Preparation of a Chitosan/Coal Gasification Slag Composite Membrane and Its Adsorption and Removal of Cr (VI) and RhB in Water
Abstract
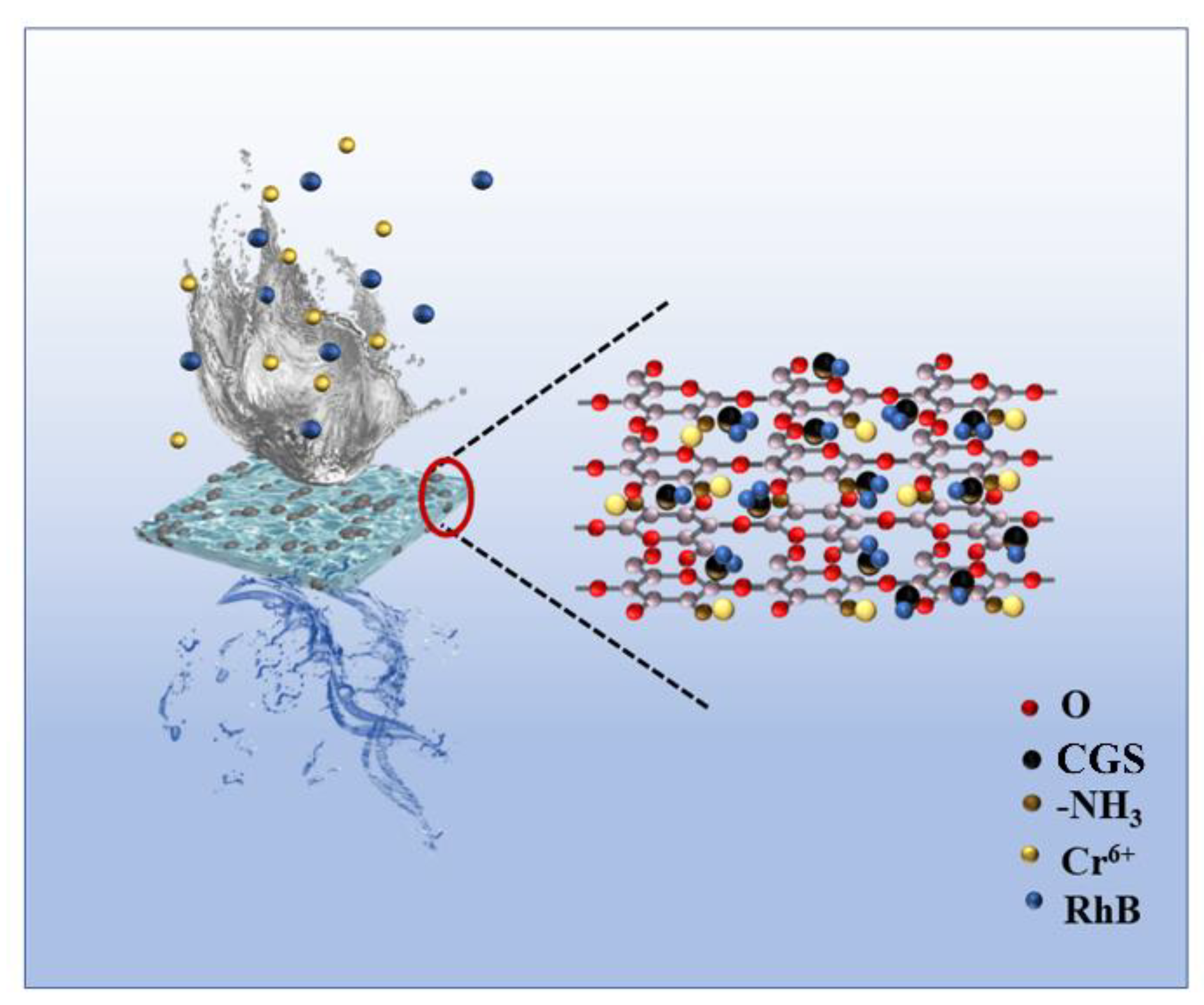
1. Introduction
2. Experimental Section
2.1. Experimental Materials
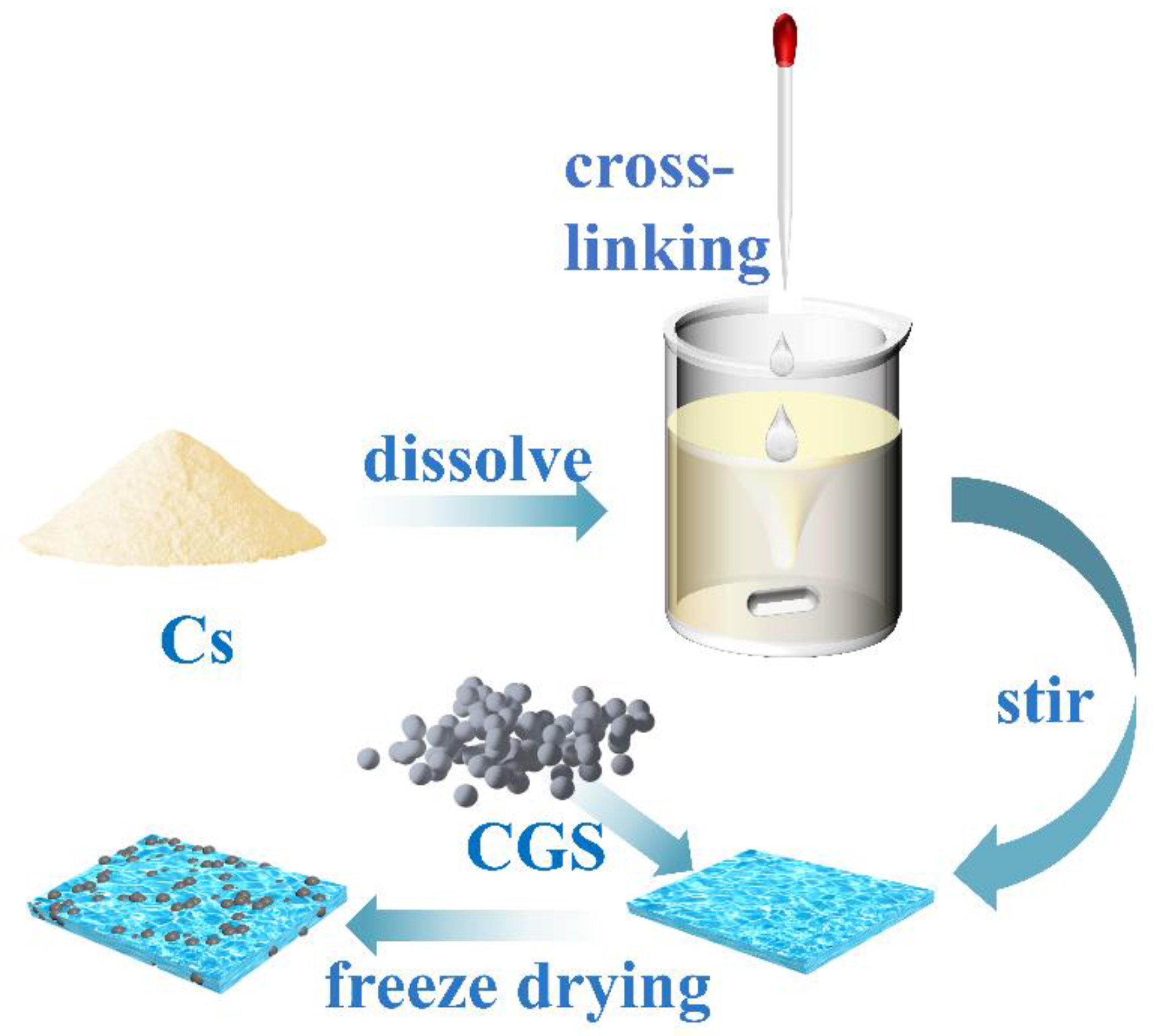
2.2. Preparation of Samples
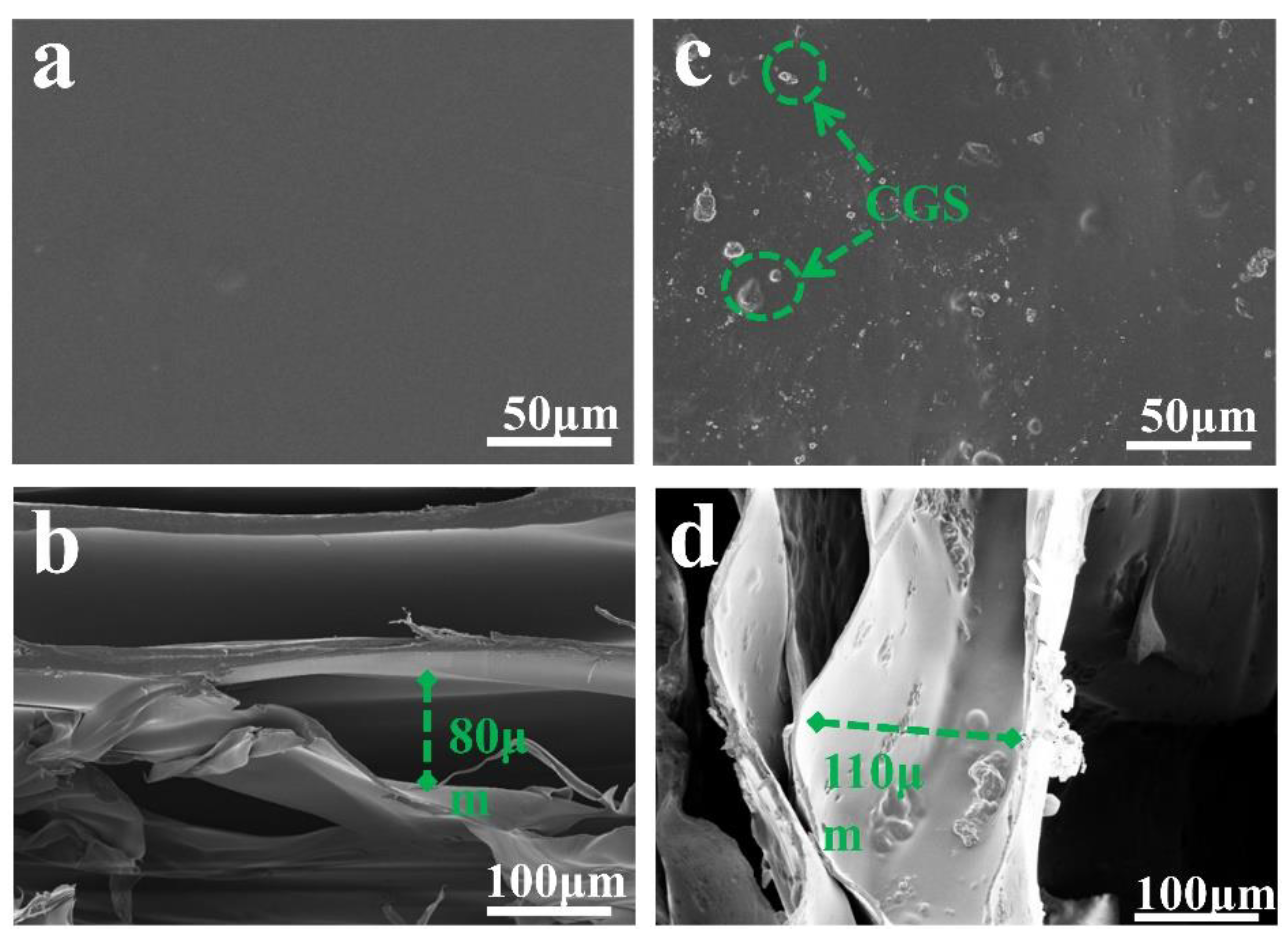
2.3. Morphological Characterization
2.4. RhB and Cr (VI) Adsorption Experiment
3. Results and Discussion
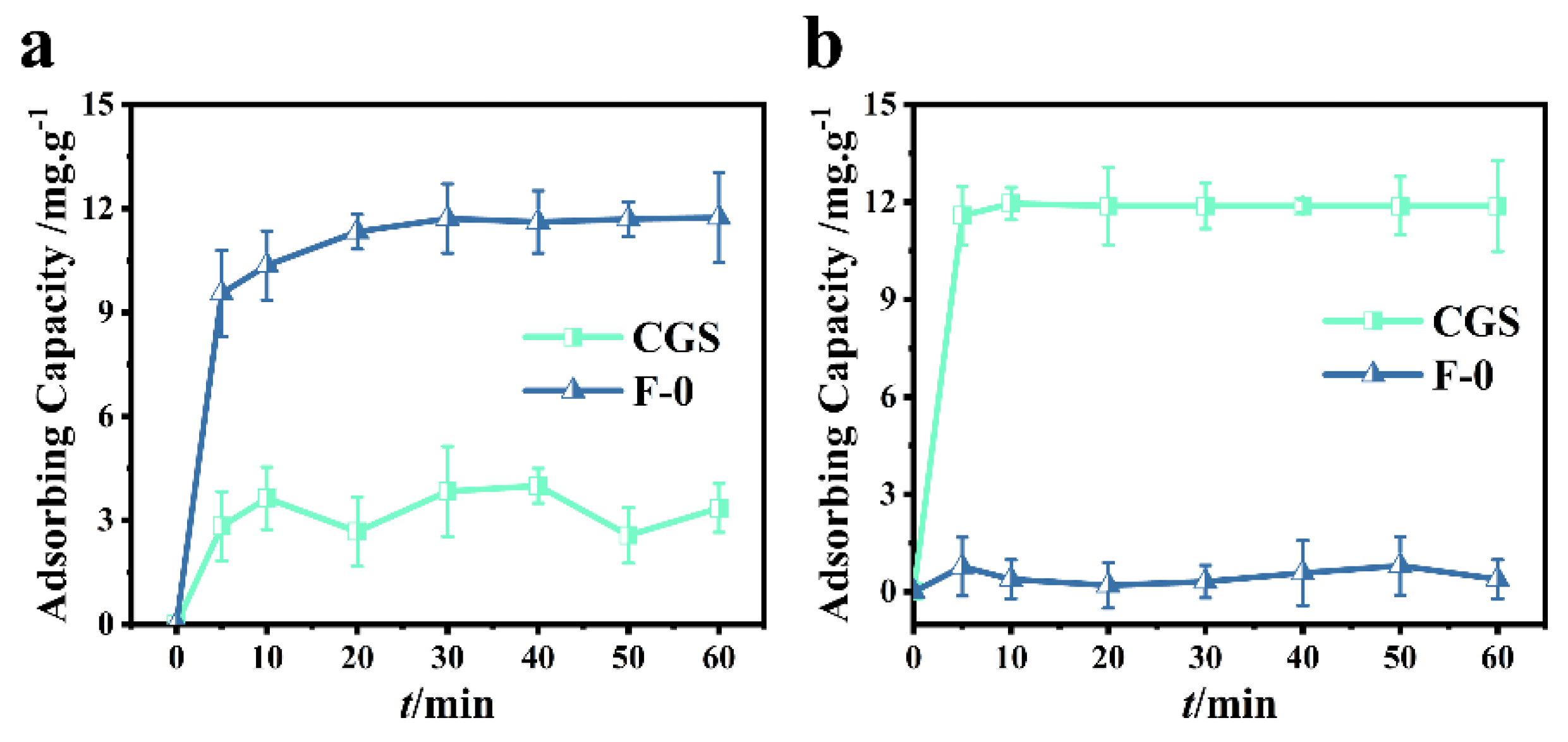
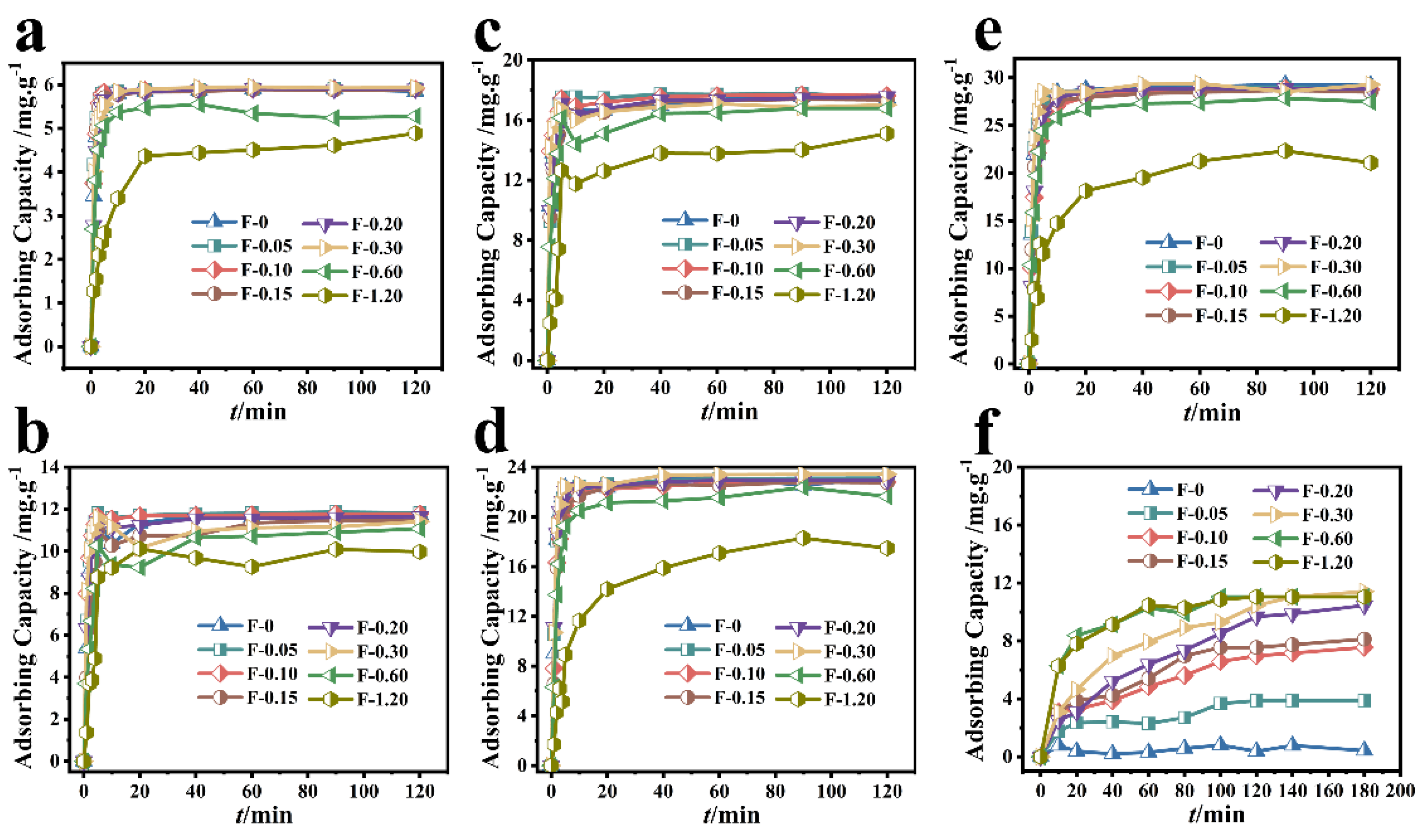
3.1. Adsorption Performance Analysis
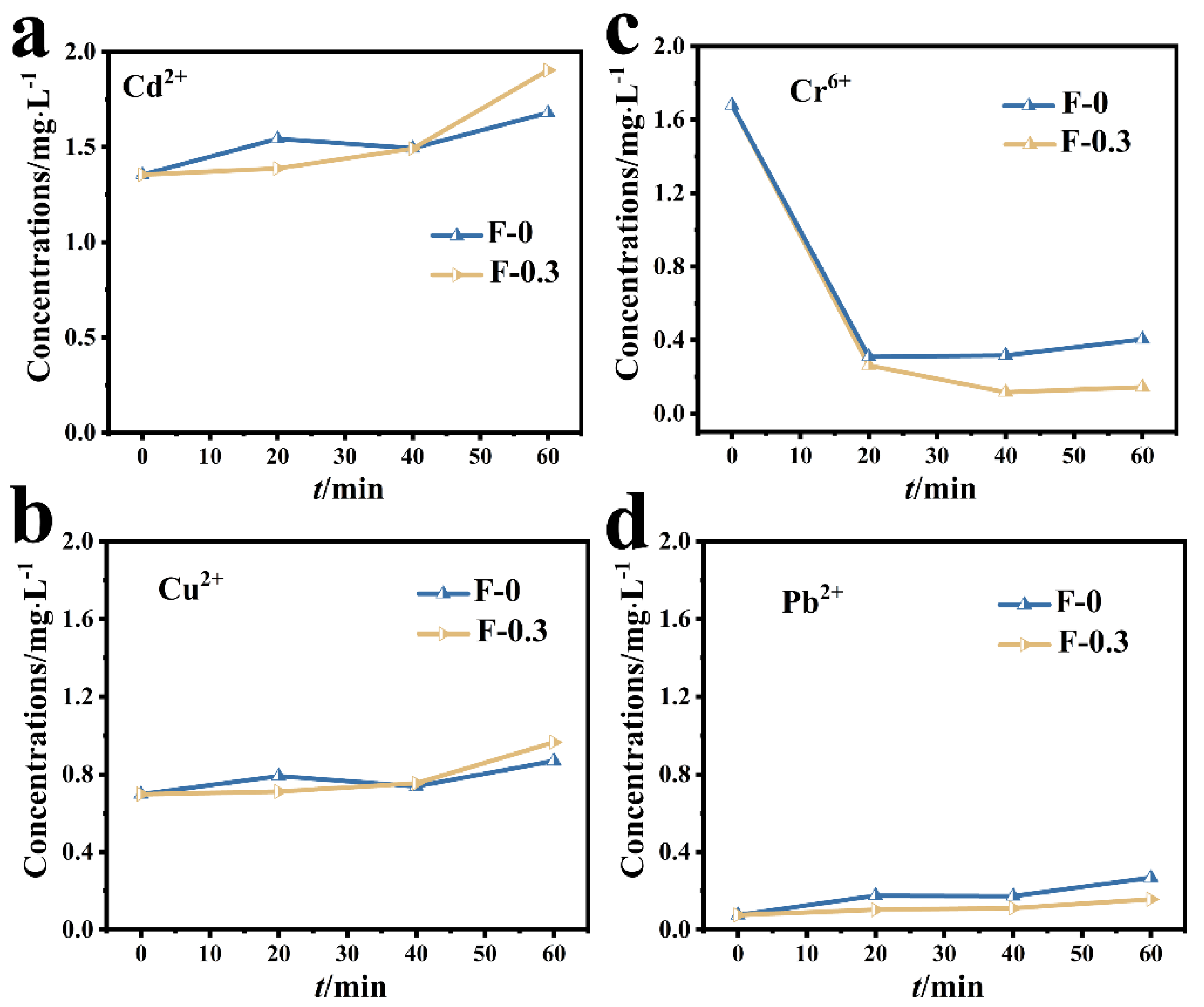
3.2. Adsorption of Cr (VI) in Mixed Metal Ions
3.3. Other Influencing Factors
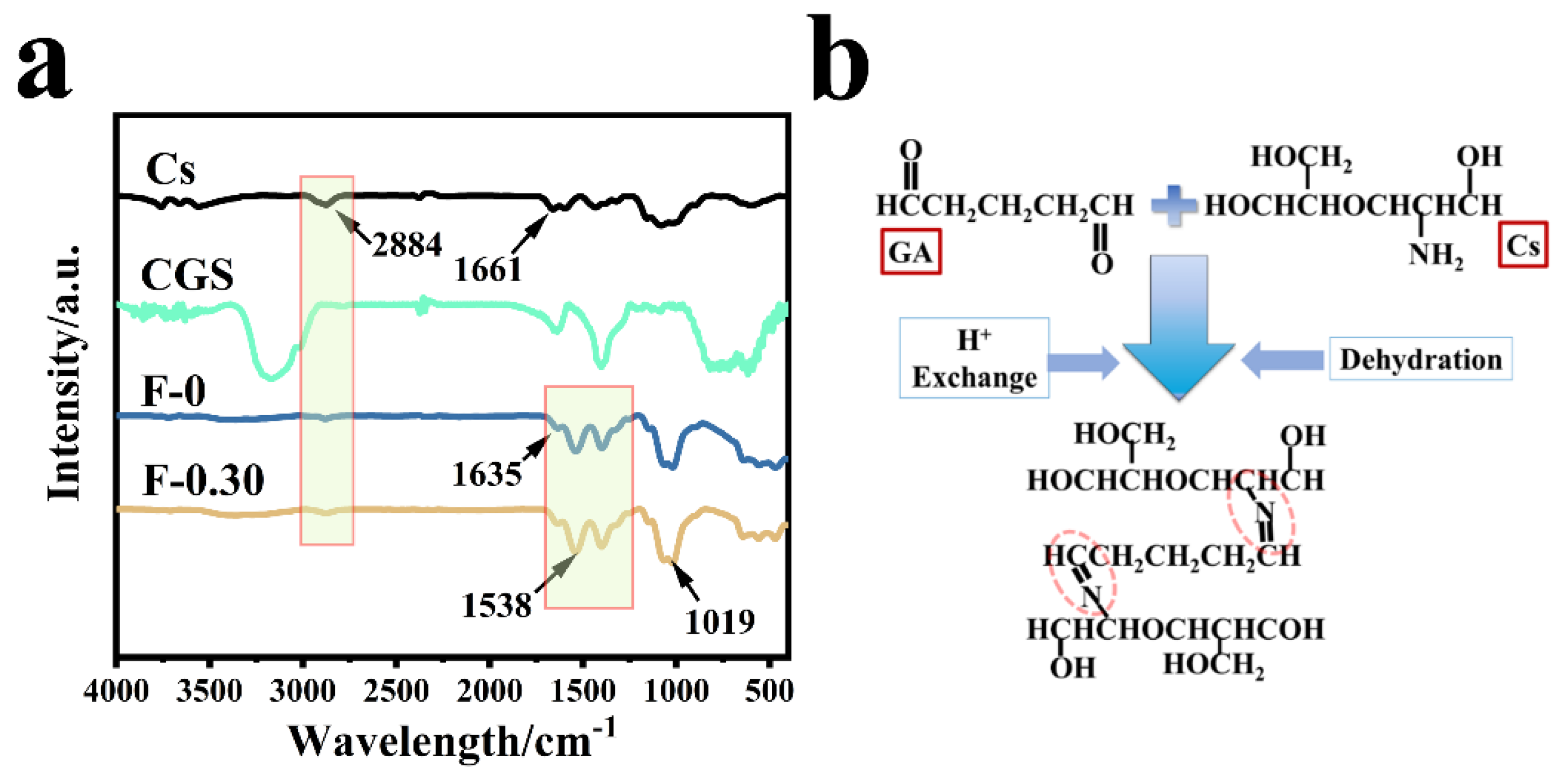
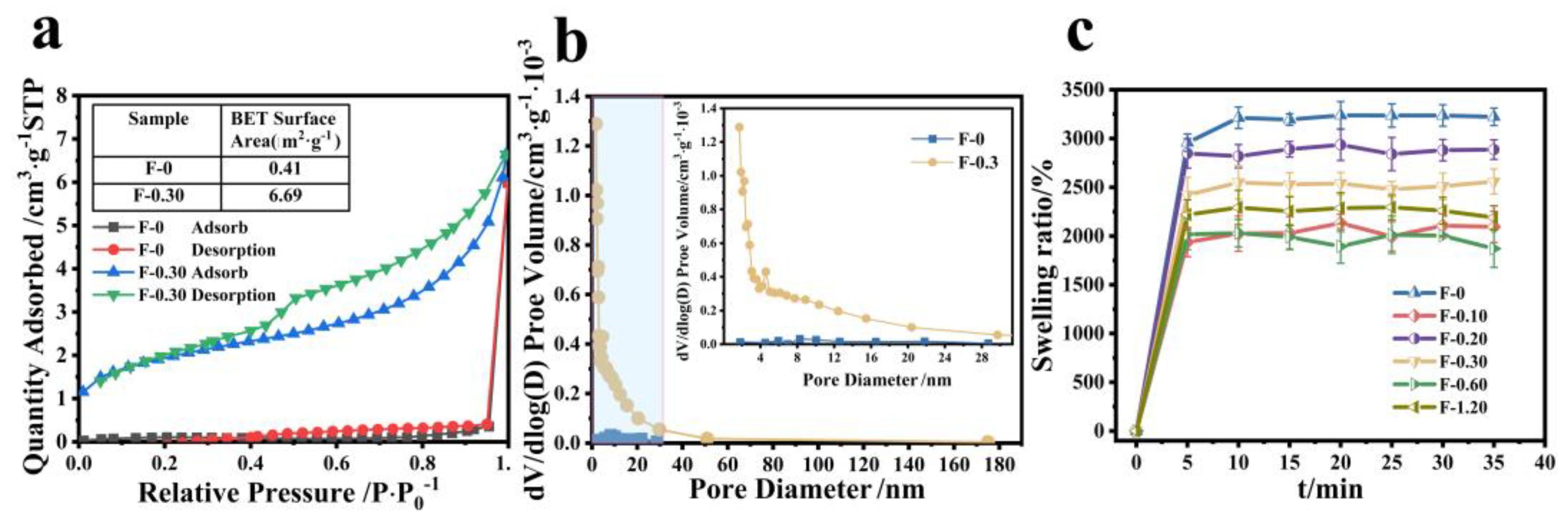
3.4. Analysis of the Relationship between Structure and Adsorption Property
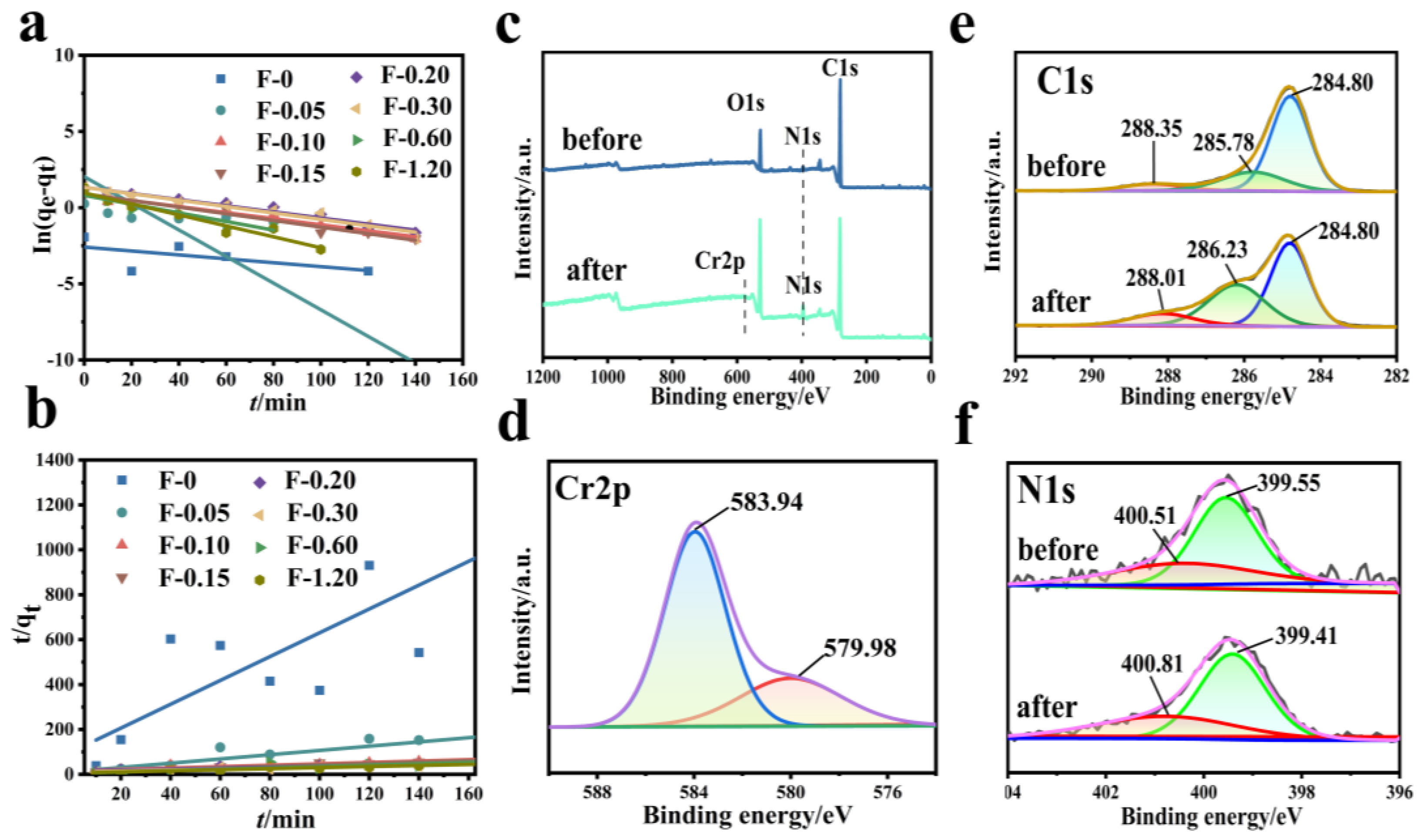
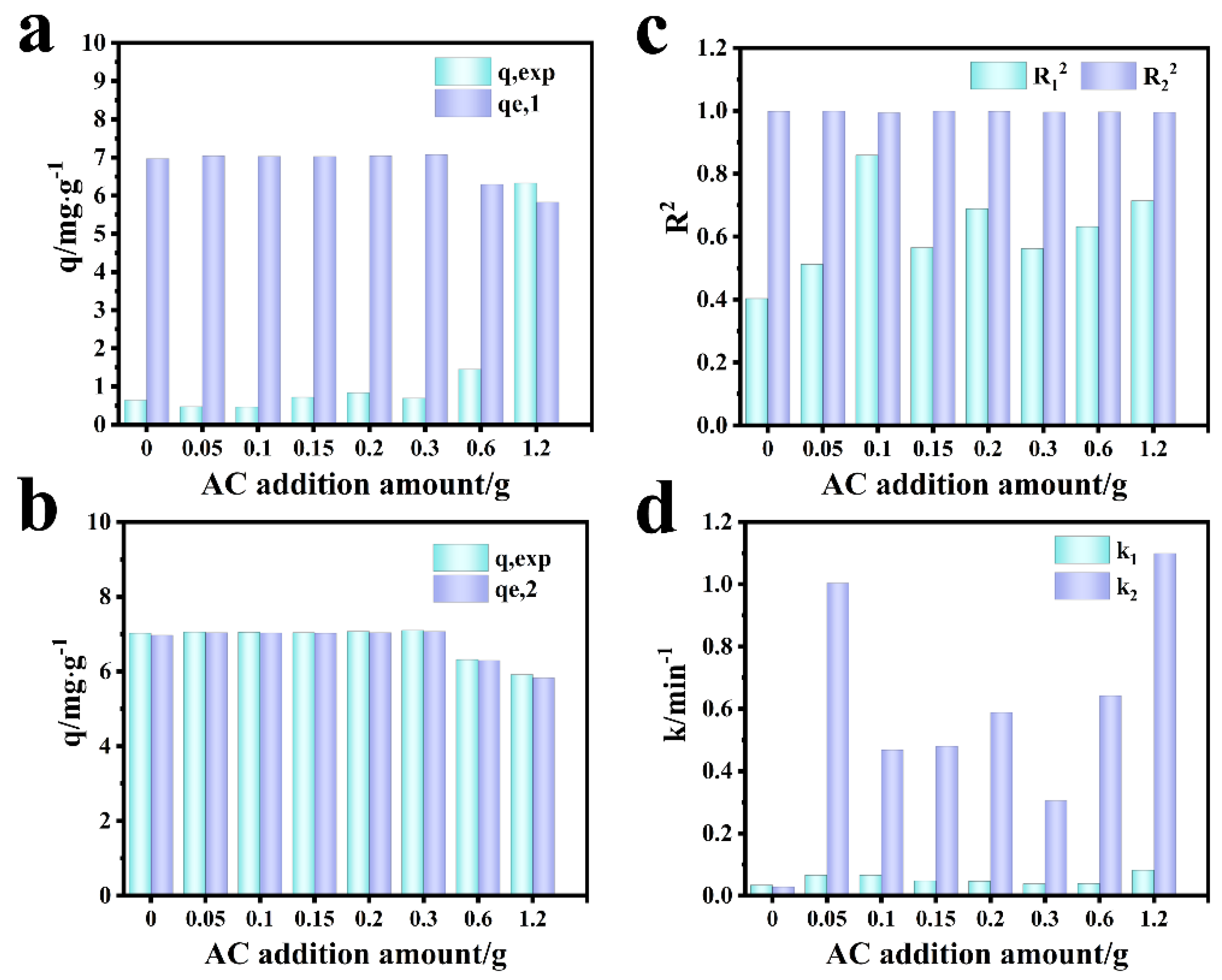
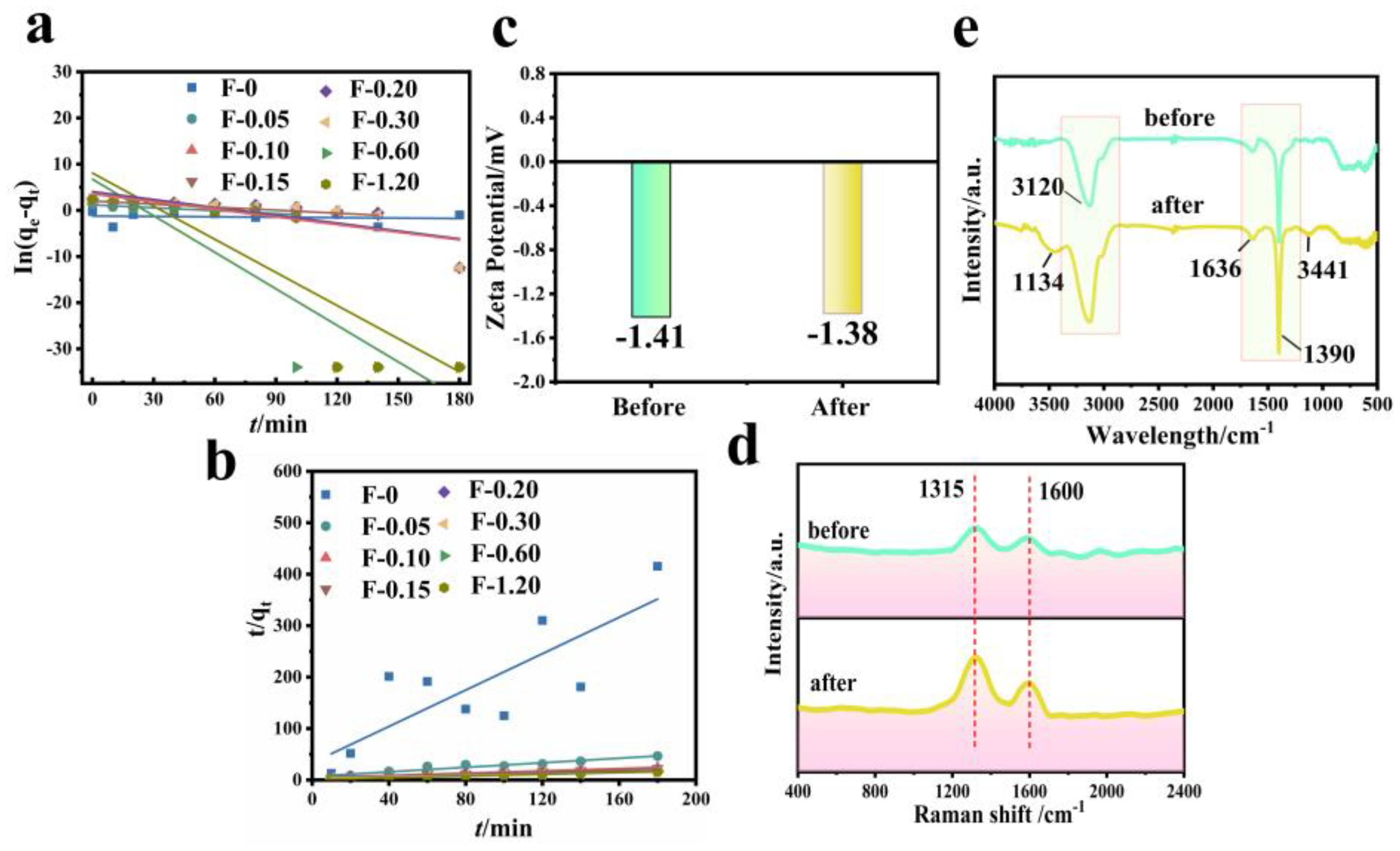
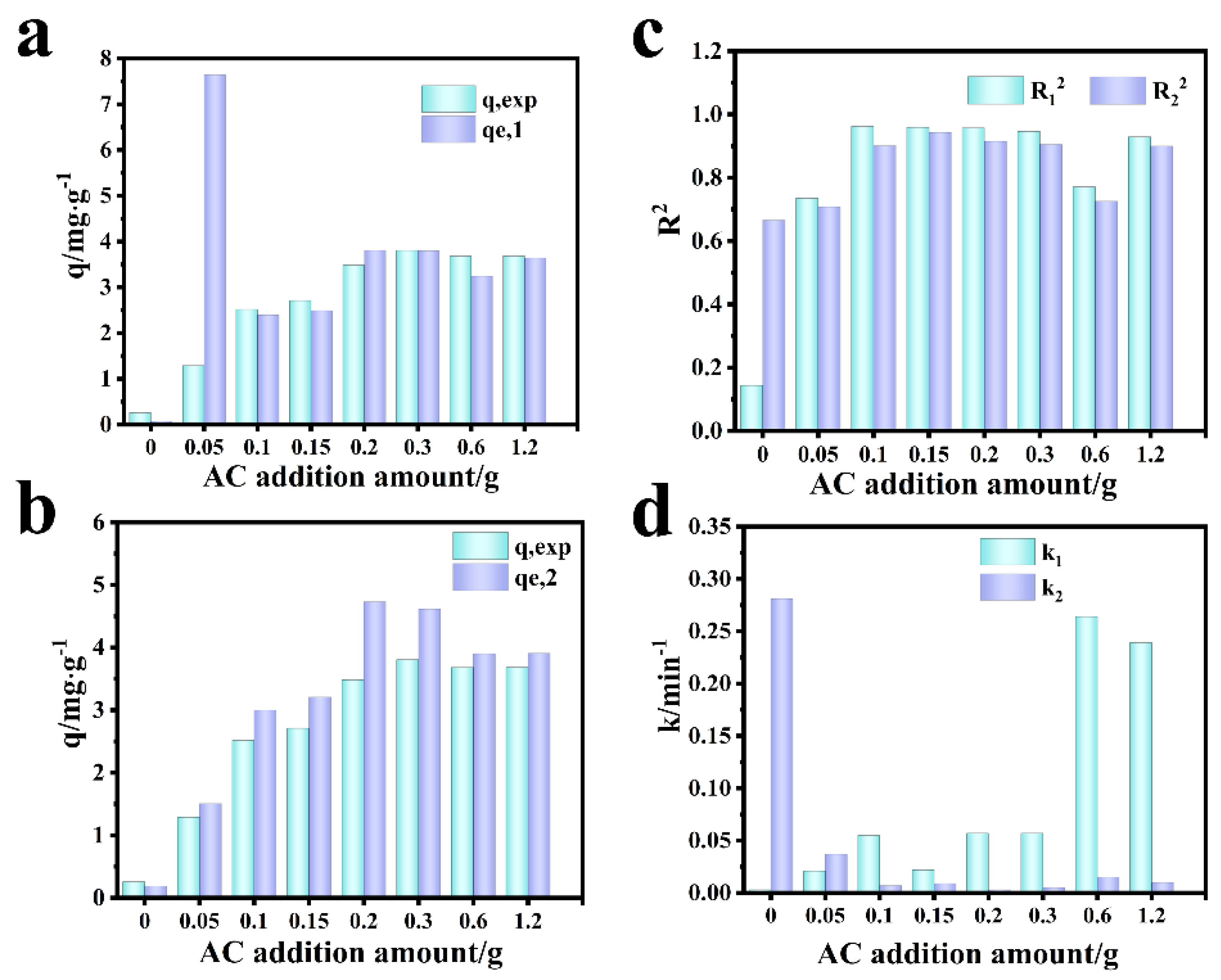
3.5. Adsorption Kinetics and Mechanism Analysis
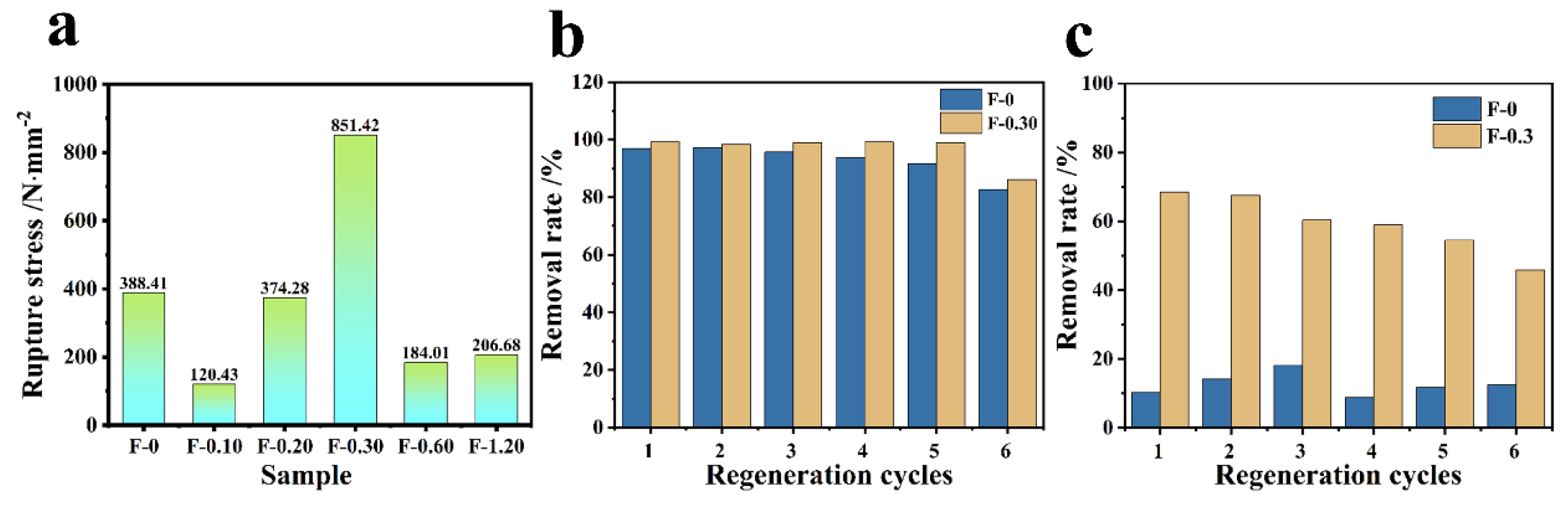
3.6. Mechanical Strength and Cyclic Performance Investigation
4. Review of Adsorption Mechanism and Comparison of Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1569. [Google Scholar] [CrossRef]
- Tu, Y.; Shao, G.; Zhang, W.; Chen, J.; Qu, Y.; Zhang, F.; Tian, S.; Zhou, Z.; Ren, Z. The degradation of printing and dyeing wastewater by manganese-based catalysts. Sci. Total Environ. 2022, 828, 154390. [Google Scholar] [CrossRef]
- Balea, A.; Monte, M.C.; Fuente, E.; Sanchez-Salvador, J.L.; Blanco, A.; Negro, C. Cellulose nanofibers and chitosan to remove flexographic inks from wastewaters. Environ. Sci. Water Res. Technol. 2019, 5, 1558–1567. [Google Scholar] [CrossRef]
- Zhang, M.H.; Chen, X.; Xu, H.L.; Dong, H. Advanced treatment of printing and dyeing wastewater by activated coke and thermal regeneration of spent activated coke. Desalination Publ. 2021, 229, 421–429. [Google Scholar] [CrossRef]
- Kumar, R.; Bhattacharya, S.; Sharma, P. Novel insights into adsorption of heavy metal ions using magnetic graphene composites. J. Environ. Chem. Eng. 2021, 9, 106212. [Google Scholar] [CrossRef]
- Al-Asad, H.A.; Parniske, J.; Qian, J.; Alex, J.; Ramaswami, S.; Kaetzl, K.; Morck, T. Development and application of a predictive model for advanced wastewater treatment by adsorption onto powdered activated carbon. Water Res. 2022, 217, 118427. [Google Scholar] [CrossRef]
- Shubhashish, S.; Amin, A.S.; Dang, Y.; Karasik, S.J.; Khanna, H.S.; Suib, S.L. Synthesis of highly porous metal oxide nanoparticles for adsorption applications. ACS Appl. Nano Mater. 2022, 5, 7078–7091. [Google Scholar] [CrossRef]
- Guo, J.; Fan, X.; Li, Y.; Yu, S.; Zhang, Y.; Wang, L.; Ren, X. Mechanism of selective gold adsorption on ion-imprinted chitosan resin modified by thiourea. J. Hazard. Mater. 2021, 415, 125617. [Google Scholar] [CrossRef]
- Li, X. Preparation of Graphene Oxide-Molecular Sieve Composite Adsorbent and its Adsorption Performance for Heavy Metals in Water. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 784, p. 012022. [Google Scholar]
- Bessa, A.; Henriques, B.; Goncalves, G.; Irurueta, G.; Pereira, E.; Marques, P.A. Marques. Graphene oxide/polyethyleneimine aerogel for high-performance mercury sorption from natural waters. Chem. Eng. J. 2020, 398, 125587. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Qiu, G.; Jia, W.; Guo, Y.; Guo, F.; Wu, J. Synthesis of Porous Material from Coal Gasification Fine Slag Residual Carbon and Its Application in Removal of Methylene Blue. Molecules 2021, 26, 6116. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Liu, K.; Wu, Y.; Ai, J.; Zhang, J. Preparation of Li4SiO4-based adsorbents with coal slag for high temperature cyclic CO2 capture. Fuel 2021, 301, 121687. [Google Scholar] [CrossRef]
- Yuan, N.; Tan, K.; Zhang, X.; Zhao, A.; Guo, R. Synthesis and adsorption performance of ultra-low silica-to-alumina ratio and hierarchical porous ZSM-5 zeolites prepared from coal gasification fine slag. Chemosphere 2022, 303, 134839. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Guo, F.; Shu, R.; Dong, K.; Qiao, Q.; Liu, S.; Xu, L.; Bai, Y. Evaluation of the high metals-containing coal gasification fine clag as a high-Performance adsorbent for malachite green adsorption. Waste Biomass Valorization 2022, 116, 01831. [Google Scholar]
- Ma, X.; Li, Y.; Xu, D.; Tian, H.; Yang, H. Simultaneous adsorption of ammonia and phosphate using ferric sulfate modified carbon/zeolite composite from coal coal gasification slag. J. Environ. Manag. 2022, 305, 114404. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 21, 175191. [Google Scholar]
- Kwok, K.; Koong, L.F.; Al Ansari, T.; McKay, G. Adsorption/desorption of arsenite and arsenate on chitosan and nanochitosan. Environ. Sci. Pollut. Res. 2018, 25, 14734–14742. [Google Scholar] [CrossRef]
- Akanno, A.; Guzmán, E.; Ortega, F.; Rubio, R.G. Behavior of the water/vapor interface of chitosan solutions with an anionic surfactant: Effect of polymer–surfactant interactions. Phys. Chem. Chem. Phys. 2020, 22, 152–173. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Meng, J.; Xin, Z.; Gong, H.; Li, J. Polydopamine-assisted grafting of chitosan on porous poly (L-lactic acid) electrospun membranes for adsorption of heavy metal ions. Int. J. Biol. Macromol. 2020, 167, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.L.; Wang, X.H.; Wang, G.H.; Hao, C.; Li, X.; Li, T.H. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metalions. Compos. B. Eng. 2019, 169, 45–54. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Jia, Z.; Ma, H.; Liu, C.; Liu, Z.; Shi, Q.; Ren, B.; Li, L.; Zhang, X.; et al. Fabrication of graphene oxide/polydopamine adsorptive membrane by stepwise in-situ growth for removal of rhodamine B from water. Desalination 2021, 516, 115220. [Google Scholar] [CrossRef]
- Zhu, D.; Shi, L.; Li, H.; Zhang, J.; Zhang, J.; Wei, C. Study of the synthesis, adsorption property, and photocatalytic activity of TiO2/coal gasification fine slag mesoporous silica glass microsphere composite. Environ. Sci. Pollut. Res. Int. 2022, 22, 22798. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.W.; Zhang, K.N.; Zou, Z.J.; Lü, Q. High specific area activated carbon derived from chitosan hydrogel coated tea saponin: One-step preparation and efficient removal of methylene blue. J. Environ. Chem. Eng. 2021, 9, 105251. [Google Scholar] [CrossRef]
- Jia, W.; Du, J.; Jiang, M.; Zhang, M.; Han, E.; Niu, H.; Wu, D. Preparation and Cr (VI) adsorption of functionalized polyimide fibers. J. Appl. Polym. Sci. 2022, 139, 52799. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Truong, T.T.; Pham, T.T.; Truong, T.T.T.; Pham, T.D. Synthesis, characterization of novel ZnO/CuO nanoparticles, and the applications in photocatalytic performance for rhodamine B dye degradation. Environ. Sci. Pollut. Res. Int. 2021, 29, 22576–22588. [Google Scholar] [CrossRef]
- Gharbani, P.; Mehrizad, A. Preparation and characterization of graphitic carbon nitrides/polyvinylidene fluoride adsorptive membrane modified with chitosan for Rhodamine B dye removal from water: Adsorption isotherms, kinetics and thermodynamics. Carbohydr. Polym. 2022, 277, 118860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, H.; Xue, P.; Tian, S.; Sun, S.; Lv, X. Highly-efficient PVDF adsorptive membrane filtration based on chitosan@CNTs-COOH simultaneous removal of anionic and cationic dyes. Carbohydr. Polym. 2021, 274, 118664. [Google Scholar] [CrossRef] [PubMed]
- Kirisenage, P.M.; Zulqarnain, S.M.; Myers, J.L.; Fahlman, B.D.; Mueller, A.; Marquez, I. Development of Adsorptive Membranes for Selective Removal of Contaminants in Water. Polymers 2022, 14, 3146. [Google Scholar] [CrossRef]
- Park, J.E.; Shin, J.H.; Oh, W.; Choi, S.J.; Kim, J.; Kim, C.; Jeon, J. Removal of Hexavalent Cr(VI) from Wastewater Using Chitosan-Coated Iron Oxide Nanocomposite Membranes. Toxics 2022, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Queirós, J.M.; Salazar, H.; Valverde, A.; Botelho, G.; de Luis, R.F.; Teixeira, J.; Martins, P.M.; Lanceros-Mendez, S. Reusable composite membranes for highly efficient chromium removal from real water matrixes. Chemosphere 2022, 307, 135922. [Google Scholar] [CrossRef] [PubMed]

| Sample | Initial Concentration (mg/L) | Adsorbing Capacity (mg/g) | |
|---|---|---|---|
| 2 min | End | ||
| F-0.3 | 20 | 5.12 | 5.85 |
| 40 | 10.06 | 11.78 | |
| 60 | 15.41 | 17.66 | |
| 80 | 19.32 | 23.45 | |
| 100 | 24.03 | 29.31 | |
| F-0 | 20 | 4.87 | 5.85 |
| 40 | 9.05 | 11.46 | |
| 60 | 13.82 | 17.51 | |
| 80 | 18.22 | 23.16 | |
| 100 | 21.96 | 29.13 | |
| Author | Membrane Materials | Contaminants | Removal Rate (%) | Adsorption Amount (mg/g) | Literature |
|---|---|---|---|---|---|
| Gharbani | CS, graphite carbon nitride/polyvinylidene difluoride | RhB | 72.7 (2 mg/L, pH = 3, and 2.0 g g-C3N4) | 4.16 | [28] |
| Zhao | PVDF, CS, CNTs-COOH | RhB | 99.0 (10 mg/L, pH = 2, dosage 0.5 g) | 2.0 | [29] |
| Kirisenage | acrylate polymer nanostructured graphitic carbon | As | 1.5 | [30] | |
| NH4+ | 0.27 | ||||
| Park | chitosan-coated iron oxide nanoparticles | Cr (VI) | 14.45 (intermittent system) | [31] | |
| 14.1 (continuous inflow system) | |||||
| Queirós | Al(OH)3/PVDF-HFP MIL-88-B(Fe)/PVDF-HFP UiO-66-NH2/PVDF-HPP | Cr (VI) | 12 | 5.0 | [32] |
| 62 | 3.0 | ||||
| 97 (5 mg/L) | 3.0 | ||||
| This article | CS/CGS | Cr (VI) | 97.7 (100 mg/L, 60 mL, Adsorbent (0.2 g)) | 50.0 | This article |
| RhB | 96.2 (40 mg/L, 60 mL, 0.2g) | 11.5 (40 mg/L, 60 mL, 0.2 g) | This article |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, D.; Zhang, S.; Wang, K.; Dong, T.; Zhang, M.; Dong, G. Preparation of a Chitosan/Coal Gasification Slag Composite Membrane and Its Adsorption and Removal of Cr (VI) and RhB in Water. Molecules 2022, 27, 7173. https://doi.org/10.3390/molecules27217173
Peng D, Zhang S, Wang K, Dong T, Zhang M, Dong G. Preparation of a Chitosan/Coal Gasification Slag Composite Membrane and Its Adsorption and Removal of Cr (VI) and RhB in Water. Molecules. 2022; 27(21):7173. https://doi.org/10.3390/molecules27217173
Chicago/Turabian StylePeng, Deqiang, Shuyun Zhang, Kai Wang, Tingting Dong, Min Zhang, and Guohui Dong. 2022. "Preparation of a Chitosan/Coal Gasification Slag Composite Membrane and Its Adsorption and Removal of Cr (VI) and RhB in Water" Molecules 27, no. 21: 7173. https://doi.org/10.3390/molecules27217173
APA StylePeng, D., Zhang, S., Wang, K., Dong, T., Zhang, M., & Dong, G. (2022). Preparation of a Chitosan/Coal Gasification Slag Composite Membrane and Its Adsorption and Removal of Cr (VI) and RhB in Water. Molecules, 27(21), 7173. https://doi.org/10.3390/molecules27217173






