Modification of Cotton Fabric with Molecularly Imprinted Polymer-Coated Carbon Dots as a Sensor for 17 α-methyltestosterone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the EDA-CDs
2.1.1. Optimum Condition for Synthesis and Characterization of the EDA-CDs
2.1.2. Characterization of the Carbon Dots Using FTIR
2.2. Preparation of the EDA-CDs Grafted Cotton Fabric (EDA-CDs/CF)
2.3. Synthesis and Characterization of the MIPs@EDA-CDs/CF
2.3.1. Optimum Conditions for Synthesis of the MIPs@EDA-CDs/CF
2.3.2. Characterization of the MIPs@EDA-CD/CF Using SEM-EDS and FTIR
2.4. RGB Fluorescence Imaging of the MIPs@EDA-CDs/CF
2.5. Detection of MT Using the MIPs@EDA-CDs/CF with Image Processing via Smartphone and ImageJ Program
2.6. Validation of the Proposed Method
2.6.1. Selectivity of the MIPs@EDA-CDs/CF
2.6.2. Reusability, Stability, and Precision of the MIPs@EDA-CDs/CF
2.7. Binding Capacity of the MIPs@EDA-CDs/CF and the NIPs@EDA-CDs/CF
2.8. Determination of MT in Real Samples
3. Materials and Methodology
3.1. Reagents and Materials
3.2. Instrumentation
3.3. Smartphone and Detection Box Set Up
3.4. Synthesis of Ethylenediamine-Carbon Dots (EDA-CDs)
3.5. Preparation of the EDA-CDs Grafted Cotton Fabric
3.6. Preparation of the MIPs@EDA-CDs/CF
3.7. Validation of the Proposed Method
3.7.1. Selectivity of the MIPs@EDA-CDs/CF
3.7.2. Reusability, Stability, and Precision of the MIPs@EDA-CDs/CF
3.8. Binding Specificity of the MIP@EDA-CDs/CF and the NIP@EDA-CDs/C
3.9. Detection of MT in Real Samples Using the MIPs@EDA-CDs/CF and Smartphone-Based Image Analysis
3.10. Fluorescence Image
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef]
- Elobeid, M.A.; Allison, D.B. Putative environmental-endocrine disruptors and obesity: A review. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Aufartová, J.; Mahugo-Santana, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Nováková, L.; Solich, P. Determination of steroid hormones in biological and environmental samples using green microextraction techniques: An overview. Anal. Chim. Acta 2011, 704, 33–46. [Google Scholar] [CrossRef]
- Liu, S.; Ying, G.G.; Zhou, L.J.; Zhang, R.Q.; Chen, Z.F.; Lai, H.J. Steroids in a typical swine farm and their release into the environment. Water Res. 2012, 46, 3754–3768. [Google Scholar] [CrossRef]
- Saadaoui, A.; Sanglar, C.; Medimagh, R.; Bonhomme, A.; Baudot, R.; Chatti, S.; Marque, S.; Prim, D.; Zina, M.S.; Casabianca, H. New biosourced chiral molecularly imprinted polymer: Synthesis, characterization, and evaluation of the recognition capacity of methyltestosterone. J. Mol. Recognit. 2017, 30, e2594. [Google Scholar] [CrossRef]
- Shen, Z.H.; Fan, Q.X.; Yang, W.; Zhang, Y.L.; Wang, H.P. Effects of 17α-methyltestosterone and aromatase inhibitor letrozole on sex reversal, gonadal structure, and growth in yellow catfish pelteobagrus fulvidraco. Biol. Bull. 2015, 228, 108–117. [Google Scholar] [CrossRef]
- Mair, G.; Little, D. Population control in farmed tilapias. Naga Iclarm Q. 1991, 14, 8–13. [Google Scholar]
- Homklin, S.; Wattanodorn, T.; Ong, S.K.; Limpiyakorn, T. Biodegradation of 17α-methyltestosterone and isolation of MT-degrading bacterium from sediment of nile tilapia masculinization pond. Water Sci. Technol. 2009, 59, 261–265. [Google Scholar] [CrossRef]
- Ong, S.K.; Chotisukarn, P.; Limpiyakorn, T. Sorption of 17α-methyltestosterone onto soils and sediment. Water Air Soil Pollut. 2012, 223, 3869–3875. [Google Scholar] [CrossRef]
- Pratush, A.; Ye, X.; Yang, Q.; Kan, J.; Peng, T.; Wang, H.; Huang, T.; Xiong, G.; Hu, Z. Biotransformation strategies for steroid estrogen and androgen pollution. Appl. Microbiol. Biotechnol. 2020, 104, 2385–2409. [Google Scholar] [CrossRef]
- Megbowon, I.; Mojekwu, T. Tilapia sex reversal using methyl testosterone (MT) and its effect on fish, man and environment. Biotechnology 2013, 13, 213–216. [Google Scholar] [CrossRef]
- Islam, M.S.; Satter, A.; Chwakravorty, P.; Rahman, H.; Asif, A.A.; Hossain, A.; Rahman, M.; Haq, M.E. Comparative growth performance between monosex and natural XY male tilapia in Noakhali region, Bangladesh. Asian J. Med. Biol. Res. 2017, 3, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Wee, S.Y.; Aris, A.Z. Occurrence and public-perceived risk of endocrine disrupting compounds in drinking water. NPJ Clean Water 2019, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Su, T.P.; Pagliaro, M.; Schmidt, P.J.; Pickar, D.; Wolkowitz, O.; Rubinow, D.R. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA 1993, 269, 2760–2764. [Google Scholar] [CrossRef]
- Wolf, S.; Diel, P.; Parr, M.K.; Rataj, F.; Schanzer, W.; Vollmer, G.; Zierau, O. Long-term detection of methyltestosterone (ab-) use by a yeast transactivation system. Arch. Toxicol. 2011, 85, 285–292. [Google Scholar] [CrossRef]
- Barbosa, I.R.; Lopes, S.; Oliveira, R.; Domingues, I.; Soares, A.M.V.M.; Nogueira, A.J.A. Determination of 17 α-methyltestosterone in freshwater samples of tilapia farming by high performance liquid chromatography. Am. J. Analyt. Chem. 2013, 4, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Lindino, C.A.; Langaro Savaris, D.; Borghezan Alberton, M.; de Matos, R.; Zalazar, M.F.; Duarte, D.J.R. Development of a spectrophotometric method for the determination of 17α-methyltestosterone. Braz. J. Anal. Chem. 2019, 6, 12–19. [Google Scholar] [CrossRef]
- Yang, M.; Gu, W.; Sun, L.; Zhang, F.; Ling, Y.; Chu, X.; Wang, D. Study on the molecularly imprinted polymers with methyl-testosterone as the template. Talanta 2010, 81, 156–161. [Google Scholar] [CrossRef]
- Leporati, M.; Bergoglio, M.; Capra, P.; Bozzetta, E.; Abete, M.C.; Vincenti, M. Development, validation and application to real samples of a multiresidue LC-MS/MS method for determination of β2-agonists and anabolic steroids in bovine hair. J. Mass Spectrom. 2014, 49, 936–946. [Google Scholar] [CrossRef]
- Xie, Z.; Ouyang, X.; Guo, L.; Lin, X.; Chen, G. Determination of methyltestosterone using flow injection with chemiluminescence detection. Luminescence 2005, 20, 231–235. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, J.; Gao, J.; Sun, L.; Chang, W.B. Determination of doping methyltestosterone by capillary electrophoresis immunological analysis with thermally reversible hydrogel and laser-induced fluorescence. Electrophoresis 1999, 20, 1998–2002. [Google Scholar] [CrossRef]
- Vashist, S.K.; Mudanyali, O.; Schneider, E.M.; Zengerle, R.; Ozcan, A. Cellphone-based devices for bioanalytical sciences. Anal. Bioanal. Chem. 2014, 406, 3263–3277. [Google Scholar] [CrossRef] [Green Version]
- Roda, A.; Michelini, E.; Zangheri, M.; Di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-based biosensors: A critical review and perspectives. TrAC Trends Anal. Chem. 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, H.; Yamada, S.; Sakamoto, W.; Yogo, T. Cellulose-based molecularly imprinted red-blood-cell-like microparticles for the selective capture of cortisol. Carbohydr. Polym. 2018, 193, 173–178. [Google Scholar] [CrossRef]
- Cai, G.; Xu, Z.; Yang, M.; Tang, B.; Wang, X. Functionalization of cotton fabrics through thermal reduction of graphene oxide. Appl. Surf. Sci. 2017, 393, 441–448. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Z.; Li, X.; Li, Y.; Tan, Z.A.; Fan, L.; Yang, S. Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-emitting diodes. Adv. Mater. 2017, 29, 1604436. [Google Scholar] [CrossRef]
- Xu, L.; Pan, M.; Fang, G.; Wang, S. Carbon dots embedded metal-organic framework@molecularly imprinted nanoparticles for highly sensitive and selective detection of quercetin. Sens. Actuators B Chem. 2019, 286, 321–327. [Google Scholar] [CrossRef]
- Pandey, R.R.; Chusuei, C.C. Carbon nanotubes, graphene, and carbon dots as electrochemical biosensing composites. Molecules 2021, 26, 6674. [Google Scholar] [CrossRef]
- Domingo-Tafalla, B.; Martinez-Ferrero, E.; Franco, F.; Palomares-Gil, E. Applications of carbon dots for the photocatalytic and electrocatalytic reduction of CO2. Molecules 2022, 27, 1081. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, R.; Abdi, O. Microwave assisted synthesis of N-doped carbon dots: An easy, fast and cheap sensor for determination of aspartic acid in sport supplements. J. Fluoresc. 2019, 29, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Masoum, S. Recent advances and future trends on molecularly imprinted polymer-based fluorescence sensors with luminescent carbon dots. Talanta 2021, 223, 121411. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Linares, A.V.; Bompart, M.; Bui, B.T.S. Molecularly imprinted polymers. In Molecular Imprinting; Haupt, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–28. [Google Scholar] [CrossRef]
- Elfadil, D.; Lamaoui, A.; Della Pelle, F.; Amine, A.; Compagnone, D. Molecularly imprinted polymers combined with electrochemical sensors for food contaminants analysis. Molecules 2021, 26, 4607. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors affecting preparation of molecularly imprinted polymer and methods on finding template-monomer interaction as the key of selective properties of the materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Dong, J.; Liu, C.; Qi, Y.; Fang, G.; Wang, S. A strong blue fluorescent nanoprobe based on Mg/N co-doped carbon dots coupled with molecularly imprinted polymer for ultrasensitive and highly selective detection of tetracycline in animal-derived foods. Sens. Actuators B Chem. 2021, 338, 129809. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Xiang, S.; Nagel, S.; Lin, C.H.; Fidalgo de Cortalezzi, M. Photonic molecularly imprinted polymer film for the detection of testosterone in aqueous samples. Polymers 2018, 10, 349. [Google Scholar] [CrossRef] [Green Version]
- Bitas, D.; Samanidou, V. Molecularly imprinted polymers as extracting media for the chromatographic determination of antibiotics in milk. Molecules 2018, 23, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saylan, Y.; Akgonullu, S.; Yavuz, H.; Unal, S.; Denizli, A. Molecularly imprinted polymer based sensors for medical applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef] [Green Version]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef]
- Kellens, E.; Bové, H.; Conradi, M.; D’Olieslaeger, L.; Wagner, P.; Landfester, K.; Junkers, T.; Ethirajan, A. Improved molecular imprinting based on colloidal particles made from miniemulsion: A case study on testosterone and its structural analogues. Macromolecules 2016, 49, 2559–2567. [Google Scholar] [CrossRef]
- Tse Sum Bui, B.; Merlier, F.; Haupt, K. Toward the Use of a Molecularly imprinted polymer in doping analysis: Selective preconcentration and analysis of testosterone and epitestosterone in human urine. Anal. Chem. 2010, 82, 4420–4427. [Google Scholar] [CrossRef] [PubMed]
- Bouvarel, T.; Delaunay, N.; Pichon, V. Molecularly imprinted polymers in miniaturized extraction and separation devices. J. Sep. Sci. 2021, 44, 1727–1751. [Google Scholar] [CrossRef] [PubMed]
- Gavrilović, I.; Mitchell, K.; Brailsford, A.D.; Cowan, D.A.; Kicman, A.T.; Ansell, R.J. A molecularly imprinted receptor for separation of testosterone and epitestosterone, based on a steroidal cross-linker. Steroids 2011, 76, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Tse Sum Bui, B.; Haupt, K. Preparation and evaluation of a molecularly imprinted polymer for the selective recognition of testosterone—Application to molecularly imprinted sorbent assays. J. Mol. Recognit. 2011, 24, 1123–1129. [Google Scholar] [CrossRef]
- Tang, X.; Li, F.; Jia, J.; Yang, C.; Liu, W.; Jin, B.; Wang, X.; Gao, R.; He, D.; Guo, P. Synthesis of magnetic molecularly imprinted polymers with excellent biocompatibility for the elective separation and inhibition of testosterone in prostate cancer cells. Int. J. Nanomed. 2017, 12, 2979–2993. [Google Scholar] [CrossRef] [Green Version]
- Mpupa, A.; Selahle, S.K.; Mizaikoff, B.; Nomngongo, P.N. Recent advances in solid-phase extraction (SPE) based on molecularly imprinted polymers (MIPs) for analysis. Chemosensors 2021, 9, 151. [Google Scholar] [CrossRef]
- Ding, H.; Li, X.H.; Chen, X.B.; Wei, J.S.; Li, X.B.; Xiong, H.M. Surface states of carbon dots and their influences on luminescence. J. Appl. Phys. 2020, 27, 231101. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimagaing. Ang. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.-C.; Suresh, R.; Ramamurthy, P. Unravelling the multiple emissive states in citric-acid-derived carbon dots. J. Phys. Chem. C 2016, 120, 1252–1261. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Su, W. Molecularly imprinted poly (methacrylamide-co-methacrylic acid) composite membranes for recognition of curcumin. Anal. Chim. Acta 2018, 615, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Jhariya, S. Selective removal of amikacin from simulated polluted water using molecularly imprinting polymer (MIP). J. Macromol. Sci. A 2015, 52, 901–911. [Google Scholar] [CrossRef]
- Fang, M.; Zhou, L.; Zhang, H.; Liu, L.; Gong, Z.Y. A molecularly imprinted polymers/carbon dots-grafted paper sensor for 3-monochloropropane-1,2-diol determination. Food Chem. 2019, 274, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Yew, B.S.; Muhamad, M.; Mohamed, S.B.; Wee, F.H. Effect of alkaline treatment on structural characterisation, thermal degradation and water absorption ability of coir fibre polymer composites. Sains Malays 2019, 48, 653–659. [Google Scholar] [CrossRef]
- Yan, L.; Kasal, B.; Huang, L. A review of recent research on the use of cellulosic fibres, their fibre fabric reinforced cementitious, geo-polymer and polymer composites in civil engineering. Compos. Part B Eng. 2016, 92, 94–132. [Google Scholar] [CrossRef]
- Cortese, B.; Caschera, D.; Padeletti, G.; Ingo, G.M.; Gigli, G. A brief review of surface-functionalized cotton fabrics. Surf. Innov. 2013, 1, 140–156. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Ismail, R.; Lee, H.Y.; Mahyudin, N.A.; Abu Bakar, F. Linearity study on detection and quantification limits for the determination of avermectins using linear regression. J. Food Drug Anal. 2014, 22, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Naenna, T.; Prachayasittikul, V. Quantitative structure-imprinting factor relationship of molecularly imprinted polymers. Biosens. Bioelectron 2007, 22, 3309–3317. [Google Scholar] [CrossRef]
- Han, S.; Li, X.; Wang, Y.; Chen, S. Graphene oxide-based fluorescence molecularly imprinted composite for recognition and separation of 2,4,6-trichlorophenol. RSC Adv. 2015, 5, 2129–2136. [Google Scholar] [CrossRef]
- Lv, P.; Xie, D.; Zhang, Z. Magnetic carbon dots based molecularly imprinted polymers for fluorescent detection of bovine hemoglobin. Talanta 2018, 188, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.; Andaç, M.; Memon, N.; Bhanger, M.I.; Nizamani, S.M.; Denizli, A. Synthesis and characterization of molecularly imprinted polymer embedded composite cryogel discs: Application for the selective extraction of cypermethrins from aqueous samples prior to GC-MS analysis. RSC Adv. 2015, 5, 26604–26615. [Google Scholar] [CrossRef]
- Ndunda, E.N. Molecularly imprinted polymers-A closer look at the control polymer used in determining the imprinting effect: A mini review. J. Mol. Recognit. 2020, 33, e2855. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P. Analyzing Fluorescence Microscopy Images with ImageJ; The Nikon Imaging Center, Heidelberg University: Heidelberg, Germany, 2014; Volume 1, pp. 10–1109. [Google Scholar]
- Hartig, S.M. Basic image analysis and manipulation in ImageJ. Curr. Protoc. Mol. Biol. 2013, 102, 14–15. [Google Scholar] [CrossRef]




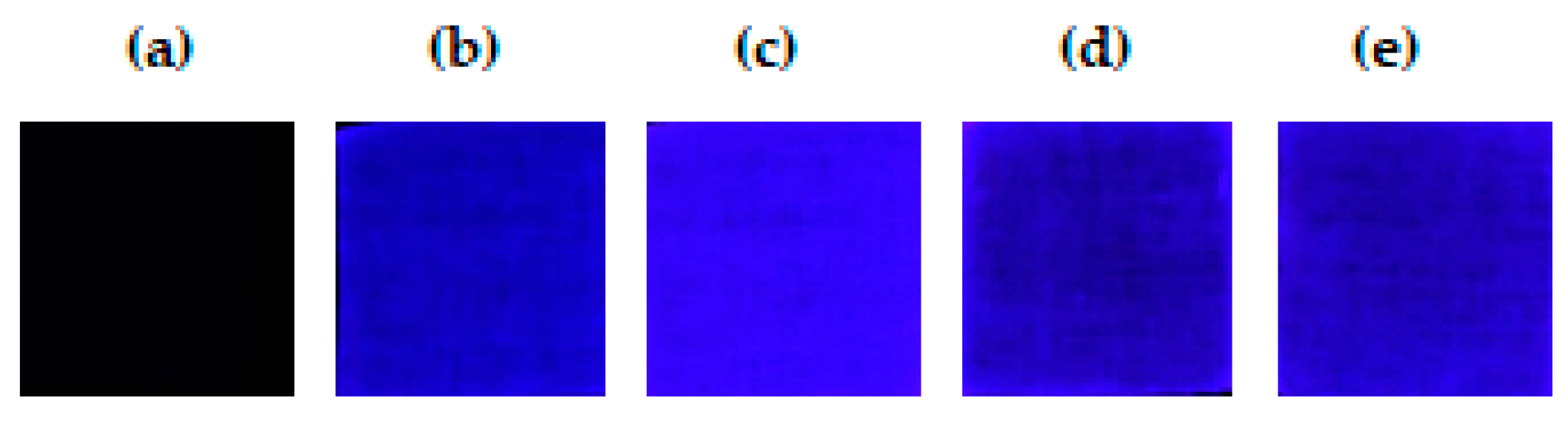




| Cotton Fabrics | Area | Min Intensity | Max Intensity | Average Intensity | Std Dev |
|---|---|---|---|---|---|
| a | 52440 | 30320 | 46478 | 38648.57 | 1539.39 |
| b | 52440 | 42342 | 56499 | 50675.78 | 1759.77 |
| c | 52440 | 46577 | 64139 | 55491.10 | 2208.76 |
| d | 52440 | 30117 | 48949 | 41239.88 | 1659.15 |
| e | 52440 | 36002 | 49510 | 42813.75 | 1685.95 |
| Concentration of MT (μg/L) | Sample | Adsorption Capacity (qt); (μg/g) | Partition Coefficient (K) |
|---|---|---|---|
| 100 | MIP 1 | 5.40 | 1.17 |
| NIP 1 | 2.99 | 0.40 | |
| 500 | MIP 2 | 28.1 | 1.19 |
| NIP 2 | 4.71 | 0.10 | |
| 1000 | MIP 3 | 60.7 | 1.24 |
| NIP 3 | 14.9 | 0.16 |
| Samples | Proposed Method | HPLC Found (μg/L) | %Relative Error | |||
|---|---|---|---|---|---|---|
| Spiked (μg/L) | Found (μg/L) | %Recovery | %RSD (n = 3) | |||
| Water | 0 | 129 ± 4 | 3 | 134 ± 1 | 4 | |
| 200 | 320 ± 12 | 96 | 4 | 330 ± 2 | 3 | |
| 400 | 535 ± 6 | 102 | 1 | 545 ± 10 | 2 | |
| 600 | 734 ± 15 | 101 | 2 | 733 ± 16 | 0 | |
| Sediment | 0 | 222 ± 13 | 6 | 218 ± 3 | 2 | |
| 200 | 420 ± 8 | 104 | 2 | 436 ± 6 | 4 | |
| 400 | 621 ± 16 | 100 | 2 | 611 ± 13 | 2 | |
| 600 | 814 ± 8 | 99 | 1 | 821 ± 16 | 1 | |
| Detection Blacklight Box | Camera Smartphone Setup | ||
|---|---|---|---|
| Variable | Value | Variable | Value |
| Box width | 20 cm | Resolution | 3024 × 4032 pixels |
| Box length | 20 cm | Wide camera | 12 MP |
| Box height | 20 cm | Operation mode | Manual |
| Lamp to the cotton fabric | 7 cm | Zoom | 2.5 |
| Smartphone to cotton fabric | 10 cm | Flash | off |
| Capture angle | 90 degrees | Aperture Av | f 1.8 |
| UVA lamp | 365–400 nm | Exposure Tv | 1/15s |
| Lamp power | 40 W | Quality | Raw photo |
| Lamp voltage | 220 V | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, M.; Thanasupsin, S.P.; Thongkon, N. Modification of Cotton Fabric with Molecularly Imprinted Polymer-Coated Carbon Dots as a Sensor for 17 α-methyltestosterone. Molecules 2022, 27, 7257. https://doi.org/10.3390/molecules27217257
Lim M, Thanasupsin SP, Thongkon N. Modification of Cotton Fabric with Molecularly Imprinted Polymer-Coated Carbon Dots as a Sensor for 17 α-methyltestosterone. Molecules. 2022; 27(21):7257. https://doi.org/10.3390/molecules27217257
Chicago/Turabian StyleLim, Monyratanak, Sudtida Pliankarom Thanasupsin, and Nisakorn Thongkon. 2022. "Modification of Cotton Fabric with Molecularly Imprinted Polymer-Coated Carbon Dots as a Sensor for 17 α-methyltestosterone" Molecules 27, no. 21: 7257. https://doi.org/10.3390/molecules27217257
APA StyleLim, M., Thanasupsin, S. P., & Thongkon, N. (2022). Modification of Cotton Fabric with Molecularly Imprinted Polymer-Coated Carbon Dots as a Sensor for 17 α-methyltestosterone. Molecules, 27(21), 7257. https://doi.org/10.3390/molecules27217257







