Chemical Composition of Tagetes patula Flowers Essential Oil and Hepato-Therapeutic Effect against Carbon Tetrachloride-Induced Toxicity (In-Vivo)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Essential Oil Extraction, and GC/MS Analysis
2.2. Molecular Networking GC Workflow
2.3. Hepatoprotection Study of Essential Oil Extracted from T. Patula
2.3.1. Antioxidant Activity
2.3.2. Biological Evaluation of the Hepatotherapy for EO of T. Patula (In Vivo)
Acute Toxicity of Extracted EO of T. Patula
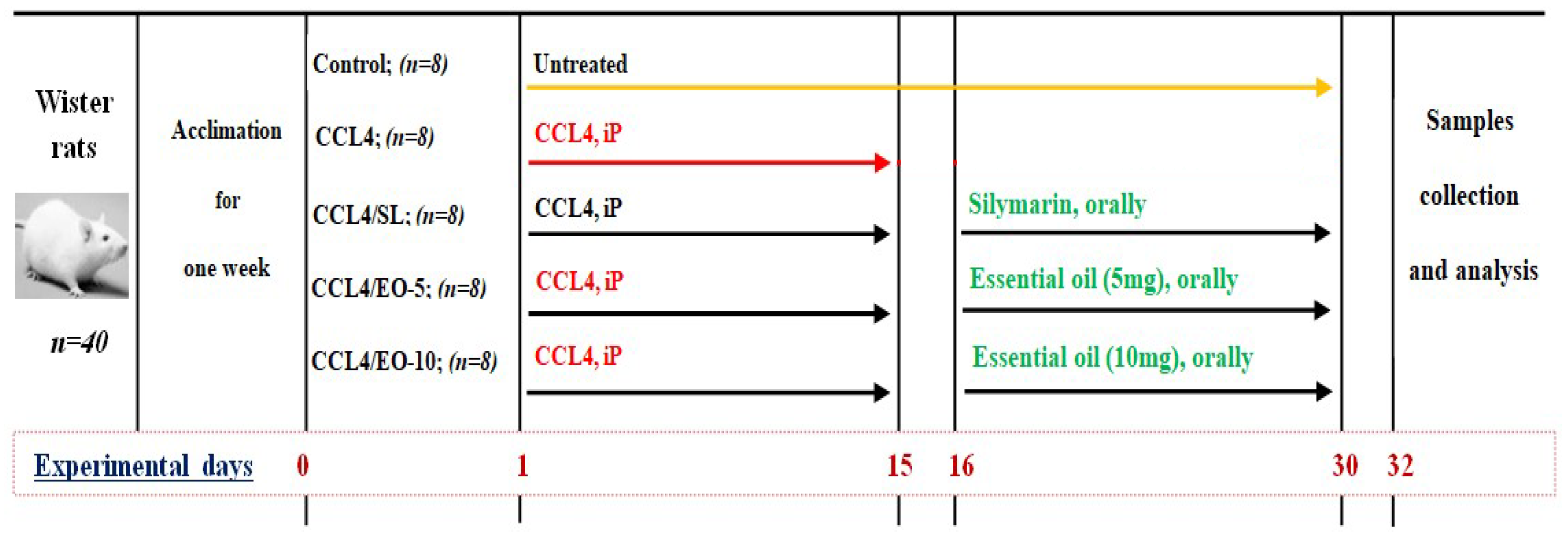
Animals and Experimental Protocol
- ○
- Control group: Untreated group.
- ○
- CCl4 group (CCL4): Rats were treated with intraperitoneal CCL4 at a dose of 1.25 mL kg−1 BW for 15 days.
- ○
- CCL4/Silymarin group (CCL4/SL): Rats received CCL4 at the abovementioned dose for 15 days, and then treated orally with silymarin at a dose of 10 mg kg−1 BW for the next 15 days.
- ○
- CCL4/Essential oil: (CCL4/EO-5): Rats were exposed to CCL4 at a dose of 1.25 mg kg−1 BW for 15 days, and then the extracted EO was administered orally by gavage at a dose of 5 mg kg−1 BW for the next 15 days.
- ○
- CCL4/Essential oil: (CCL4/EO-10): Rats received CCL4 at a dose of 1.25 mg kg−1 BW for 15 days, and then the total phenolic content (TP) of EO was administered orally by gavage at a dose of 10 mg kg−1 BW for the next 15 days.
Histopathological Inspection
Oxidative Stress Biomarkers
Serum Liver Functions Marker
Estimation of Lipid Profiles
2.4. Statistical Analysis
3. Results
3.1. T. Patula EO Chemical Constitutions
3.2. Antioxidants Activity of EO Extracted from T. Patula
3.3. Acute Oral Toxicity of Essential Oil of T. Patula
3.4. Histopathology Findings
3.5. EO Effect on MDA, TP, and NP-SH in CCL4-Treated Rats
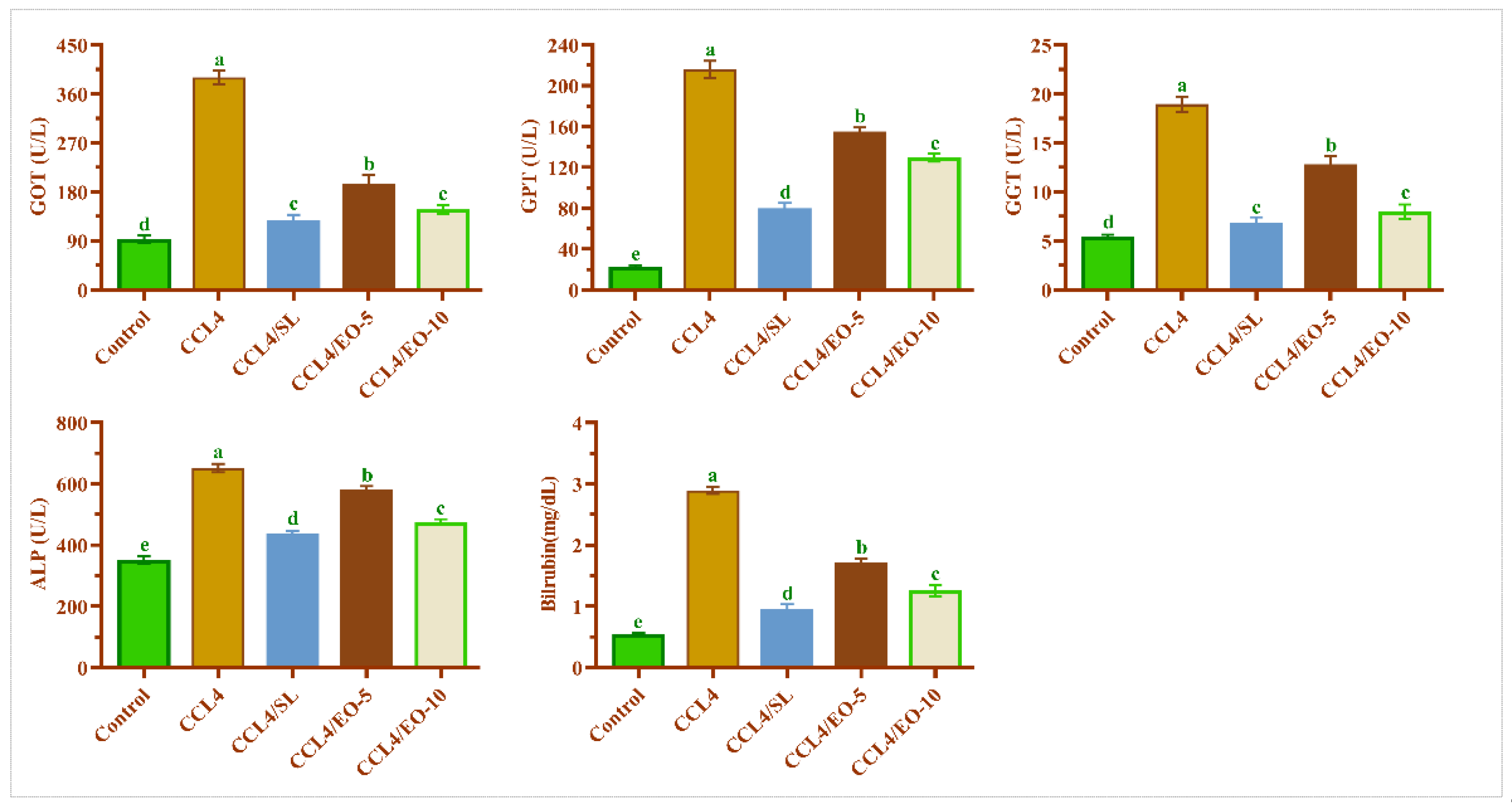
3.6. EO of T. Patula Effect on Serum Liver Functions in CCL4-Treated Rats
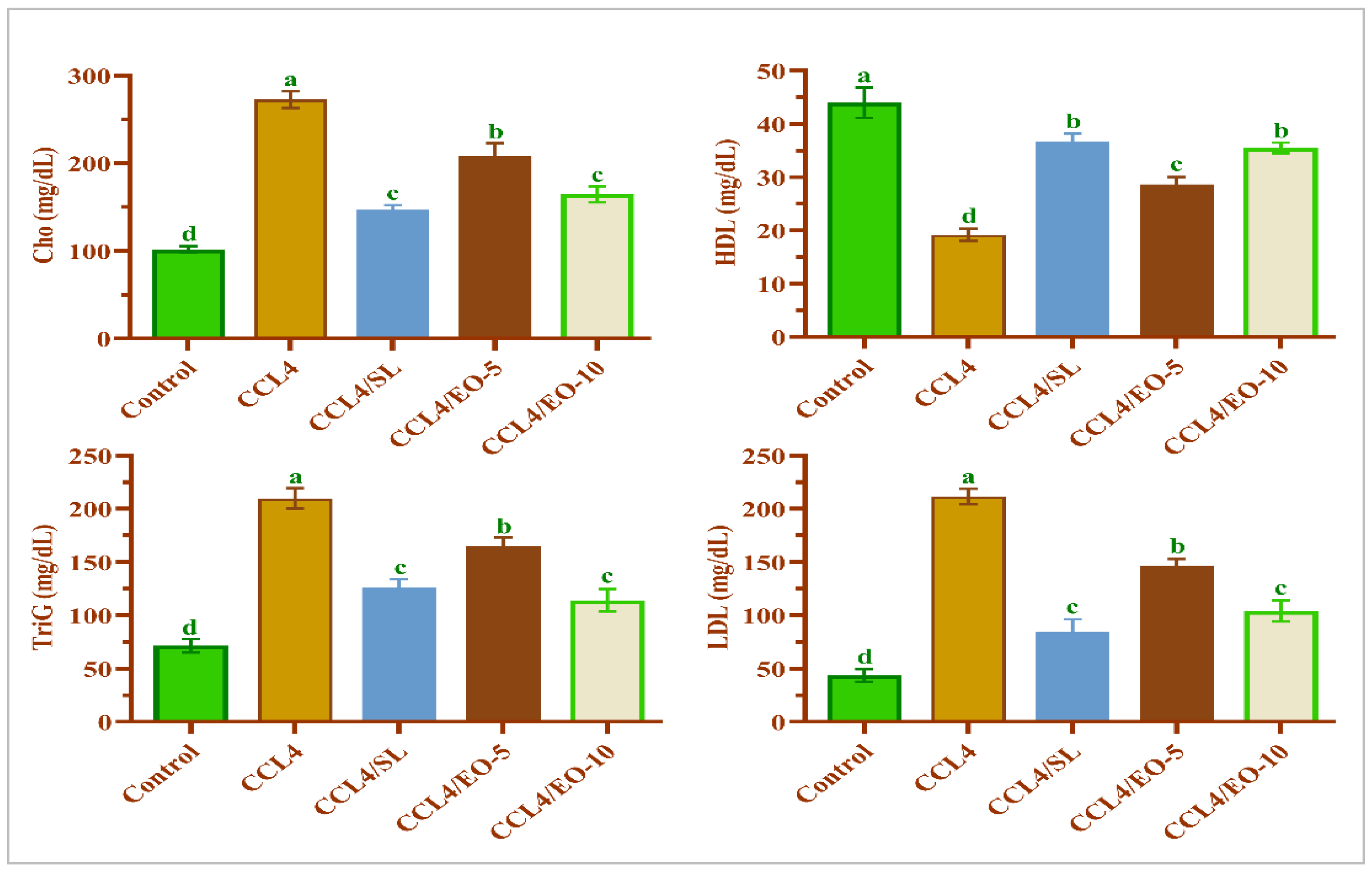
3.7. EO Effect on Lipid Profile in CCL4-Treated Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012, 14, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feijóo, M.; Túnez, I.; Ruiz, A.; Tasset, I.; Muñoz, E.; Collantes, E. Oxidative stress biomarkers as indicator of chronic inflammatory joint diseases stage. Reumatol. Clin. 2010, 6, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Chandan, B.; Saxena, A.; Shukla, S.; Sharma, N.; Gupta, D.; Singh, K.; Suri, J.; Bhadauria, M.; Qazi, G. Hepatoprotective activity of Woodfordia fruticosa Kurz flowers against carbon tetrachloride induced hepatotoxicity. J. Ethnopharmacol. 2008, 119, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, D.-H.; Kang, W.-H.; Shieh, P.-C.; Chen, F.-A.; Chang, C.-D.; Tsai, M.-L.; Cheng, A.-C.; Ho, C.-T.; Pan, M.-H. Protective effect of Pracparatum mungo extract on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem. 2010, 123, 1007–1012. [Google Scholar] [CrossRef]
- Karimi, J.; Mohammadalipour, A.; Sheikh, N.; Khodadadi, I.; Hashemnia, M.; Goudarzi, F.; Khanjarsim, V.; Solgi, G.; Hajilooi, M.; Bahabadi, M. Protective effects of combined Losartan and Nilotinib on carbon tetrachloride (CCl4)-induced liver fibrosis in rats. Drug Chem. Toxicol. 2020, 43, 468–478. [Google Scholar] [CrossRef]
- Huang, H.-L.; Wang, Y.-J.; Zhang, Q.-Y.; Liu, B.; Wang, F.-Y.; Li, J.-J.; Zhu, R.-Z. Hepatoprotective effects of baicalein against CCl4-induced acute liver injury in mice. World J. Gastroenterol. 2012, 18, 6605–6613. [Google Scholar] [CrossRef]
- Chen, X.; Meng, Q.; Wang, C.; Liu, Q.; Sun, H.; Huo, X.; Sun, P.; Yang, X.; Peng, J.; Liu, K. Protective effects of calycosin against CCl 4-induced liver injury with activation of FXR and STAT3 in mice. Pharm. Res. 2015, 32, 538–548. [Google Scholar] [CrossRef]
- Hashem, M.M.; Salama, M.M.; Mohammed, F.F.; Tohamy, A.F.; El Deeb, K.S. Metabolic profile and hepatoprotective effect of Aeschynomene elaphroxylon (Guill. & Perr.). PLoS ONE 2019, 14, 1–24. [Google Scholar]
- Unsal, V.; Cicek, M.; Sabancilar, İ. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev. Environ. Health 2020, 36, 279–295. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Liu, R.; Liu, Y.; Zhang, T.; Fu, H.; Hai, C. Oleanolic acid co-administration alleviates ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing modulating in rats. Chem.-Biol. Interact. 2014, 221, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; Deabes, M.; El-Askary, A.; El-Kott, A.F.; Albadrani, G.M.; Seif, A.; Wang, Z. Ephedra sinica mitigates hepatic oxidative stress and inflammation via suppressing the TLR4/MyD88/NF-κB pathway in fipronil-treated rats. Environ. Sci. Pollut. Res. 2021, 28, 62943–62958. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T. Aromatherapy: Overview, safety and quality issues. OA Altern. Med. 2013, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Faizi, S.; Fayyaz, S.; Bano, S.; Yawar Iqbal, E.; Siddiqi, H.; Naz, A. Isolation of nematicidal compounds from Tagetes patula L. yellow flowers: Structure–activity relationship studies against cyst nematode Heterodera zeae infective stage larvae. J. Agric. Food Chem. 2011, 59, 9080–9093. [Google Scholar] [CrossRef] [PubMed]
- Aati, H.Y.; Perveen, S.; Orfali, R.; Al-Taweel, A.M.; Aati, S.; Wanner, J.; Khan, A.; Mehmood, R. Chemical composition and antimicrobial activity of the essential oils of Artemisia absinthium, Artemisia scoparia, and Artemisia sieberi grown in Saudi Arabia. Arab. J. Chem. 2020, 13, 8209–8217. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Koksal, E.; Bursal, E.; Dikici, E.; Tozoglu, F.; Gulcin, I. Antioxidant activity of Melissa officinalis leaves. J. Med. Plants Res. 2011, 5, 217–222. [Google Scholar]
- Rao, M. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Mamza, U.; Sodipo, O.; Abdulrahman, F.; Khan, I. Evaluation of phytochemical, antimicrobial and toxicity studies of ethanolic stem extract of Phyllanthus amarus Thonn and Schum (Euphorbiacea). Int. J. Sci. Technoledge 2015, 3, 262–268. [Google Scholar]
- Ojeaburu, S.; Oriakhi, K. Hepatoprotective, antioxidant and, anti-inflammatory potentials of gallic acid in carbon tetrachloride-induced hepatic damage in Wistar rats. Toxicol. Rep. 2021, 8, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.S.; Reddy, H.; Deng, S.; Hoffmann, J.; Spangsmark, D.; Madsen, L.B.; Holm-Nielsen, J.B.; Rosendahl, L.A. Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresour. Technol. 2013, 131, 413–419. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Lowery, G.H. A Quantitative Study of The Nocturnal Migration of Birds; University of Kansas: Lawrence, KS, USA, 1951. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Otto, A.; Oliver, H.; Jane, M. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J. Biol. Chem. 1946, 164, 321–329. [Google Scholar]
- Whitfield, J. Gamma glutamyl transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef]
- Doumas, B.T.; Kwok-Cheung, P.P.; Perry, B.W.; Jendrzejczak, B.; McComb, R.B.; Schaffer, R.; Hause, L.L. Candidate reference method for determination of total bilirubin in serum: Development and validation. Clin. Chem. 1985, 31, 1779–1789. [Google Scholar] [CrossRef]
- Davidson, M.H.; Rosenson, R.S. Novel targets that affect high-density lipoprotein metabolism: The next frontier. Am. J. Cardiol. 2009, 104, 52E–57E. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.-S.S.; Salman, M.S.; Fadl, M.A.; Elkhateeb, A.; Hassan, M.M. Chemical composition and biological activity of Mentha longifolia L. essential oil growing in Taif, KSA extracted by hydrodistillation, solvent free microwave and microwave hydrodistillation. J. Essent. Oil Bear. Plants 2018, 21, 1–14. [Google Scholar] [CrossRef]
- Rondon, M.; Velasco, J.; Hernandez, J.; Pecheneda, M.; Rojas, J.; Morales, A.; Carmona, J.; Diaz, T. Chemical composition and antibacterial activity of the essential oil of Tagetes patula L. (Asteraceae) collected from the Venezuela Andes. Rev. Latinoam. Quím. 2006, 34, 32–36. [Google Scholar]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A sesquiterpene with countless biological properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef] [Green Version]
- Mughal, T.A.; Saleem, M.Z.; Ali, S.; Anwar, K.K.; Bashir, M.M.; Babar, M.; Khan, M.A. Evaluation of Hepatotoxicity of Carbon Tetrachloride and Pharmacological Intervention by Vitamin E in Balb C Mice. Pak. J. Zool. 2019, 51, 755–761. [Google Scholar] [CrossRef]
- El-Hadary, A.E.; Elsanhoty, R.M.; Ramadan, M.F. In vivo protective effect of Rosmarinus officinalis oil against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats. PharmaNutrition 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Fadda, L.M.; Ali, H.M.; Abdel Baky, N.A.; El-Orabi, N.F.; Al-Rasheed, N.M.; Yacoub, H.I. New mechanism in the modulation of carbon tetrachloride hepatotoxicity in rats using different natural antioxidants. Toxicol. Mech. Methods 2016, 26, 243–250. [Google Scholar] [CrossRef]
- Moreira, P.R.; Maioli, M.A.; Medeiros, H.C.; Guelfi, M.; Pereira, F.T.; Mingatto, F.E. Protective effect of bixin on carbon tetrachloride-induced hepatotoxicity in rats. Biol. Res. 2014, 47, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Weber, L.W.; Boll, M.; Stampfl, A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef]
- Lombardi, B.; Ugazio, G.; Raick, A. Choline-deficiency fatty liver: Relation of plasma phospholipids to liver triglycerides. Am. J. Physiol.-Leg. Content 1966, 210, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Ullah, H.; Khan, A.; Baig, M.W.; Ullah, N.; Ahmed, N.; Tipu, M.K.; Ali, H.; Khan, S. Poncirin attenuates CCL4-induced liver injury through inhibition of oxidative stress and inflammatory cytokines in mice. BMC Complement. Med. Ther. 2020, 20, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.-Q.; Ding, J.; Zhang, L.; Liu, C.-M. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway. Environ. Toxicol. Pharmacol. 2014, 37, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, R.O.; Glende, E.A.; Britton, R.S. Free radical damage and lipid peroxidation. In Hepatotoxicology; CRC Press: Boca Raton, FL, USA, 2020; pp. 401–436. [Google Scholar]
- Li, L.; Li, W.; Kim, Y.-h.; Lee, Y.W. Chlorella vulgaris extract ameliorates carbon tetrachloride-induced acute hepatic injury in mice. Exp. Toxicol. Pathol. 2013, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Jeong, H.G. Protective effect of Platycodi radix on carbon tetrachloride-induced hepatotoxicity. Food Chem. Toxicol. 2002, 40, 517–525. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, M.; Ahmad, R.; Rahman, N.U.; Khan, Z.; Dou, D.; Sechel, G.; Manea, R. Traditional uses, Phyto-chemistry and pharmacological activities of Tagetes patula L. J. Ethnopharmacol. 2020, 255, 1–16. [Google Scholar] [CrossRef]
- Singh, N.; Thakur, R. A Review on Pharmacological aspects of Tagetes erecta Linn. PharmaTutor 2019, 7, 16–24. [Google Scholar]
- Adeyemi, O.S.; Fambegbe, M.; Daniyan, O.R.; Nwajei, I. Yoyo Bitters, a polyherbal formulation influenced some biochemical parameters in Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2012, 23, 135–138. [Google Scholar] [CrossRef]
- Zafar, R.; Ali, S.M. Anti-hepatotoxic effects of root and root callus extracts of Cichorium intybus L. J. Ethnopharmacol. 1998, 63, 227–231. [Google Scholar] [CrossRef]
- Sadeghi, H.; Jahanbazi, F.; Sadeghi, H.; Omidifar, N.; Alipoor, B.; Kokhdan, E.P.; Mousavipoor, S.M.; Mousavi-Fard, S.H.; Doustimotlagh, A.H. Metformin attenuates oxidative stress and liver damage after bile duct ligation in rats. Res. Pharm. Sci. 2019, 14, 122–129. [Google Scholar] [CrossRef]
- Li, R.; Yang, W.; Yin, Y.; Ma, X.; Zhang, P.; Tao, K. 4-OI Attenuates Carbon Tetrachloride-Induced Hepatic Injury via Regulating Oxidative Stress and the Inflammatory Response. Front. Pharmacol. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- He, J.; Bai, K.; Hong, B.; Zhang, F.; Zheng, S. Docosahexaenoic acid attenuates carbon tetrachloride-induced hepatic fibrosis in rats. Int. Immunopharmacol. 2017, 53, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Bartikova, H.; Hanusova, V.; Skalova, L.; Ambroz, M.; Bousova, I. Antioxidant, pro-oxidant and other biological activities of sesquiterpenes. Curr. Top. Med. Chem. 2014, 14, 2478–2494. [Google Scholar] [CrossRef] [PubMed]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef] [PubMed]
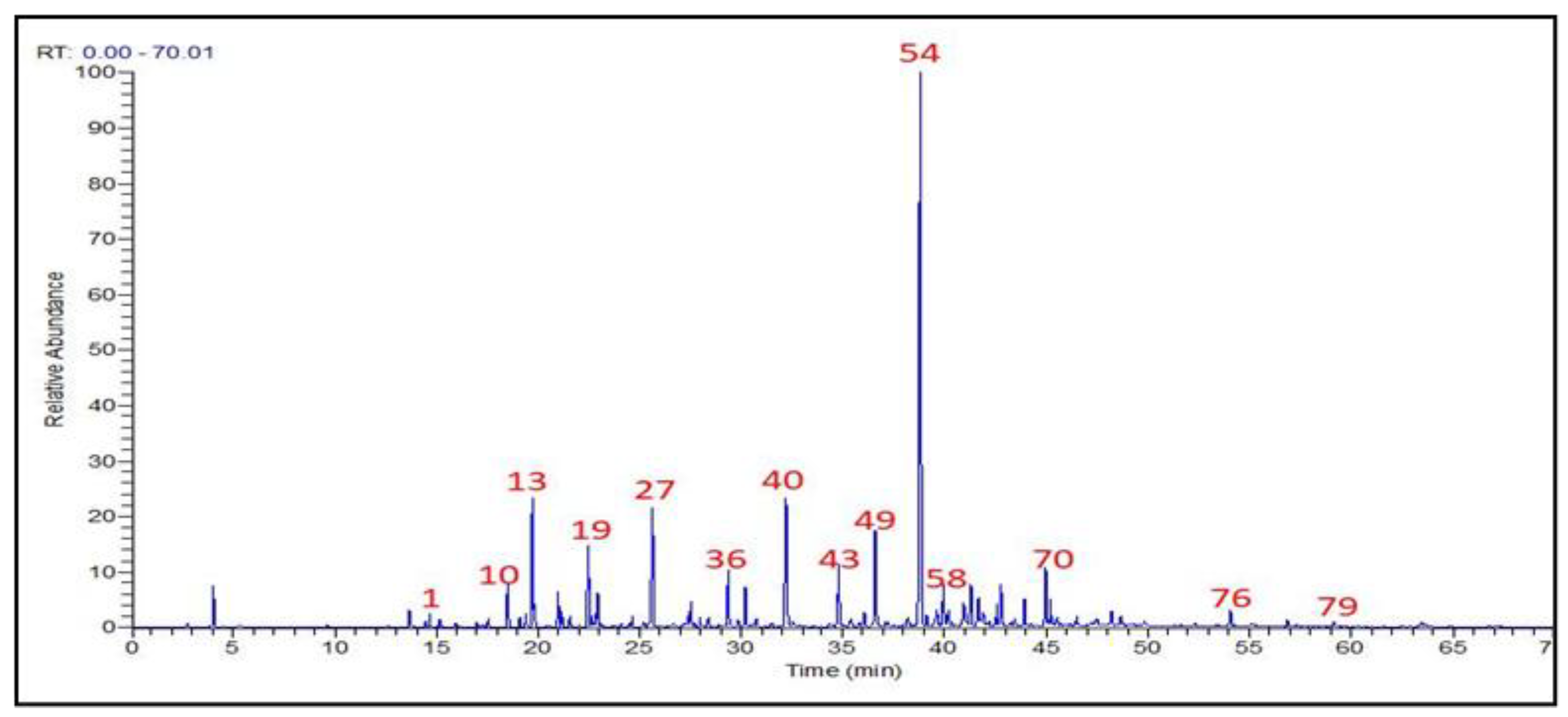
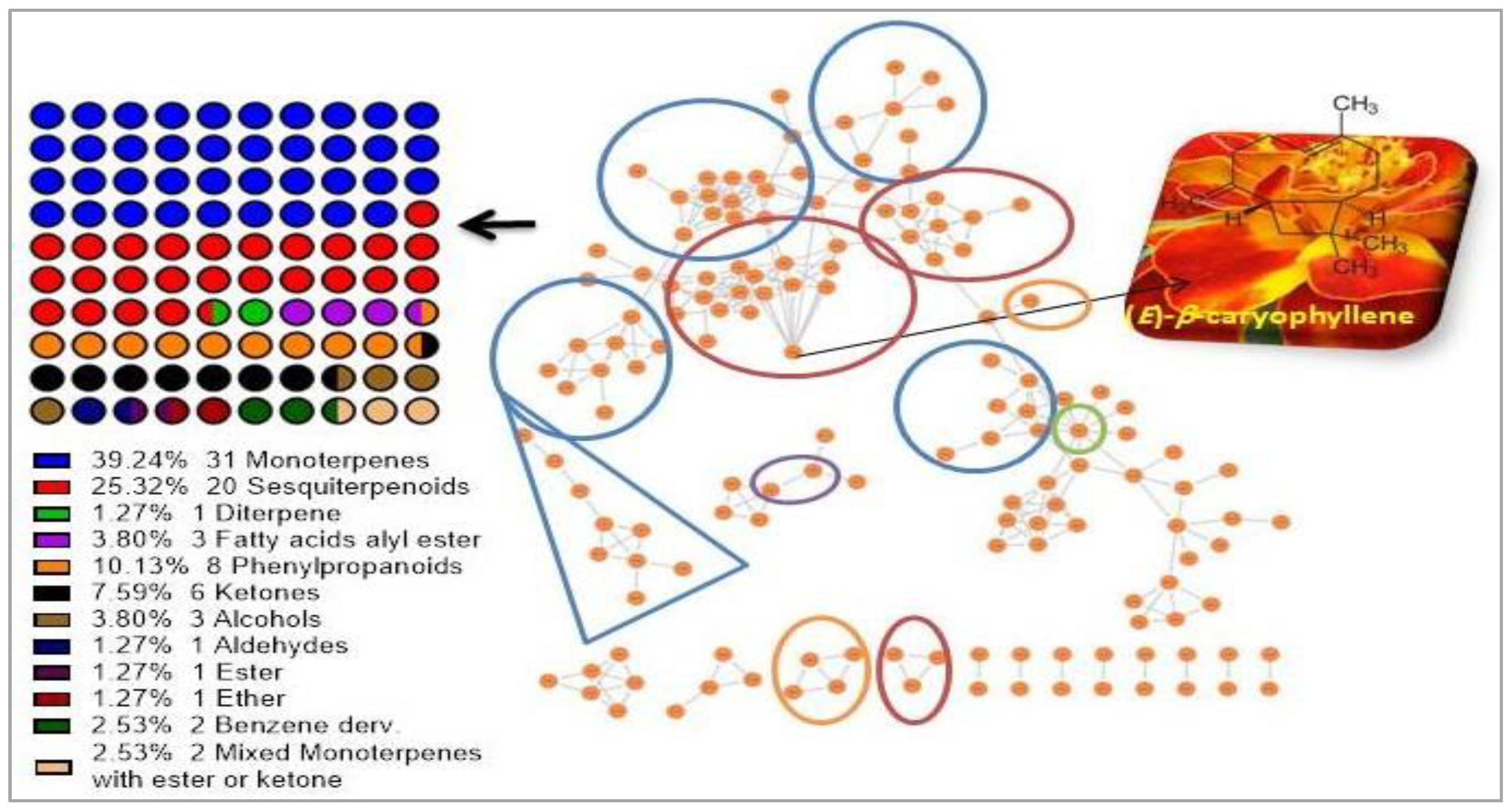


| No. | Apex RT | RRT | Compound | RI | % Area | NP Class |
|---|---|---|---|---|---|---|
| 1 | 14.73 | 0.38 | α-thujene | 932 | 0.3 | Monoterpene |
| 2 | 15.08 | 0.39 | 5-methyl-3-heptanone | 939 | tr. | ketone |
| 3 | 15.17 | 0.39 | α-pinene | 941 | 0.2 | Monoterpene |
| 4 | 15.95 | 0.41 | camphene | 957 | 0.1 | Monoterpene |
| 5 | 16.17 | 0.42 | benzaldehyde | 961 | 0.1 | Shikimates and Phenylpropanoids |
| 6 | 17.01 | 0.44 | sabinene | 979 | 0.1 | Monoterpene |
| 7 | 17.19 | 0.44 | 6-methyl-5-hepten-2-one | 983 | tr. | ketone |
| 8 | 17.31 | 0.45 | β-pinene | 985 | 0.1 | Monoterpene |
| 9 | 17.59 | 0.45 | myrcene | 991 | 0.3 | Monoterpene |
| 10 | 18.52 | 0.48 | α-phellandrene | 1010 | 0.9 | Monoterpene |
| 11 | 19.12 | 0.49 | α-terpinene | 1022 | 0.2 | Monoterpene |
| 12 | 19.45 | 0.50 | p-cymene | 1029 | 0.3 | Monoterpene |
| 13 | 19.75 | 0.51 | limonene | 1035 | 4.3 | Monoterpene |
| 14 | 19.84 | 0.51 | β-phellandrene | 1036 | 0.6 | Monoterpene |
| 15 | 20.42 | 0.53 | (E)-β-ocimene | 1048 | tr. | Monoterpene |
| 16 | 20.6 | 0.53 | dihydrotagetone | 1052 | 0.1 | Acyclic monoterpene |
| 17 | 21.17 | 0.54 | γ-terpinene | 1063 | 0.4 | Monoterpene |
| 18 | 21.61 | 0.56 | Cis-sabinene hydrate (thujanol or 4-thujanol) | 1072 | 0.4 | Monoterpene |
| 19 | 22.48 | 0.58 | 2-nonanone | 1089 | 9.7 | ketone |
| 20 | 22.7 | 0.58 | terpinolene | 1094 | 0.4 | Monoterpene |
| 21 | 22.87 | 0.59 | 2-nonanol | 1097 | 0.8 | fatty alcohols |
| 22 | 22.97 | 0.59 | linalool | 1099 | 1.2 | Monoterpene |
| 23 | 23.1 | 0.59 | nonanal | 1102 | 0.2 | saturated fatty aldehyde |
| 24 | 23.8 | 0.61 | phenylethyl alcohol | 1116 | 0.1 | Volatile alcohol |
| 25 | 24.48 | 0.63 | allo-ocimene | 1130 | 0.1 | Acyclic Monoterpene |
| 26 | 25.19 | 0.65 | (E)-tagetone | 1145 | 0.2 | unsaturated ketone |
| 27 | 25.65 | 0.66 | camphor | 1154 | 4.4 | Monoterpene |
| 28 | 26.7 | 0.69 | isoborneol | 1176 | 0.1 | Monoterpene |
| 29 | 27.18 | 0.70 | terpinen-4-ol | 1185 | 0.2 | Monoterpene |
| 30 | 27.42 | 0.71 | 2-decanone | 1190 | 0.8 | Methyl ketone |
| 31 | 27.56 | 0.71 | dill ether | 1193 | 0.7 | Benzofurans |
| 32 | 27.73 | 0.71 | α-terpineol | 1197 | 0.1 | Monoterpene |
| 33 | 28.02 | 0.72 | cis-dihydrocarvone | 1203 | 0.4 | Monoterpene |
| 34 | 28.42 | 0.73 | trans-dihydrocarvone | 1211 | 0.4 | Monoterpene |
| 35 | 28.92 | 0.74 | shisofuran | 1222 | 0.1 | Monoterpene |
| 36 | 29.41 | 0.76 | 2-nonyl acetate + cis-ocimenone | 1232 | 2.3 | Ester + Monoterpene |
| 37 | 29.83 | 0.77 | trans-ocimenone | 1241 | 0.3 | Monoterpene |
| 38 | 30.23 | 0.78 | carvone | 1250 | 1.2 | Monoterpene |
| 39 | 30.78 | 0.79 | piperitone | 1261 | 0.2 | Monoterpene |
| 40 | 32.23 | 0.83 | 2-undecanone + bornyl acetate | 1292 | 12.2 | dialkyl ketone + acetate ester Monoterpene |
| 41 | 32.52 | 0.84 | 2-undecanol | 1299 | 0.4 | Saturated fatty alcohol |
| 42 | 32.91 | 0.85 | (Z)-methyl cinnamate | 1307 | tr. | Phenylpropanoids |
| 43 | 34.83 | 0.89 | piperitenone | 1350 | 2.0 | Monoterpene |
| 44 | 35.04 | 0.90 | terpinyl acetate | 1355 | 0.1 | Monoterpene |
| 45 | 35.46 | 0.91 | 6-dodecanone | 1364 | 0.5 | Ketone |
| 46 | 35.84 | 0.92 | piperitenone oxide | 1373 | 0.2 | Monoterpene |
| 47 | 36.08 | 0.93 | (Z)-ethyl cinnamate | 1378 | 0.4 | Phenylpropanoids |
| 48 | 36.52 | 0.94 | (E)-methyl cinnamate | 1388 | 0.2 | Phenylpropanoids |
| 49 | 36.64 | 0.94 | biphenyl (I.S.) | 1391 | 3.2 | Shikimates and Phenylpropanoids |
| 50 | 37.13 | 0.96 | methyl eugenol | 1402 | 0.2 | Aromatic ether (Phenylpropanoid) |
| 51 | 37.27 | 0.96 | β-elemene | 1405 | 0.2 | Sesquiterpenoids |
| 52 | 38.04 | 0.98 | cyperene | 1424 | 0.1 | Sesquiterpenoids |
| 53 | 38.24 | 0.98 | 2-undecyl acetate | 1428 | 0.4 | Ester |
| 54 | 38.85 | 1 | (E)-β-caryophyllene | 1443 | 24.1 | Sesquiterpenoids |
| 55 | 39.12 | 1.01 | β-ylangene | 1449 | 0.4 | Sesquiterpenoids |
| 56 | 39.29 | 1.01 | geranyl acetone | 1453 | 0.1 | Monoterpene ketone |
| 57 | 39.63 | 1.02 | aromadendren | 1461 | 0.5 | Sesquiterpenoids |
| 58 | 40 | 1.03 | (Z)-ethyl cinnamate | 1470 | 1.2 | Phenylpropanoids |
| 59 | 40.24 | 1.04 | α-humulene | 1476 | 0.5 | Sesquiterpenoids |
| 60 | 41.34 | 1.06 | germacrene D | 1502 | 1.3 | Sesquiterpenoids |
| 61 | 41.69 | 1.07 | (E,E)-α-farnesene | 1511 | 1.1 | Acyclic Sesquiterpenoids |
| 62 | 41.89 | 1.08 | ledene | 1516 | 0.6 | Sesquiterpenoids |
| 63 | 41.98 | 1.08 | bicyclogermacrene | 1518 | 0.4 | Sesquiterpenoids |
| 64 | 42.57 | 1.09 | γ-cadinene | 1533 | 0.7 | Sesquiterpenoids |
| 65 | 42.81 | 1.10 | delta-cadinene | 1539 | 1.3 | Sesquiterpenoids |
| 66 | 43.29 | 1.11 | cadina-2,4-diene | 1551 | 0.1 | Sesquiterpenoids |
| 67 | 43.49 | 1.12 | α-cadinene | 1556 | 0.2 | Sesquiterpenoids |
| 68 | 43.73 | 1.13 | α-calacorene | 1562 | tr. | Sesquiterpenoids |
| 69 | 43.98 | 1.13 | (E)-nerolidol | 1568 | 0.9 | Sesquiterpene alcohol |
| 70 | 44.99 | 1.16 | cis-davanone | 1593 | 1.8 | Sesquiterpene |
| 71 | 45.26 | 1.16 | spathulenol | 1600 | 1.0 | Sesquiterpene |
| 72 | 45.58 | 1.17 | caryophyllene oxide | 1608 | 0.3 | Sesquiterpenoids |
| 73 | 46.5 | 1.20 | dill apiole | 1632 | 0.3 | Benzodioxoles |
| 74 | 49.85 | 1.28 | davanone-2-ol | 1722 | 0.1 | Phenylpropene |
| 75 | 52.34 | 1.35 | ethyl myristate | 1791 | 0.1 | Fatty acid ethyl ester |
| 76 | 54.09 | 1.39 | neophytadiene | 1841 | 0.6 | Acyclic diterpene |
| 77 | 54.25 | 1.40 | phytone (fitone) | 1846 | 0.1 | Acyclic Sesquiterpe |
| 78 | 56.88 | 1.46 | methyl palmitate | 1922 | 0.2 | Fatty acid methyl ester |
| 79 | 59.2 | 1.52 | ethyl palmitate | 1990 | 0.2 | Fatty acid ethyl ester |
| SUM | 89.8% |
| Treatments | DPPH (µg/mL) | Nitric Oxide (µg/mL) | FRAP FE (μg/mL) |
|---|---|---|---|
| EO T. patula | 29.85 ± 4.53 | 33.19 ± 3.8 | 30.22 ± 2.12 |
| Ascorbic Acid | 21.52 ± 2.02 | 34.63 ± 1.57 | 35.01 ± 2.59 |
| Group | Number of Animals | Dose (mg/kg) | Dose Differences (a) | Dead (n.) | Mean of Mortality (b) | Product (a × b) |
|---|---|---|---|---|---|---|
| 1 | 6 | 10 | - | 0 | - | - |
| 2 | 6 | 20 | 10 | 0 | 0 | 0 |
| 3 | 6 | 50 | 30 | 1 | 0.5 | 15 |
| 4 | 6 | 100 | 50 | 2 | 1.5 | 75 |
| 5 | 6 | 200 | 100 | 3 | 3.5 | 350 |
| 440 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aati, H.Y.; Emam, M.; Al-Qahtani, J.; Aati, S.; Aati, A.; Wanner, J.; Seif, M.M. Chemical Composition of Tagetes patula Flowers Essential Oil and Hepato-Therapeutic Effect against Carbon Tetrachloride-Induced Toxicity (In-Vivo). Molecules 2022, 27, 7242. https://doi.org/10.3390/molecules27217242
Aati HY, Emam M, Al-Qahtani J, Aati S, Aati A, Wanner J, Seif MM. Chemical Composition of Tagetes patula Flowers Essential Oil and Hepato-Therapeutic Effect against Carbon Tetrachloride-Induced Toxicity (In-Vivo). Molecules. 2022; 27(21):7242. https://doi.org/10.3390/molecules27217242
Chicago/Turabian StyleAati, Hanan Y., Mahmoud Emam, Jawaher Al-Qahtani, Sultan Aati, Abdulrahman Aati, Juergen Wanner, and Mohamed M. Seif. 2022. "Chemical Composition of Tagetes patula Flowers Essential Oil and Hepato-Therapeutic Effect against Carbon Tetrachloride-Induced Toxicity (In-Vivo)" Molecules 27, no. 21: 7242. https://doi.org/10.3390/molecules27217242
APA StyleAati, H. Y., Emam, M., Al-Qahtani, J., Aati, S., Aati, A., Wanner, J., & Seif, M. M. (2022). Chemical Composition of Tagetes patula Flowers Essential Oil and Hepato-Therapeutic Effect against Carbon Tetrachloride-Induced Toxicity (In-Vivo). Molecules, 27(21), 7242. https://doi.org/10.3390/molecules27217242





