Sources, Transformations, Syntheses, and Bioactivities of Monoterpene Pyridine Alkaloids and Cyclopenta[c]pyridine Derivatives
Abstract
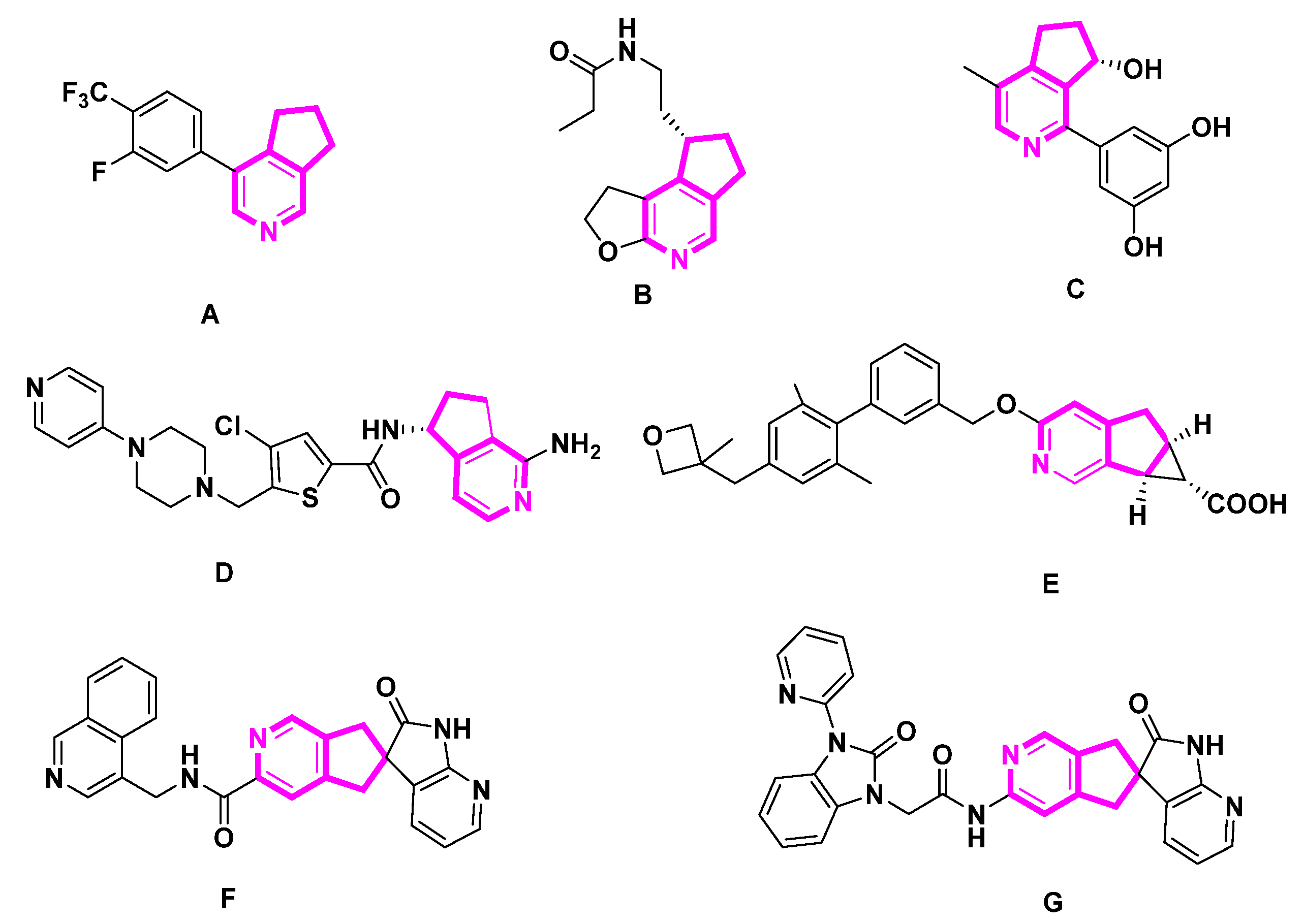
1. Introduction
2. MTPAs and Their Activities
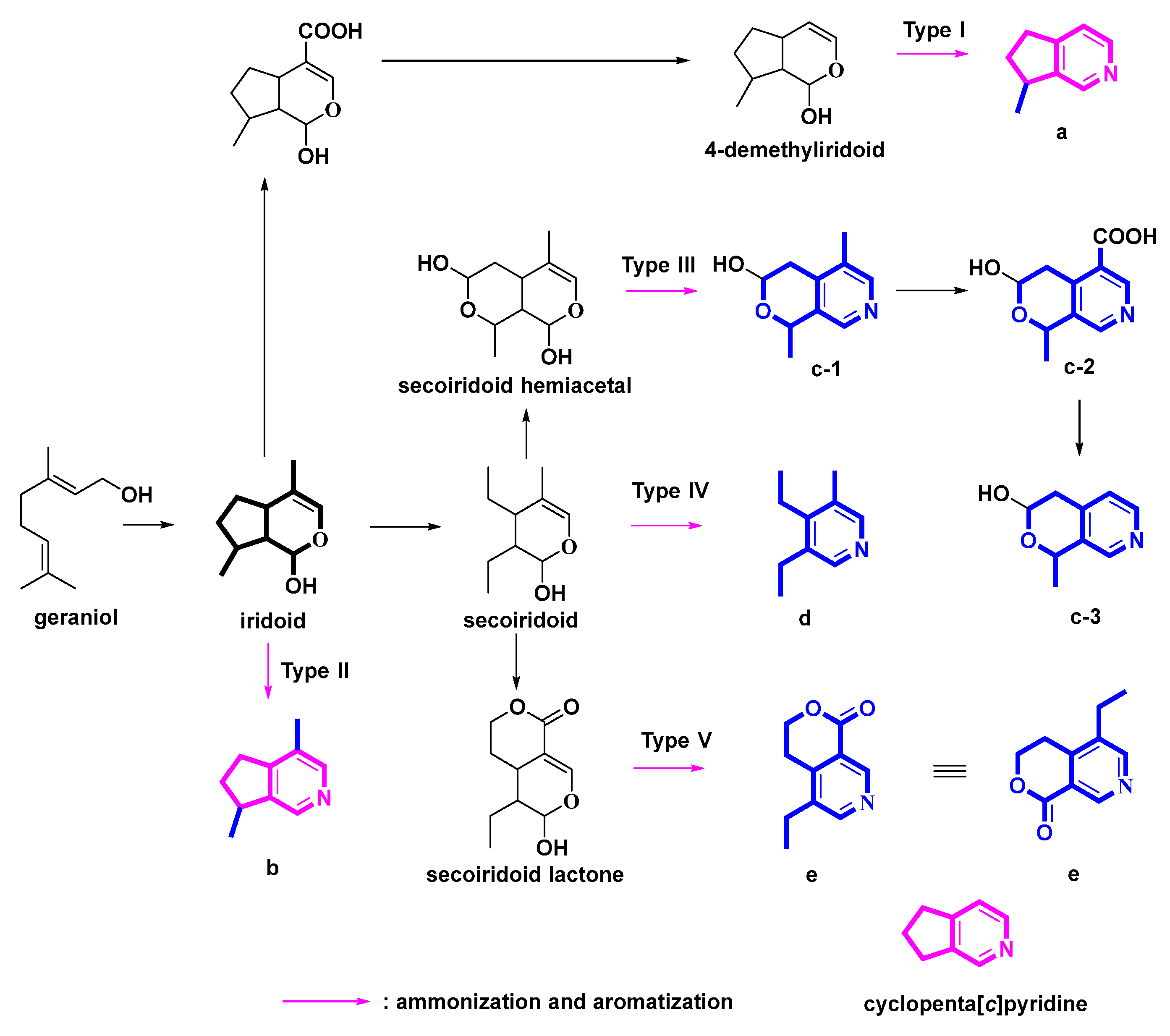
2.1. Pyridine Alkaloids Derived from 4-Demethyliridoids (Figure 2, Table 1, Type I)
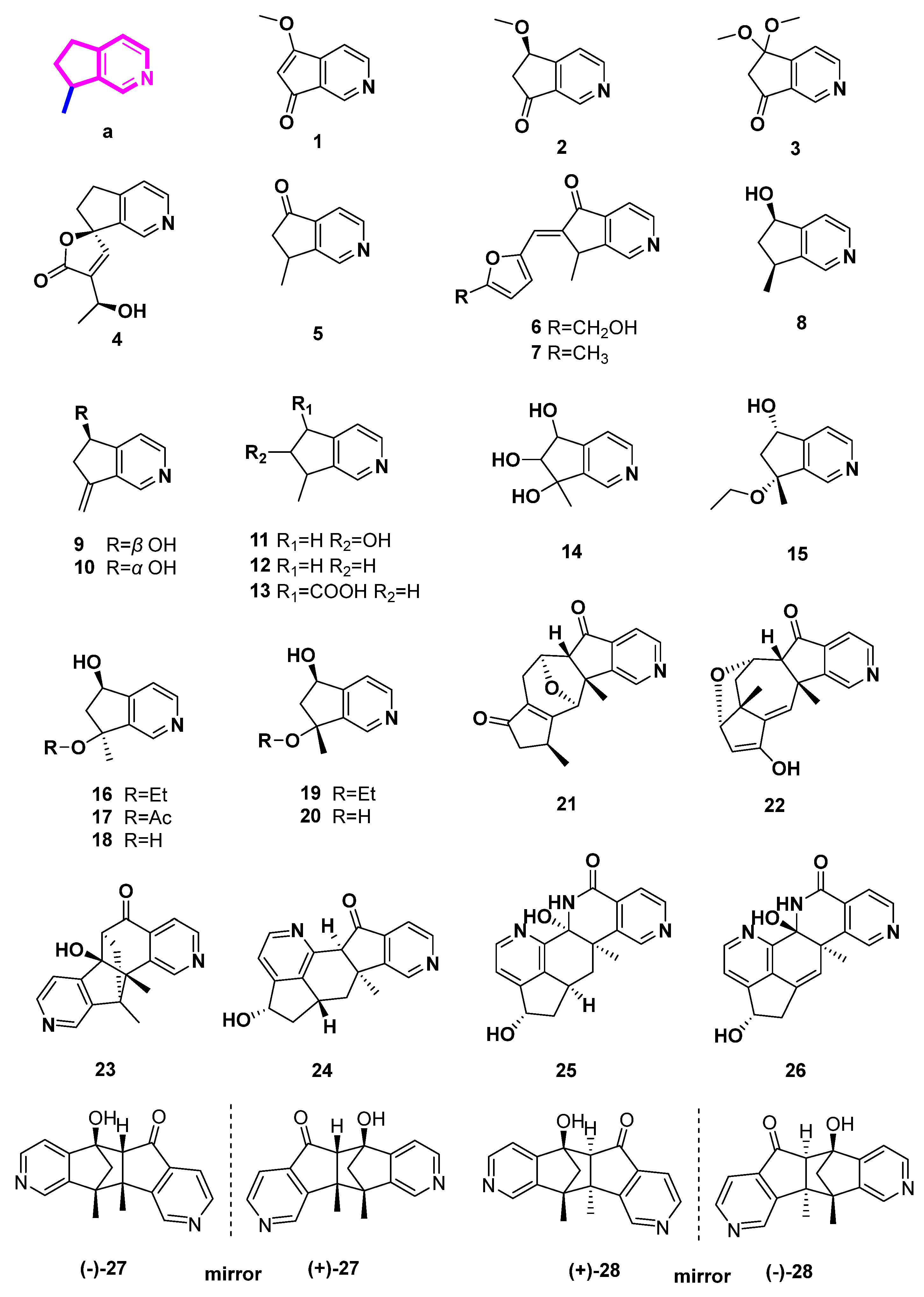
| Number | Compound Name | Source | Reference |
|---|---|---|---|
| 1 | scrophularianine A | Scrophularia ningpoensis | [10] |
| 2 | scrophularianine B | Scrophularia ningpoensis | [10] |
| 3 | scrophularianine C | Scrophularia ningpoensis | [10] |
| 4 | plumerianine | Plumeria acutifolia | [11] |
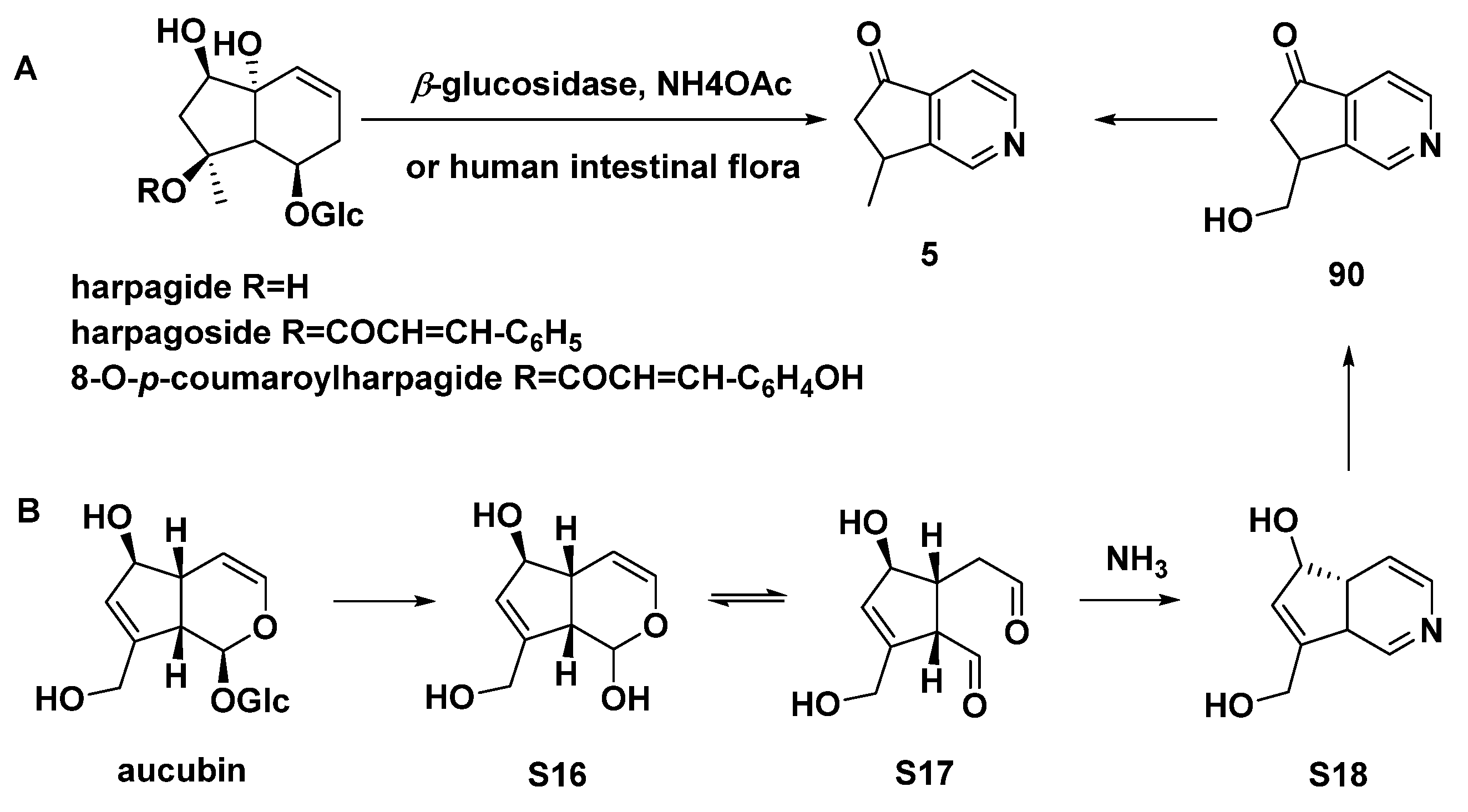
| 5 | aucubinine B | Harpagophytum procumbens, Ceolospermum billardieri, Caryopteris mongolica Bunge with basification of NH4OH | [12,13] |
| 6 | beatrine A | Harpagophytum procumbens | [12] |
| 7 | beatrine B | Harpagophytum procumbens | [12] |
| 8 | coelobillardine | Ceolospermum billardieri | [13] |
| 9 | dehydro 7-8 coelobillardierine | Ceolospermum billardieri | [13] |
| 10 | (S)-7-Methylene-6,7-dihydro-5H-cyclopenta[c]pyridine-5-ol | Caryopteris glutinosa with basification of NH4OH | [16] |
| 11 | cantleyine I | Castilleja miniata | [14] |
| 12 | 4-noractinidine | Penstemon whippleanus | [14] |
| 13 | pedicularine | Penstemon whippleanus | [14] |
| 14 | salviadiginine A | Salvia digitaloids | [15] |
| 15 | (5S*,7R*)-7-ethoxy-6,7-dihydro-7-methyl-5H-cyclopenta[c]pyridin-5-ol | Caryopteris mongolica Bunge with basification of NH4OH | [17] |
| 16 | caryopterisines F | Caryopteris glutinosa with basification of NH4OH | [16] |
| 17 | caryopterisines G | Caryopteris glutinosa with basification of NH4OH | [16] |
| 18 | caryopterisines H | Caryopteris glutinosa with basification of NH4OH | [16] |
| 19 | caryopterisines I | Caryopteris glutinosa with basification of NH4OH | [16] |
| 20 | Oxerine | Caryopteris glutinosa with basification of NH4OH | [16] |
| 21 | (5aR*,6S*,10S*,11R*,11aR*)-10,11a-dimethyl-6,7,9,10,11,11a-hexahydro-5H-6,11-epoxycyclopenta [6,7]azuleno[1,2-c]pyridin-5,8(5aH)-dione | Caryopteris mongolica Bunge with basification of NH4OH | [17] |
| 22 | (5aR*,6S*,7aR*,8S*,11aR*)-10-hydroxy-7a,11a-dimethyl-5a,6,7,7a,8,11a-hexahydro-5H-6,8-epoxycyclopenta[6,7]azuleno[1,2-c]pyridin-5-one | Caryopteris mongolica Bunge with basification of NH4OH | [17] |
| 23 | (6S*,6aR*,11R*,11aS*)-6a-hydroxy-11,11a-dimethyl-6,6a,11,11a-tetrahydro-5H-6,11-methanopyrido[3′,4′:4,5]cyclopenta[1,2-h]isoquinolin-5-one | Caryopteris mongolica Bunge with basification of NH4OH | [17] |
| 24 | caryopterisines C | Caryopteris glutinosa with basification of NH4OH | [16] |
| 25 | caryopterisines D | Caryopteris glutinosa with basification of NH4OH | [16] |
| 26 | caryopterisines E | Caryopteris glutinosa with basification of NH4OH | [16] |
| 27 |
(5R*,5aR*,10bS*,11R*)-5-hydroxy-10b,11-dimethyl-5,5a,10b,11-tetrahydro-6H-5,11-methanopyrido[3′,4′:3,4]cyclopenta[1,2-g]isoquinolin-6-one (±)-caryopterisines B |
Caryopteris mongolica
Bunge with basification of NH4OH Caryopteris glutinosa with basification of NH4OH | [17,18] |
| 28 | (±)-caryopterisines A | Caryopteris glutinosa with basification of NH4OH | [18] |
2.2. Pyridine Alkaloids Derived from Iridoids (Figure 3, Table 2, Type II)

| Number | Compound Name | Source | Reference |
|---|---|---|---|
| 29 | actinidine | Actinidia polygama; Valeriana officinalis | [19,21] |
| 30 | valerianine | Valeriana officinalis | [19] |
| 31 | trans- p-coumaroyl -9-cantleyine | Ceolospermum billardieri | [13] |
| 32 | cis- p-coumaroyl -9-cantleyine | Ceolospermum billardieri | [13] |
| 33 | deoxyrhexifolin | Castilleja rbexifolia; A purported hybrid of Castilleja rbexifolia and Castilleja miniata | [22] |
| 34 | rhexifoline |
A purported hybrid of Castilleja rbexifolia and Castilleja miniate; Transform of Penstemonoside | [22,23] |
| 35 | cantleyine II | Strychnos trinervis | [24] |
| 36 | euphrosine | Orthocarpus sp. | [25] |
| 37 | 10-acetoxy-actinidine | Argylia radiata | [26] |
| 38 | (+)-boscbniakine | Penstemon whippleanus | [14] |
| 39 | carbomethoxypedicularine | Penstemon whippleanus | [14] |
| 40 | a MTPA Dimer | transformation from geniposide | [27] |
| 41 | lindeniamine | transformation from Lindenia austro-caledonica Brongn | [28] |
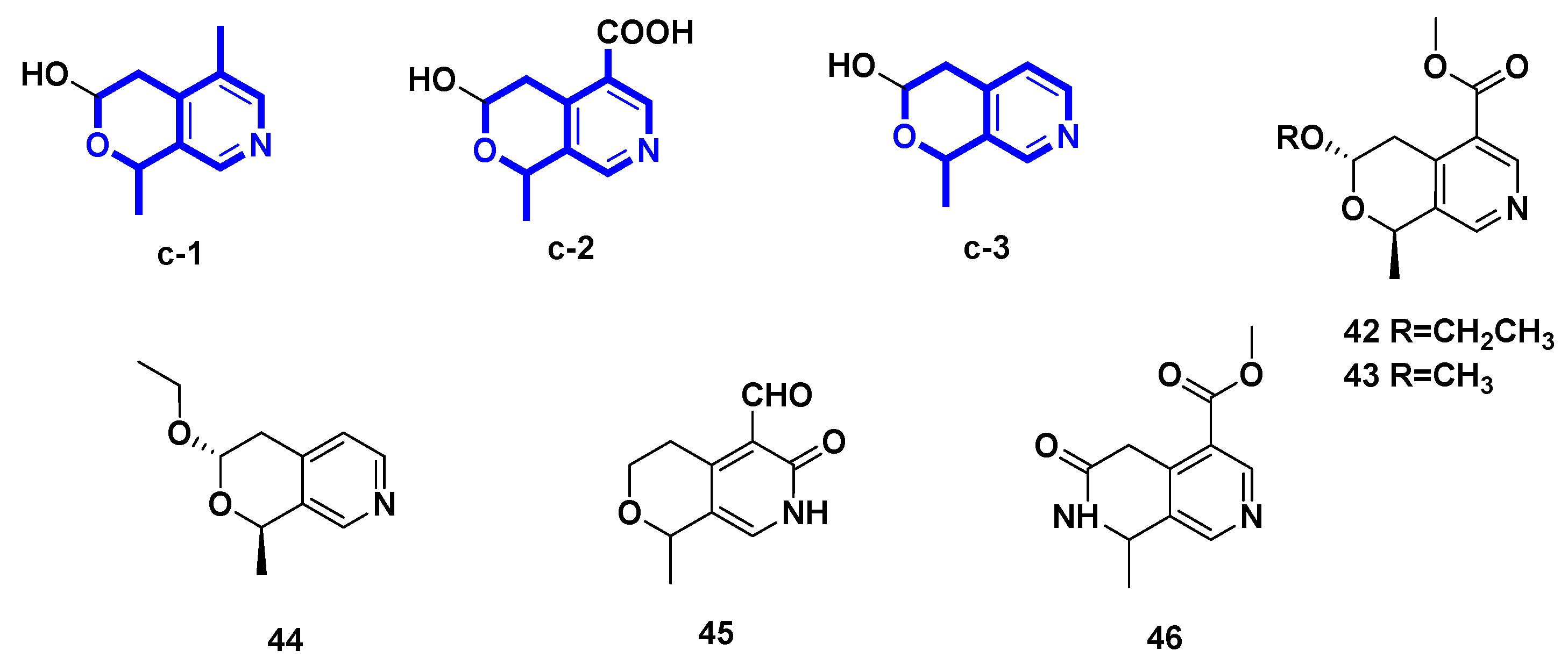
2.3. Pyridine Alkaloids Derived from Hemiacetal Secoiridoids (Figure 4, Table 3, Type III)
| Number | Compound Name | Source | Reference |
|---|---|---|---|
| 42 | (–)-vincapyridine A | Vinca major | [29] |
| 43 | (–)-vincapyridine B | Vinca major | [29] |
| 44 | (–)-vincapyridine C | Vinca major | [29] |
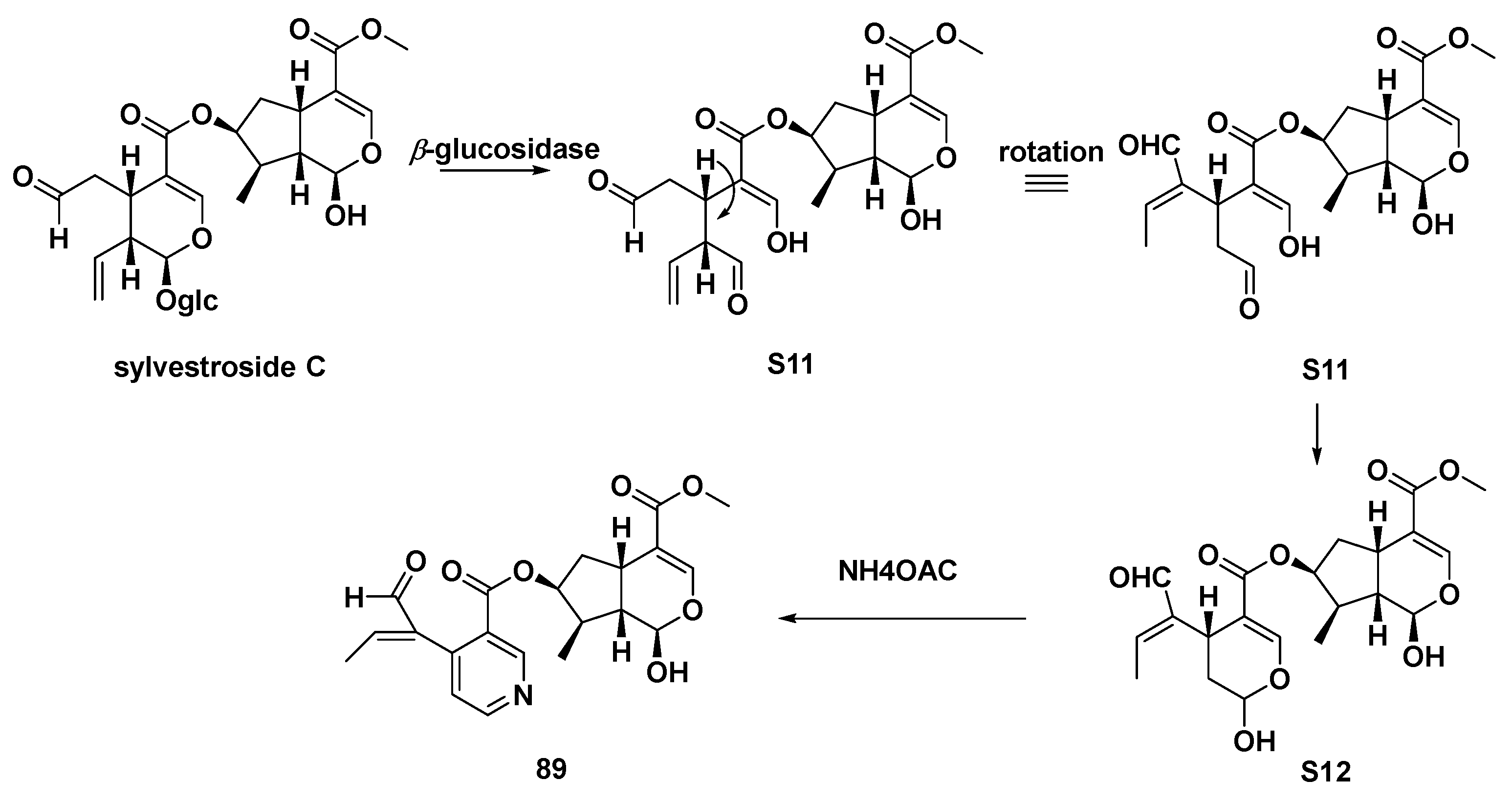
| 45 | gentianal | transformation of gentiopicroside | [30] |
| 46 | jasminin | transformation of secoiridoid glucosides from Ligustrum vulgare L. | [31] |
2.4. Pyridine Alkaloids Derived from Secoiridoids (Figure 5, Table 4, Type IV)
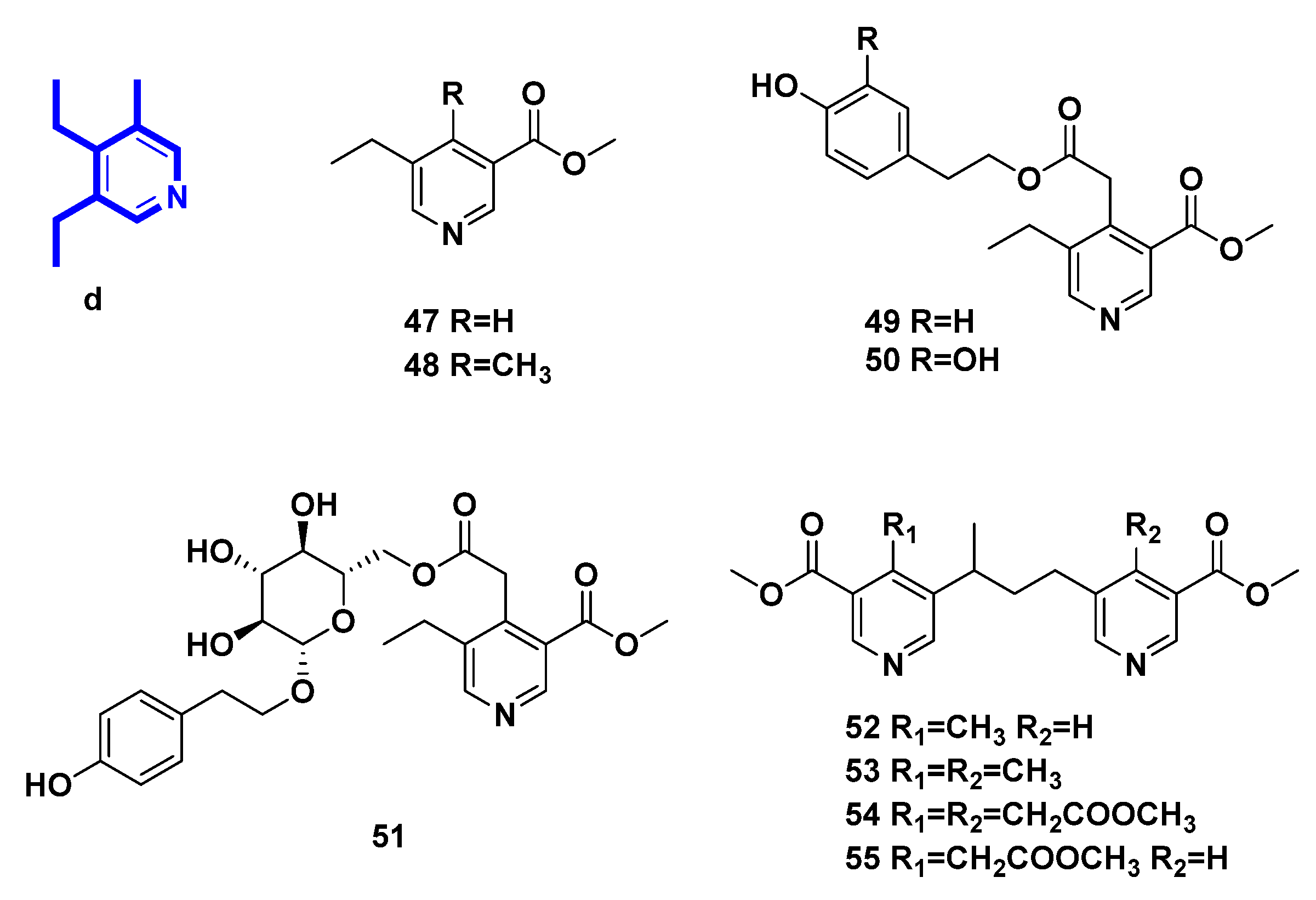
| Number | Compound Name | Source | Reference |
|---|---|---|---|
| 47 | methyl 5-ethylnicotinate | transformation of secoiridoid glucosides from Ligustrum vulgare L. | [31] |
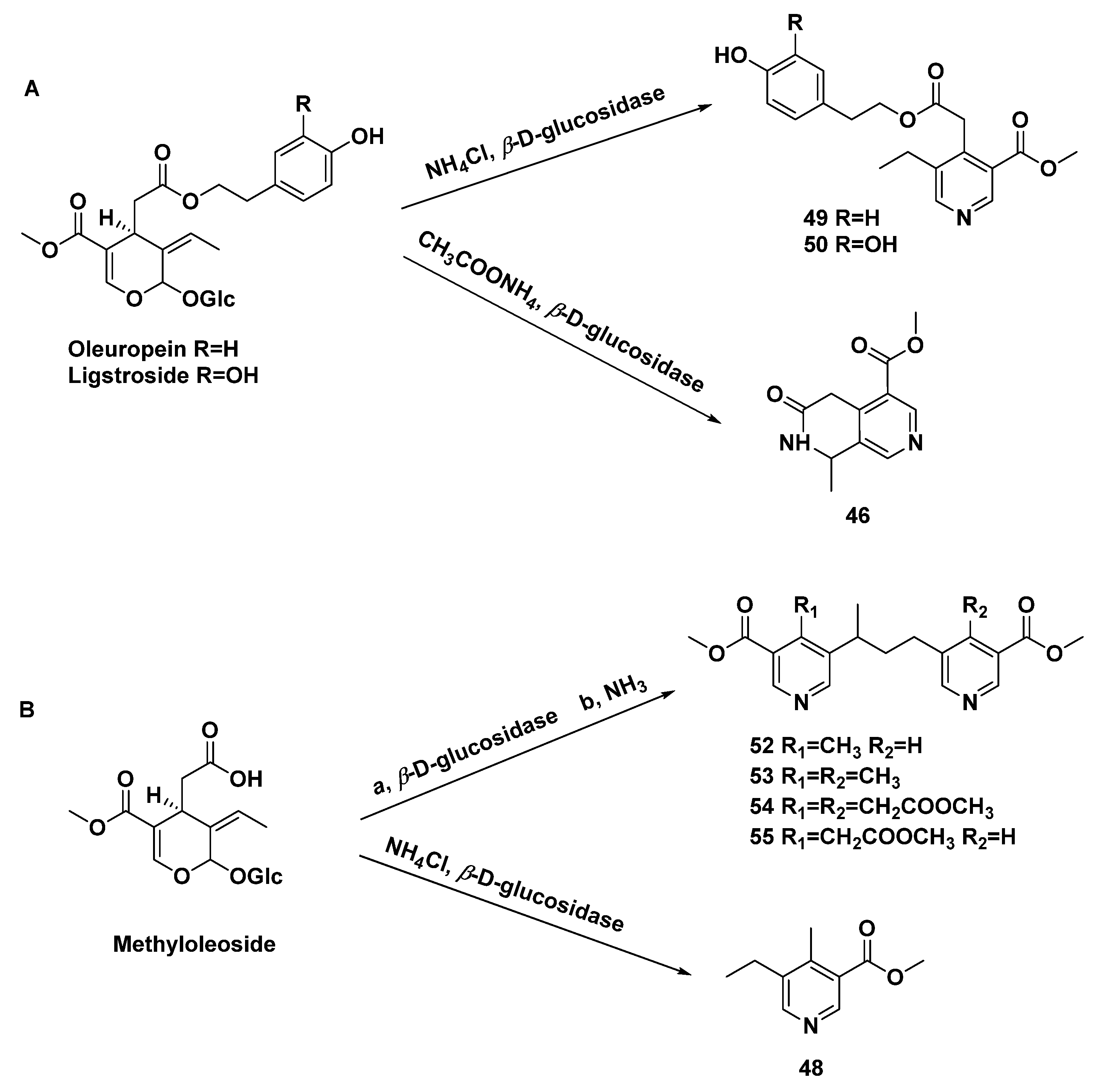
| 48 | methyI5-ethyl-4-methyl-nicotinate | transformation of secoiridoid glucosides from Ligustrum vulgare L. | [31,32,33] |
| 49 | p-hydroxy-β-phenethyI5-ethyl-3-methoxycarbonyl-4-pyridinylacetate | transformation of secoiridoid glucosides from Ligustrum vulgare L. | [31,32,33] |
| 50 | 3,4-dihydroxy-β-phenylethyl-5-ethyl-3-methoxycarbonyl-4-pyridinylacetate | transformation of secoiridoid glucosides | [33] |
| 51 | p-hydroxy-β-phenetyl-PD-glucopyranoside 6-(5-ethyl-3-methoxycarbonyl)-4-pyridineacetate | transformation of secoiridoid glucosides from Ligustrum vulgare L. | [32] |
| 52 | 4-methyl-5,5′-[(1-methyltrimethylen)di](methylnicotinate) | transformation of secoiridoid glucosides from Ligustrum vulgare L. | [33,34] |
| 53 | 4.4′-bis-methyl-5,5′-[(l-methyltrimethylen)di](methylnicotinate). | transformation of secoiridoid glucosides from Ligustrum vulgare L. | [33,34] |
| 54 | methyl 3,3′-bis-methoxycarbony1-5,5′-[(1-methyltrimethylene)di]-4,4′-bis-piridinylacetate | transformation of secoiridoid glucosides | [33] |
| 55 | methyl 5-[3-(3-methoxycarbonyI-5-pyridinyl)-l-methylpropyl]-3-methoxycarbonyl-4-pyridinylacetate | transformation of secoiridoid glucosides | [33] |
2.5. Pyridines Alkaloids Derived from Lactone Secoiridoids (Figure 6, Table 5, Type V)
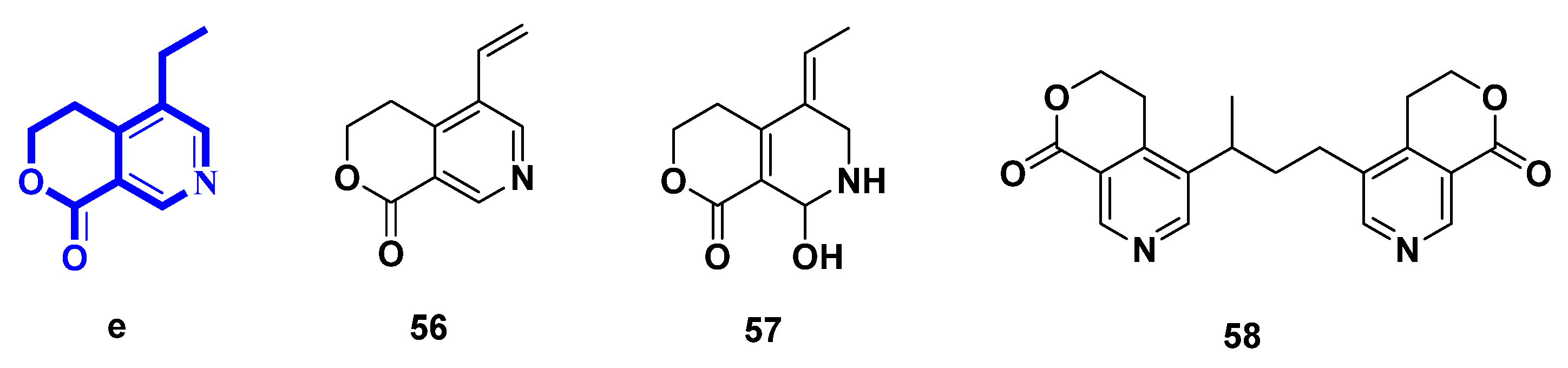
| Number | Compound Name | Source | Reference |
|---|---|---|---|
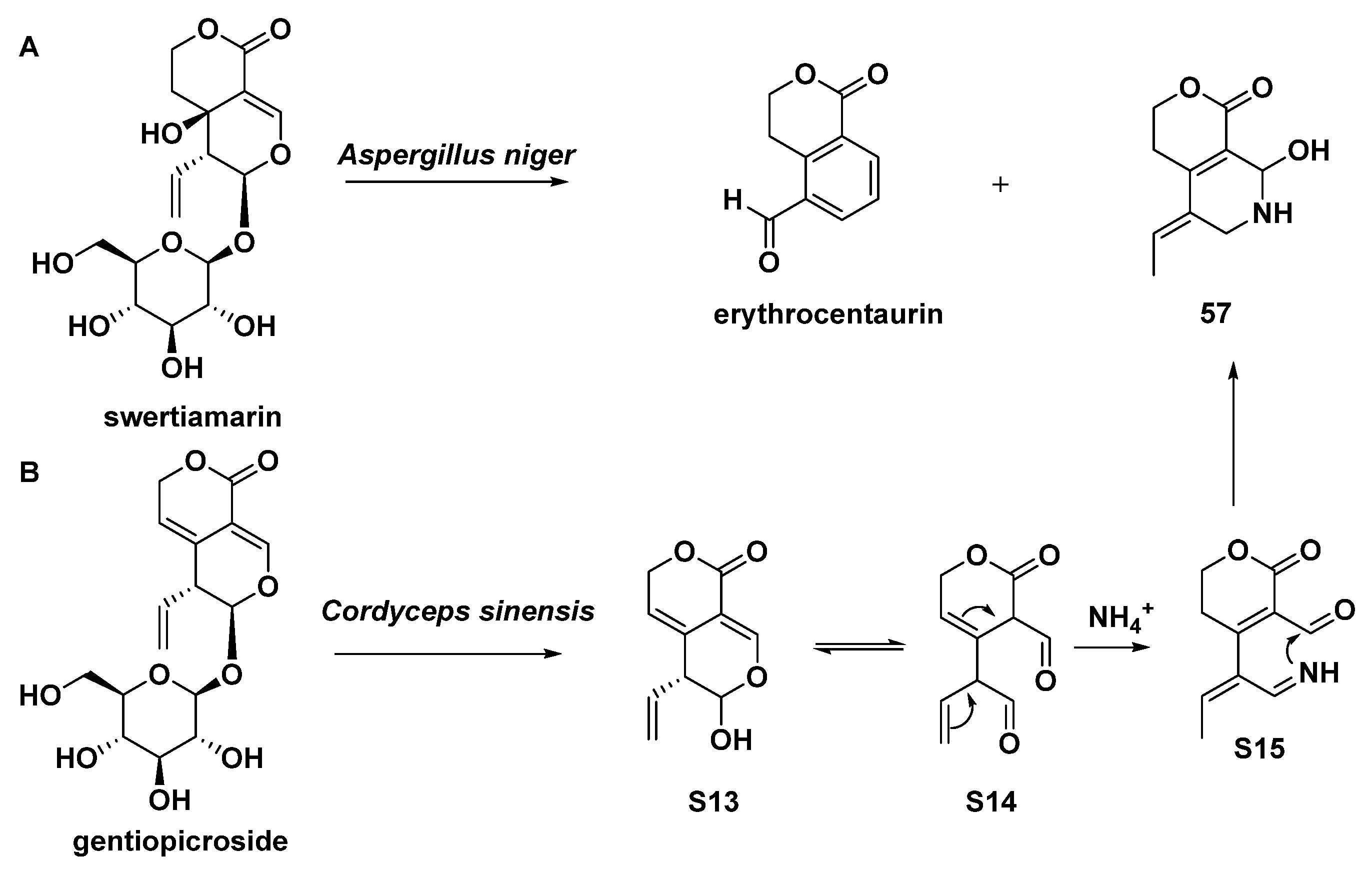
| 56 | gentianine | transformation of gentiopicroside | [30] |
| 57 | (Z)-5-ethylidene-8-hydroxy-3,4,5,6,7,8-hexahydropyrano[3,4-c]pyridine-1-one | biotransformation of gentiopicroside by asexual mycelia of Cordyceps sinensis; biotransformation of swertiamarin by Aspergillus niger | [30,35] |
| 58 | oliveramine | transformation from Gentiana olivieui | [34] |
2.6. Phenyl-Substituted Cyclopenta[c]pyridine Derivatives (Figure 7, Table 6, Type VI)
| Number | Compound Name | Source | Reference |
|---|---|---|---|
| 59 | ganocochlearine C | Ganoderma cochlear | [37,38] |
| 60 | ganoapplanatumine B | Ganoderma cochlear | [37,38] |
| 61 | ganocochlearine D | Ganoderma cochlear | [37,38] |
| 62 | ganocochlearine E | Ganoderma cochlear | [37,38] |
| 63 | ganocochlearine F | Ganoderma cochlear | [37,38] |
| 64 | ganocochlearine G | Ganoderma cochlear | [37,38] |
| 65 | ganocochlearine H | Ganoderma cochlear | [37,38] |
| 66 | ganocochlearine I | Ganoderma cochlear | [37,38] |
| 67 | sinensine E | Ganoderma cochlear | [37,38] |
| 68 | lucidimine C | Ganoderma cochlear | [37,38] |
| 69 | ganocochlearine A | Ganoderma cochlear, Ganoderma lucidum, Ganoderma austral | [37,38,44,45] |
| 70 | ganocochlearine B | Ganoderma cochlear | [37,38] |
| 71 | 6-hydroxyganocochlearine A | Ganoderma luteomarginatum | [39] |
| 72 | sinensine A | Ganoderma sinense | [40] |
| 73 | sinensine B | Ganoderma sinense | [40] |
| 74 | sinensine C | Ganoderma sinense | [40] |
| 75 | sinensine D | Ganoderma sinense | [40] |
| 76 | sinensine E | Ganoderma sinense | [40] |
| 77 | ganoapplanatumine A | Ganoderma applanatum | [42] |
| 78 | ganoapplanatumine B | Ganoderma applanatum | [42] |
| 79 | epi-ganoapplanatumine B | Ganoderma applanatum | [42] |
| 80 | lucidimine A | Ganoderma lucidum | [43] |
| 81 | lucidimine B/ganocalicine B | Ganoderma lucidum, Ganoderma calidophilum | [43,46] |
| 82 | lucidimine C | Ganoderma lucidum | [43] |
| 83 | lucidimine D | Ganoderma lucidum | [43] |
| 84 | ganocalicine A | Ganoderma calidophilum | [46] |

3. Generation of MTPAs and Their Activities
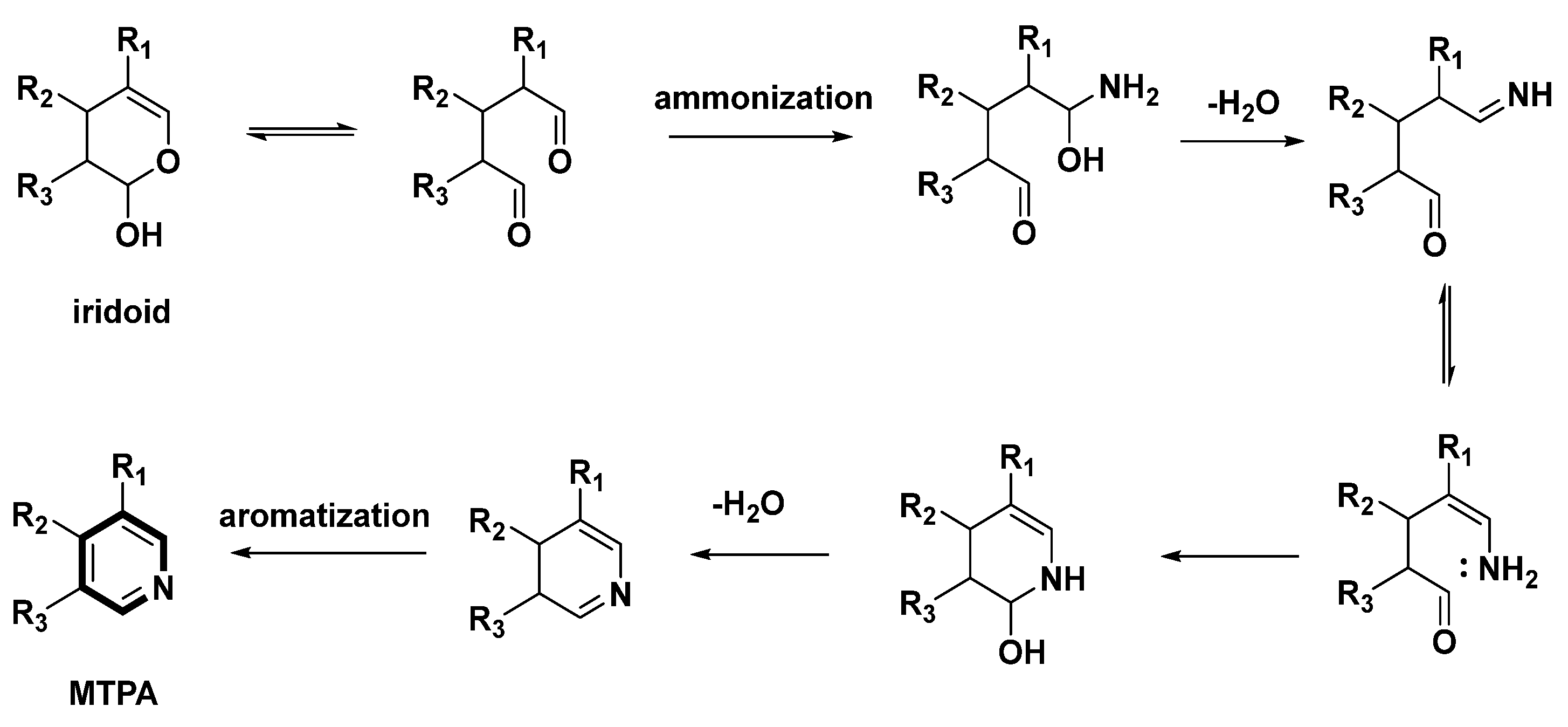
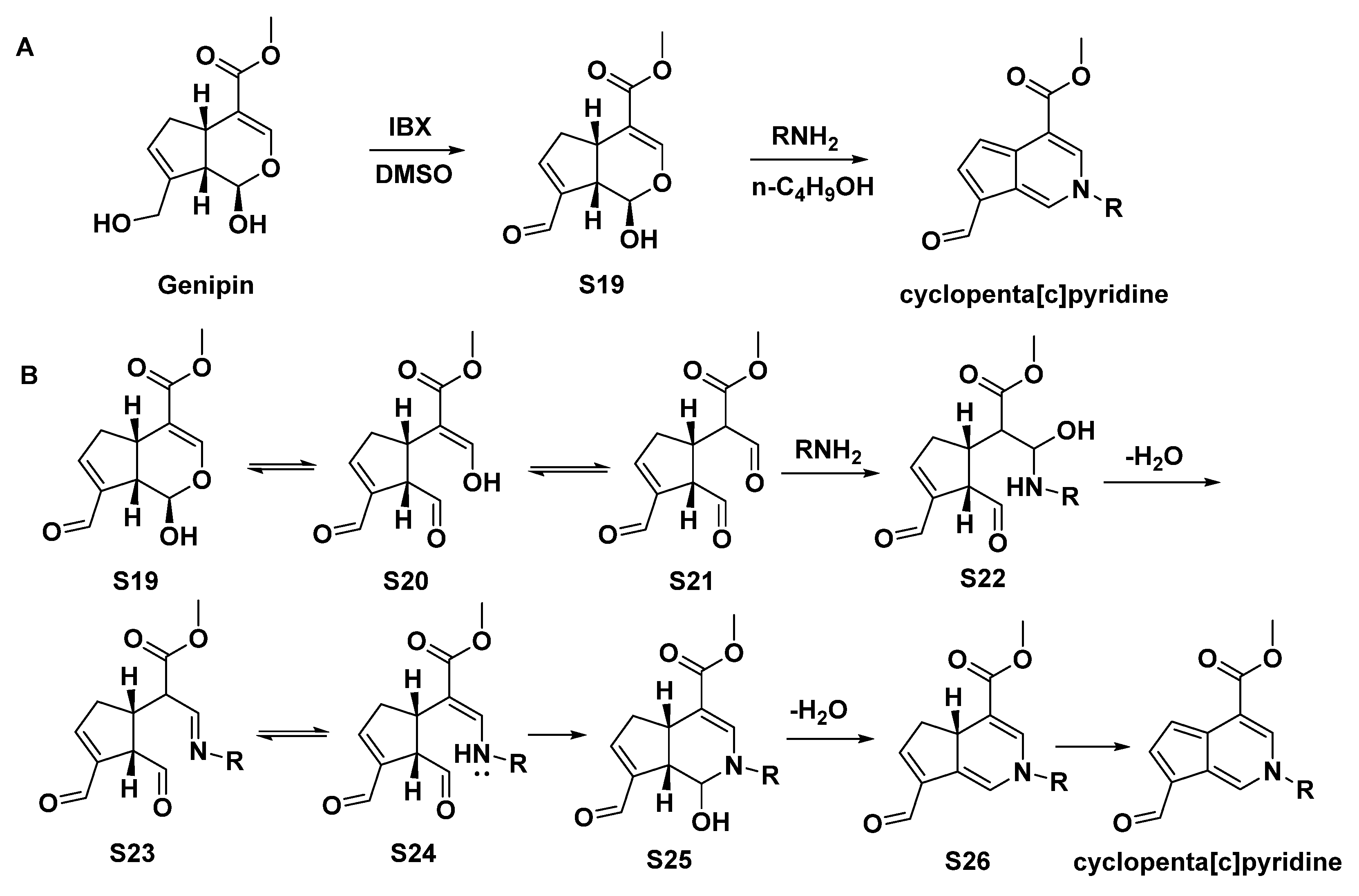
3.1. MTPAs Yielded by Chemical Transformation of IGs
3.2. MTPAs Generated from the Biotransformation of IGs
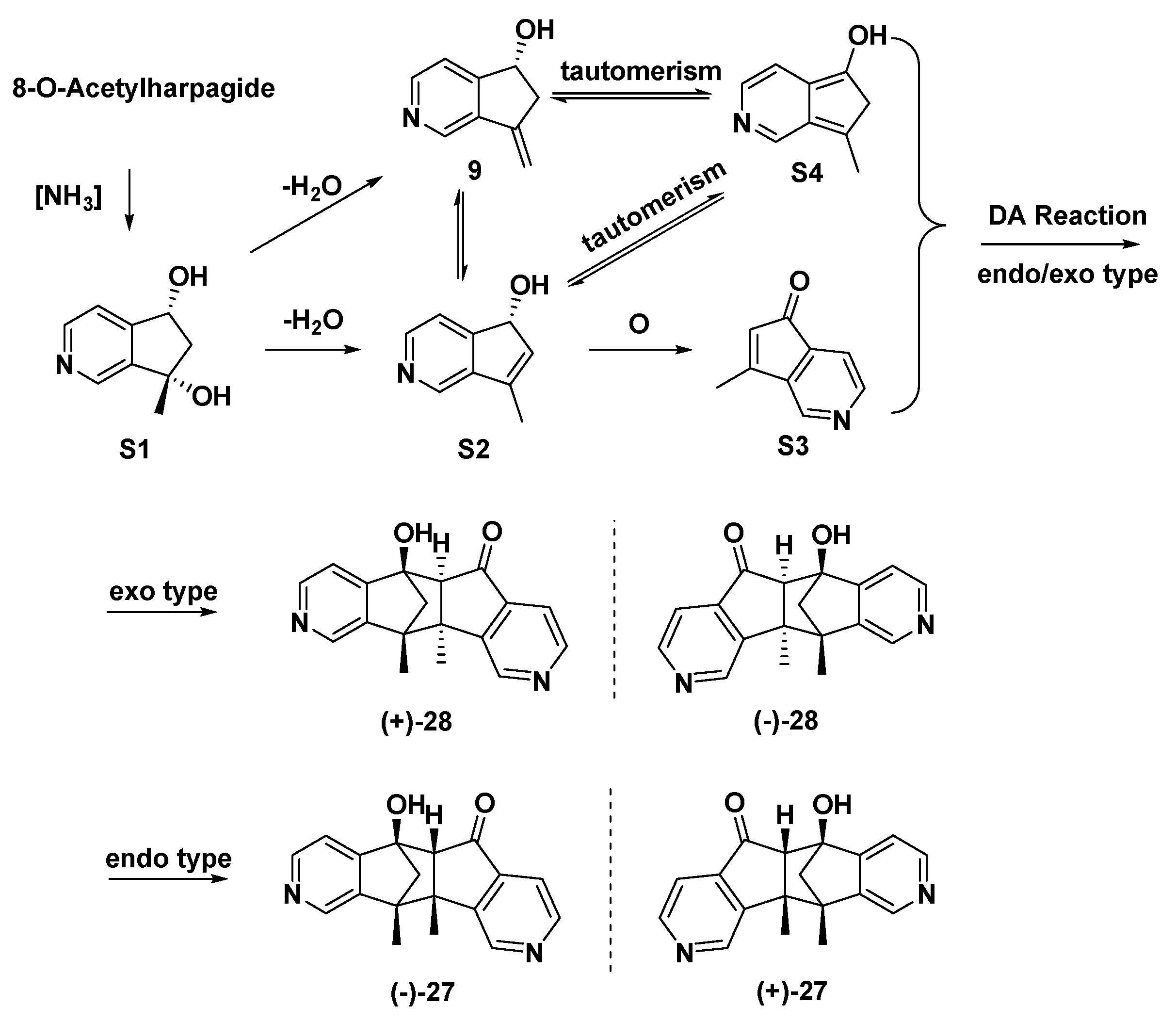
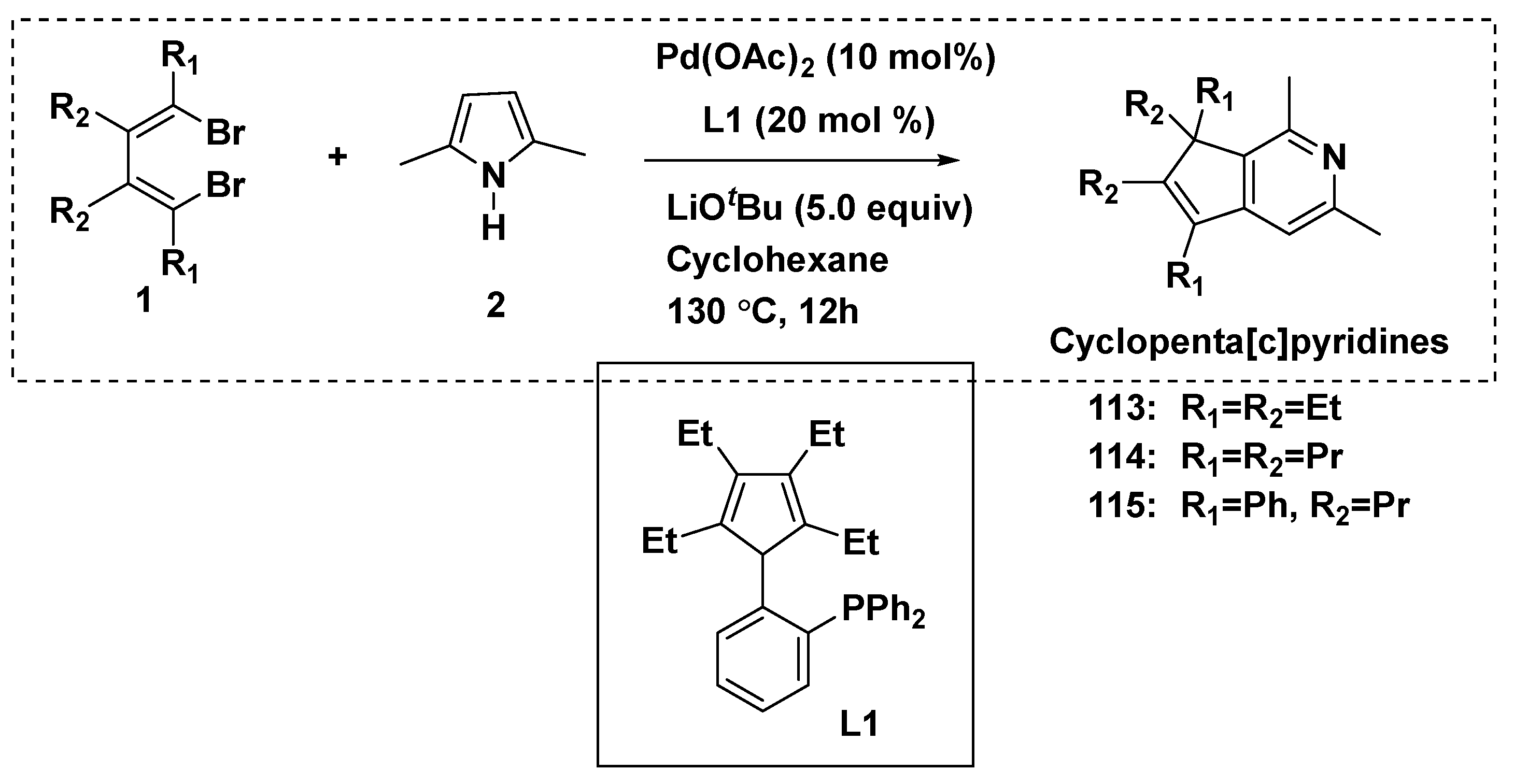
3.3. Chemical Synthesis of MPTAs/Cyclopenta[c]pyridines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among USA FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.-Y.; You, S.; Deng, Z.; Liu, Y.; Wang, Q. Natural product cerbinal and its analogues cyclopenta [c] pyridines: Synthesis and discovery as novel pest control agents. J. Agric. Food Chem. 2019, 67, 10498–10504. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, I.V.; Dyachenko, V.D. Cycloalka [c] pyridine derivatives. Methods of synthesis and chemical properties. Russ. J. Org. Chem. 2017, 53, 1769–1787. [Google Scholar] [CrossRef]
- Martin, R.E.; Lehmann, J.; Alzieu, T.; Lenz, M.; Corrales, M.A.C.; Aebi, J.D.; Märki, H.P.; Kuhn, B.; Amrein, K.; Mayweg, A.V.; et al. Synthesis of annulated pyridines as inhibitors of aldosterone synthase (CYP11B2). Org. Biomol. Chem. 2016, 14, 5922–5927. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Alzieu, T.; Martin, R.E.; Britton, R. The Kondrat’eva reaction in flow: Direct access to annulated pyridines. Org. Lett. 2013, 15, 3550–3553. [Google Scholar] [CrossRef] [PubMed]
- Childs, M.L.; Davie, R.L.; Edwards, H.J.; Evans, D.M.; Hodgson, S.T.; Mazzacani, A.; Clark, D.E.; Hinchliffe, P.S.; Baker, T.M.; Sambrook Smith, C.P.; et al. Carboxamides as Enzyme Inhibitors and Their Preparation. WO2021032938A1, 25 February 2021. [Google Scholar]
- Hagmann, W.K.; Nargund, R.P.; Blizzard, T.A.; Josien, H.; Biju, P.; Plummer, C.W.; Dang, Q.; Li, B.; Lin, L.S.; Cui, M.; et al. Preparation of Tricyclic Compounds as GPR40 Agonists for Use in Treating Diabetes and Associated Conditions. WO2014019186A1, 6 February 2014. [Google Scholar]
- Bell, I.; Selnick, H.; Zartman, C.B. 2’-Oxo-1’, 2’, 5, 7-Tetrahydrospiro [Cyclopenta [c] Pyridine-6,3’-Pyrrolo [2,3-b] Pyridine]-3-Carboxamide Derivatives as CGRP Receptor Antagonists and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Headaches. WO2008153849A1, 18 December 2008. [Google Scholar]
- Bell, I.M.; Selnick, H.G.; Stump, C.A. Preparation of Oxotetrahydrospiro[Cyclopentapyridinepyrrolo [2,3-b] Pyridinyl] Acetamides and Analogs Thereof as CGRP Receptor Antagonists. WO2008130512A1, 30 October 2008. [Google Scholar]
- Zhu, L.-J.; Hou, Y.-L.; Shen, X.-Y.; Pan, X.-D.; Zhang, X.; Yao, X.-S. Monoterpene pyridine alkaloids and phenolics from Scrophularia ningpoensis and their cardioprotective effect. Fitoterapia 2013, 88, 44–49. [Google Scholar] [CrossRef]
- Hassan, E.M.; Shahat, A.A.; Ibrahim, N.A.; Vlietinck, A.J.; Apers, S.; Pieters, L. A new monoterpene alkaloid and other constituents of Plumeria acutifolia. Planta Medica 2008, 74, 1749–1750. [Google Scholar] [CrossRef]
- Baghdikian, B.; Ollivier, E.; Faure, R.; Debrauwer, L.; Rathelot, P.; Balansard, G. Two new pyridine monoterpene alkaloids by chemical conversion of a commercial extract of Harpagophytum procumbens. J. Nat. Prod. 1998, 62, 211–213. [Google Scholar] [CrossRef]
- Lopez, J.L.; Pusset, J.; Feliciano, A.S. Plants of new-caledonia. 115. Monoterpene alkaloids from Coelospermum billardieri. J. Nat. Prod. 1988, 51, 829–835. [Google Scholar] [CrossRef]
- McCoy, J.W.; Stermitz, F.R. Chemistry of the scrophulariaceae. 2. Alkaloids from Castilleja miniata and Penstemon whippleanus, two host species for the plume moth, Amblyptilia (Platyptilia) pica. J. Nat. Prod. 1983, 46, 902–907. [Google Scholar] [CrossRef]
- Wu, S.-J.; Huang, C.-H.; Chan, Y.-Y.; Liao, Y.-R.; Hwang, T.-L.; Wu, T.-S. Two diterpenoids and a cyclopenta [c] pyridine derivative from roots of Salvia digitaloids. Int. J. Mol. Sci. 2014, 15, 11566–11577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, Y.; Pan, D.; Yao, X.; Wang, F.; Zhang, G.; Luo, Y. Antifibrotic pyridine-containing monoterpene alkaloids from Caryopteris glutinosa. Phytochemistry 2022, 203, 113378. [Google Scholar] [CrossRef] [PubMed]
- Mishig, D.; Gruner, M.; Lübken, T.; Ganbaatar, C.; Regdel, D.; Knölker, H.-J. Isolation and structure elucidation of pyridine alkaloids from the aerial parts of the Mongolian medicinal plant Caryopteris mongolica Bunge. Sci. Rep. 2021, 11, 13740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, G.; Cao, Y.; Mishig, D.; Lodonjav, M.; Pan, D.; Yao, X.; Wang, F.; Zhang, G.; Luo, Y. (±)-Caryopterisines A and B, dimeric monoterpene alkaloids with unprecedented 6/5/5/5/6 pentacyclic rings scaffold from Caryopteris glutinosa. Bioorg. Chem. 2021, 116, 105364. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Jaki, J. Biomedically relevant chemical constituents of Valeriana officinalis. J. Appl. Biomed. 2010, 8, 11–18. [Google Scholar] [CrossRef]
- O’Connor, S.E.; Maresh, J.J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 2006, 23, 532–547. [Google Scholar] [CrossRef]
- Shi, Q.; He, Y.; Chen, J.; Lu, L. Thermally induced actinidine production in biological samples. J. Agric. Food Chem. 2020, 68, 12252–12258. [Google Scholar] [CrossRef]
- Roby, M.R.; Stermitz, F.R. Pyrrolizidine and pyridine monoterpene alkaloids from 2 castilleja plant hosts of the plume moth, Platyptilia pica. J. Nat. Prod. 1984, 47, 846–853. [Google Scholar] [CrossRef]
- Roby, M.R.; Stermitz, F.R. Chemistry of the scrophulariaceae. 4. Penstemonoside and other iridoids from Castilleja rhexifolia. Conversion of penstemonoside to the pyridine monoterpene alkaloid rhexifoline. J. Nat. Prod. 1984, 47, 854–857. [Google Scholar] [CrossRef]
- Da Silva, T.; Da Silva, B.; Mukherjee, R. The monoterpene alkaloid cantleyine from Strychnos trinervis root and its spasmolytic properties. Phytomedicine 1999, 6, 169–176. [Google Scholar] [CrossRef]
- Boros, C.A.; Stermitz, F.R.; Harris, G.H. Iridoid glycosides and a pyridine monoterpene alkaloid from Orthocarpus. New artifactual iridoid dienals. J. Nat. Prod. 1990, 53, 72–80. [Google Scholar] [CrossRef]
- Bianco, A.; Bonadies, F.; Cianciolo, V.; Melchioni, C.; Ramunno, A.; Dezzi, S.; Nicoletti, M.; Serafini, M.; Ballero, M. Monoterpene alkaloids from Argylia radiata. Nat. Prod. Lett. 2002, 16, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, S.M.; Stermitz, F.R. Pyridine monoterpene alkaloid formation from iridoid glycosides. A novel pmta dimer from geniposide. J. Nat. Prod. 1996, 59, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Saad, H.; Anton, R.; Quirion, J.; Chauvière, G.; Pusset, J. New-caledonian plants.116.(1) Lindenialine and lindeniamine, two new iridoids from Lindenia austro-caledonica brongn. Tetrahedron Lett. 1988, 29, 615–618. [Google Scholar] [CrossRef]
- Wei, X.; Khan, A.; Song, D.; Dai, Z.; Liu, Y.-P.; Yu, H.-F.; Wang, B.; Zhu, P.-F.; Ding, C.-F.; Zhao, X.-D.; et al. Three new pyridine alkaloids from Vinca major cultivated in Pakistan. Nat. Prod. Bioprospect. 2017, 7, 323–327. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Xu, M.; Zhu, H.-T.; Chen, K.-K.; Zhang, Y.-J.; Yang, C.-R. Biotransformation of gentiopicroside by asexual mycelia of Cordyceps sinensis. Bioorganic Med. Chem. Lett. 2007, 17, 3195–3197. [Google Scholar] [CrossRef]
- Willems, M. Transformation of Secoiridoid Glucosides to Pyridine Alkaloids. Arch. der Pharm. 1987, 320, 1245–1248. [Google Scholar] [CrossRef]
- Willems, M. A Glucosidic alkaloid artifact, originated from secoiridoid glucosides from fruits of Ligustrum vulgare L. Arch. der Pharm. 1988, 321, 357–358. [Google Scholar] [CrossRef]
- Ranarivelo, Y.; Magiatis, P.; Skaltsounis, A.-L.; Tillequin, F. Selective amination of secoiridoid glycosides to give monomeric pyridine, dimeric pyridine, and naphthyridine alkaloids. Nat. Prod. Lett. 2001, 15, 131–137. [Google Scholar] [CrossRef]
- Willems, M. Dimeric pyridine alkaloids. Artifacts, originating from secoiridoid glucosides from Ligustrum vulgare L. Arch. Pharm. 1988, 321, 229–330. [Google Scholar] [CrossRef]
- Chang, J.; Zhou, B. Biotransformation of swertiamarin by Aspergillus niger. Pak. J. Pharm. Sci. 2015, 28, 1933–1937. [Google Scholar]
- Bhattacharya, S.K.; Ghosal, S.; Chaudhuri, R.K.; Singh, A.K.; Sharma, P.V. Letter: Chemical constituents of gentianaceae. XI. Antipsychotic activity of gentianine. J. Pharm. Sci. 1974, 63, 1341–1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Dou, M.; Luo, Q.; Cheng, L.-Z.; Yan, Y.-M.; Li, R.-T.; Cheng, Y.-X. Racemic alkaloids from the fungus Ganoderma cochlear. Fitoterapia 2017, 116, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, X.L.; Wang, Y.Z.; Cheng, Y.X. Two new alkaloids from Ganoderma cochlear. Nat. Prod. Res. Dev. 2015, 27, 1325–1328. [Google Scholar]
- Li, X.-C.; Liu, F.; Su, H.-G.; Guo, L.; Zhou, Q.-M.; Huang, Y.-J.; Peng, C.; Xiong, L. Two pairs of alkaloid enantiomers from Ganoderma luteomarginatum. Biochem. Syst. Ecol. 2019, 86, 103930. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Wang, C.-F.; Peng, X.-R.; Qiu, M.-H. New alkaloids from the fruiting bodies of Ganoderma sinense. Nat. Prod. Bioprospect. 2011, 1, 93–96. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, F.; Chen, R. A novel alkaloid from the fruiting bodies of Ganoderma sinense Zhao, Xu et Zhang. Chin. Chem. Lett. 2010, 21, 197–199. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, X.-H.; Yang, Z.-L.; Tu, Z.-C.; Cheng, Y.-X. Miscellaneous meroterpenoids from Ganoderma applanatum. Tetrahedron 2016, 72, 4564–4574. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Chen, H.P.; Feng, T.; Li, Z.H.; Dong, Z.J.; Liu, J.K. Lucidimine A-D, four new alkaloids from the fruiting bodies of Ganoderma lucidum. J. Asian Nat. Prod. Res. 2015, 17, 1160–1165. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Peng, X.-R.; Dong, J.-R.; Yan, H.; Kong, Q.-H.; Shi, Q.-Q.; Li, D.-S.; Zhou, L.; Li, Z.-R.; Qiu, M.-H. Aromatic constituents from Ganoderma lucidum and their neuroprotective and anti-inflammatory activities. Fitoterapia 2019, 134, 58–64. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, Y.; Qin, F.-Y.; Yan, Y.-M.; Cheng, Y.-X. Meroterpenoids and alkaloids from Ganoderma australe. Nat. Prod. Res. 2019, 35, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Cheng, B.H.; Ma, Q.Y.; Wang, Q.; Kong, F.D.; Dai, H.F.; Qiu, S.Q.; Zheng, P.Y.; Liu, Z.Q.; Zhao, Y.X. Anti-allergic Prenylated Hydroquinones and Alkaloids from the fruiting body of Ganoderma calidophilum. RSC Adv. 2016, 6, 21139–21147. [Google Scholar] [CrossRef]
- Zhang, J.F.; Huang, S.; Shan, L.H.; Chen, L.; Zhang, Y.; Zhou, X.L. New iridoid glucoside from Pterocephalus hookeri. Chin. J. Org. Chem. 2015, 35, 2441–2444. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Guo, C.-X.; Zhu, Y.-Z.; Li, Y.-M.; Guo, F.-J.; Zhu, G.-F. Four new bis-iridoids isolated from the traditional Tibetan herb Pterocephalus hookeri. Fitoterapia 2014, 98, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tanaka, K.; Watanabe, S.; Tezuka, Y.; Saiki, I. Dipasperoside A, a novel pyridine alkaloid-coupled iridoid glucoside from the roots of Dipsacus asper. Chem. Pharm. Bull. 2013, 61, 1318–1322. [Google Scholar] [CrossRef][Green Version]
- Huang, J.-G.; Zhou, L.-J.; Xu, H.-H. Two new constituents from Torricellia tiliifolia stem barks. Helv. Chim. Acta 2011, 94, 327–330. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants: 6. Biologically active marine and terrestrial alkaloid glycosides. Lipids 2005, 40, 1081–1105. [Google Scholar] [CrossRef]
- Saxena, J.; Mathela, C.S. Antifungal activity of new compounds from Nepeta leucoyhylla and Nepeta clarkei. Appl. Environ. Microbiol. 1996, 62, 702–704. [Google Scholar] [CrossRef]
- Takahashi, A.; Ikeda, D.; Nakamura, H.; Naganawa, H.; Kurasawa, S.; Okami, Y.; Takeuchi, T.; Iitaka, Y. Altemicidin, a new acaricidal and antitumor substance. II. Structure determination. J. Antibiot. 1989, 42, 1562–1566. [Google Scholar] [CrossRef]
- Baghdikian, B.; Guiraud-Dauriac, H.; Ollivier, E.; N’Guyen, A.; Dumenil, G.; Balansard, G. Formation of nitrogen-containing metabolites from the main iridoids of Harpagophytum procumbens and H. zeyheri by human intestinal bacteria. Planta Med. 1999, 65, 164–166. [Google Scholar] [CrossRef]
- Ghisalberti, E. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine 1998, 5, 147–163. [Google Scholar] [CrossRef]
- Kawata, Y.; Hattori, M.; Akao, T.; Kobashi, K.; Namba, T. Formation of nitrogen-containing metabolites from geniposide and gardenoside by human intestinal bacteria. Planta Med. 1991, 57, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Kawata, Y.; Inoue, K.; Shu, Y.; Che, Q.; Namba, T.; Kobashi, K. Transformation of aucubin to new pyridine monoterpene alkaloids, aucubinines A and B, by human intestinal bacteria. Phytother. Res. 1990, 4, 66–70. [Google Scholar] [CrossRef]
- Cheng, G.G.; Li, D.; Hou, B.; Li, X.N.; Liu, L.; Chen, Y.Y.; Lunga, P.K.; Khan, A.; Liu, Y.P.; Zuo, Z.L.; et al. Melokhanines A-J, bioactive monoterpenoid indole alkaloids with diverse skeletons from Melodinus khasianus. J. Nat. Prod. 2016, 79, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Uredi, D.; Motati, D.R.; Watkins, E.B. A simple, tandem approach to the construction of pyridine derivatives under metal-free conditions: A one-step synthesis of the monoterpene natural product, (−)-actinidine. Chem. Commun. 2019, 55, 3270–3273. [Google Scholar] [CrossRef]
- Yin, J.; Ye, Q.; Hao, W.; Du, S.; Gu, Y.; Zhang, W.X.; Xi, Z. Formation of cyclopenta [c] pyridine derivatives from 2,5-Disubstituted pyrroles and 1,4-Dibromo-1,3-butadienes via Pyrrole-Ring One-Carbon expansion. Org. Lett. 2017, 19, 138–141. [Google Scholar] [CrossRef]
- Beckett, J.S.; Beckett, J.D.; Hofferberth, J.E. A divergent approach to the diastereoselective synthesis of several ant-associated iridoids. Org. Lett. 2010, 12, 1408–1411. [Google Scholar] [CrossRef]
- Ohba, M.; Izuta, R.; Shimizu, E. Use of the oxazole-olefin diels-alder reaction in the total synthesis of the monoterpene alkaloids (-)-plectrodorine and (+)-oxerine. Chem. Pharm. Bull. 2006, 54, 63–67. [Google Scholar] [CrossRef][Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tao, F.; Cui, T.; Luo, C.; Zhou, Z.; Huang, Y.; Tan, L.; Peng, W.; Wu, C. Sources, Transformations, Syntheses, and Bioactivities of Monoterpene Pyridine Alkaloids and Cyclopenta[c]pyridine Derivatives. Molecules 2022, 27, 7187. https://doi.org/10.3390/molecules27217187
Zhang X, Tao F, Cui T, Luo C, Zhou Z, Huang Y, Tan L, Peng W, Wu C. Sources, Transformations, Syntheses, and Bioactivities of Monoterpene Pyridine Alkaloids and Cyclopenta[c]pyridine Derivatives. Molecules. 2022; 27(21):7187. https://doi.org/10.3390/molecules27217187
Chicago/Turabian StyleZhang, Xuejian, Feiyan Tao, Tao Cui, Cheng Luo, Zhigang Zhou, Yuchuan Huang, Lanlan Tan, Wei Peng, and Chunjie Wu. 2022. "Sources, Transformations, Syntheses, and Bioactivities of Monoterpene Pyridine Alkaloids and Cyclopenta[c]pyridine Derivatives" Molecules 27, no. 21: 7187. https://doi.org/10.3390/molecules27217187
APA StyleZhang, X., Tao, F., Cui, T., Luo, C., Zhou, Z., Huang, Y., Tan, L., Peng, W., & Wu, C. (2022). Sources, Transformations, Syntheses, and Bioactivities of Monoterpene Pyridine Alkaloids and Cyclopenta[c]pyridine Derivatives. Molecules, 27(21), 7187. https://doi.org/10.3390/molecules27217187






