2′-Fucosyllactose Suppresses Angiogenesis and Alleviates Toxic Effects of 5-Fu in a HCT116 Colon Tumor-Bearing Model
Abstract
1. Introduction
2. Results
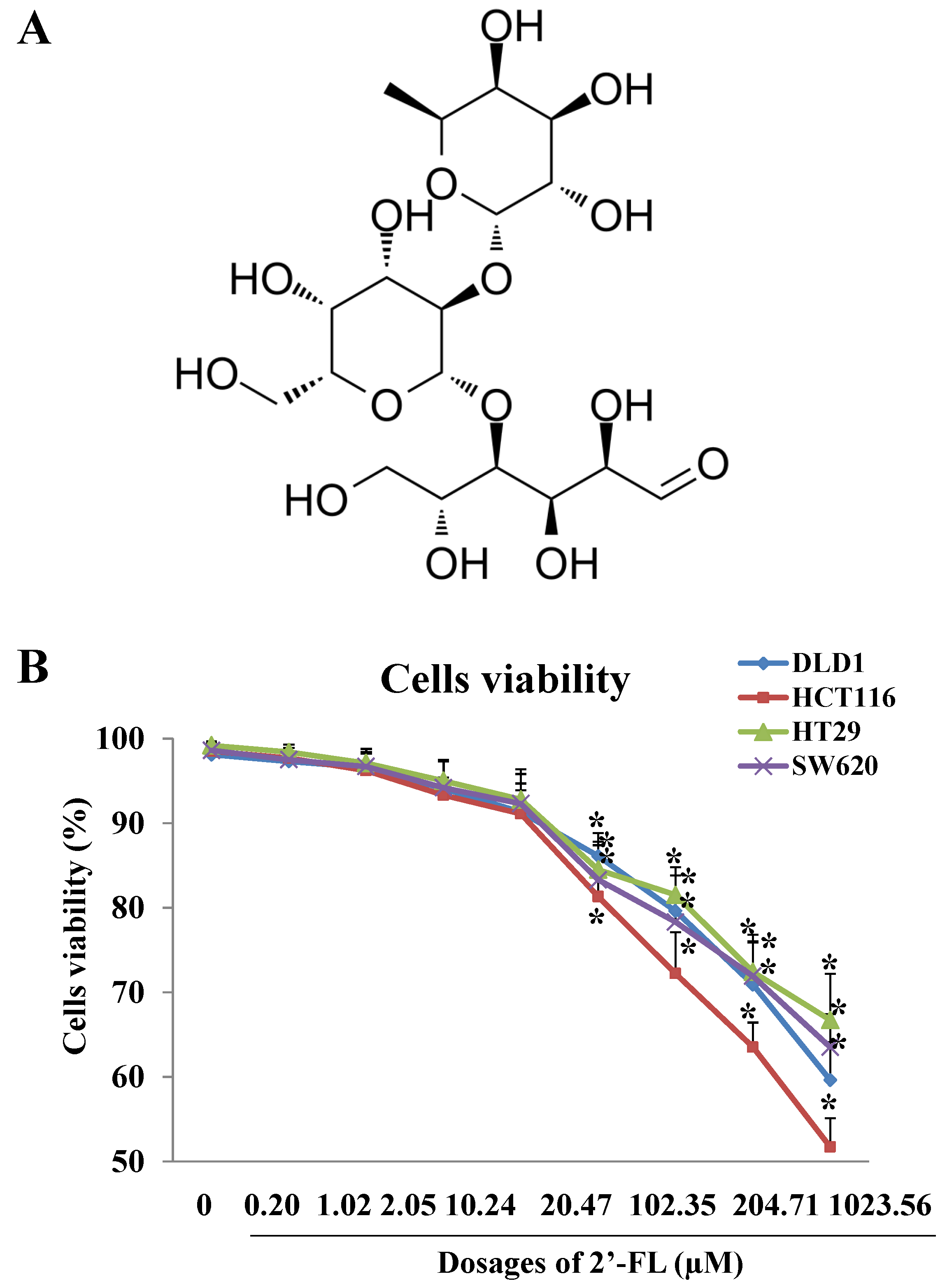
2.1. Cells Viability Assay and Dosages Selection
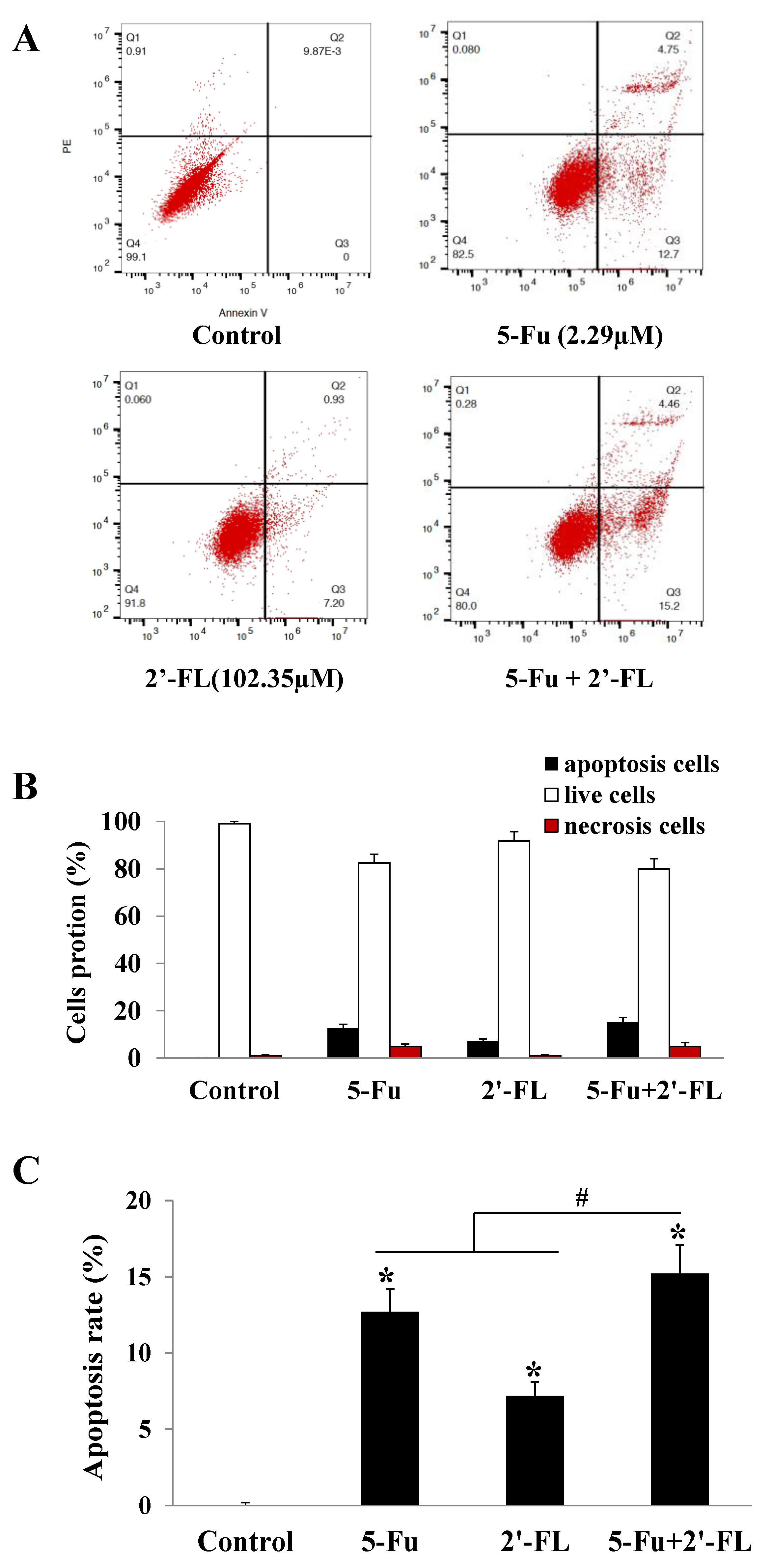
2.2. The Apoptosis Effect of 5-Fu and 2′-FL on HCT116 Cells
2.3. 2′-FL Suppressed Xenografted Colon Tumors in a Nude Mice Model
2.4. 2′-FL Reduced the Degrees of Tumor Malignancy and Angiogenesis
2.5. 2′-FL Alleviated the Toxic Effects When Combined with 5-Fu
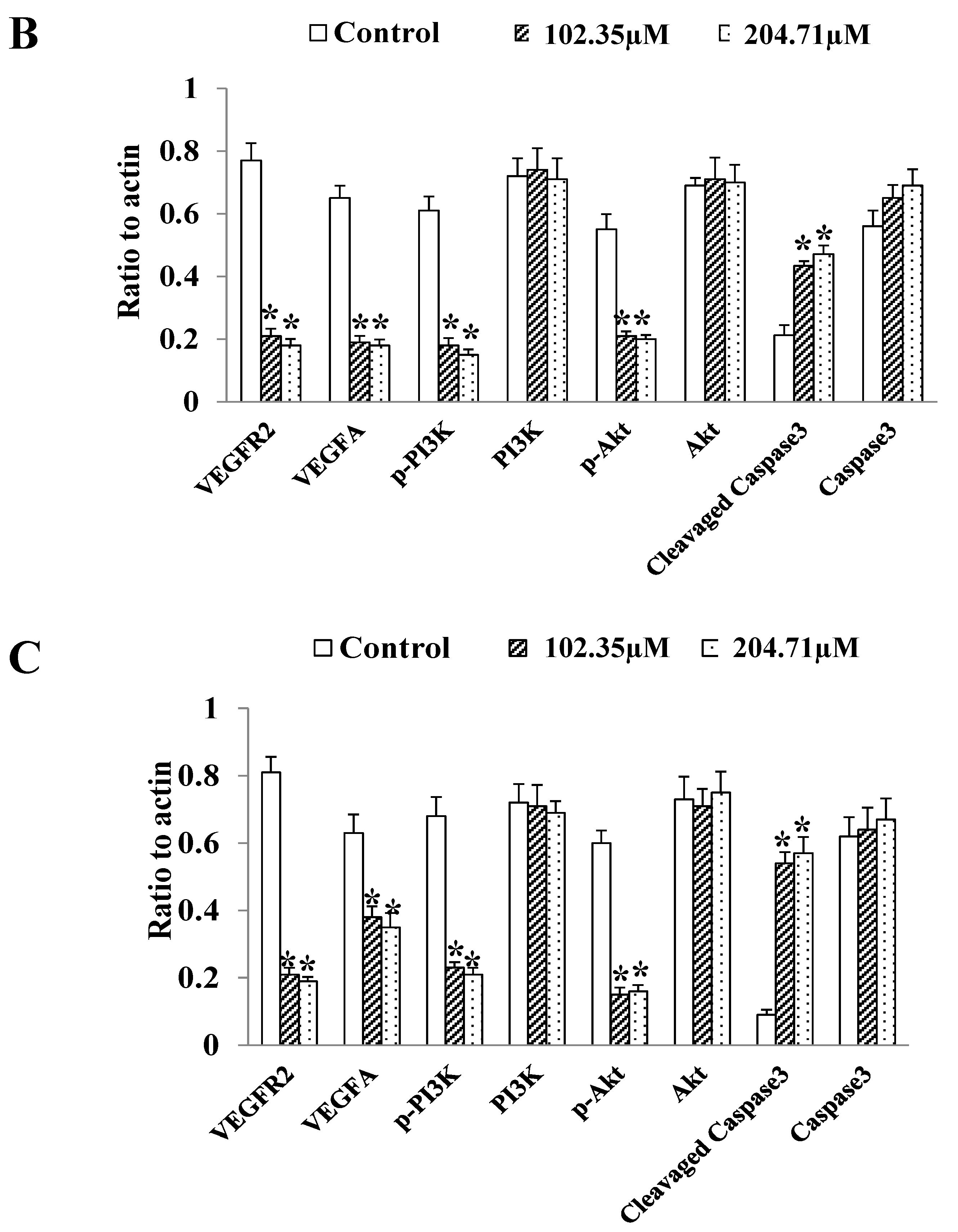
2.6. 2′-FL Regulates the Angiogenesis Pathway of VEGFA/VEGFR2 and Induces Apoptosis in HCT116 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Activity Detection
4.3. Apoptosis Detection
4.4. Animal Tests
4.5. HE and IHC Methods
4.6. Western Blotting
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yang, Z.; Chunhua, G.; Huayan, Y.; Jianguo, Y.; Yong, C. Anatomical basis for the choice of laparoscopic surgery for low rectal cancer through the pelvic imaging data—a cohort study. World J. Surg. Oncol. 2018, 16, 199. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.D.; Song, L.R.; Lu, H.C.; Zheng, X. Prediction of different stages of rectal cancer: Texture analysis based on diffusion-weighted images and apparent diffusion coefficient maps. World J. Gastroenterol. 2020, 26, 2082–2096. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.D.; Li, H.; Li, Y.C.; Zeng, H. Naphthazarin suppresses cell proliferation and induces apoptosis in human colorectal cancer cells via the B-cell lymphoma 2/B-cell associated X protein signaling pathway. Oncol. Lett. 2016, 12, 5211–5216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, C.; Wen, T.; Zhao, Q. Probiotics Used for Postoperative Infections in Patients Undergoing Colorectal Cancer Surgery. Biomed. Res. Int. 2020, 2020, 5734718. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Colangelo, T.; Panza, A.; Rubino, R.; Tiberio, C.; Palumbo, O.; Carella, M.; Trombetta, D.; Gentile, A.; Tavano, F.; et al. Analysis of clock gene-miRNA correlation networks reveals candidate drivers in colorectal cancer. Oncotarget 2016, 7, 45444–45461. [Google Scholar] [CrossRef]
- Olsen, D.A.; Thomsen, C.E.B.; Andersen, R.F.; Madsen, J.S.; Jakobsen, A.; Brandslund, I. Decreased concentrations of intracellular signaling proteins in colon cancer patients with BRAF mutations. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, Y.; Fan, J.; Fan, J.; Deng, L.; Deng, L.; Huang, B.; Zhou, B.; Zhou, B. Antitumor efficacy of cytosine deaminase-armed vaccinia virus plus 5-fluorocytosine in colorectal cancers. Cancer Cell Int. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Ho, S.Y.; Wu, W.S.; Lin, L.C.; Wu, Y.H.; Chiu, H.W.; Yeh, Y.L.; Huang, B.M.; Wang, Y.J. Cordycepin enhances radiosensitivity in oral squamous carcinoma cells by inducing autophagy and apoptosis through cell cycle arrest. Int. J. Mol. Sci. 2019, 20, 5336. [Google Scholar] [CrossRef]
- Tang, M.; Lu, X.; Zhang, C.; Du, C.; Cao, L.; Hou, T.; Li, Z.; Tu, B.; Cao, Z.; Li, Y.; et al. Downregulation of SIRT7 by 5-fluorouracil induces radiosensitivity in human colorectal cancer. Theranostics 2017, 7, 1346–1359. [Google Scholar] [CrossRef]
- Yang, H.; Huang, S.; Wei, Y.; Cao, S.; Pi, C.; Feng, T.; Liang, J.; Zhao, L.; Ren, G. Curcumin enhances the anticancer effect of 5-fluorouracil against gastric cancer through down-regulation of COX-2 and NF-κB signaling pathways. J. Cancer 2017, 8, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Zoli, W.; Ulivi, P.; Tesei, A.; Fabbri, F.; Rosetti, M.; Maltoni, R.; Giunchi, D.C.; Ricotti, L.; Brigliadori, G.; Vannini, I.; et al. Addition of 5-fluorouracil to doxorubicin-paclitaxel sequence increases caspase-dependent apoptosis in breast cancer cell lines. Breast Cancer Res. 2005, 7, R681. [Google Scholar] [CrossRef] [PubMed]
- Moodley, T.; Singh, M. Polymeric mesoporous silica nanoparticles for enhanced delivery of 5-fluorouracil in vitro. Pharmaceutics 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Manthey, C.F.; Autran, C.A.; Eckmann, L.; Bode, L. Human milk oligosaccharides protect against enteropathogenic escherichia coli attachment in vitro and EPEC colonization in suckling mice. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.M.; Lodge, C.J.; Dharmage, S.C.; Dai, X.; Bode, L.; Lowe, A.J. Human milk oligosaccharides and associations with immune-mediated disease and infection in childhood: A systematic review. Front. Pediatr. 2018, 6, 91. [Google Scholar] [CrossRef]
- Bode, L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef]
- Salli, K.; Hirvonen, J.; Siitonen, J.; Ahonen, I.; Anglenius, H.; Maukonen, J. Selective Utilization of the Human Milk Oligosaccharides 2′-Fucosyllactose, 3-Fucosyllactose, and Difucosyllactose by Various Probiotic and Pathogenic Bacteria. J. Agric. Food Chem. 2021, 69, 170–182. [Google Scholar] [CrossRef]
- Facinelli, B.; Marini, E.; Magi, G.; Zampini, L.; Santoro, L.; Catassi, C.; Monachesi, C.; Gabrielli, O.; Coppa, G. V Breast milk oligosaccharides: Effects of 2′-fucosyllactose and 6′-sialyllactose on the adhesion of Escherichia coli and Salmonella fyris to Caco-2 cells. J. Matern. Neonatal Med. 2019, 32, 2950–2952. [Google Scholar] [CrossRef]
- Good, M.; Sodhi, C.P.; Yamaguchi, Y.; Jia, H.; Lu, P.; Fulton, W.B.; Martin, L.Y.; Prindle, T.; Nino, D.F.; Zhou, Q.; et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 2016, 116, 1175–1187. [Google Scholar] [CrossRef]
- Wu, R.Y.; Li, B.; Koike, Y.; Määttänen, P.; Miyake, H.; Cadete, M.; Johnson-Henry, K.C.; Botts, S.R.; Lee, C.; Abrahamsson, T.R.; et al. Human Milk Oligosaccharides Increase Mucin Expression in Experimental Necrotizing Enterocolitis. Mol. Nutr. Food Res. 2019, 63, 1800658. [Google Scholar] [CrossRef]
- He, Y.Y.; Liu, S.B.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Coulet, M.; Phothirath, P.; Allais, L.; Schilter, B. Pre-clinical safety evaluation of the synthetic human milk, nature-identical, oligosaccharide 2′-O-Fucosyllactose (2′FL). Regul. Toxicol. Pharmacol. 2014, 68, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.J.; Monteagudo-Mera, A.; Contractor, N.; Gibson, G.R. Impact of 2′-Fucosyllactose on gut microbiota composition in adults with chronic gastrointestinal conditions: Batch culture fermentation model and pilot clinical trial findings. Nutrients 2021, 13, 938. [Google Scholar] [CrossRef]
- Zhao, G.; Williams, J.; Washington, M.K.; Yang, Y.; Long, J.R.; Townsend, S.D.; Yan, F. 2’-Fucosyllactose ameliorates chemotherapy-induced intestinal mucositis by protecting intestinal epithelial cells against apoptosis. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.; Cha, B.; Jang, D.; Park, C.; Park, G.; Kwak, B.; Bin, B. Human milk oligosaccharide 2’-fucosyllactose promotes melanin degradation via the autophagic AMPK–ULK1 signaling axis. Sci. Rep. 2022, 12, 13983. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Mayneris-Perxachs, J.; Knipping, K.; Pérez-Cano, F.J. Immunomodulatory and prebiotic effects of 2’-Fucosyllactose in suckling rats. Front. Immunol. 2019, 10, 1773. [Google Scholar] [CrossRef]
- Lakshmikanthan, S.; Sobczak, M.; Chun, C.; Henschel, A.; Dargatz, J.; Ramchandran, R.; Chrzanowska-Wodnicka, M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin αvβ3. Blood 2011, 118, 2015–2026. [Google Scholar] [CrossRef]
- Hida, K.; Maishi, N.; Annan, D.A.; Hida, Y. Contribution of tumor endothelial cells in cancer progression. Int. J. Mol. Sci. 2018, 19, 1272. [Google Scholar] [CrossRef]
- Abou Faycal, C.; Gazzeri, S.; Eymin, B. A VEGF-A/SOX2/SRSF2 network controls VEGFR1 pre-mRNA alternative splicing in lung carcinoma cells. Sci. Rep. 2019, 9, 336. [Google Scholar] [CrossRef]
- Oh, M.; Rho, S.B.; Son, C.; Park, K.; Song, S.Y. Non-proteolytic calpain-6 interacts with VEGFA and promotes angiogenesis by increasing VEGF secretion. Sci. Rep. 2019, 9, 15771. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, H.I.; Ismail, M.F.; Shalaby, F.M.; Abou-El-Magd, R.F.; Gaur, R.L.; Fernando, A.; Raj, M.H.G.; Ouhtit, A. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int. J. Biol. Sci. 2009, 5, 466–473. [Google Scholar] [CrossRef] [PubMed]



| Group (mg/Kg) | Heart Index | Liver Index | Kidney Index | Spleen Index | Thymus Index |
|---|---|---|---|---|---|
| Control | 0.55 ± 0.06 | 6.02 ± 0.29 | 1.55 ± 0.20 | 0.51 ± 0.04 | 0.065 ± 0.007 |
| 5-Fu(0.5) | 0.67 ± 0.07 * | 6.85 ± 0.41 * | 2.03 ± 0.37 * | 0.35 ± 0.07 * | 0.042 ± 0.009 * |
| 2′-FL(100) | 0.56 ± 0.04 # | 6.05 ± 0.33 # | 1.52 ± 0.17 # | 0.52 ± 0.07 # | 0.063 ± 0.010 # |
| 2′-FL + 5-Fu | 0.63 ± 0.05 * | 6.43 ± 0.45 *,# | 1.68 ± 0.33 *,# | 0.44 ± 0.09 *,# | 0.052 ± 0.009 *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, B.; Wang, Y. 2′-Fucosyllactose Suppresses Angiogenesis and Alleviates Toxic Effects of 5-Fu in a HCT116 Colon Tumor-Bearing Model. Molecules 2022, 27, 7255. https://doi.org/10.3390/molecules27217255
Li H, Wang B, Wang Y. 2′-Fucosyllactose Suppresses Angiogenesis and Alleviates Toxic Effects of 5-Fu in a HCT116 Colon Tumor-Bearing Model. Molecules. 2022; 27(21):7255. https://doi.org/10.3390/molecules27217255
Chicago/Turabian StyleLi, Huiying, Bingyuan Wang, and Yang Wang. 2022. "2′-Fucosyllactose Suppresses Angiogenesis and Alleviates Toxic Effects of 5-Fu in a HCT116 Colon Tumor-Bearing Model" Molecules 27, no. 21: 7255. https://doi.org/10.3390/molecules27217255
APA StyleLi, H., Wang, B., & Wang, Y. (2022). 2′-Fucosyllactose Suppresses Angiogenesis and Alleviates Toxic Effects of 5-Fu in a HCT116 Colon Tumor-Bearing Model. Molecules, 27(21), 7255. https://doi.org/10.3390/molecules27217255





