Microplastic-Free Microcapsules to Encapsulate Health-Promoting Limonene Oil
Abstract
1. Introduction
2. Results & Discussion
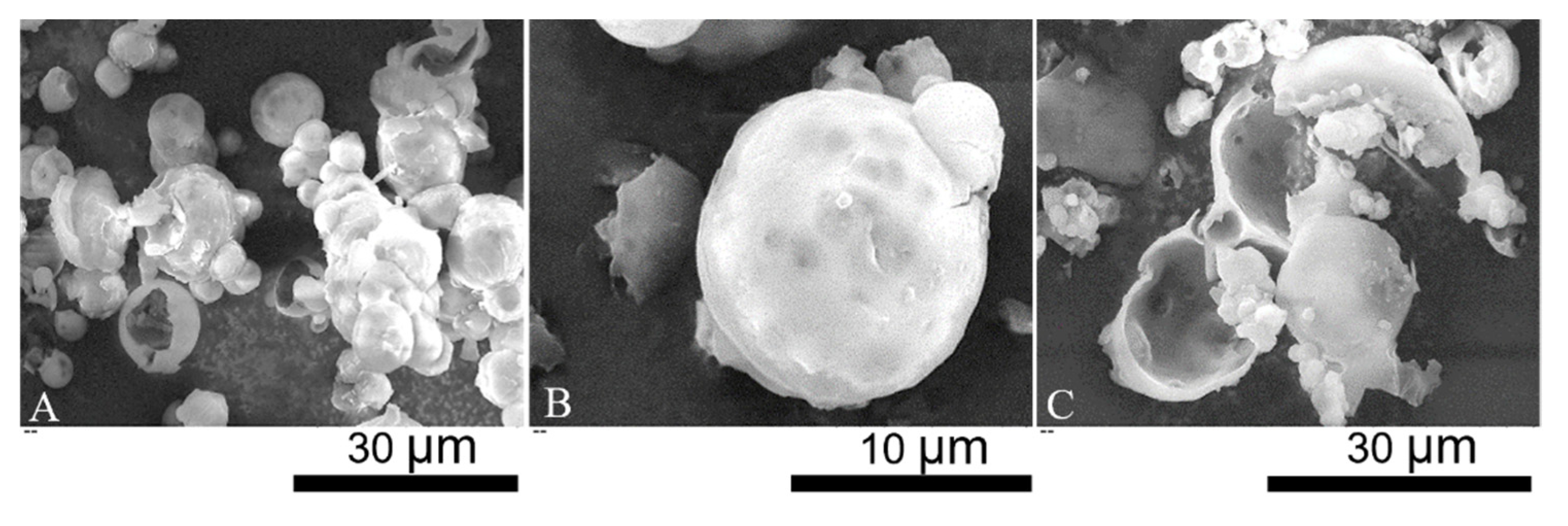
2.1. Morphology
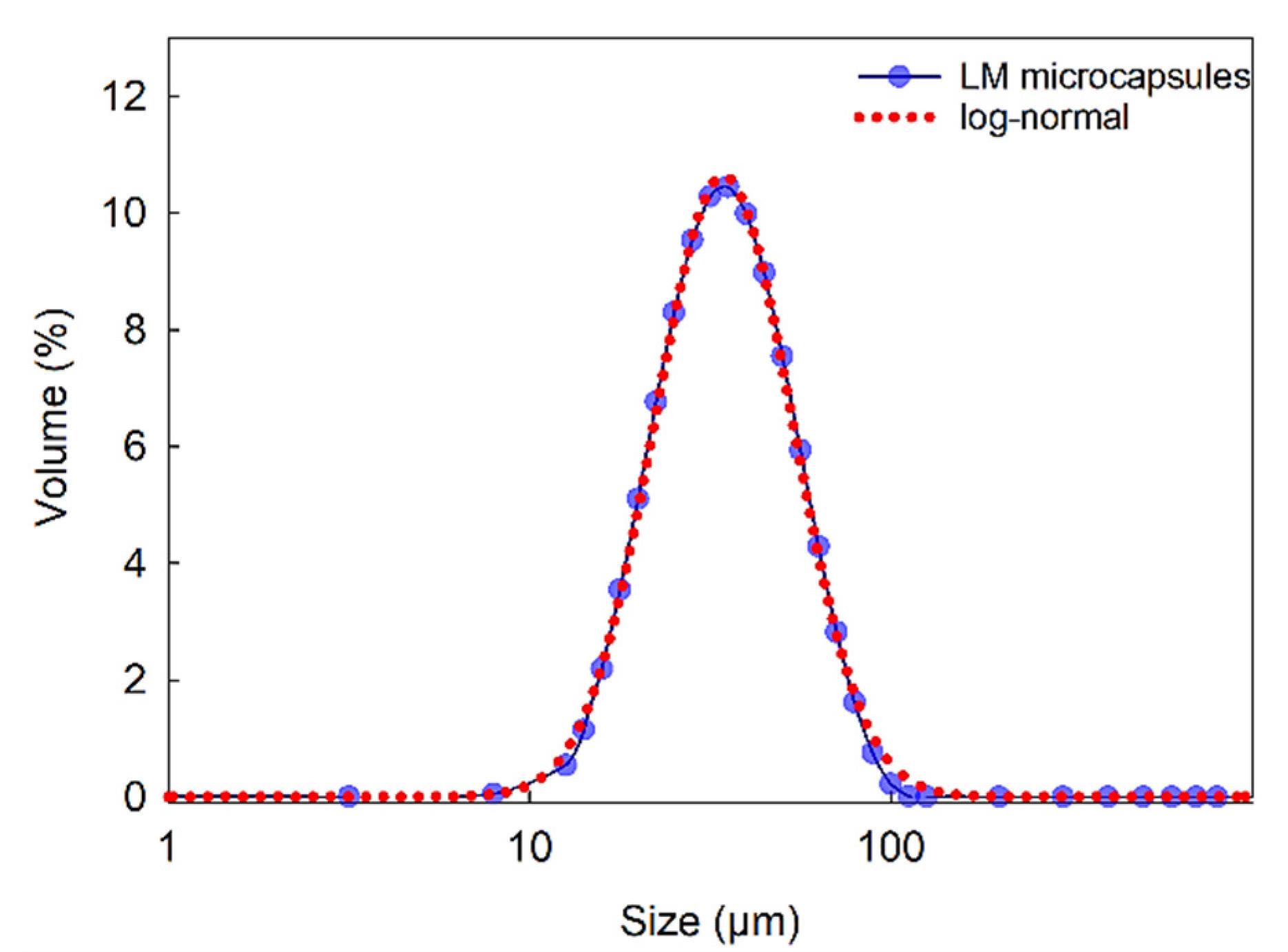
2.2. Particle Size Distribution
2.3. Encapsulation Efficiency and Payload
2.4. Mechanical Properties
| Mechanical Property Parameter | LM-Microcapsules | HS-Microcapsules [10] | HB40-Microcapsules [27,41,42] |
|---|---|---|---|
| Mean Diameter (μm) | 24.4 ± 1.2 | 27.5 ± 1.5 | 4.0–24.0 ± 1.0 |
| Rupture Force (mN) | 0.93 ± 0.1 | 2.0 ± 0.1 | 0.7 ± 0.1– 2.8 ± 0.1 |
| Rupture Tension (N/m) | 37.7 ± 3.0 | 71.6 ± 3.9 | - |
| Nominal Rupture Stress (MPa) | 2.1 ± 0.2 | 3.6 ± 0.3 | 4.2 ± 0.4 |
| Displacement at Rupture (μm) | 3.4 ± 0.4 | 6.3 ± 3.1 | 3.5 ± 0.2 |
| Rupture Deformation (%) | 14.5 ± 1.0 | 22.7 ± 1.5 | 24.8 ± 1.5 |
| Number of particles compressed | 30 | 30 | - |
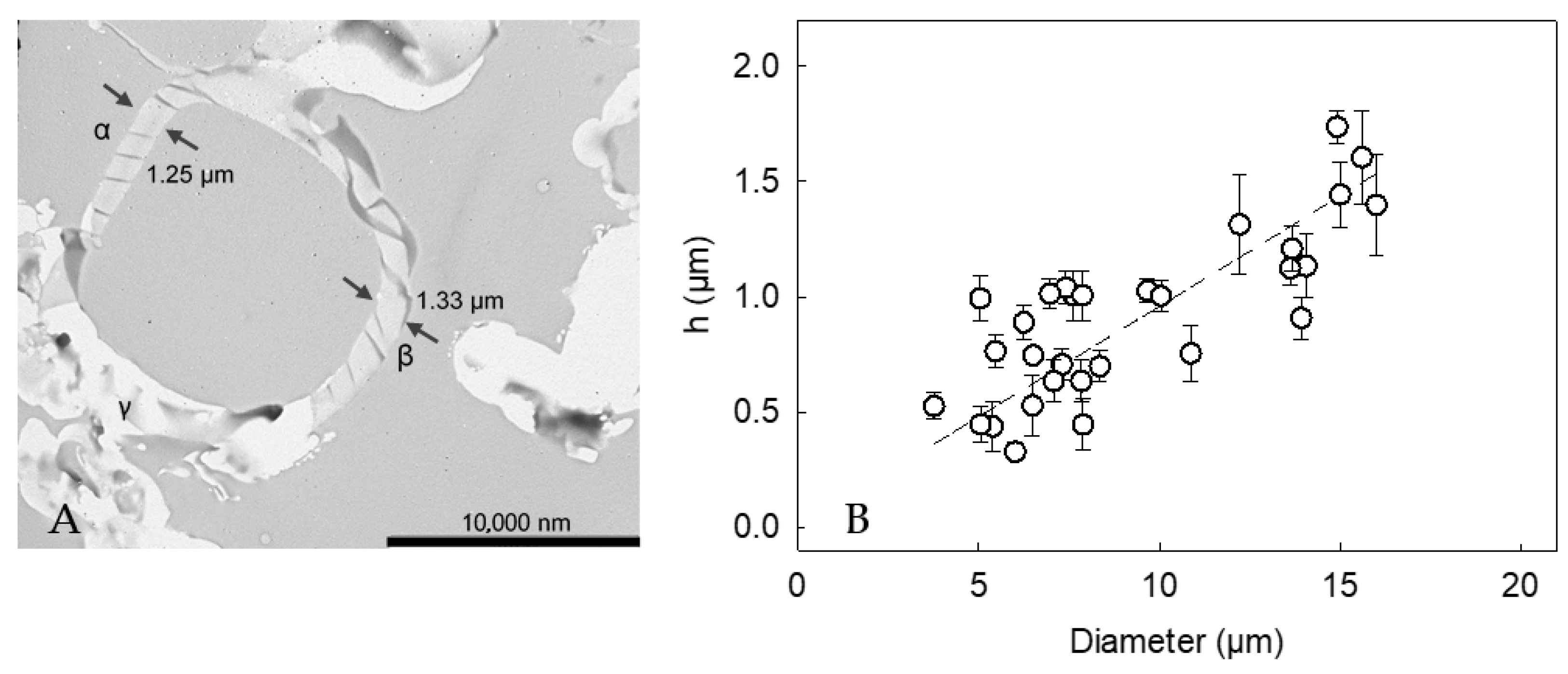
2.5. Shell Thickness
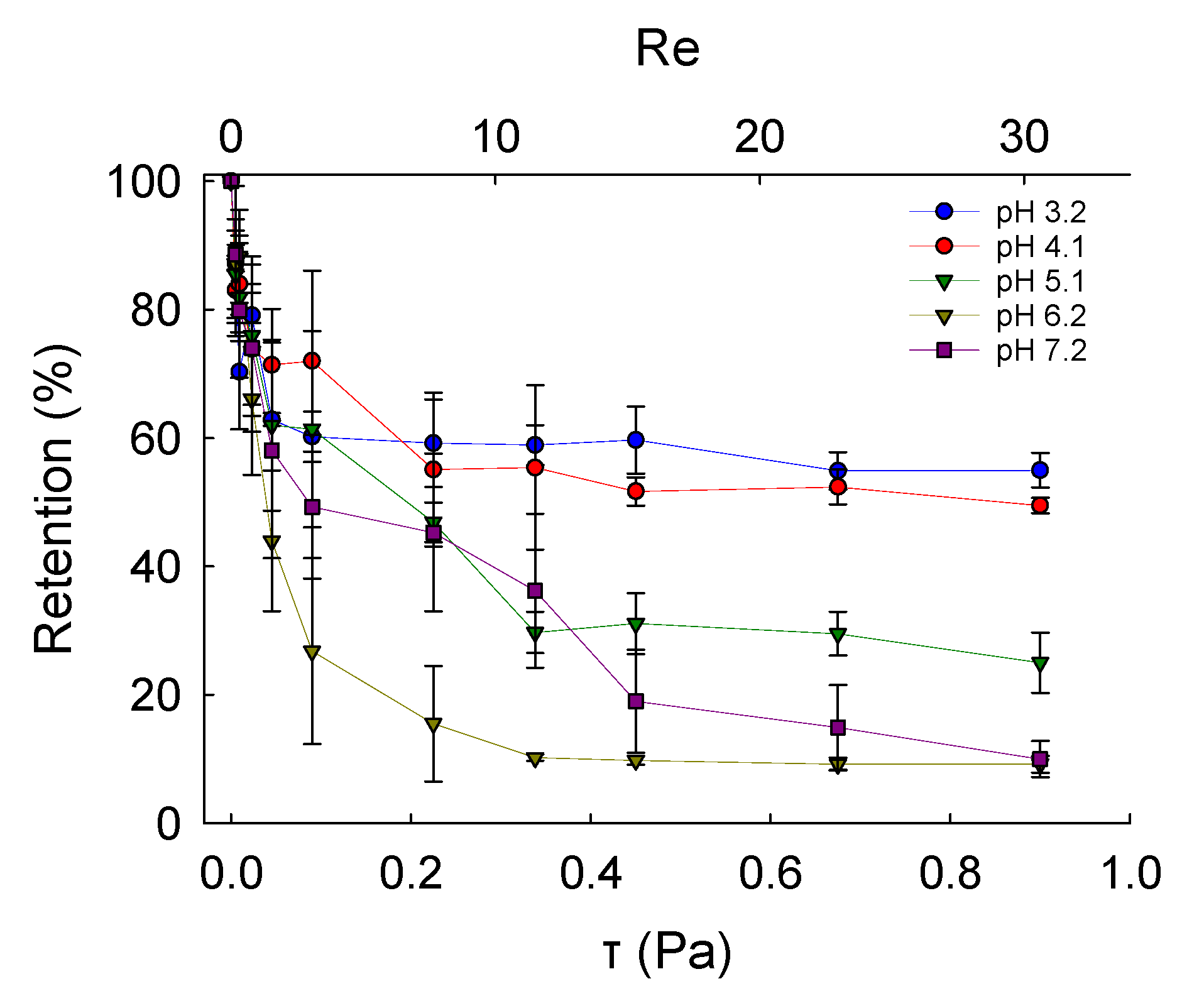
2.6. Adhesive Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of Microcapsules
3.3. Analytical Techniques
3.3.1. Laser Diffraction Particle Size Analysis
3.3.2. Bright-Field Optical Microscopy
3.3.3. Scanning Electron Microscopy (SEM)
3.3.4. Transmission Electron Microscopy (TEM)
3.3.5. Gas Chromatography (GC)
3.3.6. Micromanipulation
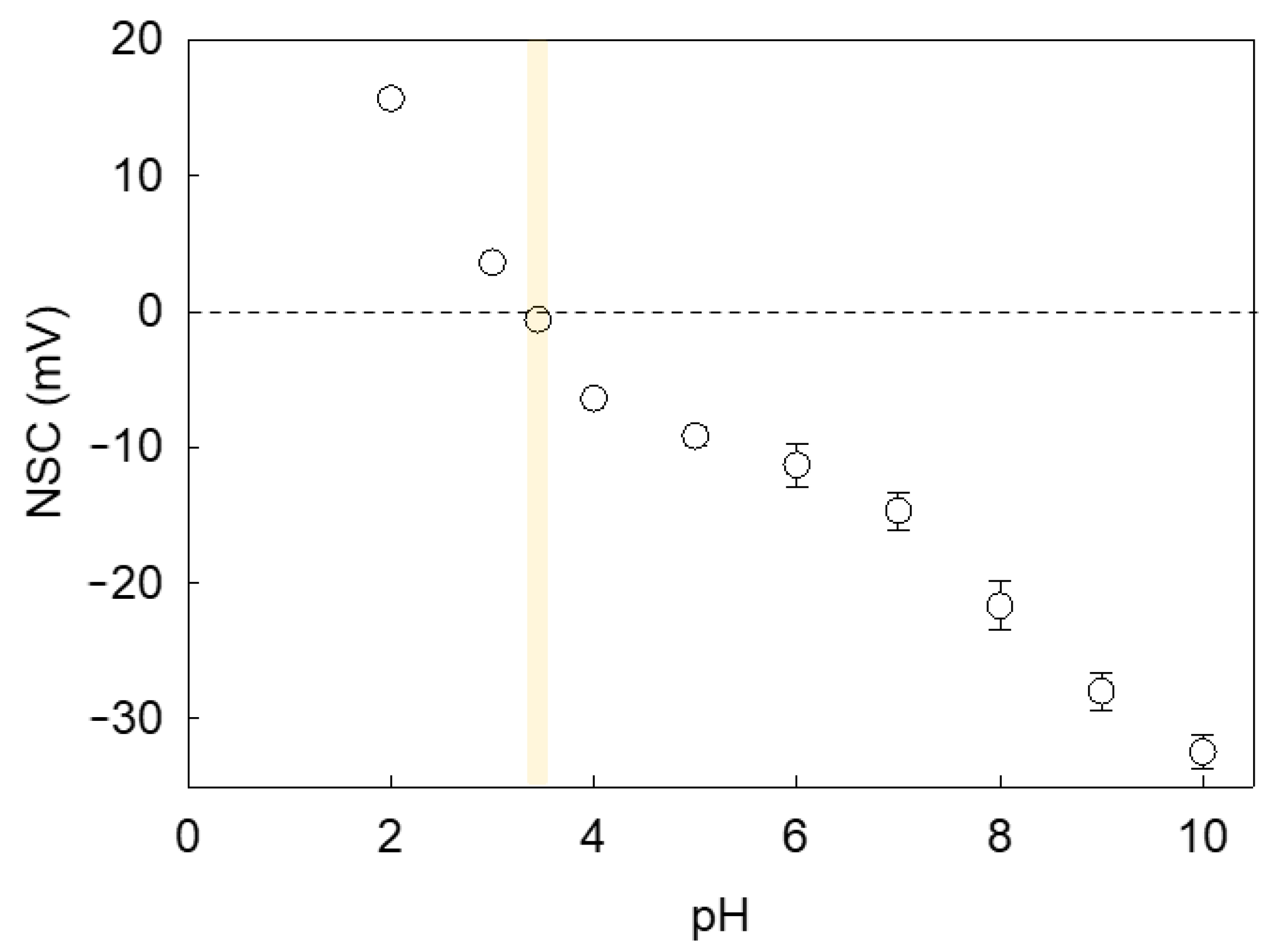
3.3.7. Zeta Potentiometry
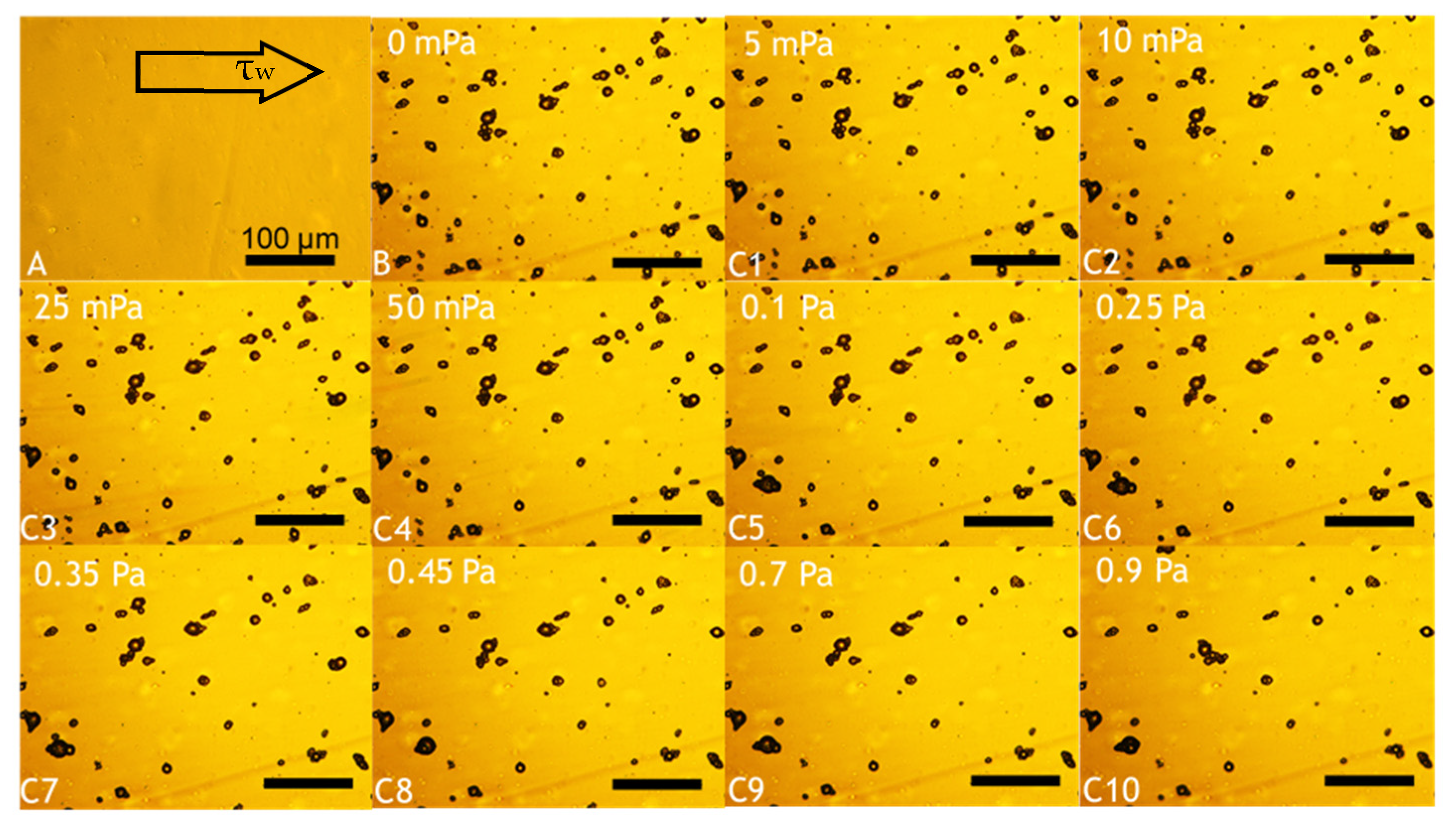
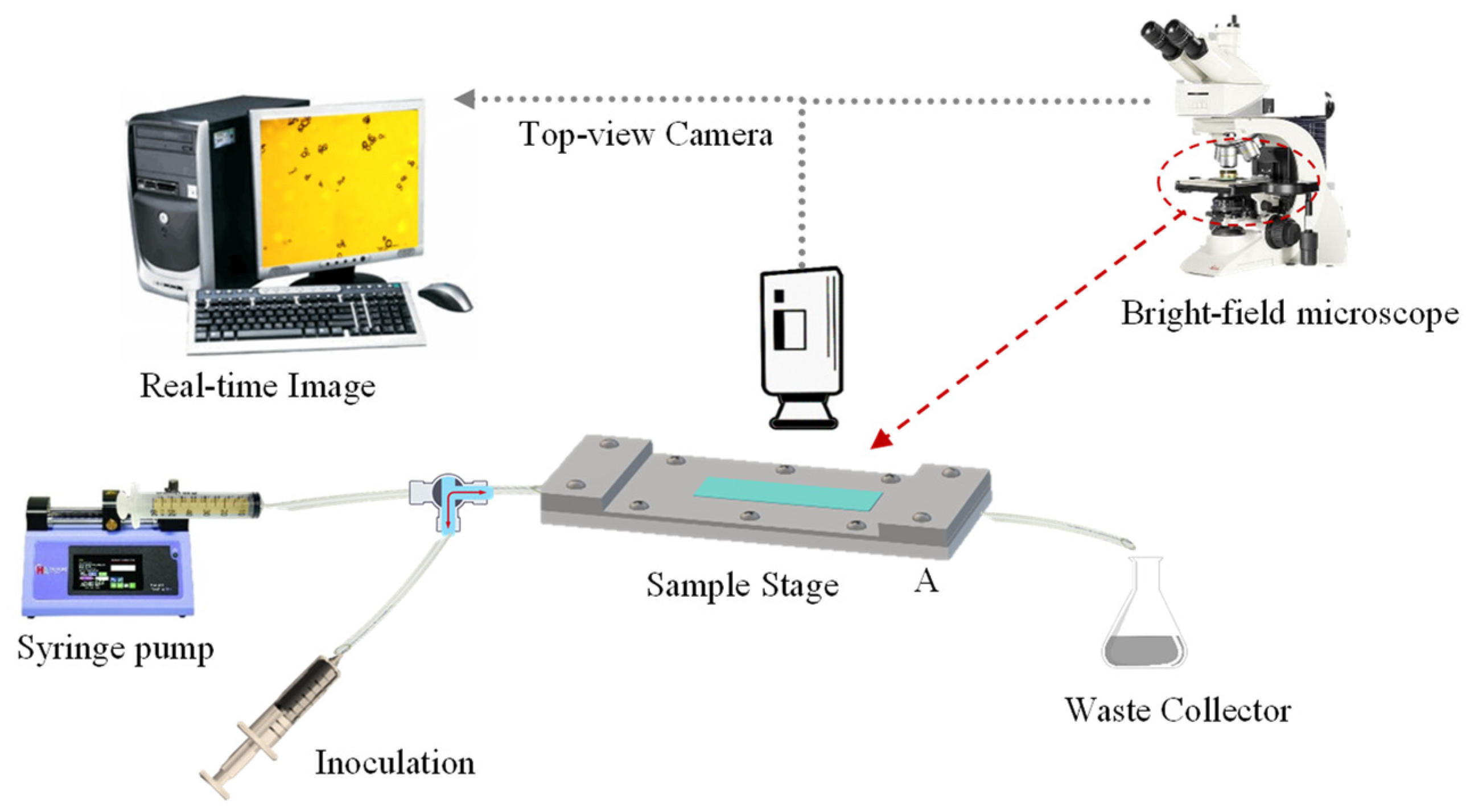
3.3.8. Microfluidic Flow Chamber
Assembly of a Flow Chamber System
Flow Chamber Methodology
Testing Solution
Testing Specimen
Investigation of Microcapsules Deposition and Retention onto PET Films
- i.
- Loading: The test solution at the required pH was infused into the chamber with a syringe pump (5 mL·min−1; 3 min). The outlet of the flow chamber was slightly tilted upwards to achieve no air bubbles within the system. The chamber was then secured onto the flat microscope stage, and configured downwards according to ρr,LM;
- ii.
- Image focusing: The PET film was adjusted into focus onto the lower surface of the PET film in agreement with ρr,LM. The focus included the cross-sectional area of the chamber;
- iii.
- Deposition: A specimen aliquot (4 mL) containing suspended microcapsules was inoculated into the flower chamber. Microcapsules were left to rest to equilibrium (30 min), which was monitored via digitalised optical microscopy;
- iv.
- Cleaning: The test slurry at the required pH and flowrate (15 μL·min−1) was fed into the system (5 min). This step helped to remove free oil droplets, which may be due to microcapsules rupturing when inoculated;
- v.
- Flushing: Increasing flowrates were selected, leading to a gradual increase of the shear stress. Each flowrate (0.065, 0.125, 0.3, 0.6, 1.2, 3.0, 4.5, 6.0, 9.0, 12.0 mL·min−1) was held for 3 min to afford the equilibrium between microcapsules and the PET film. Accordingly, images of microcapsules adhering to the PET film were captured every 3 min from a fixed position via bright-field optical microscopy.
3.3.9. Image Analysis
3.3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated limonene: A pleasant lemon-like aroma with promising application in the agri-food industry. A review. Molecules 2020, 25, 2589. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Ota, Y.; Ito, M. Diversity in principal constituents of plants with a lemony scent and the predominance of citral. J. Nat. Med. 2022, 76, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef] [PubMed]
- Baiocco, D.; Preece, J.A.; Zhang, Z. Microcapsules with a fungal chitosan-gum arabic-maltodextrin shell to encapsulate health-beneficial peppermint oil. Food Hydrocoll. Health 2021, 1, 100016. [Google Scholar] [CrossRef]
- Miller, J.A.; Thompson, P.A.; Hakim, I.A.; Chow, H.H.S.; Thomson, C.A. D-Limonene: A bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Oncol. Rev. 2011, 5, 31–42. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Sun, Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef]
- Chaimovitsh, D.; Shachter, A.; Abu-Abied, M.; Rubin, B.; Sadot, E.; Dudai, N. Herbicidal activity of monoterpenes is associated with disruption of microtubule functionality and membrane integrity. Weed Sci. 2017, 65, 19–30. [Google Scholar] [CrossRef]
- Walia, S.; Saha, S.; Tripathi, V.; Sharma, K.K. Phytochemical biopesticides: Some recent developments. Phytochem. Rev. 2017, 16, 989–1007. [Google Scholar] [CrossRef]
- Baiocco, D.; Preece, J.A.; Zhang, Z. Encapsulation of hexylsalicylate in an animal-free chitosan-gum arabic shell by complex coacervation. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 625, 126861. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Mustapha, A.N.; Zhang, X.; Baiocco, D.; Wellio, G.; Davies, T.; Zhang, Z.; Li, Y. Improved volatile cargo retention and mechanical properties of capsules via sediment-free in situ polymerization with cross-linked poly(vinyl alcohol) as an emulsifier. J. Colloid Interface Sci. 2020, 568, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Baiocco, D.; Mustapha, A.N.; Zhang, X.; Yu, Q.; Wellio, G.; Zhang, Z.; Li, Y. Hydrocolloids: Nova materials assisting encapsulation of volatile phase change materials for cryogenic energy transport and storage. Chem. Eng. J. 2020, 382, 123028. [Google Scholar] [CrossRef]
- Katsumi, N.; Kusube, T.; Nagao, S.; Okochi, H. Accumulation of microcapsules derived from coated fertilizer in paddy fields. Chemosphere 2021, 267, 129185. [Google Scholar] [CrossRef]
- Souza, J.M.; Caldas, A.L.; Tohidi, S.D.; Molina, J.; Souto, A.P.; Fangueiro, R.; Zille, A. Properties and controlled release of chitosan microencapsulated limonene oil. Rev. Bras. De Farmacogn. 2014, 24, 691–698. [Google Scholar] [CrossRef]
- Leclercq, S.; Harlander, K.R.; Reineccius, G.A. Formation and characterization of microcapsules by complex coacervation with liquid or solid aroma cores. Flavour Fragr. J. 2009, 24, 17–24. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, Z.; Li, J.; Li, S.; Kong, L.; Li, P.; Wang, Q. Development and evaluation of novel flavour microcapsules containing vanilla oil using complex coacervation approach. Food Chem 2014, 145, 272–277. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Wang, Q.; Zhao, X.; Yang, H.; Gong, F.; Guo, H. Preparation and antimicrobial activity of pectin-chitosan embedding nisin microcapsules. Eur. Polym. J. 2021, 157, 110676. [Google Scholar] [CrossRef]
- Valle, J.A.B.; Valle, R.d.C.S.C.; Bierhalz, A.C.K.; Bezerra, F.M.; Hernandez, A.L.; Lis Arias, M.J. Chitosan microcapsules: Methods of the production and use in the textile finishing. J. Appl. Polym. Sci. 2021, 138, 50482. [Google Scholar] [CrossRef]
- Haave, M.; Gomiero, A.; Schönheit, J.; Nilsen, H.; Olsen, A.B. Documentation of microplastics in tissues of wild coastal animals. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Han, Z.; Jiang, T.; Xie, L.; Zhang, R. Microplastics impact shell and pearl biomineralization of the pearl oyster Pinctada fucata. Environ. Pollut. 2022, 293, 118522. [Google Scholar] [CrossRef] [PubMed]
- Cverenkárová, K.; Valachovičová, M.; Mackuľak, T.; Žemlička, L.; Bírošová, L. Microplastics in the food chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Do, U.T.; Kim, J.W.; Jo, D.; Luu, Q.S.; Jung, J.; Lee, Y. Biodegradability evaluation of hydroxyethylcellulose-based microcapsules by 1h nuclear magnetic resonance spectroscopy. J. Ind. Eng. Chem. 2021, 95, 51–56. [Google Scholar] [CrossRef]
- Baiocco, D. Fabrication and Characterisation of Vegetable Chitosan Derived Microcapsules. PhD Thesis, University of Birmingham, UK, 2021. [Google Scholar]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A review of crustacean and fungal chitin in wound treatment. Mar Drugs 2020, 18, 64. [Google Scholar] [CrossRef]
- Sánchez-Navarro, M.M.; Pérez-Limiñana, M.Á.; Arán-Ais, F.; Orgilés-Barceló, C. Scent properties by natural fragrance microencapsulation for footwear applications. Polym. Int. 2015, 64, 1458–1464. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, Z. Mechanical properties of melamine-formaldehyde microcapsules. J. Microencapsul. 2001, 18, 593–602. [Google Scholar] [PubMed]
- Lelevic, A.; Souchon, V.; Moreaud, M.; Lorentz, C.; Geantet, C. Gas chromatography vacuum ultraviolet spectroscopy: A review. J. Sep. Sci. 2020, 43, 150–173. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.N.; Fernandes, I.; Martins, I.M.; Mata, V.G.; Barreiro, F.; Rodrigues, A.E. Microencapsulation of limonene for textile application. Ind. Eng. Chem. Res. 2008, 47, 4142–4147. [Google Scholar] [CrossRef]
- Alexandridou, S.; Kiparissides, C. Production of oil-containing polyterephthalamide microcapsules by interfacial polymerization. An experimental investigation of the effect of process variables on the microcapsule size distribution. J. Microencapsul. 1994, 11, 603–614. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Chapter III—Emulsions and microemulsions for topical and transdermal drug delivery. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 59–94. [Google Scholar]
- Liu, E.-H.; McGrath, K.M. Emulsion microstructure and energy input, roles in emulsion stability. Colloids Surf. A: Physicochem. Eng. Asp. 2005, 262, 101–112. [Google Scholar] [CrossRef]
- Qin, Z.H.; Lewandowski, M.; Sun, Y.N.; Shaikhutdinov, S.; Freund, H.J. Encapsulation of Pt nanoparticles as a result of strong metal−support interaction with Fe3O4 (111). J. Phys. Chem. C 2008, 112, 10209–10213. [Google Scholar] [CrossRef]
- Tasker, A.L.; Hitchcock, J.P.; He, L.; Baxter, E.A.; Biggs, S.; Cayre, O. J, The effect of surfactant chain length on the morphology of poly(methyl methacrylate) microcapsules for fragrance oil encapsulation. J. Colloid Interface Sci. 2016, 484, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Szczotok, A.M.; Garrido, I.; Carmona, M.; Kjøniksen, A.-L.; Rodriguez, J.F. Predicting microcapsules morphology and encapsulation efficiency by combining the spreading coefficient theory and polar surface energy component. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 554, 49–59. [Google Scholar] [CrossRef]
- Sharkawy, A.; Fernandes, I.P.; Barreiro, M.F.; Rodrigues, A.E.; Shoeib, T. Aroma-loaded microcapsules with antibacterial activity for eco-friendly textile application: Synthesis, characterization, release, and green grafting. Ind. Eng. Chem. Res. 2017, 56, 5516–5526. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Wu, T.; Wang, L.; Chen, S.; Ding, T.; Hu, Y. Preparation and characterization of citrus essential oils loaded in chitosan microcapsules by using different emulsifiers. J. Food Eng. 2018, 217, 108–114. [Google Scholar] [CrossRef]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef]
- Yow, H.N.; Routh, A.F. Formation of liquid core–polymer shell microcapsules. Soft Matter R. Soc. Chem. 2006, 2, 940–949. [Google Scholar] [CrossRef]
- Spruijt, E.; Sprakel, J.; Cohen Stuart, M.A.; van der Gucht, J. Interfacial tension between a complex coacervate phase and its coexisting aqueous phase. Soft Matter R. Soc. Chem. 2010, 6, 172–178. [Google Scholar] [CrossRef]
- Long, Y.; Song, K.; York, D.; Zhang, Z.; Preece, J.A. Composite microcapsules with enhanced mechanical stability and reduced active ingredient leakage. Particuology 2016, 26, 40–46. [Google Scholar] [CrossRef]
- Long, Y.; York, D.; Zhang, Z.; Preece, J.A. Microcapsules with low content of formaldehyde: Preparation and characterization. J. Mater. Chem. 2009, 19, 6882–6887. [Google Scholar] [CrossRef]
- Mercadé-Prieto, R.; Nguyen, B.; Allen, R.; York, D.; Preece, J.A.; Goodwin, T.E.; Zhang, Z. Determination of the elastic properties of single microcapsules using micromanipulation and finite element modeling. Chem. Eng. Sci. 2011, 66, 2042–2049. [Google Scholar] [CrossRef]
- Gray, A.; Egan, S.; Bakalis, S.; Zhang, Z. Determination of microcapsule physicochemical, structural, and mechanical properties. Particuology 2016, 24, 32–43. [Google Scholar] [CrossRef]
- Endruweit, A.; Zeng, X.; Long, A.C. Effect of specimen history on structure and in-plane permeability of woven fabrics. J. Compos. Mater. 2014, 49, 1563–1578. [Google Scholar] [CrossRef]
- Liu, S. Understanding Molecular Interactions to Enhance Deposition of Perfume Microcapsules on Fabric Surfaces. PhD Thesis, University of Birmingham, Birmingham, UK, 2018. [Google Scholar]
- Butstraen, C.; Salaün, F. Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr. Polym. 2014, 99, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V. Dynamic light scattering based microelectrophoresis: Main prospects and limitations. J. Dispers. Sci. Technol. 2012, 33, 1762–1786. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kaminska, I. Effect of pH and surfactants on the electrokinetic properties of nanoparticles dispersions and their application to the PET fibres modification. J. Mol. Liq. 2020, 320, 114426. [Google Scholar] [CrossRef]
- Moleon, J.A.; Ontiveros-Ortega, A.; Gimenez-Martin, E.; Plaza, I. Effect of N-cetylpyridinium chloride in adsorption of graphene oxide onto polyester. Dye. Pigment. 2015, 122, 310–316. [Google Scholar] [CrossRef]
- Liu, K.M.; Preece, J.A.; York, D.; Bowen, J.; Zhang, Z. Measurement of the adhesion between single melamine-formaldehyde resin microparticles and a flat fabric surface using AFM. J. Adhes. Sci. Technol. 2013, 27, 973–987. [Google Scholar] [CrossRef]
- Giesbers, M.; Kleijn, J.M.; Cohen Stuart, M.A. Interactions between acid- and base-functionalized surfaces. J. Colloid Interface Sci. 2002, 252, 138–148. [Google Scholar] [CrossRef]
- Lane, W.O.; Jantzen, A.E.; Carlon, T.A.; Jamiolkowski, R.M.; Grenet, J.E.; Ley, M.; Haseltine, M.; Galinat, L.J.L.F.-H.; Allen, J.D.; Truskey, G.A.; et al. Parallel-plate flow chamber and continuous flow circuit to evaluate endothelial progenitor cells under laminar flow shear stress. J. Vis. Exp. 2012, 59, 3349. [Google Scholar] [CrossRef]
- Guillemot, G.; Lorthois, S.; Schmitz, P.; Mercier-Bonin, M. Evaluating the adhesion force between saccharomyces cerevisiae yeast cells and polystyrene from shear-flow induced detachment experiments. Chem. Eng. Res. Des. 2007, 85, 800–807. [Google Scholar] [CrossRef]
- Wang, P.; Yang, L.; Hsieh, A.H.; Yang, L.; Hsieh, A.H. Nucleus pulposus cell response to confined and unconfined compression implicates mechanoregulation by fluid shear stress. Ann. Biomed. Eng. 2011, 39, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Bruyninckx, K.; Dusselier, M. Sustainable chemistry considerations for the encapsulation of volatile compounds in laundry-type applications. ACS Sustain. Chem. Eng. 2019, 7, 8041–8054. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baiocco, D.; Zhang, Z. Microplastic-Free Microcapsules to Encapsulate Health-Promoting Limonene Oil. Molecules 2022, 27, 7215. https://doi.org/10.3390/molecules27217215
Baiocco D, Zhang Z. Microplastic-Free Microcapsules to Encapsulate Health-Promoting Limonene Oil. Molecules. 2022; 27(21):7215. https://doi.org/10.3390/molecules27217215
Chicago/Turabian StyleBaiocco, Daniele, and Zhibing Zhang. 2022. "Microplastic-Free Microcapsules to Encapsulate Health-Promoting Limonene Oil" Molecules 27, no. 21: 7215. https://doi.org/10.3390/molecules27217215
APA StyleBaiocco, D., & Zhang, Z. (2022). Microplastic-Free Microcapsules to Encapsulate Health-Promoting Limonene Oil. Molecules, 27(21), 7215. https://doi.org/10.3390/molecules27217215





