Mechanistic Insights into the Ameliorative Effect of Cichoriin on Diabetic Rats—Assisted with an In Silico Approach
Abstract
1. Introduction
2. Results
2.1. Effect of Cichoriin on Blood Glucose Level and Serum Insulin of Diabetic Rats
2.2. Effect of Cichoriin on Triglycerides and Total Cholesterol
2.3. Effect of Cichoriin on Oxidative Stress Markers
2.4. Histopathological Analysis of Pancreatic Tissue
2.5. Effect of Cichoriin on Pancreatic Insulin Immunoexpression in Diabetic Rats
2.6. Effect of Cichoriin on mRNA and Protein Expression of GLUT4, AMPK, and PI3K in Skeletal Muscle of Diabetic Rats
2.7. In Silico Binding of Cichoriin and GLUT4, AMPK, and PI3K Markers
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Induction of Type 2 DM
4.3. Design of Study
4.4. Measurement of Blood Glucose Level, Serum Triglycerides, Total Cholesterol and Insulin
4.5. Measurement of Antioxidant and Oxidative Stress Parameters
4.6. Histopathological Analysis of Pancreatic Tissue
4.7. Immunohistochemistry of Insulin in Pancreas
4.8. RT-PCR for GLUT4, AMPK, and PI3K in Skeletal Muscle
4.9. Western Blot for GLUT4, AMPK, and PI3K in Skeletal Muscle
4.10. Computational Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Patle, D.; Vyas, M.; Khatik, G.L. A review on natural products and herbs used in the management of diabetes. Curr. Diabetes Rev. 2021, 17, 186–197. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.-Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O. Natural products in diabetes research: Quantitative literature analysis. Nat. Prod. Res. 2021, 35, 5813–5827. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Rahaman, M.S.; Islam, F.; Ahmed, M.; Mitra, S.; Khandaker, M.U.; Idris, A.M. The multifunctional role of herbal products in the management of diabetes and obesity: A comprehensive review. Molecules 2022, 27, 1713. [Google Scholar] [CrossRef] [PubMed]
- Vivó-Barrachina, L.; Rojas-Chacón, M.J.; Navarro-Salazar, R.; Belda-Sanchis, V.; Pérez-Murillo, J.; Peiró-Puig, A.; Herran-González, M.; Pérez-Bermejo, M.; Pérez-Bermejo, M. The Role of Natural Products on Diabetes Mellitus Treatment: A Systematic Review of Randomized Controlled Trials. Pharmaceutics 2022, 14, 101. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, B.; Zhou, Z. Preclinical Studies of Natural Products Targeting the Gut Microbiota: Beneficial Effects on Diabetes. J. Agric. Food Chem. 2022, 70, 8569–8581. [Google Scholar] [CrossRef]
- Hatware, K.V.; Sharma, S.; Patil, K.; Shete, M.; Karri, S.; Gupta, G. Evidence for gastroprotective, anti-inflammatory and antioxidant potential of methanolic extract of Cordia dichotoma leaves on indomethacin and stress induced gastric lesions in Wistar rats. Biomed. Pharmacother. 2018, 103, 317–325. [Google Scholar] [CrossRef]
- Das, R.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Hossain, M.J.; Alqahtani, A.M.; Alghazwani, Y.; Dhama, K.; Simal-Gandara, J. Medicinal plants used against hepatic disorders in Bangladesh: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114588. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Van Anil Kumar, N.; Sharopov, F.; Ramirez-Alarcon, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Valere Tsouh Fokou, P.; Kobarfard, F.; Amiruddin Zakaria, Z. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Chen, L.; Lu, X.; El-Seedi, H.; Teng, H. Recent advances in the development of sesquiterpenoids in the treatment of type 2 diabetes. Trends Food Sci. Technol. 2019, 88, 46–56. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida Junior, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef]
- Qin, H.-L.; Zhang, Z.-W.; Ravindar, L.; Rakesh, K. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef]
- Detsi, A.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2015–2016). Expert Opin. Ther. Pat. 2017, 27, 1201–1226. [Google Scholar] [CrossRef]
- Bansal, Y.; Sethi, P.; Bansal, G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]
- Rawat, A.; Reddy, A.V.B. Recent advances on anticancer activity of coumarin derivatives. Eur. J. Med. Chem. Rep. 2022, 5, 100038. [Google Scholar] [CrossRef]
- Ibrar, A.; Shehzadi, S.A.; Saeed, F.; Khan, I. Developing hybrid molecule therapeutics for diverse enzyme inhibitory action: Active role of coumarin-based structural leads in drug discovery. Bioorg. Med. Chem. 2018, 26, 3731–3762. [Google Scholar] [CrossRef]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Li, L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Ranđelović, S.; Bipat, R. A Review of Coumarins and Coumarin-Related Compounds for Their Potential Antidiabetic Effect. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Park, H.S.; Lee, A.; Min, J.; Park, Y.; Cha, J. Synthesis of α-cichoriin Using Deinococcus geothermalis Amylosucrase and Its Antiproliferative Effect. Microbiol. Biotechnol. Lett. 2022, 50, 218–227. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and Antioxidant Activities of Coumarins from the Roots of Ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Yao, Z.L.; Yang, D.; Ke, J.; Wu, Q.L.; Li, J.K.; Zhou, X.D. Chemical constituents from Fraxinus hupehensis and their antifungal and herbicidal activities. Biomolecules 2020, 10, 74. [Google Scholar] [CrossRef]
- Rivero-Segura, N.A.; Gomez-Verjan, J.C. In silico screening of natural products isolated from Mexican herbal medicines against COVID-19. Biomolecules 2021, 11, 216. [Google Scholar] [CrossRef]
- Khalil, H.E.; Kamel, M.S. Phytochemical and biological studies of Cichorium endivia L. leaves. J. Pharm. Sci. Res. 2015, 7, 509. [Google Scholar]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Seregheti, T.; Pinto, A.; Gonçalves, M.d.C.; Antunes, A.d.S.; Almeida, W.d.S.; Machado, R.; Silva, J.; Ferreira, P.; Pessoa, C.; Dos Santos, V. Antiproliferative and photoprotective activities of the extracts and compounds from Calea fruticosa. Braz. J. Med. Biol. Res. 2020, 53, e9375. [Google Scholar] [CrossRef]
- El-Huneidi, W.; Anjum, S.; Saleh, M.A.; Bustanji, Y.; Abu-Gharbieh, E.; Taneera, J. Carnosic Acid Protects INS-1 β-Cells against Streptozotocin-Induced Damage by Inhibiting Apoptosis and Improving Insulin Secretion and Glucose Uptake. Molecules 2022, 27, 2102. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Król, E.; Krejpcio, Z. Evaluation of anti-diabetic potential of chromium (III) propionate complex in high-fat diet fed and STZ injected rats. Food Chem. Toxicol. 2011, 49, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, J.; Chen, X.-Q.; Zan, K.; Wen, X.-D.; Chen, H.; Wang, Q.; Lai, M.-X. Antidiabetic and antioxidant effects of extracts from Potentilla discolor Bunge on diabetic rats induced by high fat diet and streptozotocin. J. Ethnopharmacol. 2010, 132, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 114. [Google Scholar] [CrossRef]
- Murali, R.; Srinivasan, S.; Ashokkumar, N. Antihyperglycemic effect of fraxetin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Biochimie 2013, 95, 1848–1854. [Google Scholar] [CrossRef]
- Onder, A.; Cinar, A.S.; Baran, M.Y.; Kuruüzüm-Uz, A.; Trendafilova, A. Coumarins from Seseli petraeum M. Bieb. (Apiaceae) and their α-glucosidase inhibitory activity. S. Afr. J. Bot. 2022, 144, 458–463. [Google Scholar] [CrossRef]
- Lee, S.O.; Choi, S.Z.; Lee, J.H.; Chung, S.H.; Park, S.H.; Kang, H.C.; Yang, E.Y.; Cho, H.J.; Lee, K.R. Antidiabetic coumarin and cyclitol compounds from Peucedanum japonicum. Arch. Pharm. Res. 2004, 27, 1207–1210. [Google Scholar] [CrossRef]
- Ramesh, B.; Pugalendi, K. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J. Med. Food 2006, 9, 562–566. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, Z.; Zhang, L.; Liu, Y.; Ding, H.; Yin, S. Isofraxidin, a coumarin component improves high-fat diet induced hepatic lipid homeostasis disorder and macrophage inflammation in mice. Food Funct. 2017, 8, 2886–2896. [Google Scholar] [CrossRef]
- Chang, W.C.; Wu, S.C.; Xu, K.D.; Liao, B.C.; Wu, J.F.; Cheng, A.S. Scopoletin protects against methylglyoxal-induced hyperglycemia and insulin resistance mediated by suppression of advanced glycation endproducts (AGEs) generation and anti-glycation. Molecules 2015, 20, 2786–2801. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Park, J.E.; Han, J.S. Scopoletin increases glucose uptake through activation of PI3K and AMPK signaling pathway and improves insulin sensitivity in 3T3-L1 cells. Nutr. Res. 2020, 74, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Li, L.; Yu, H.; Wu, Y.; Li, H. Coumarins ameliorate diabetogenic action of dexamethasone via Akt activation and AMPK signaling in skeletal muscle. J. Pharmacol. Sci. 2019, 139, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.E.; Abdelwahab, M.F.; Emeka, P.M.; Badger-Emeka, L.I.; Thirugnanasambantham, K.; Ibrahim, H.-I.M.; Naguib, S.M.; Matsunami, K.; Abdel-Wahab, N.M. Ameliorative Effect of Ocimum forskolei Benth on Diabetic, Apoptotic, and Adipogenic Biomarkers of Diabetic Rats and 3T3-L1 Fibroblasts Assisted by In Silico Approach. Molecules 2022, 27, 2800. [Google Scholar] [CrossRef]
- Pari, L.; Rajarajeswari, N. Efficacy of coumarin on hepatic key enzymes of glucosemetabolism in chemical induced type 2 diabetic rats. Chem. Biol. Interact. 2009, 3, 292–296. [Google Scholar] [CrossRef]
- Salmanoglu, D.S.; Gurpinar, T.; Vural, K.; Ekerbicer, N.; Darıverenli, E.; Var, A. Melatonin and L-Carnitin Improves Endothelial Disfunction and Oxidative Stress in Type 2 Diabetic Rats. Redox Biol. 2016, 8, 199–204. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Khalil, H.E.; Abdelwahab, M.F.; Emeka, P.M.; Badger-Emeka, L.I.; Abdel Hafez, S.M.N.; AlYahya, K.A.; Ahmed, A.-S.F.; Anter, A.F.; Abdel-Wahab, N.M.; Matsunami, K.; et al. Chemical Composition and Valorization of Broccoli Leaf By-Products (Brassica oleracea L. Variety: Italica) to Ameliorate Reno-Hepatic Toxicity Induced by Gentamicin in Rats. Appl. Sci. 2022, 12, 6903. [Google Scholar] [CrossRef]
- Khalil, H.E.; Abdelwahab, M.F.; Emeka, P.M.; Badger-Emeka, L.I.; Ahmed, A.-S.F.; Anter, A.F.; Abdel Hafez, S.M.N.; AlYahya, K.A.; Ibrahim, H.-I.M.; Thirugnanasambantham, K.; et al. Brassica oleracea L. var. botrytis Leaf Extract Alleviates Gentamicin-Induced Hepatorenal Injury in Rats—Possible Modulation of IL-1β and NF-κB Activity Assisted with Computational Approach. Life 2022, 12, 1370. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–127. [Google Scholar] [PubMed]
- Culling, C.F.A. Handbook of Histopathological and Histochemical Techniques, 3rd ed.; Butterworths: London, UK, 2013. [Google Scholar]
- Elsayed, H.E.; Ebrahim, H.Y.; Mady, M.S.; Khattab, M.A.; El-Sayed, E.K.; Moharram, F.A. Ethnopharmacological impact of Melaleuca rugulosa (Link) Craven leaves extract on liver inflammation. J. Ethnopharmacol. 2022, 292, 115215. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Hu, M.; Li, R.; Li, B.; Huang, D.; Ma, W.; Jia, X.; Lv, Z. Insulin on Changes in Expressions of Aquaporin-1, Aquaporin-5, and Aquaporin-8 in Submandibular Salivary Glands of Rats with Streptozotocin-Induced Diabetes. Int. J. Clin. Exp. Pathol. 2021, 14, 221–229. [Google Scholar]
- Ibrahim, H.-I.M.; Darrag, H.M.; Alhajhoj, M.R.; Khalil, H.E. Biomolecule from Trigonella stellata from Saudi Flora to Suppress Osteoporosis via Osteostromal Regulations. Plants 2020, 9, 1610. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.E.; Ibrahim, H.-I.M.; Ahmed, E.A.; Emeka, P.M.; Alhaider, I.A. Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via miR-26b and miR-146a. Pharmaceuticals 2022, 15, 154. [Google Scholar] [CrossRef]
- Khalil, H.E.; Ibrahim, H.-I.M.; Darrag, H.M.; Matsunami, K. Insight into Analysis of Essential Oil from Anisosciadium lanatum Boiss.—Chemical Composition, Molecular Docking, and Mitigation of Hepg2 Cancer Cells through Apoptotic Markers. Plants 2022, 11, 66. [Google Scholar] [CrossRef]


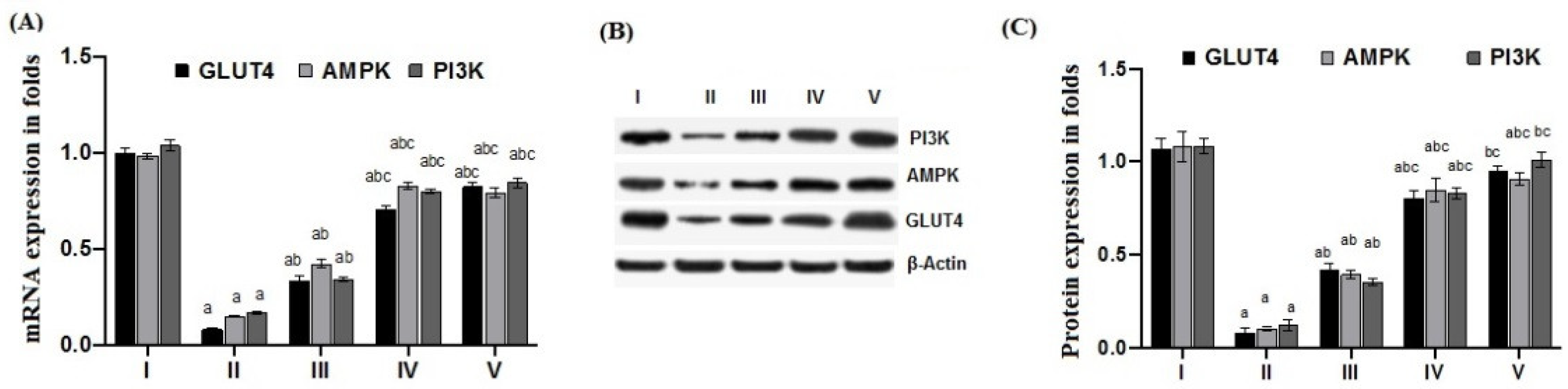
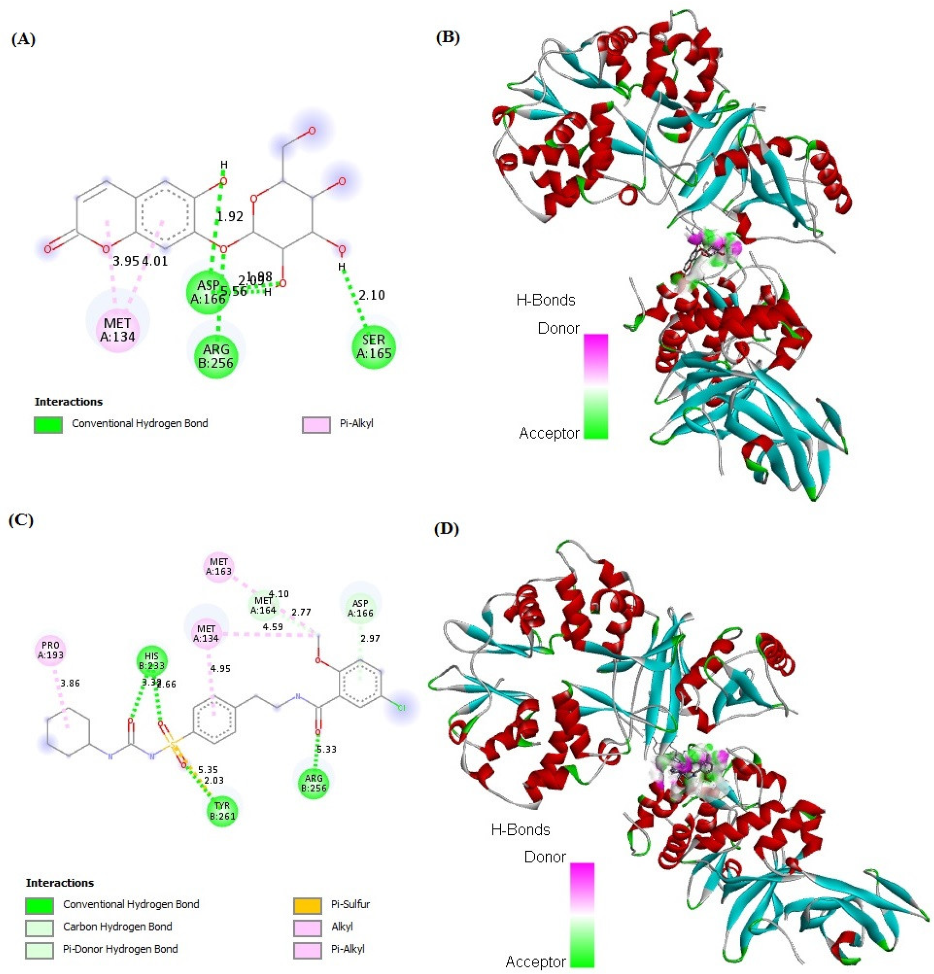
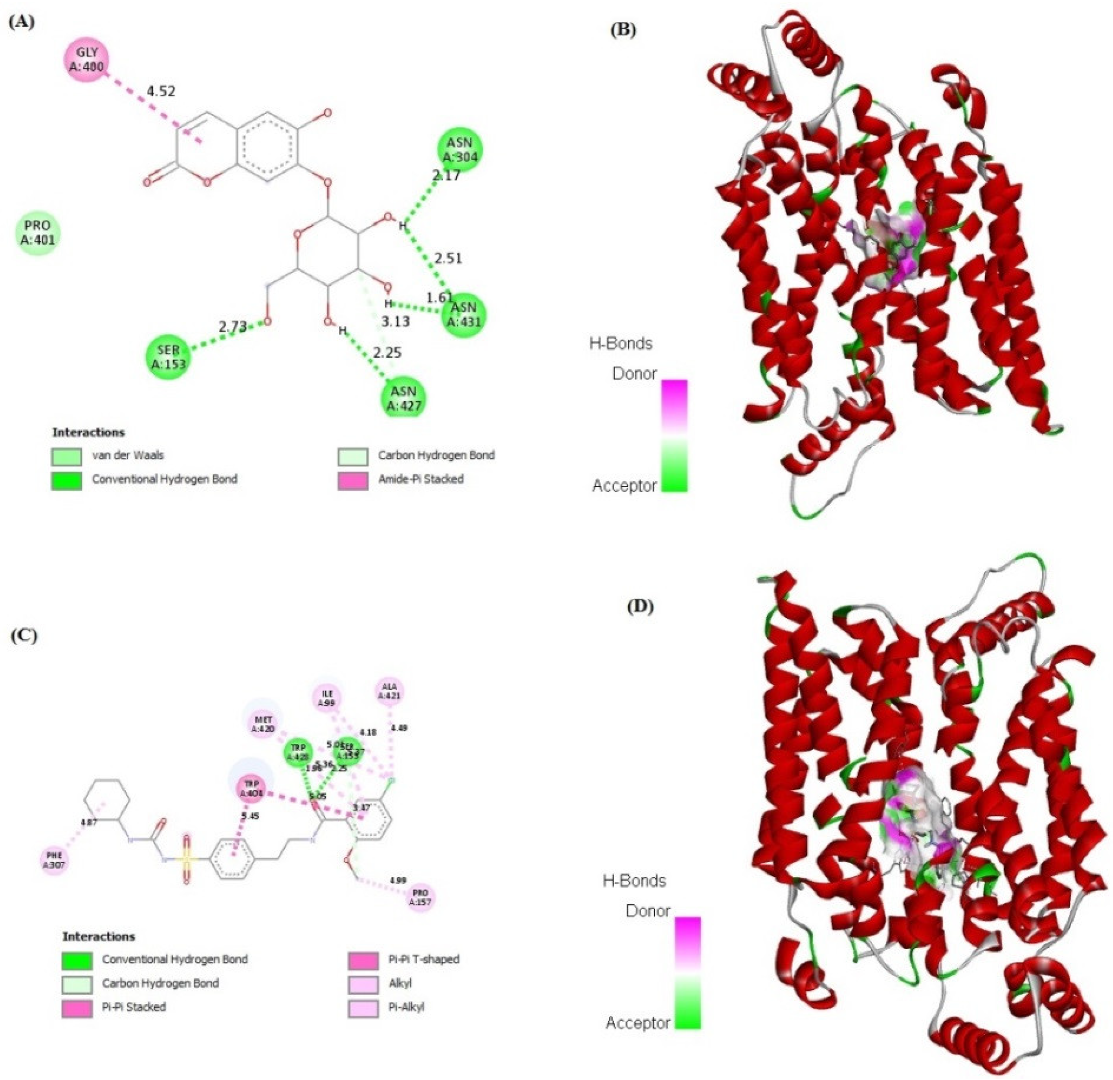

| Groups | Group I (Control) | Group II (Diabetic) | Group III (50 mg/kg, Cichoriin) | Group IV (100 mg/kg, Cichoriin) | Group V (5 mg/kg, GB) |
|---|---|---|---|---|---|
| BGL (mg/dL) | 103 ± 4 | 452 ± 44 a | 206 ± 7 a,b | 127 ± 3 b | 128 ± 5 b |
| Serum Insulin (nIU/mL) | 7.8 ± 0.2 | 2.6 ± 0.1 a | 4.8 ± 0.2 a,b | 7.0 ± 0.2 a,b,c | 7.2 ± 0.1 b,c |
| TG (mg/dL) | 64.7 ± 9.7 | 127.7 ± 19.4 a | 99.5 ± 4.8 | 76.9 ± 9.2 b | 81.9 ± 5.6 |
| TC (mg/dL) | 65.4 ± 5.5 | 99.5 ± 5.6 a | 83.8 ± 2.1 | 78.0 ± 4.9 b | 77.6 ± 4.4 b |
| Group I (Control) | Group II (Diabetic) | Group III (50 mg/kg, Cichoriin) | Group IV (100 mg/kg, Cichoriin) | Group V (5 mg/kg, GB) | |
|---|---|---|---|---|---|
| TAC (mM/L) | 1.70 ± 0.01 | 0.60 ± 0.05 a | 0.75 ± 0.03 a | 0.79 ± 0.06 a,b | 0.83 ± 0.04 a,b |
| Catalase (U/L) | 371.4 ± 31.5 | 151.0 ± 24.9 a | 217.5 ± 15.7 a | 395.9 ± 18.5 b,c | 429.9 ± 29.2 b,c |
| SOD (U/g tissue) | 1476 ± 64.4 | 1236 ± 32.1 a | 1314 ± 34.0 | 1526 ± 25.2 b,c | 1554 ± 17.2 b,c |
| MDA (nmol/g tissue) | 39.4 ± 1.9 | 64.0 ± 5.7 a | 53.04 ± 3.8 | 38.7 ± 1.5 b,c | 37.8 ± 1.7 b,c |
| Gene Symbol | Primer Sequence From 5′–3′ |
|---|---|
| GLUT 4 | F: GGTTCCATCCATGAGAGTTATGTGTC R: CTAAAGAGAGAAGGTGTCCGTCG |
| AMPK | F: GTGCCTATGAGCACCAAGTCAG R: TTCATGCTCTGGTTAGGGTGAG |
| PI3K | F: TTAAACGCGAAGGCAACGA R: CAGTCTCCTCCTGCTGTCGAT |
| GAPDH | F: CCTCGTCTCATAGACAAGATGGT R: GGGTAGAGTCATACTGGAACATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, H.E.; Abdelwahab, M.F.; Ibrahim, H.-I.M.; AlYahya, K.A.; Mohamed, A.A.; Radwan, A.S.; Waz, S. Mechanistic Insights into the Ameliorative Effect of Cichoriin on Diabetic Rats—Assisted with an In Silico Approach. Molecules 2022, 27, 7192. https://doi.org/10.3390/molecules27217192
Khalil HE, Abdelwahab MF, Ibrahim H-IM, AlYahya KA, Mohamed AA, Radwan AS, Waz S. Mechanistic Insights into the Ameliorative Effect of Cichoriin on Diabetic Rats—Assisted with an In Silico Approach. Molecules. 2022; 27(21):7192. https://doi.org/10.3390/molecules27217192
Chicago/Turabian StyleKhalil, Hany Ezzat, Miada F. Abdelwahab, Hairul-Islam Mohamed Ibrahim, Khalid A. AlYahya, Ahmed Adel Mohamed, Amira Samir Radwan, and Shaimaa Waz. 2022. "Mechanistic Insights into the Ameliorative Effect of Cichoriin on Diabetic Rats—Assisted with an In Silico Approach" Molecules 27, no. 21: 7192. https://doi.org/10.3390/molecules27217192
APA StyleKhalil, H. E., Abdelwahab, M. F., Ibrahim, H.-I. M., AlYahya, K. A., Mohamed, A. A., Radwan, A. S., & Waz, S. (2022). Mechanistic Insights into the Ameliorative Effect of Cichoriin on Diabetic Rats—Assisted with an In Silico Approach. Molecules, 27(21), 7192. https://doi.org/10.3390/molecules27217192





