Yang, J.; Chu, Y.; Huang, S.; Yang, Y.; Liu, C. Intervention mechanism of repeated oral GABA administration on anxiety-like behaviors induced by emotional stress in rats. Psychiatry Res. 2019, 271, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.B.; Feitosa, M.L.; Silva, M.I.G.; Noronha, E.C.; Moura, B.A.; Venâncio, E.T.; Rios, E.R.V.; de Sousa, D.P.; de Vasconcelos, S.M.M.; de França Fonteles, M.M. Anxiolytic-like Effect of the Monoterpene 1,4-Cineole in Mice. Pharmacol. Biochem. Behav. 2010, 96, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Mizushige, T.; Kanegawa, N.; Yamada, A.; Ota, A.; Kanamoto, R.; Ohinata, K. Aromatic Amino Acid-Leucine Dipeptides Exhibit Anxiolytic-like Activity in Young Mice. Neurosci. Lett. 2013, 543, 126–129. [Google Scholar] [CrossRef]
- Reimus, P.W.; Dangelmayr, M.A.; Clay, J.T.; Chamberlain, K.R. Uranium Natural Attenuation Downgradient of an in Situ Recovery Mine Inferred from a Cross-Hole Field Test. Environ. Sci. Technol. 2019, 53, 7483–7493. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Freitas, R.M.; Oliveira, G.L.S.; Guha, B. Neuropharmacological Screenings of Hydroalcoholic Fractions of Urena lobata L. World J. Pharm. Pharm. Sci 2014, 3, 62–71. [Google Scholar]
- Watanabe, M.; Maemura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. GABA and GABA Receptors in the Central Nervous System and Other Organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar]
- Li, Y.; Song, D.; Bo, F.; Deng, M.; Tang, X. Diazepam Inhibited Lipopolysaccharide (LPS)-Induced Pyroptotic Cell Death and Alleviated Pulmonary Fibrosis in Mice by Specifically Activating GABAA Receptor A4-Subunit. Biomed. Pharmacother. 2019, 118, 109239. [Google Scholar] [CrossRef]
- Calcaterra, N.E.; Barrow, J.C. Classics in Chemical Neuroscience: Diazepam (Valium). ACS Chem. Neurosci. 2014, 5, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, S.D.; Norman, A.; Hince, D.A.; Melichar, J.K.; Hulse, G.K. Benzodiazepine Dependence and Its Treatment with Low Dose Flumazenil. Br. J. Clin. Pharmacol. 2014, 77, 285–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, R.; Al-Khafaji, K.; Khan, R.A.; Sarkar, C.; Islam, M.S.; Dey, D.; Jain, D.; Faria, F.; Akbor, R.; Atolani, O. Quercetin and/or Ascorbic Acid Modulatory Effect on Phenobarbital-Induced Sleeping Mice Possibly through Gabaa and Gabab Receptor Interaction Pathway. Pharmaceuticals 2021, 14, 721. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Azam, S.; Haque, M.E.; Jo, S.-H.; Uddin, M.S.; Kim, I.-S.; Choi, D.-K. Taurine and Its Analogs in Neurological Disorders: Focus on Therapeutic Potential and Molecular Mechanisms. Redox Biol. 2019, 24, 101223. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Hascoët, M. The Mouse Light/Dark Box Test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Ishola, I.O.; Chatterjee, M.; Tota, S.; Tadigopulla, N.; Adeyemi, O.O.; Palit, G.; Shukla, R. Antidepressant and Anxiolytic Effects of Amentoflavone Isolated from Cnestis Ferruginea in Mice. Pharmacol. Biochem. Behav. 2012, 103, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.R.; Rudolph, U.; Lüscher, C. Hooked on Benzodiazepines: GABAA Receptor Subtypes and Addiction. Trends Neurosci. 2011, 34, 188–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, J. Tests for Emotionality in Rats and Mice: A Review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- de Almeida, A.A.C.; Costa, J.P.; de Carvalho, R.B.F.; de Sousa, D.P.; de Freitas, R.M. Evaluation of Acute Toxicity of a Natural Compound (+)-Limonene Epoxide and Its Anxiolytic-like Action. Brain Res. 2012, 1448, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Watanabe, M.; SAITO, H. Studies of the Spontaneous Movement of Animals by the Hole Cross Test; Effect of 2-Dimethyl-Aminoethanol and Its Acyl Esters on the Central Nervous System. Jpn. J. Pharmacol. 1971, 21, 797–810. [Google Scholar] [CrossRef]
- Subhan, N.; Alam, M.A.; Ahmed, F.; Shahid, I.J.; Nahar, L.; Sarker, S.D. Bioactivity of Excoecaria Agallocha. Rev. Bras. Farmacogn. 2008, 18, 521–526. [Google Scholar] [CrossRef]
- Crawley, J.N. Neuropharmacologic Specificity of a Simple Animal Model for the Behavioral Actions of Benzodiazepines. Pharmacol. Biochem. Behav. 1981, 15, 695–699. [Google Scholar] [CrossRef]

| Treatment Groups | Number of Squares Crossed | Number of Grooming | Number of Rearing |
|---|---|---|---|
| Vehicle | 49.13 ± 2.56 | 2.67 ± 0.44 | 35.17 ± 1.85 |
| DZP | 23.60 ± 2.03 a | 2.00 ± 0.36 a | 19.20 ± 7.92 a |
| QUR | 42.80 ± 3.80 a | 2.60 ± 0.76 | 27.80 ± 6.83 a |
| DZP + QUR | 30.40 ± 1.30 ac | 2.00 ± 0.80 ac | 22.00 ± 10.30 ac |
| FLU | 50.75 ± 0.71 | 3.27 ± 0.52 | 39.78 ± 1.09 |
| DZP + FLU | 49.97 ± 1.20 bc | 1.57 ± 0.88 abc | 40.09 ± 1.41 abc |
| QUR + FLU | 47.00 ± 3.30 bc | 3.43 ± 0.35 abc | 43.00 ± 2.98 abc |
| DZP + FLU + QUR | 28.8 ± 2.87 abc | 2.40 ± 0.57 abc | 15.60 ± 2.14 abc |
| Treatment Groups | Number of Hole Cross |
|---|---|
| Vehicle | 25.67 ± 1.37 |
| DZP | 15.80 ± 3.86 a |
| QUR | 17.03 ± 1.27 a |
| DZP + QUR | 18.01 ± 2.15 ab |
| FLU | 26.07 ± 1.08 |
| DZP + FLU | 20.67 ± 2.61 abc |
| QUR + FLU | 22.00 ± 1.37 abc |
| DZP + FLU + QUR | 12.60 ± 2.41 abc |
| Treatment Groups | Number of Swings |
|---|---|
| Vehicle | 32.07 ± 2.08 |
| DZP | 19.60 ± 3.73 a |
| QUR | 22.40 ± 2.56 a |
| DZP + QUR | 24.00 ± 2.45 a |
| FLU | 33.08 ± 2.78 |
| DZP + FLU | 30.21 ± 2.54 abc |
| QUR + FLU | 29.60 ± 1.21 bc |
| DZP + FLU + QUR | 16.60 ± 2.80 abc |
| Treatment Groups | Time Spent in Light Box (s) |
|---|---|
| Vehicle | 104.20 ± 1.48 |
| DZP | 118.60 ± 2.92 a |
| QUR | 126.20 ± 4.27 ab |
| DZP + QUR | 129.00 ± 2.09 abc |
| FLU | 113.30 ± 1.01 a |
| DZP + FLU | 106.50 ± 0.65 a |
| QUR + FLU | 104.60 ± 1.08 |
| DZP + FLU + QUR | 122.00 ± 4.67 ab |
| Protein (Receptor) | Binding Affinity (Kcal/mol) | No. of H-Bond | H-Bond Residues | H-Bond Length (Å) | Other Bond Residues |
|---|---|---|---|---|---|
| GABA α5–QUR | −6.8 | 1 | Thr59 | 1.99 | Asp58 Pro281 |
| GABA β1–QUR | −8.5 | 0 | - | - | Glu92 Thr271 Tyr220 |
| GABA β2–QUR | −8.1 | 2 | Glu436 Lys427 | 2.06 2.11 | Lys 353 Pro287 |
| Protein (Receptor) | Binding Affinity (Kcal/mol) | No. of H-Bond | H-Bond Residues | Bond Length (Å) | Other Bond Residues |
|---|---|---|---|---|---|
| GABA α5-DZP | −6.2 | 2 | Asn192 Lys225 | 2.20 2.37 | Try228 |
| GABA β1-DZP | −7.0 | 0 | - | - | Ala276, Cys232, Gly254, Trp381, val304 |
| GABA β2-DZP | −6.4 | 1 | Lys397 | 2.78 | Arg394, Glu395, Pro245, Val396 |
| Protein (Receptor) | Binding Affinity (Kcal/mol) | No. of H-Bond | H-Bond Residues | Bond Length (Å) | Other Bond Residues |
|---|---|---|---|---|---|
| GABA α5-FLU | −6.0 | 0 | - | - | Asn278, Tyr228, Ser 279 |
| GABA β1-FLU | −6.4 | 1 | Ser233 | 2.25 | Cys232, Trp168, Trp381 |
| GABA β2-FLU | −7.9 | 2 | Cys690 Tyr620 | 2.55 2.55 | Ala526, Cys662, ILE687, Leu519, Ser691, Val 658, Val686 |
| Complex Name | ∆GvdW (kJ/mol) | ∆Gelec (kJ/mol) | ∆Gpol (kJ/mol) | ∆Gnonpol (kJ/mol) | ∆E (MM-PBSA) (kJ/mol) |
|---|---|---|---|---|---|
| GABA α5–QUR | −59.798 | −39.976 | 83.475 | −8.699 | −27.071 |
| GABA β1–QUR | −75.562 | −80.536 | 145.703 | −13.908 | −25.083 |
| GABA β2–QUR | −83.053 | −54.634 | 127.317 | −11.403 | −16.850 |
| Treatment Groups | Description | Dose |
|---|---|---|
| Gr.-I: Negative control | Vehicle 0.5% tween 80 + 0.9% NaCl solution | 10 mL/kg |
| Gr.-II: DZP | Diazepam Standard 1: Benzodiazepine receptor agonist | 2 mg/kg |
| Gr.-III: QUR | Quercetin Test sample | 50 mg/kg |
| Gr.-IV: DZP + QUR | Diazepam + Quercetin | 2 mg/kg + 50 mg/kg |
| Gr.-V: FLU | Flumazenil Standard 2: Benzodiazepine receptor antagonist | 2.5 mg/kg |
| Gr.-V-I: DZP + FLU | Diazepam + Flumazenil | 2 mg/kg + 2.5 mg/kg |
| Gr.-VII: QUR + FLU | Quercetin + Flumazenil | 50 mg/kg + 2.5 mg/kg |
| Gr.-VIII: DZP + FLU + QUR | Diazepam + Flumazenil + Quercetin | 2 mg/kg + 2.5 mg/kg + 50 mg/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S.; Hossain, R.; Ahmed, T.; Rahaman, M.M.; Al-Khafaji, K.; Khan, R.A.; Sarkar, C.; Bappi, M.H.; de Andrade, E.M.; Araújo, I.M.; et al. Anxiolytic-like Effect of Quercetin Possibly through GABA Receptor Interaction Pathway: In Vivo and In Silico Studies. Molecules 2022, 27, 7149. https://doi.org/10.3390/molecules27217149
Islam MS, Hossain R, Ahmed T, Rahaman MM, Al-Khafaji K, Khan RA, Sarkar C, Bappi MH, de Andrade EM, Araújo IM, et al. Anxiolytic-like Effect of Quercetin Possibly through GABA Receptor Interaction Pathway: In Vivo and In Silico Studies. Molecules. 2022; 27(21):7149. https://doi.org/10.3390/molecules27217149
Chicago/Turabian StyleIslam, Md. Shahazul, Rajib Hossain, Taukir Ahmed, Md. Mizanur Rahaman, Khattab Al-Khafaji, Rasel Ahmed Khan, Chandan Sarkar, Mehedi Hasan Bappi, Edlane Martins de Andrade, Isaac Moura Araújo, and et al. 2022. "Anxiolytic-like Effect of Quercetin Possibly through GABA Receptor Interaction Pathway: In Vivo and In Silico Studies" Molecules 27, no. 21: 7149. https://doi.org/10.3390/molecules27217149
APA StyleIslam, M. S., Hossain, R., Ahmed, T., Rahaman, M. M., Al-Khafaji, K., Khan, R. A., Sarkar, C., Bappi, M. H., de Andrade, E. M., Araújo, I. M., Coutinho, H. D. M., Kowalska, G., Kowalski, R., Hanif, M. A., & Islam, M. T. (2022). Anxiolytic-like Effect of Quercetin Possibly through GABA Receptor Interaction Pathway: In Vivo and In Silico Studies. Molecules, 27(21), 7149. https://doi.org/10.3390/molecules27217149













