Copper-Catalyzed Diboron-Mediated cis-Semi-Hydrogenation of Alkynes under Facile Conditions
Abstract
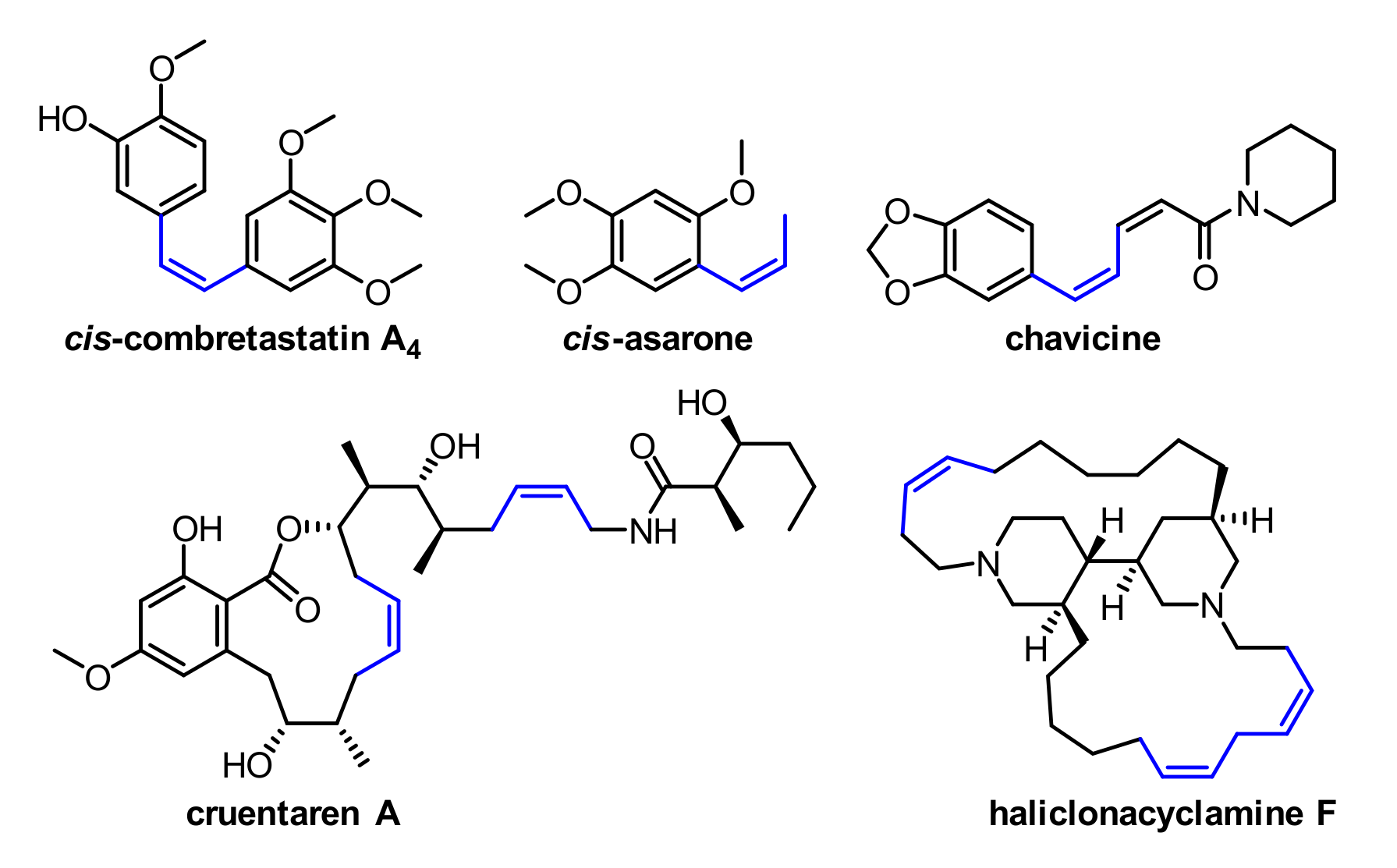
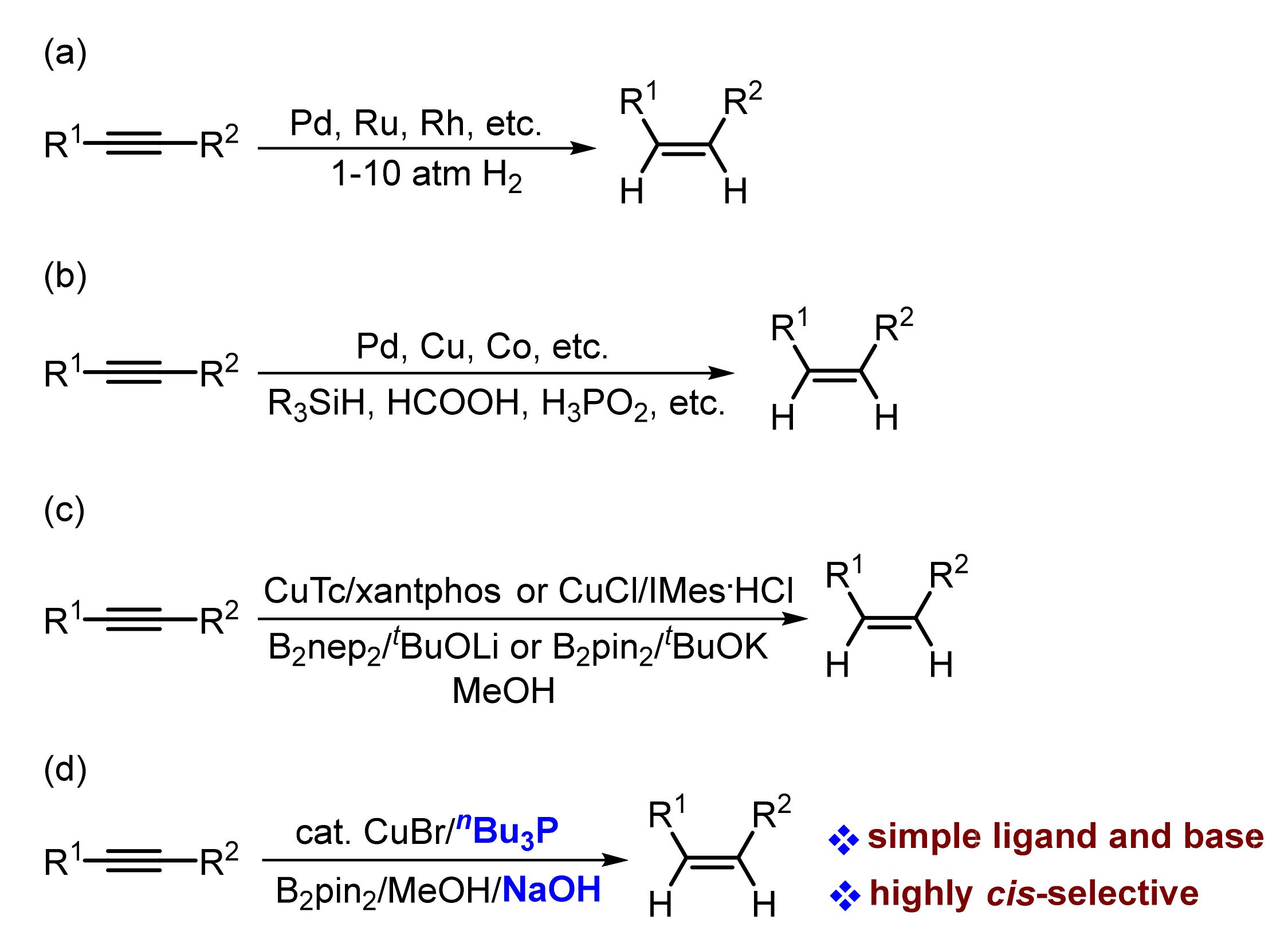
1. Introduction
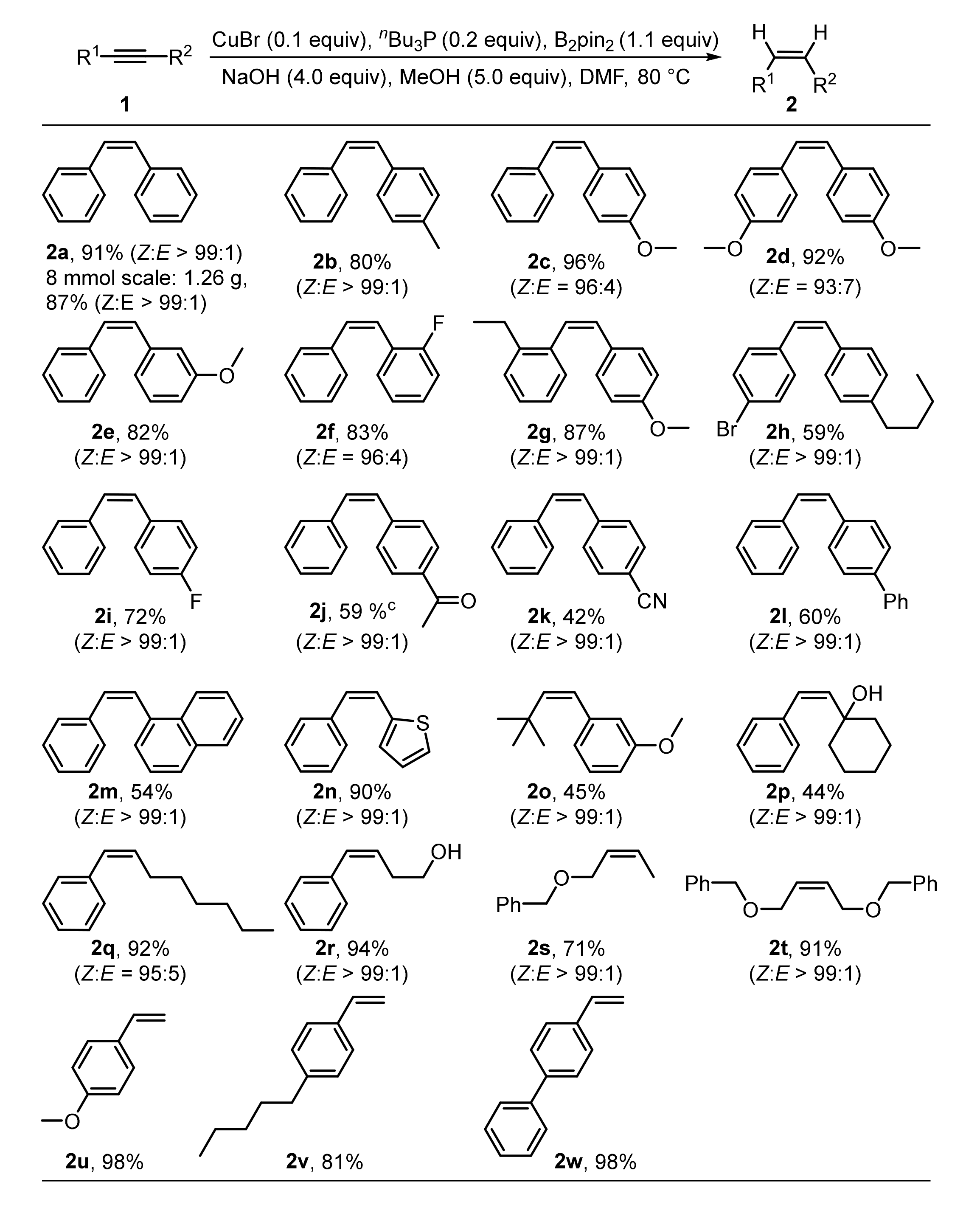
2. Results and Discussions
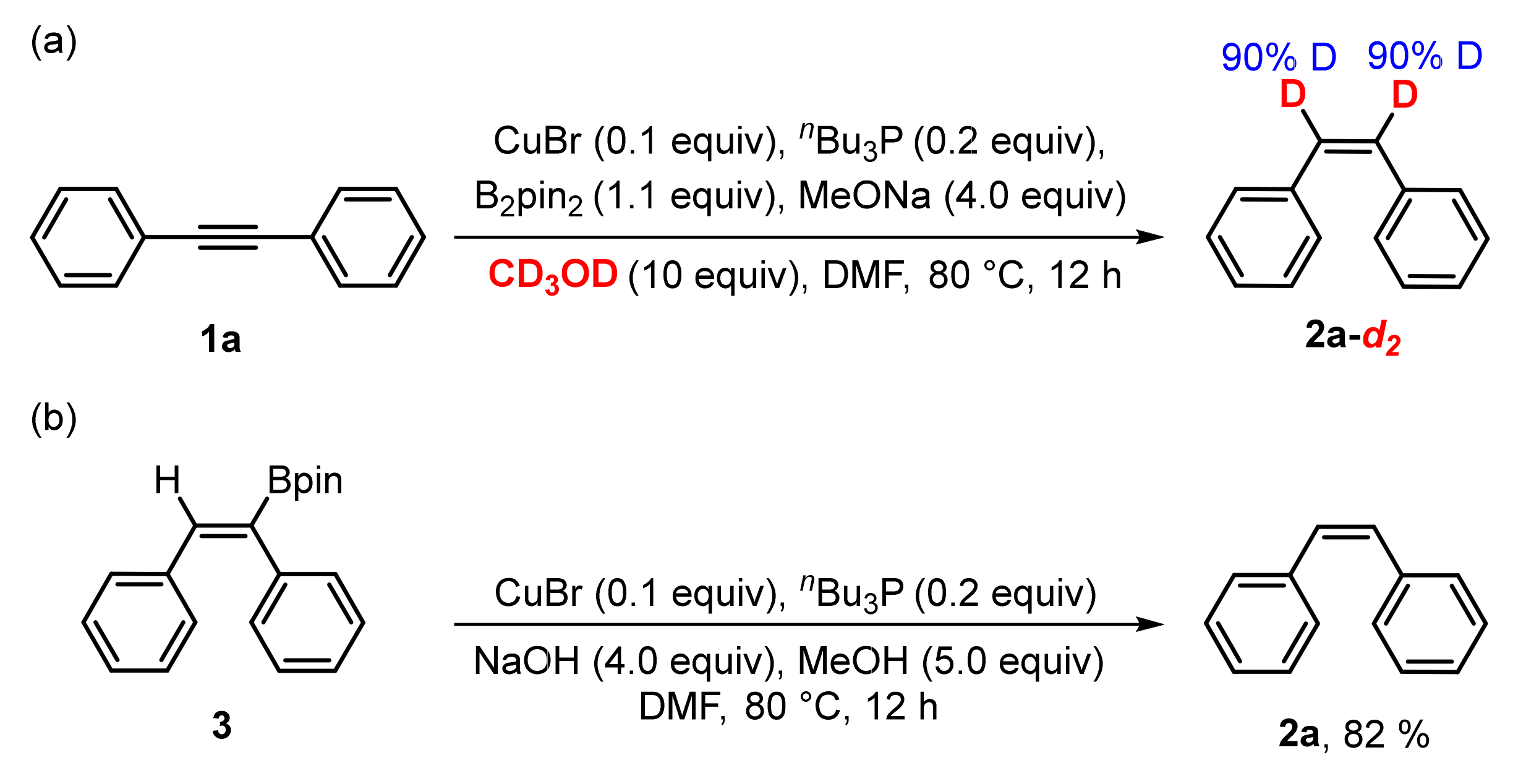
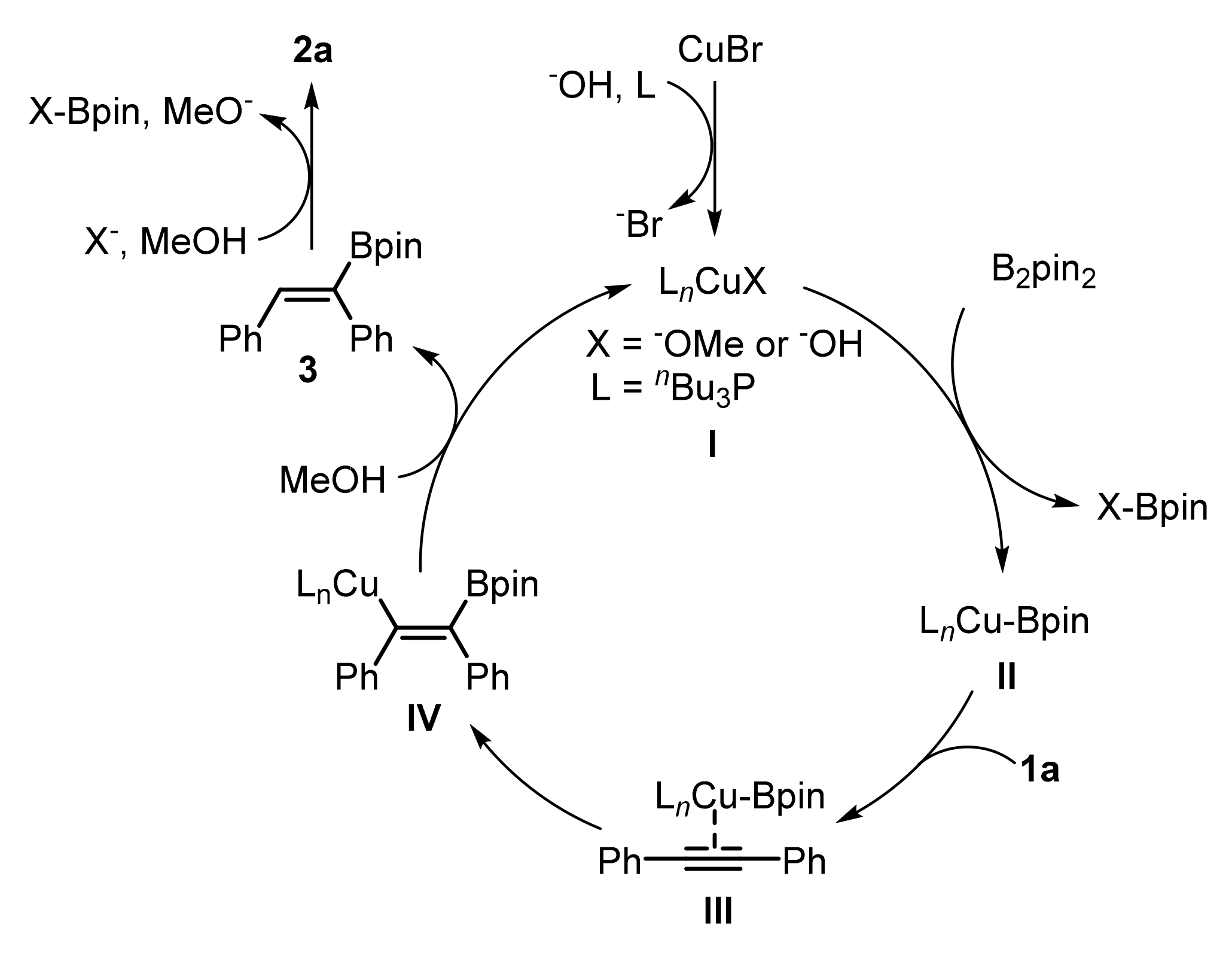
3. Mechanistic Study
4. Materials and Methods
Experimental Procedures and Characterization of Products
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pettit, G.R.; Singh, S.B.; Boyd, M.R.; Hamel, E.; Pettit, R.K.; Schmidt, J.M.; Hogan, F. Antineoplastic Agents. 291. Isolation and Synthesis of Combretastatins A-4, A-5, and A-6. J. Med. Chem. 1995, 38, 1666–1672. [Google Scholar] [CrossRef]
- Oger, C.; Balas, L.; Durand, T.; Galano, J.M. Are Alkyne Reductions Chemo-, Regio-, and Stereoselective Enough to Provide pure (Z)-olefins in Polyfunctionalized Bioactive Molecules? Chem. Rev. 2013, 113, 1313–1350. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Urabe, D.; Masuda, K.; Isobe, Y.; Arita, M.; Inoue, M. Total Synthesis of Four Stereoisomers of (4Z,7Z,10Z,12E,16Z,18E)-14,20-Dihydroxy-4,7,10,12,16,18-Docosahexaenoic Acid and Their Anti-inflammatory Activities. J. Org. Chem. 2015, 80, 7713–7726. [Google Scholar] [CrossRef] [PubMed]
- Kunkalkar, R.A.; Fernandes, R.A. Protecting-Group-Free Total Synthesis of Chatenaytrienin-2. J. Org. Chem. 2019, 84, 12216–12220. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Quesada, M.; Cárdenas-Lizana, F.; Dessimoz, A.-L.; Kiwi-Minsker, L. Modern Trends in Catalyst and Process Design for Alkyne Hydrogenations. ACS Catal. 2012, 2, 1773–1786. [Google Scholar] [CrossRef]
- Parker, G.L.; Smith, L.K.; Baxendale, I.R. Development of the Industrial Synthesis of Vitamin A. Tetrahedron 2016, 72, 1645–1652. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Sorba, G.; Pagliai, F.; Busacca, S.; Genazzani, A.A. Medicinal Chemistry of Combretastatin A4: Present and Future Directions. J. Med. Chem. 2006, 49, 3033–3044. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.Y.; Yun, B.-S.; Hwang, B.K. Antifungal Activity of β-Asarone from Rhizomes of Acorus gramineus. J. Agric. Food Chem. 2004, 52, 776–780. [Google Scholar] [CrossRef]
- Vintonyak, V.V.; Maier, M.E. Total Synthesis of Cruentaren A. Angew. Chem. Int. Ed. 2007, 46, 5209–5211. [Google Scholar] [CrossRef]
- Hall, J.A.; Kusuma, B.R.; Brandt, G.E.L.; Blagg, B.S.J. Cruentaren A Binds F1F0 ATP Synthase to Modulate the Hsp90 Protein Folding Machinery. ACS Chem. Biol. 2014, 9, 976–985. [Google Scholar] [CrossRef]
- Iqbal, G.; Iqbal, A.; Mahboob, A.; Farhat, M.S.; Ahmed, T. Memory Enhancing Effect of Black Pepper in the AlCl3 Induced Neurotoxicity Mouse Model is Mediated Through Its Active Component Chavicine. Curr. Pharm. Biotechnol. 2016, 17, 962–973. [Google Scholar] [CrossRef]
- De Oliveira, J.H.H.L.; Nascimento, A.M.; Kossuga, M.H.; Cavalcanti, B.C.; Pessoa, C.O.; Moraes, M.O.; Macedo, M.L.; Ferreira, A.G.; Hajdu, E.; Pinheiro, U.S.; et al. Cytotoxic Alkylpiperidine Alkaloids from the Brazilian Marine Sponge Pachychalina Alcaloidifera. J. Nat. Prod. 2007, 70, 538–543. [Google Scholar] [CrossRef]
- Maryanoff, B.E.; Reitz, A.B. The Wittig Olefination Reaction and Modifications Involving Phosphoryl-stabilized Carbanions. Stereochemistry, Mechanism, and Selected Synthetic Aspects. Chem. Rev. 1989, 89, 863–927. [Google Scholar] [CrossRef]
- Wadsworth, W.S.; Emmons, W.D. The Utility of Phosphonate Carbanions in Olefin Synthesis. J. Am. Chem. Soc. 1961, 83, 1733–1738. [Google Scholar] [CrossRef]
- Baudin, J.B.; Hareau, G.; Julia, S.A.; Ruel, O. A Direct Synthesis of Olefins by Reaction of Carbonyl Compounds with Lithio Derivatives of 2-[alkyl- or (2′-alkenyl)- or benzyl-sulfonyl]-benzothiazoles. Tetrahedron Lett. 1991, 32, 1175–1178. [Google Scholar] [CrossRef]
- Peterson, D.J. Carbonyl Olefination Reaction Using Silyl-substituted Organometallic Compounds. J. Org. Chem. 1968, 33, 780–784. [Google Scholar] [CrossRef]
- Takai, K.; Nitta, K.; Utimoto, K. Simple and Selective Method for RCHO → (E)-RCH=CHX Conversion by Means of a CHX3-CrCl2 system. J. Am. Chem. Soc. 1986, 108, 7408–7410. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef]
- Chen, X.; Engle, K.M.; Wang, D.-H.; Yu, J.-Q. Palladium(II)-Catalyzed C-H Activation/C-C Cross-Coupling Reactions: Versatility and Practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [Google Scholar] [CrossRef]
- Trost, B.M.; Ball, Z.T.; Jöge, T. A Chemoselective Reduction of Alkynes to (E)-Alkenes. J. Am. Chem. Soc. 2002, 124, 7922–7923. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. Eliminations. In March’s Advanced Organic Chemistry, 5th ed.; Smith, M.B., March, J., Eds.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2006; pp. 1477–1558. [Google Scholar]
- Lindlar, H. Ein neuer Katalysator für selektive Hydrierungen. Helv. Chim. Acta. 1952, 35, 446–450. [Google Scholar] [CrossRef]
- Campos, K.R.; Cai, D.; Journet, M.; Kowal, J.J.; Larsen, R.D.; Reider, P.J. Controlled Semihydrogenation of Aminoalkynes Using Ethylenediamine as a Poison of Lindlar’s Catalyst. J. Org. Chem. 2001, 66, 3634–3635. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Kango, H.; Yamashita, H. Pd Nanoparticles and Aminopolymers Confined in Hollow Silica Spheres as Efficient and Reusable Heterogeneous Catalysts for Semihydrogenation of Alkynes. ACS Catal. 2019, 9, 1993–2006. [Google Scholar] [CrossRef]
- Siau, W.-Y.; Zhang, Y.; Zhao, Y. Stereoselective Synthesis of Z-Alkenes. In Stereoselective Alkene Synthesis, 1st ed.; Wang, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 327, pp. 33–58. [Google Scholar]
- Li, J.; Hua, R.; Liu, T. Highly Chemo- and Stereoselective Palladium-Catalyzed Transfer Semihydrogenation of Internal Alkynes Affording cis-Alkenes. J. Org. Chem. 2010, 75, 2966–2970. [Google Scholar] [CrossRef] [PubMed]
- Radkowski, K.; Sundararaju, B.; Fürstner, A. A Functional-Group-Tolerant Catalytic trans Hydrogenation of Alkynes. Angew. Chem. Int. Ed. 2013, 52, 355–360. [Google Scholar] [CrossRef]
- Karunananda, M.K.; Mankad, N.P. E-Selective Semi-Hydrogenation of Alkynes by Heterobimetallic Catalysis. J. Am. Chem. Soc. 2015, 137, 14598–14601. [Google Scholar] [CrossRef]
- Jagtap, S.A.; Bhanage, B.M. Ligand Assisted Rhodium Catalyzed Selective Semi-hydrogenation of Alkynes Using Syngas and Molecular Hydrogen. ChemistrySelect 2018, 3, 713–718. [Google Scholar] [CrossRef]
- Gilloux, T.; Jacob, G.; Labarthe, E.; Delalu, H.; Darwich, C. Study of the Reactivity of 1,1′-dimethylbistetrazole towards Catalytic Hydrogenation and Chemical Reduction. React. Kinet. Mech. Catal. 2021, 134, 851–865. [Google Scholar] [CrossRef]
- Higashida, K.; Mashima, K. E-Selective Semi-hydrogenation of Alkynes with Dinuclear Iridium Complexes under Atmospheric Pressure of Hydrogen. Chem. Lett. 2016, 45, 866–868. [Google Scholar] [CrossRef]
- La Pierre, H.S.; Arnold, J.; Toste, F.D. Z-Selective Semihydrogenation of Alkynes Catalyzed by a Cationic Vanadium Bisimido Complex. Angew. Chem. Int. Ed. 2011, 50, 3900–3903. [Google Scholar] [CrossRef]
- Gianetti, T.L.; Tomson, N.C.; Arnold, J.; Bergman, R.G. Z-Selective, Catalytic Internal Alkyne Semihydrogenation under H2/CO Mixtures by a Niobium(III) Imido Complex. J. Am. Chem. Soc. 2011, 133, 14904–14907. [Google Scholar] [CrossRef]
- Tokmic, K.; Fout, A.R. Alkyne Semihydrogenation with a Well-Defined Nonclassical Co–H2 Catalyst: A H2 Spin on Isomerization and E-Selectivity. J. Am. Chem. Soc. 2016, 138, 13700–13705. [Google Scholar] [CrossRef]
- Gregori, B.J.; Nowakowski, M.; Schoch, A.; Pöllath, S.; Zweck, J.; Bauer, M.; Jacobi von Wangelin, A. Stereoselective Chromium-Catalyzed Semi-Hydrogenation of Alkynes. ChemCatChem 2020, 12, 5359–5363. [Google Scholar] [CrossRef]
- Brzozowska, A.; Azofra, L.M.; Zubar, V.; Atodiresei, I.; Cavallo, L.; Rueping, M.; El-Sepelgy, O. Highly Chemo- and Stereoselective Transfer Semihydrogenation of Alkynes Catalyzed by a Stable, Well-Defined Manganese(II) Complex. ACS Catal. 2018, 8, 4103–4109. [Google Scholar] [CrossRef]
- Srimani, D.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Iron Pincer Complex Catalyzed, Environmentally Benign, E-Selective Semi-Hydrogenation of Alkynes. Angew. Chem. Int. Ed. 2013, 52, 14131–14134. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Gladiali, S.; Alberico, E. Asymmetric Transfer Hydrogenation: Chiral Ligands and Applications. Chem. Soc. Rev. 2006, 35, 226–236. [Google Scholar] [CrossRef]
- Enthaler, S.; Haberberger, M.; Irran, E. Highly Selective Iron-Catalyzed Synthesis of Alkenes by the Reduction of Alkynes. Chem. Asian J. 2011, 6, 1613–1623. [Google Scholar] [CrossRef]
- Johnson, C.; Albrecht, M. Z-Selective Alkyne Semi-hydrogenation Catalysed by Piano-Stool N-heterocyclic Carbene Iron Complexes. Catal. Sci. Technol. 2018, 8, 2779–2783. [Google Scholar] [CrossRef]
- Drost, R.M.; Bouwens, T.; van Leest, N.P.; de Bruin, B.; Elsevier, C.J. Convenient Transfer Semihydrogenation Methodology for Alkynes Using a PdII-NHC Precatalyst. ACS Catal. 2014, 4, 1349–1357. [Google Scholar] [CrossRef]
- Kaicharla, T.; Zimmermann, B.M.; Oestreich, M.; Teichert, J.F. Using Alcohols as Simple H2-Equivalents for Copper-Catalysed Transfer Semihydrogenations of Alkynes. Chem. Commun. 2019, 55, 13410–13413. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, S.; Liang, Z.; Sun, Y.; Cheng, R.; Yang, B.; Liu, Y.; Yang, J.; Sun, F. Ligand-Promoted Iridium-Catalyzed Transfer Hydrogenation of Terminal Alkynes with Ethanol and Its Application. ACS Omega 2019, 4, 16045–16051. [Google Scholar] [CrossRef] [PubMed]
- Korytiaková, E.; Thiel, N.O.; Pape, F.; Teichert, J.F. Copper(I)-Catalysed Transfer Hydrogenations with Ammonia Borane. Chem. Commun. 2017, 53, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Lim, T.; Han, M.S. A Simple and Efficient in situ Generated Copper Nanocatalyst for Stereoselective Semihydrogenation of Alkynes. Chem. Commun. 2021, 57, 6891–6894. [Google Scholar] [CrossRef]
- Li, J.; Hua, R. Stereodivergent Ruthenium-Catalyzed Transfer Semihydrogenation of Diaryl Alkynes. Chem.-Eur. J. 2011, 17, 8462–8465. [Google Scholar] [CrossRef]
- Guyon, C.; Métay, E.; Popowycz, F.; Lemaire, M. Synthetic Applications of Hypophosphite Derivatives in Reduction. Org. Biomol. Chem. 2015, 13, 7879–7906. [Google Scholar] [CrossRef]
- Chen, T.; Xiao, J.; Zhou, Y.; Yin, S.; Han, L.-B. Nickel-Catalyzed (E)-Selective Semihydrogenation of Internal Alkynes with Hypophosphorous Acid. J. Organomet. Chem. 2014, 749, 51–54. [Google Scholar] [CrossRef]
- Tian, W.-F.; He, Y.-Q.; Song, X.-R.; Ding, H.-X.; Ye, J.; Guo, W.-J.; Xiao, Q. Cis-Selective Transfer Semihydrogenation of Alkynes by Merging Visible-Light Catalysis with Cobalt Catalysis. Adv. Synth. Catal. 2020, 362, 1032–1038. [Google Scholar] [CrossRef]
- Neeve, E.C.; Geier, S.J.; Mkhalid, I.A.; Westcott, S.A.; Marder, T.B. Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse. Chem. Rev. 2016, 116, 9091–9161. [Google Scholar] [CrossRef]
- Wang, M.; Shi, Z. Methodologies and Strategies for Selective Borylation of C-Het and C-C Bonds. Chem. Rev. 2020, 120, 7348–7398. [Google Scholar] [CrossRef]
- Rao, S.; Prabhu, K.R. Stereodivergent Alkyne Reduction by using Water as the Hydrogen Source. Chem.-Eur. J. 2018, 24, 13954–13962. [Google Scholar] [CrossRef] [PubMed]
- Ros, A.; Fernandez, R.; Lassaletta, J.M. Functional Group Directed C-H Borylation. Chem. Soc. Rev. 2014, 43, 3229–3243. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Wen, H.; Ouyang, L.; Luo, N.; Liao, J.; Luo, R. Substrate-Controlled Cu(OAc)2-Catalyzed Stereoselective Semi-Reduction of Alkynes with MeOH as the Hydrogen Source. ACS Omega 2021, 6, 11740–11749. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.P.; Le, T.-N.; Fernandez, G.E.; Quiambao, L.G.; Stokes, B.J. Tetrahydroxydiboron-Mediated Palladium-Catalyzed Transfer Hydrogenation and Deuteriation of Alkenes and Alkynes Using Water as the Stoichiometric H or D Atom Donor. J. Am. Chem. Soc. 2016, 138, 6107–6110. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Zhou, B.; Jin, H.; Liu, Y. Diboron-Assisted Copper-Catalyzed Z-Selective Semihydrogenation of Alkynes Using Ethanol as a Hydrogen Donor. J. Org. Chem. 2019, 84, 3579–3589. [Google Scholar] [CrossRef]
- Han, X.; Hu, J.; Chen, C.; Yuan, Y.; Shi, Z. Copper-Catalysed, Diboron-Mediated cis-Dideuterated Semihydrogenation of Alkynes with Heavy Water. Chem. Commun. 2019, 55, 6922–6925. [Google Scholar] [CrossRef]
- Sun, Z.-Y.; Zhou, S.; Yang, K.; Guo, M.; Zhao, W.; Tang, X.; Wang, G. Tetrahydroxydiboron-Promoted Radical Addition of Alkynols. Org. Lett. 2020, 22, 6214–6219. [Google Scholar] [CrossRef]
- Yang, K.; Wang, P.; Sun, Z.-Y.; Guo, M.; Zhao, W.; Tang, X.; Wang, G. Hydrogen-Bonding Controlled Nickel-Catalyzed Regioselective Cyclotrimerization of Terminal Alkynes. Org. Lett. 2021, 23, 3933–3938. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Wang, G. Tetrahydroxydiboron-Initiated Atom-Transfer Radical Cyclization. Synthesis 2021, 53, 3555–3563. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, L.; Sun, Q.; Guo, M.; Wang, G.; Zhao, W.; Tang, X. Tetrahydroxydiboron and Nickel Chloride Cocatalyzed Rapid Radical Cyclization toward Pyrrolizidine and Indolizidine Alkaloids. J. Org. Chem. 2022, 87, 3788–3793. [Google Scholar] [CrossRef]
- Laitar, D.S.; Müller, P.; Sadighi, J.P. Efficient Homogeneous Catalysis in the Reduction of CO2 to CO. J. Am. Chem. Soc. 2005, 127, 17196–17197. [Google Scholar] [CrossRef]
- Fujihara, T.; Sawada, A.; Yamaguchi, T.; Tani, Y.; Terao, J.; Tsuji, Y. Boraformylation and Silaformylation of Allenes. Angew. Chem. Int. Ed. 2017, 56, 1539–1543. [Google Scholar] [CrossRef]
- Belger, C.; Neisius, N.M.; Plietker, B. A Selective Ru-Catalyzed Semireduction of Alkynes to Z-Olefins under Transfer-Hydrogenation Conditions. Chem.-Eur. J. 2010, 16, 12214–12220. [Google Scholar] [CrossRef]
- Shimasaki, T.; Konno, Y.; Tobisu, M.; Chatani, N. Nickel-Catalyzed Cross-Coupling Reaction of Alkenyl Methyl Ethers with Aryl Boronic Esters. Org. Lett. 2009, 11, 4890–4892. [Google Scholar] [CrossRef]
- Oh, K.-B.; Kim, S.-H.; Lee, J.; Cho, W.-J.; Lee, T.; Kim, S. Discovery of Diarylacrylonitriles as a Novel Series of Small Molecule Sortase A Inhibitors. J. Med. Chem. 2004, 47, 2418–2421. [Google Scholar] [CrossRef]
- Roberts, J.C.; Pincock, J.A. The Photochemical Addition of 2,2,2-Trifluoroethanol to Methoxy-Substituted Stilbenes. J. Org. Chem. 2004, 69, 4279–4282. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.-J.; Li, H.-H.; Tian, S.-K. A Highly Tunable Stereoselective Olefination of Semistabilized Triphenylphosphonium Ylides with N-Sulfonyl Imines. J. Am. Chem. Soc. 2010, 132, 5018–5020. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, J.; Zhong, C.; Izumi, K.-J.; Zhang, C. A Concise and Convenient Synthesis of Stilbenes via Benzils and Arylmethyldiphenylphosphine Oxides. Chin. J. Chem. 2007, 25, 1866–1870. [Google Scholar] [CrossRef]
- Alacid, E.; Nájera, C. Aqueous Sodium Hydroxide Promoted Cross-Coupling Reactions of Alkenyltrialkoxysilanes under Ligand-Free Conditions. J. Org. Chem. 2008, 73, 2315–2322. [Google Scholar] [CrossRef]
- Kim, I.S.; Dong, G.R.; Jung, Y.H. Palladium(II)-Catalyzed Isomerization of Olefins with Tributyltin Hydride. J. Org. Chem. 2007, 72, 5424–5426. [Google Scholar] [CrossRef]
- Deyris, P.-A.; Cañeque, T.; Wang, Y.; Retailleau, P.; Bigi, F.; Maggi, R.; Maestri, G.; Malacria, M. Catalytic Semireduction of Internal Alkynes with All-Metal Aromatic Complexes. ChemCatChem. 2015, 7, 3266–3269. [Google Scholar] [CrossRef]
- Wu, F.-L.; Ross, B.P.; McGeary, R.P. New Methodology for the Conversion of Epoxides to Alkenes. Eur. J. Org. Chem. 2010, 2010, 1989–1998. [Google Scholar] [CrossRef]
- Su, W.; Urgaonkar, S.; Verkade, J.G. Pd2(dba)3/P(i-BuNCH2CH2)3N-Catalyzed Stille Cross-Coupling of Aryl Chlorides. Org. Lett. 2004, 6, 1421–1424. [Google Scholar] [CrossRef]
- Fäh, C.; Mathys, R.; Hardegger, L.A.; Meyer, S.; Bur, D.; Diederich, F. Enantiomerically Pure and Highly Substituted Alicyclic α,α-Difluoro Ketones: Potential Inhibitors for Malarial Aspartic Proteases, the Plasmepsins. Chem. Eur. J. 2010, 2010, 4617–4629. [Google Scholar] [CrossRef]
- Kee, C.H.; Ariffin, A.; Awang, K.; Takeya, K.; Morita, H.; Hussain, S.I.; Chan, K.M.; Wood, P.J.; Threadgill, M.D.; Lim, C.G.; et al. Challenges Associated with the Synthesis of Unusual o-Carboxamido Stilbenes by the Heck Protocol: Intriguing Substituent Effects, Their Toxicological and Chemopreventive Implications. Org. Biomol. Chem. 2010, 8, 5646–5660. [Google Scholar] [CrossRef]
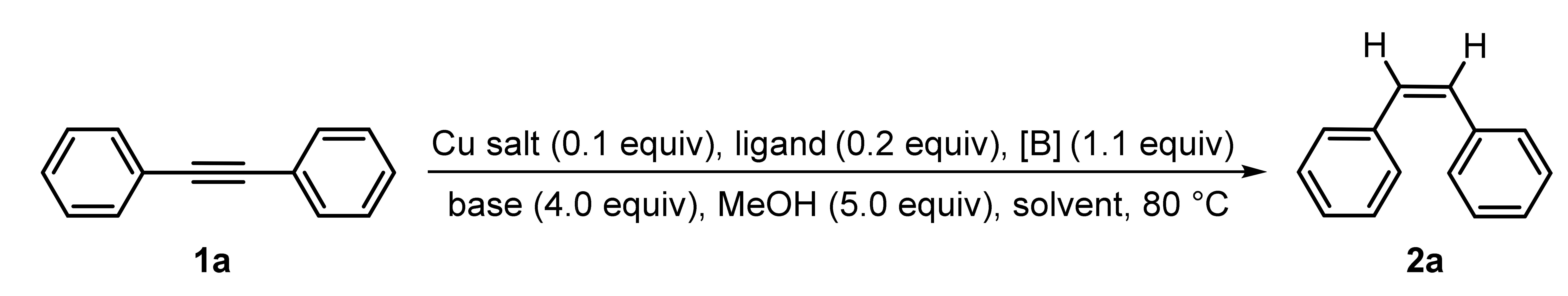
| Entry | Catalyst | Ligand | [B] | Base | Solvent | Yield (%) (Z/E) b |
|---|---|---|---|---|---|---|
| 1 | CuBr | nBu3P | B2pin2 | NaOH | DMF | >98 (91 c, >99:1) |
| 2 | CuI | nBu3P | B2pin2 | NaOH | DMF | 59 (6:1) |
| 3 | CuCl | nBu3P | B2pin2 | NaOH | DMF | 40 (2.4:1) |
| 4 | CuBr2 | nBu3P | B2pin2 | NaOH | DMF | 61 (7.6:1) |
| 5 | CuBr | Ph3P | B2pin2 | NaOH | DMF | 33 (>99:1) |
| 6 | CuBr | DPPB | B2pin2 | NaOH | DMF | 49 (>99:1) |
| 7 | CuBr | Cy3P | B2pin2 | NaOH | DMF | 85 (>99:1) |
| 8 | CuBr | - | B2pin2 | NaOH | DMF | 79 (4.2:1) |
| 9 | CuBr | nBu3P | B2(OH)4 | NaOH | DMF | 17 (>99:1) |
| 10 | CuBr | nBu3P | B2pin2 | LiOtBu | DMF | 92 (19:1) |
| 11 | CuBr | nBu3P | B2pin2 | Na2CO3 | DMF | 56 (12:1) |
| 12 | CuBr | nBu3P | B2pin2 | DBU | DMF | 75 (>99:1) |
| 13 | CuBr | nBu3P | B2pin2 | NaOH | DMSO | 91 (>99:1) |
| 14 | CuBr | nBu3P | B2pin2 | NaOH | THF | 72 (15:1) |
| 15 | CuBr | nBu3P | B2pin2 | NaOH | MeCN | 77 (>99:1) |
| 16 | CuBr | nBu3P | B2pin2 | NaOH | dioxane | 91 (9.5:1) |
| 17 | CuBr | nBu3P | B2pin2 | NaOH | DME | 80 (10:1) |
| 18 d | CuBr | nBu3P | B2pin2 | NaOH | DMF | 20 (7.3:1) |
| 19 e | CuBr | nBu3P | B2pin2 | NaOH | DMF | 64 (21:1) |
| 20 | - | nBu3P | B2pin2 | NaOH | DMF | 0 |
| 21 | CuBr | nBu3P | - | NaOH | DMF | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Zhang, H.; Ma, D.; Wang, G. Copper-Catalyzed Diboron-Mediated cis-Semi-Hydrogenation of Alkynes under Facile Conditions. Molecules 2022, 27, 7213. https://doi.org/10.3390/molecules27217213
Zeng Y, Zhang H, Ma D, Wang G. Copper-Catalyzed Diboron-Mediated cis-Semi-Hydrogenation of Alkynes under Facile Conditions. Molecules. 2022; 27(21):7213. https://doi.org/10.3390/molecules27217213
Chicago/Turabian StyleZeng, Yuxi, Honggang Zhang, Daofan Ma, and Guangwei Wang. 2022. "Copper-Catalyzed Diboron-Mediated cis-Semi-Hydrogenation of Alkynes under Facile Conditions" Molecules 27, no. 21: 7213. https://doi.org/10.3390/molecules27217213
APA StyleZeng, Y., Zhang, H., Ma, D., & Wang, G. (2022). Copper-Catalyzed Diboron-Mediated cis-Semi-Hydrogenation of Alkynes under Facile Conditions. Molecules, 27(21), 7213. https://doi.org/10.3390/molecules27217213






