Water Saturated with Pressurized CO2 as a Tool to Create Various 3D Morphologies of Composites Based on Chitosan and Copper Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
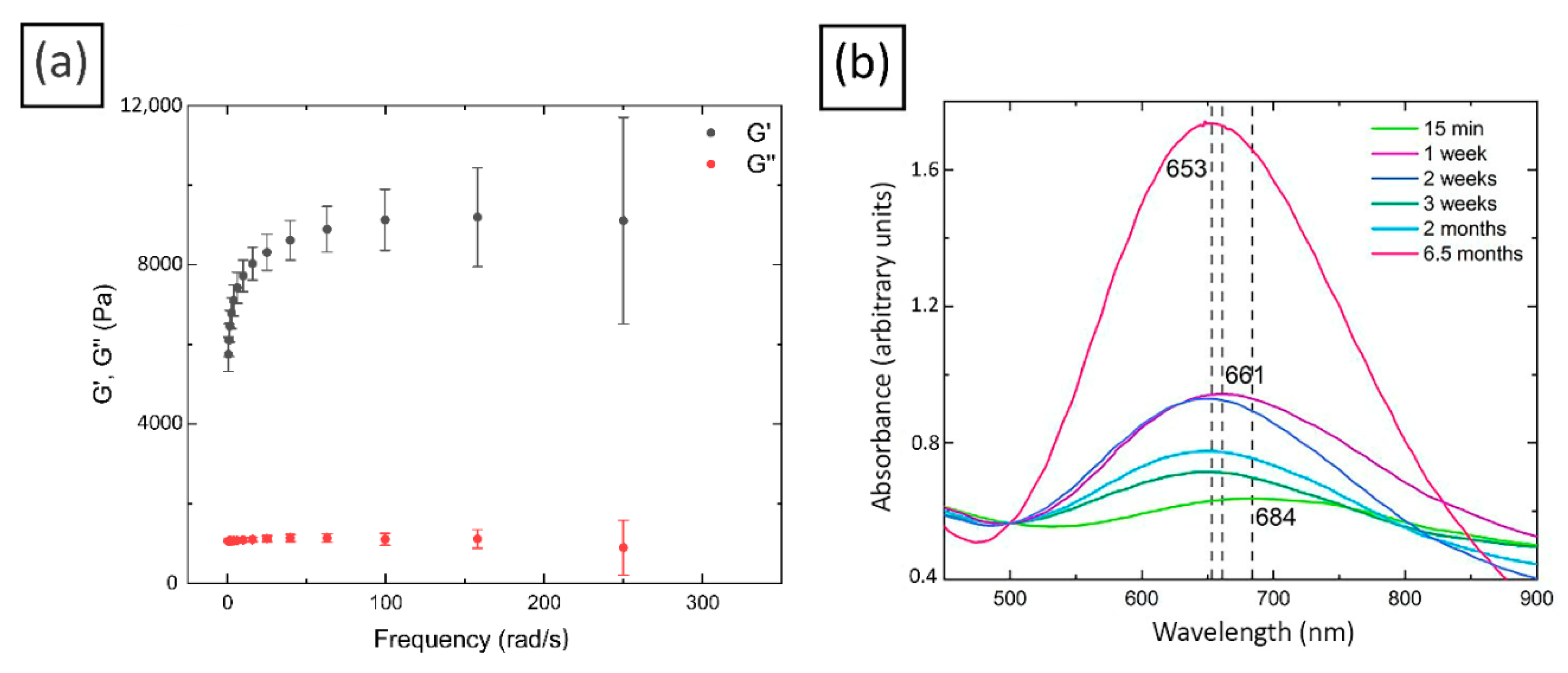
2.1. Rheological Study
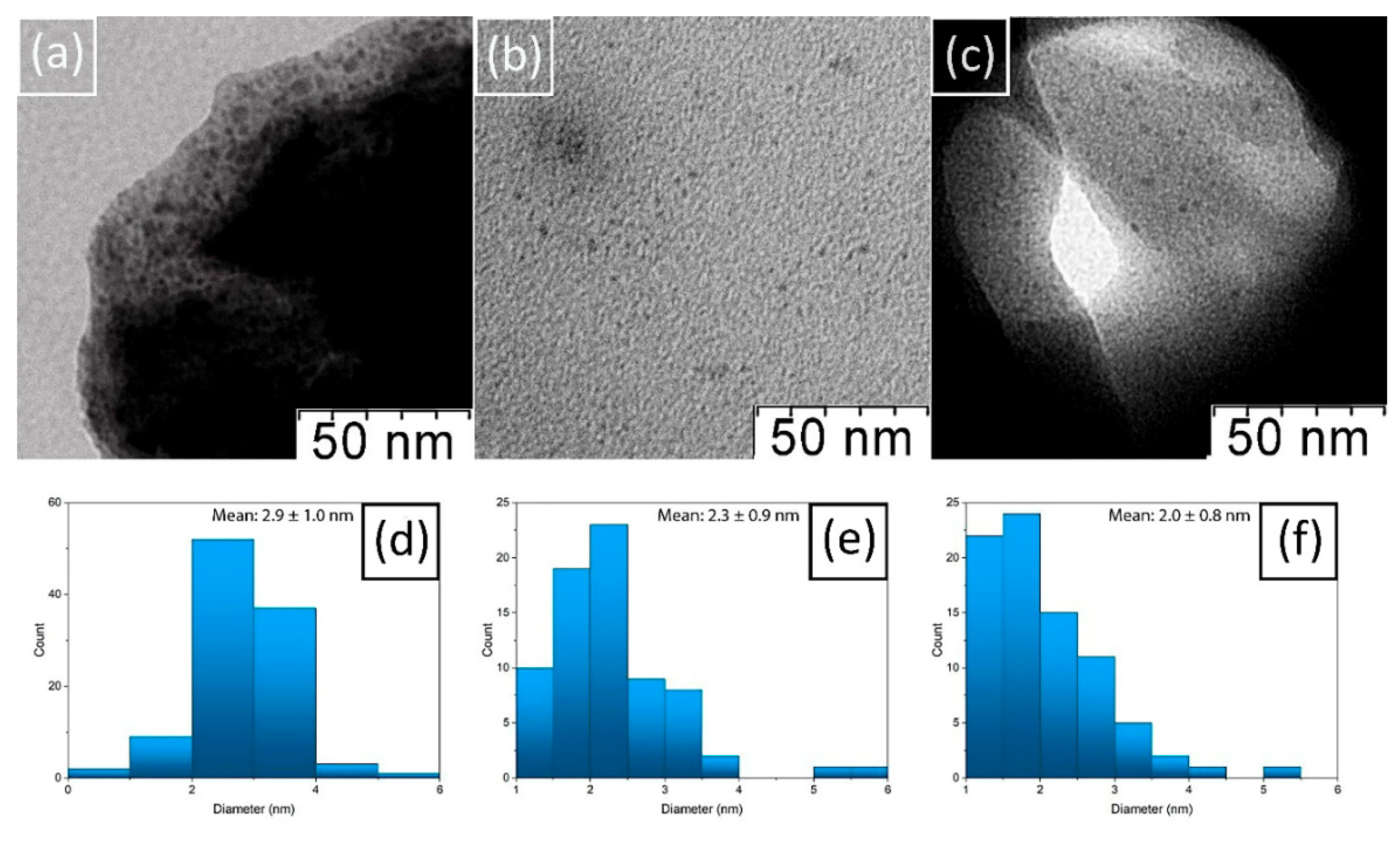
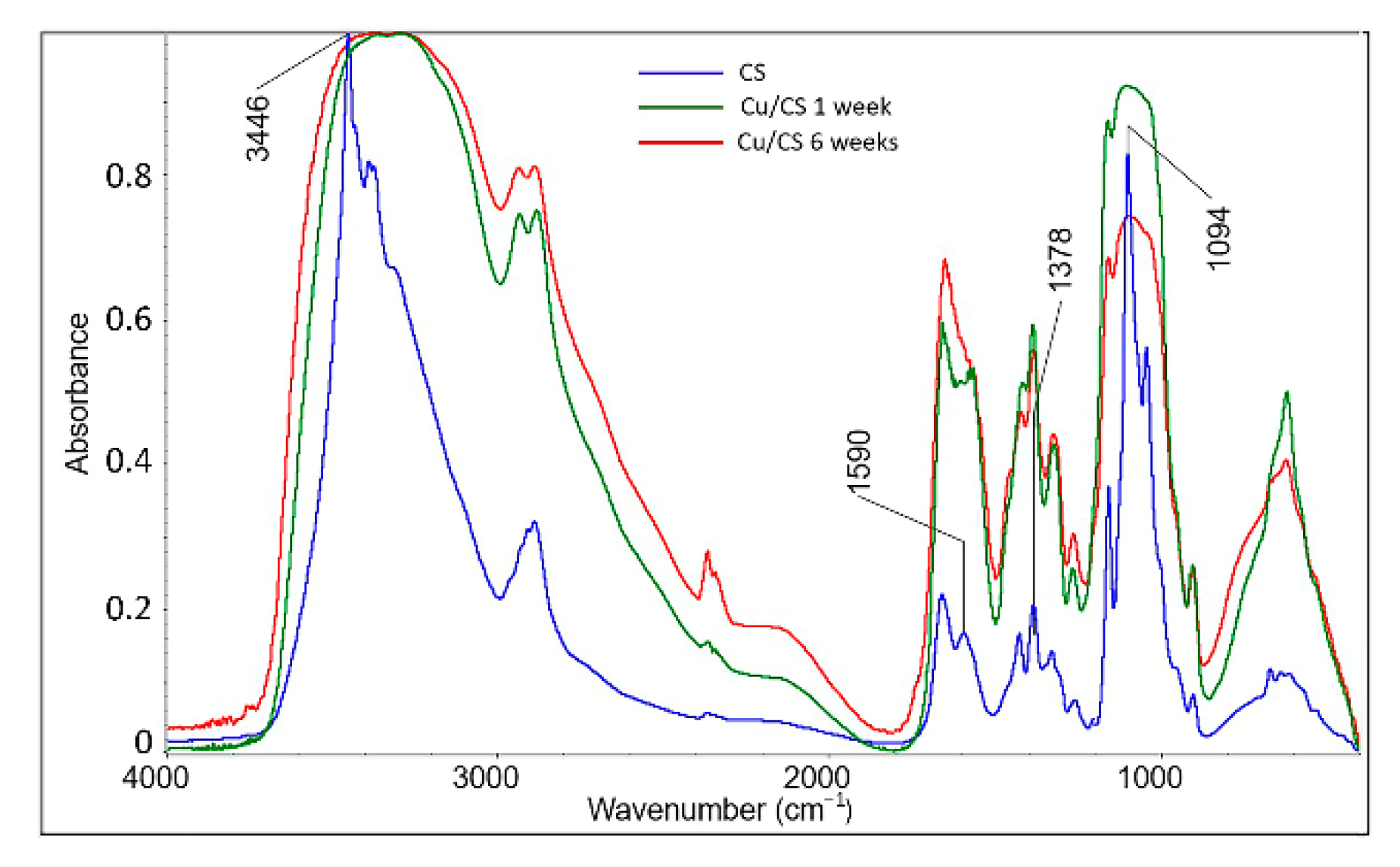
2.2. Characterization of Cu/CS Composite Hydrogel
2.3. Antimicrobial Activity
2.4. Porous Gel Modification
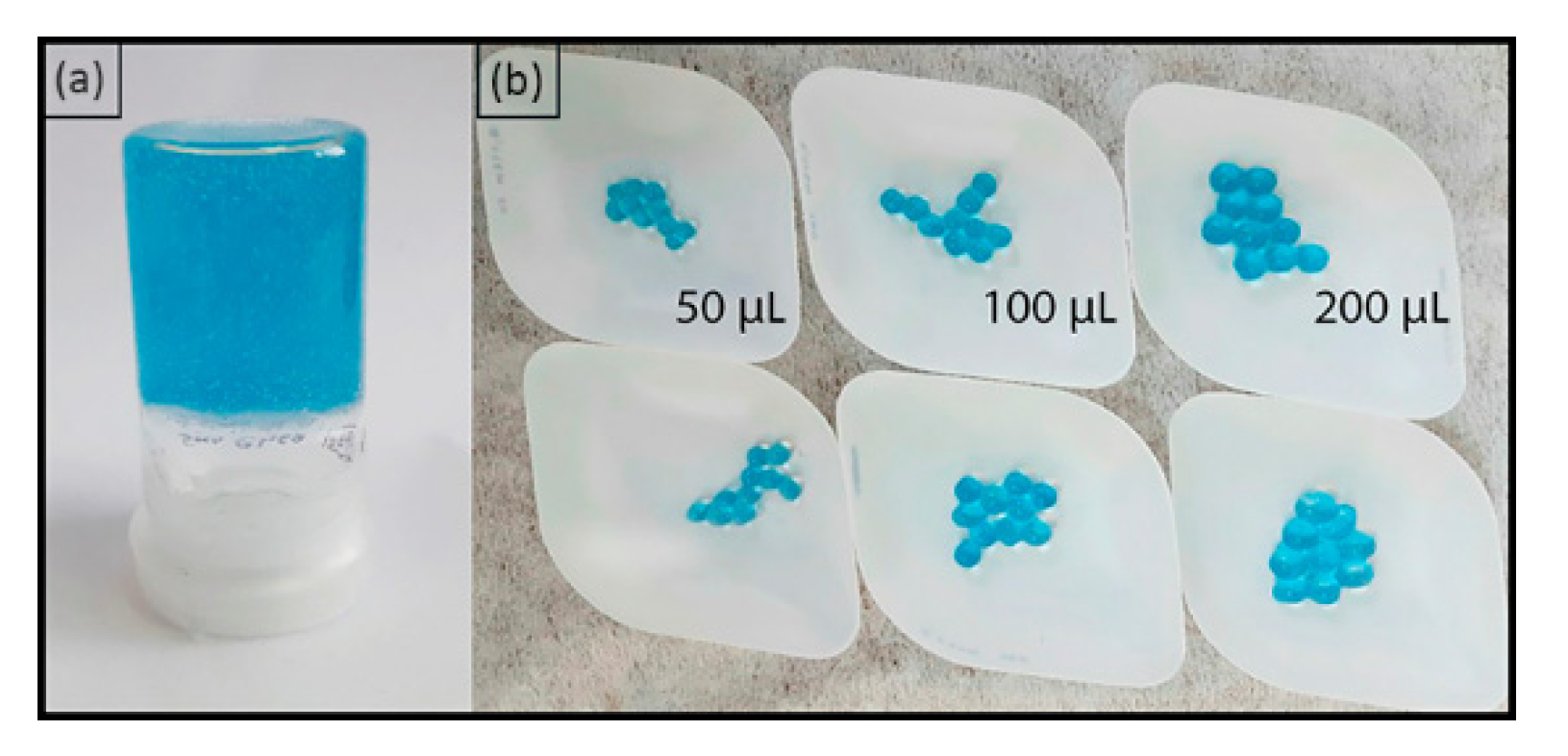
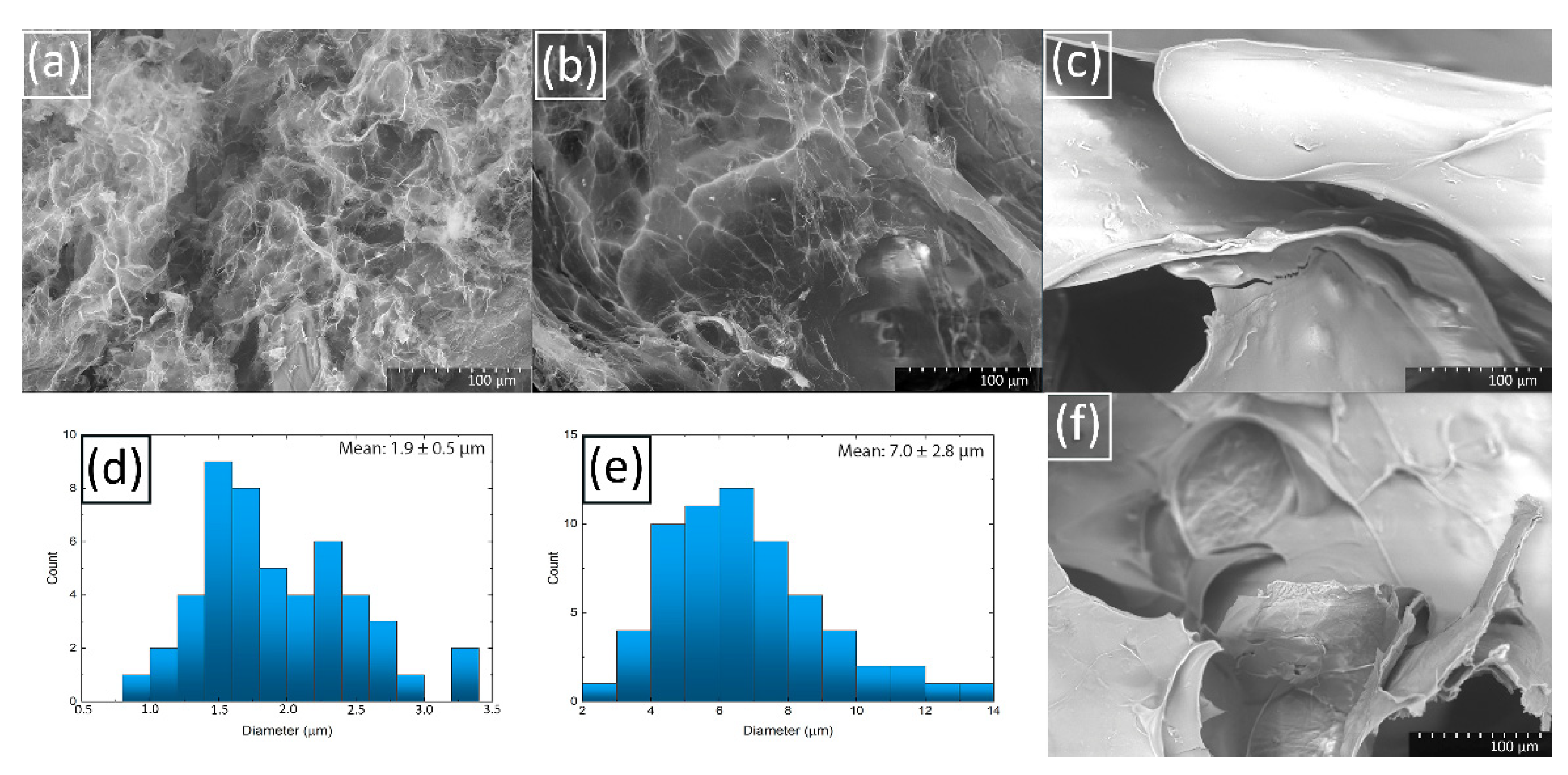
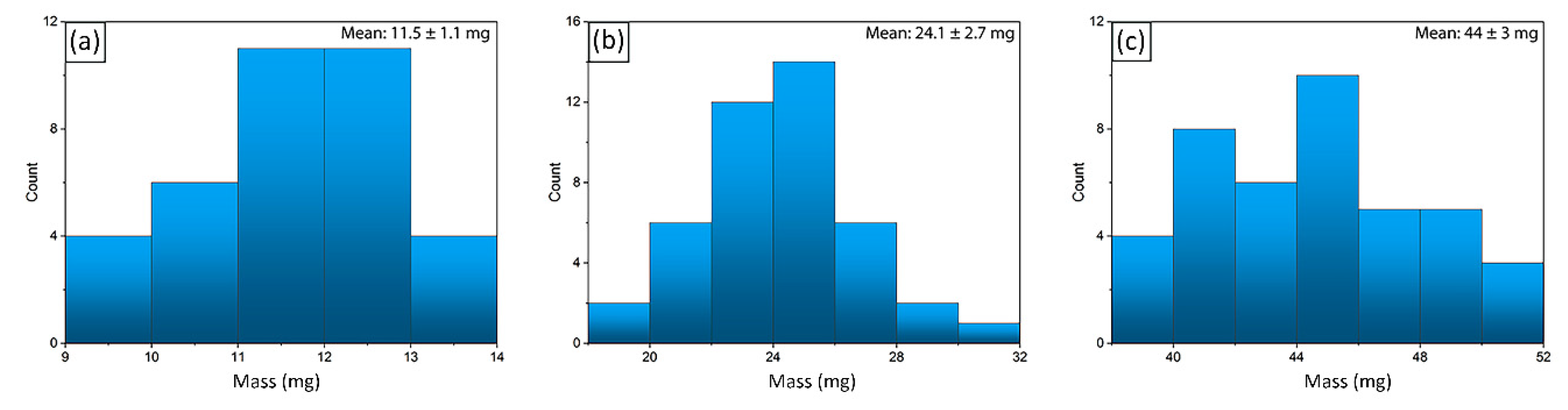
2.5. Characteristics of Hydrogel Spheres
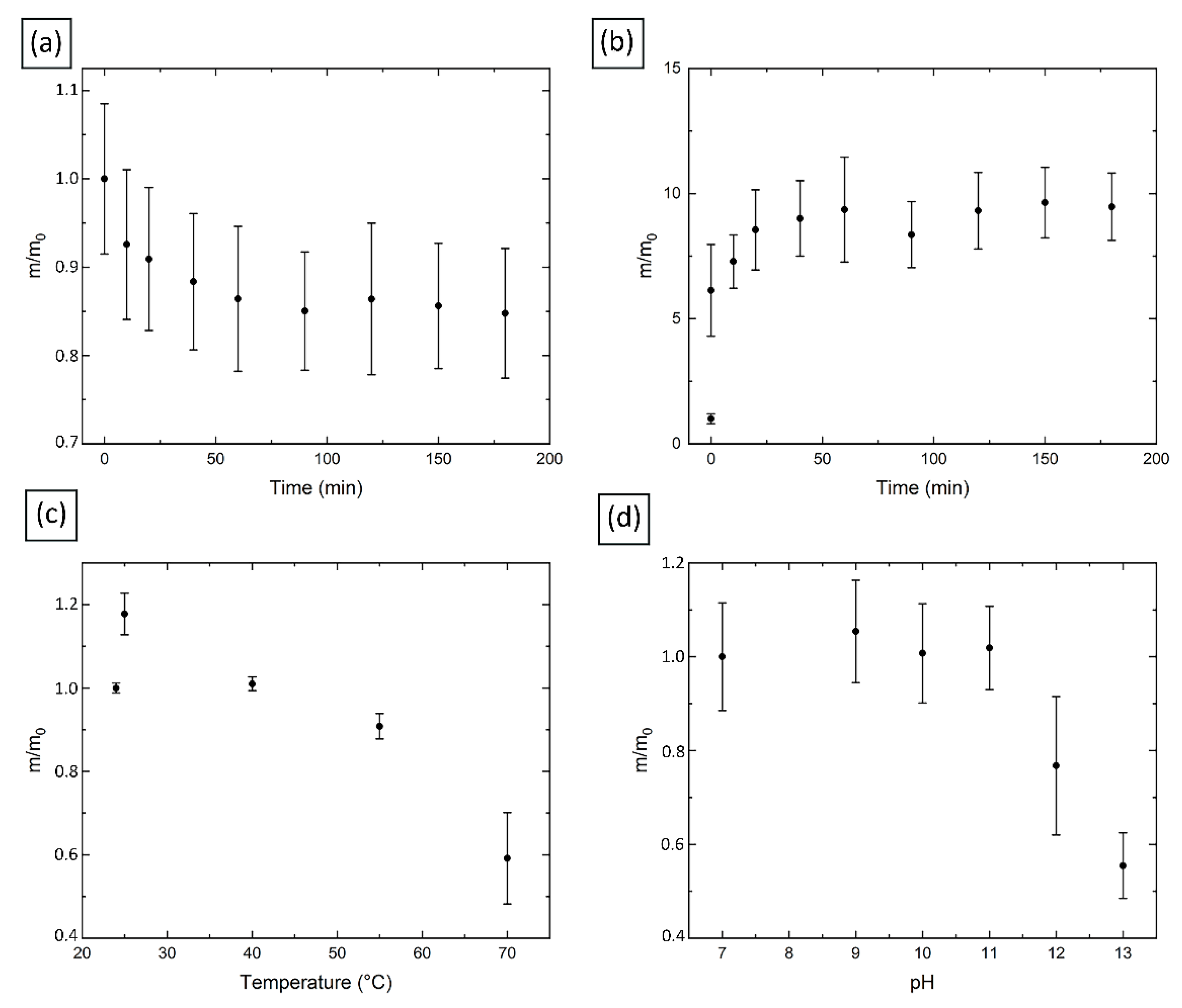
2.6. Properties of Hydrogel Spheres
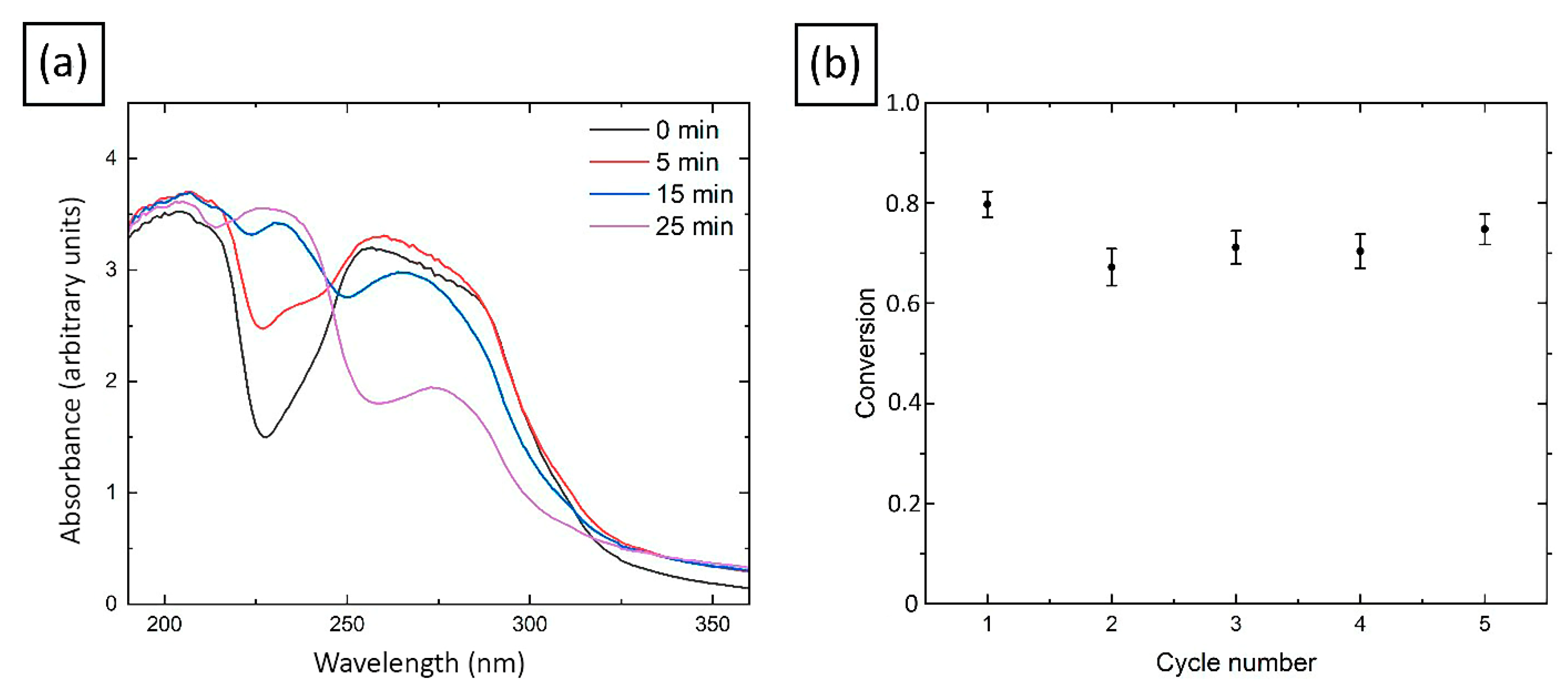
2.7. Catalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Gel Preparation
3.3. Micrporous Gel Preparation
3.4. Synthesis of Cu/CS Spherical Composites
3.5. Characterization
3.6. Antimicrobial Activity
3.7. Model Catalysis Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper nanoparticles with high antimicrobial activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- Madkour, M.; Bumajdad, A.; Al-Sagheer, F. To what extent do polymeric stabilizers affect nanoparticles characteristics? Adv. Colloid Interface Sci. 2019, 270, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Kraynov, A.; Müller, T.E. Concepts for the Stabilization of Metal Nanoparticles in Ionic Liquids. In Applications of Ionic Liquids in Science and Technology; Handy, S.T., Ed.; InTech: Rijeka, Croatia, 2011; pp. 235–260. [Google Scholar] [CrossRef] [Green Version]
- Benelmekki, M.; Vernieres, J.; Kim, J.-H.; Diaz, R.-E.; Grammatikopoulos, P.; Sowwan, M. On the formation of ternary metallic-dielectric multicore-shell nanoparticles by inert-gas condensation method. Mater. Chem. Phys. 2015, 151, 275–281. [Google Scholar] [CrossRef]
- Vollmer, C.; Janiak, C. Naked metal nanoparticles from metal carbonyls in ionic liquids: Easy synthesis and stabilization. Coord. Chem. Rev. 2011, 255, 2039–2057. [Google Scholar] [CrossRef]
- Schroder, K.A.; McCool, S.C.; Furlan, W.R. Broadcast Photonic Curing of Metallic Nanoparticle Films. In Proceedings of the 2006 NSTI Nanotechnology Conference and Trade Show—NSTI Nanotech 2006 Technical Proceedings, Boston, MA, USA, 7–11 May 2006; Volume 3, pp. 198–201. [Google Scholar]
- Sekhon, J.S.; Verma, S.S. Refractive Index Sensitivity Analysis of Ag, Au, and Cu Nanoparticles. Plasmonics 2011, 6, 311–317. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xia, M.; Ye, Y.; Hu, C. Antimicrobial ability of Cu2+-montmorillonite. Appl. Clay Sci. 2004, 27, 215–218. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Dodane, V.; Vilivalam, V.D. Pharmaceutical applications of chitosan. Pharm. Sci. Technol. Today 1998, 1, 246–253. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 8, 981–1014. [Google Scholar] [CrossRef]
- Kaminski, W.; Tomczak, E.; Jaros, K. Interactions of metal ions sorbed on chitosan beads. Desalination 2008, 218, 281–286. [Google Scholar] [CrossRef]
- Ahmad, M.; Zhang, B.; Wang, J.; Xu, J.; Manzoor, K.; Ahmad, S.; Ikram, S. New method for hydrogel synthesis from diphenylcarbazide chitosan for selective copper removal. Int. J. Biol. Macromol. 2019, 136, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Elsabee, M.Z.; Naguib, H.F.; Morsi, R.E. Chitosan based nanofibers, review. Mater. Sci. Eng. C 2012, 32, 1711–1726. [Google Scholar] [CrossRef]
- Irikura, K.; Ekapakul, N.; Choochottiros, C.; Chanthaset, N.; Yoshida, H.; Ajiro, H. Fabrication of flexible blend films using a chitosan derivative and poly(trimethylene carbonate). Polym. J. 2021, 53, 823–833. [Google Scholar] [CrossRef]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef]
- Robitzer, M.; Quignard, F. Marine Polysaccharides and their Conversion into Functional Materials. CHIMIA 2011, 65, 81–84. [Google Scholar] [CrossRef] [PubMed]
- El Kadib, A. Green and Functional Aerogels by Macromolecular and Textural Engineering of Chitosan Microspheres. Chem. Rec. 2020, 20, 753–772. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Alghuthaymi, M.A.; Shami, A.; Rubina, M.S.; Abramchuk, S.S.; Shtykova, E.V.; Vasil’Kov, A.Y. Copper-Chitosan Nanocomposite Hydrogels against Aflatoxigenic Aspergillus Flavus from Dairy Cattle Feed. J. Fungi 2020, 6, 112. [Google Scholar] [CrossRef]
- Sun, L.; Li, A.; Hu, Y.; Li, Y.; Shang, L.; Zhang, L. Self-Assembled Fluorescent and Antibacterial GHK-Cu Nanoparticles for Wound Healing Applications. Part. Part. Syst. Charact. 2019, 36, 1800420. [Google Scholar] [CrossRef]
- Huang, X.; Xu, C.; Li, Y.; Cheng, H.; Wang, X.; Sun, R. Quaternized chitosan-stabilized copper sulfide nanoparticles for cancer therapy (clinically translatable cancer treatment). Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 129–137. [Google Scholar] [CrossRef]
- Khan, S.B.; Ali, F.; Akhtar, K. Chitosan nanocomposite fibers supported copper nanoparticles based perceptive sensor and active catalyst for nitrophenol in real water. Carbohydr. Polym. 2019, 207, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, E.M.; Ali, F.; Khan, S.B.; Marwani, H.M.; Danish, E.Y.; Asiri, A.M. Copper nanoparticles embedded chitosan for efficient detection and reduction of nitroaniline. Int. J. Biol. Macromol. 2019, 131, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Heydari-Soureshjani, E.; Afiyuni, S.S.; Rezaei, B. Copper nanoparticles immobilized on a hybrid chitosan derivative-graphite substrate as a novel electrocatalyst for the oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 16497–16506. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.; Kumar, K.D.; Akbari-Fakhrabadi, A.; Mangalaraja, R.V.; Amalraj, J. Chitosan capped copper oxide/copper nanoparticles encapsulated microbial resistant nanocomposite films. Int. J. Biol. Macromol. 2019, 128, 499–508. [Google Scholar] [CrossRef]
- Ti, S.; Choudhary, M.K.; Joshi, A.; Saharan, V. Assessment of Cu- Chitosan Nanoparticles for its Antibacterial Activity against Pseudomonas syringae pv. glycinea. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1335–1350. [Google Scholar] [CrossRef]
- Anh, T.T.L.; Dat, D.V. Green synthesis of Cu-chitosan nanocomposite by the extract of aganonerion polymorphum leaves for antibacterial application. J. Eng. Sci. Technol. Rev. 2019, 14, 3361. [Google Scholar]
- de Souza, J.F.; da Silva, G.T.; Fajardo, A.R. Chitosan-based film supported copper nanoparticles: A potential and reusable catalyst for the reduction of aromatic nitro compounds. Carbohydr. Polym. 2017, 161, 187–196. [Google Scholar] [CrossRef]
- Wuethrich, B. Allergic and intolerance reactions to wine. Allergology 2011, 34, 427–436. [Google Scholar] [CrossRef]
- Ernstgård, L.; Iregren, A.; Sjögren, B.; Johanson, G. Acute effects of exposure to vapours of acetic acid in humans. Toxicol. Lett. 2006, 165, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Pigaleva, M.A.; Elmanovich, I.V.; Kononevich, Y.N.; Gallyamov, M.O.; Muzafarov, A.M. A biphase H2O/CO2system as a versatile reaction medium for organic synthesis. RSC Adv. 2015, 5, 103573–103608. [Google Scholar] [CrossRef]
- Pigaleva, M.A.; Portnov, I.V.; Rudov, A.A.; Blagodatskikh, I.V.; Grigoriev, T.E.; Gallyamov, M.O.; Potemkin, I.I. Stabilization of Chitosan Aggregates at the Nanoscale in Solutions in Carbonic Acid. Macromolecules 2014, 47, 5749–5758. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kato, Y.; Yoshida, Y.; Isogai, A. SEC-MALS study on aggregates of chitosan molecules in aqueous solvents: Influence of residual N-acetyl groups. Carbohydr. Polym. 2006, 66, 192–198. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Geeraerd, A.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Novikov, I.V.; Pigaleva, M.A.; Abramchuk, S.S.; Molchanov, V.S.; Philippova, O.E.; Gallyamov, M.O. Chitosan composites with Ag nanoparticles formed in carbonic acid solutions. Carbohydr. Polym. 2018, 190, 103–112. [Google Scholar] [CrossRef]
- Pigaleva, M.A.; Novikov, I.V.; Nikolaev, A.Y.; Vasil’Ev, V.G.; Abramchuk, S.S.; Naumkin, A.V.; Arkharova, N.A.; Sadykova, V.S.; Kuvarina, A.E.; Gallyamov, M.O. Platinum cross-linked chitosan hydrogels synthesized in water saturated with CO2 under high pressure. J. Appl. Polym. Sci. 2020, 138, 50006. [Google Scholar] [CrossRef]
- Stamer, K.S.; Pigaleva, M.A.; Abramchuk, S.S.; Gallyamov, M.O. Principles of Gold Nanoparticles Stabilization with Chitosan in Carbonic Acid Solutions Under High CO2 Pressure. Dokl. Phys. Chem. 2020, 495, 166–170. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, T.; Qi, Z.; Li, F.; Yang, K.; Ding, S.; Lin, S.; Tian, F. Fabrication of effective mesoporous silica materials for emergency hemostasis application. Silicon 2022. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Li, P.; Hao, X.; Yang, X.; Xi, G.; Liu, W.; Feng, Y.; He, H.; Shi, C. Polysaccharide Based Hemostatic Strategy for Ultrarapid Hemostasis. Macromol. Biosci. 2020, 20, e1900370. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal Nanoparticles as Green Catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Cheng, X.; Li, Y.; Yang, G. Constructing biodegradable nanochitin-contained chitosan hydrogel beads for fast and efficient removal of Cu(II) from aqueous solution. Carbohydr. Polym. 2019, 211, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Prasher, S.O.; Simpson, B.K. Removal of Selected Metal Ions from Aqueous Solutions Using Chitosan Flakes. Sep. Sci. Technol. 2000, 35, 547–560. [Google Scholar] [CrossRef]
- Paulino, A.T.; Belfiore, L.A.; Kubota, L.T.; Muniz, E.C.; Almeida, V.C.; Tambourgi, E.B. Effect of magnetite on the adsorption behavior of Pb(II), Cd(II), and Cu(II) in chitosan-based hydrogels. Desalination 2011, 275, 187–196. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Reddy, K.K.; Pyarasani, R.D.; Akbari-Fakhrabadi, A.; Amalraj, J. Chitosan-pluronic based Cu nanocomposite hydrogels for prototype antimicrobial applications. Int. J. Biol. Macromol. 2020, 143, 825–832. [Google Scholar] [CrossRef]
- Nishinari, K. Rheological and DSC study of sol-gel transition in aqueous dispersions of industrially important polymers and colloids. Colloid Polym. Sci. 1997, 275, 1093–1107. [Google Scholar] [CrossRef]
- Zoon, P.; Roefs, S.P.F.M.; de Cindio, B.; van Vliet, T. Rheological properties of skim milk gels at various temperatures; interrelation between the dynamic moduli and the relaxation modulus. Rheol. Acta 1990, 29, 223–230. [Google Scholar] [CrossRef]
- Gabriele, D.; de Cindio, B.; D’Antona, P. A weak gel model for foods. Rheol. Acta 2001, 40, 120–127. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.; Akhter, R. Preparation and characterization of chitosan flake and chitosan nanopowder gels: A comparative study of rheological, thermal and morphological perspectives. LWT-Food Sci. Technol. 2021, 148, 111771. [Google Scholar] [CrossRef]
- Hernández, R.; Zamora-Mora, V.; Sibaja-Ballestero, M.; Vega-Baudrit, J.; López, D.; Mijangos, C. Influence of iron oxide nanoparticles on the rheological properties of hybrid chitosan ferrogels. J. Colloid Interface Sci. 2007, 339, 53–59. [Google Scholar] [CrossRef]
- Shaheen, A.; Maswal, M.; Dar, A.A. Synergistic effect of various metal ions on the mechanical, thixotropic, self-healing, swelling and water retention properties of bimetallic hydrogels of alginate. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127223. [Google Scholar] [CrossRef]
- Khanna, P.; Gaikwad, S.; Adhyapak, P.; Singh, N.; Marimuthu, R. Synthesis and characterization of copper nanoparticles. Mater. Lett. 2007, 61, 4711–4714. [Google Scholar] [CrossRef]
- Kazakevich, P.; Simakin, A.; Voronov, V.; Shafeev, G. Laser induced synthesis of nanoparticles in liquids. Appl. Surf. Sci. 2006, 252, 4373–4380. [Google Scholar] [CrossRef]
- Sakthisabarimoorthi, A.; Jose, M.; Dhas, S.A.M.B.; Das, S.J. Fabrication of Cu@Ag core–shell nanoparticles for nonlinear optical applications. J. Mater. Sci. Mater. Electron. 2016, 28, 4545–4552. [Google Scholar] [CrossRef]
- Majhi, J.K.; Mandal, A.C.; Kuiri, P.K. Theoretical Calculation of Optical Absorption of Noble Metal Nanoparticles Using a Simple Model: Effects of Particle Size and Dielectric Function. J. Comput. Theor. Nanosci. 2015, 12, 2997–3005. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Li, X.; Rui, M.; Zeng, H. Localized surface plasmon resonance of Cu nanoparticles by laser ablation in liquid media. RSC Adv. 2015, 5, 79738–79745. [Google Scholar] [CrossRef]
- Ershov, B.G. Formation of metal nanoparticles in aqueous solutions: Atoms and clusters, fast nucleation reactions. Microsyst. Technol. 2003, 12, 31–41. [Google Scholar]
- Song, Y.; Doomes, E.E.; Prindle, J.; Tittsworth, R.; Hormes, A.J.; Kumar, C.S.S.R. Investigations into Sulfobetaine-Stabilized Cu Nanoparticle Formation: Toward Development of a Microfluidic Synthesis. J. Phys. Chem. B 2005, 109, 9330–9338. [Google Scholar] [CrossRef]
- Muthukrishnan, A.; Sathiyabama, M. Green Synthesis of Copper-Chitosan Nanoparticles and Study of its Antibacterial Activity. J. Nanomed. Nanotechnol. 2015, 6, 251. [Google Scholar] [CrossRef]
- Said-Galiev, E.E.; Gamzazade, A.I.; Grigor’Ev, T.E.; Khokhlov, A.R.; Bakuleva, N.P.; Lyutova, I.G.; Shtykova, E.; Dembo, K.A.; Volkov, V.V. Synthesis of Ag and Cu-chitosan metal-polymer nanocomposites in supercritical carbon dioxide medium and study of their structure and antimicrobial activity. Nanotechnol. Russ. 2011, 6, 341–352. [Google Scholar] [CrossRef]
- Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M.K.; Sharma, S.; Pal, A.; Raliya, R.; Biswas, P. Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 2015, 75, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Anthonsen, M.W.; Vårum, K.M.; Hermansson, A.M.; Smidsrød, O.; Brant, D.A. Aggregates in acidic solutions of chitosans detected by static laser light scattering. Carbohydr. Polym. 1994, 25, 13–23. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A. Contribution to the study of the complexation of copper by chitosan and oligomers. Polymer 2002, 43, 1267. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA 300 Database; Wiley: Chichester, UK, 1992; p. 280. [Google Scholar]
- Novikov, I.V.; Pigaleva, M.A.; Levin, E.E.; Abramchuk, S.S.; Naumkin, A.V.; Li, H.; Pich, A.; Gallyamov, M.O. The mechanism of stabilization of silver nanoparticles by chitosan in carbonic acid solutions. Colloid Polym. Sci. 2020, 298, 1135–1148. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Anicuta, S.G.; Dobre, L.; Stroescu, M.; Jipa, I. Fourier transform infrared (FTIR) spectroscopy for characterization of antimicrobial films containing chitosan. An. Univ. Din Oradea Fasc. Ecotoxicologie Zooteh. Și Tehnol. Ind. Aliment. 2010, 11, 815–822. [Google Scholar]
- Kraskouski, A.N.; Nikalaichuk, V.V.; Kulikouskaya, V.I.; Hileuskaya, K.S.; Kalatskaja, J.N.; Nedved, E.L.; Laman, N.A.; Agabekov, V.E. Preparation and Properties of Hydrogel Microparticles Based on Chitosan. Theor. Exp. Chem. 2020, 56, 243–251. [Google Scholar] [CrossRef]
- Monier, M.; Ayad, D.; Wei, Y.; Sarhan, A. Adsorption of Cu(II), Co(II), and Ni(II) ions by modified magnetic chitosan chelating resin. J. Hazard. Mater. 2010, 177, 962–970. [Google Scholar] [CrossRef]
- Wahid, F.; Hu, X.-H.; Chu, L.-Q.; Jia, S.-R.; Xie, Y.-Y.; Zhong, C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int. J. Biol. Macromol. 2019, 122, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Trimukhe, K.D.; Varma, A.J. A morphological study of heavy metal complexes of chitosan and crosslinked chitosans by SEM and WAXRD. Carbohydr. Polym. 2008, 71, 698–702. [Google Scholar] [CrossRef]
- Beppu, M.M.; Arruda, E.J.; Vieira, R.S.; Santos, N.N. Adsorption of Cu (II) on porous chitosan membranes functionalized with histidine. J. Membr. Sci. 2004, 240, 227–235. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q.; Wang, K.; Fu, Q.; Zhang, C. Water transport behavior of chitosan porous membranes containing multi-walled carbon nanotubes (MWNTs). J. Membr. Sci. 2009, 337, 240–247. [Google Scholar] [CrossRef]
- Zeng, X.; Ruckenstein, E. Cross-linked macroporous chitosan anion-exchange membranes for protein separations. J. Membr. Sci. 1998, 148, 195–205. [Google Scholar] [CrossRef]
- Zeng, X.; Ruckenstein, E. Control of Pore Sizes in Macroporous Chitosan and Chitin Membranes. Ind. Eng. Chem. Res. 1996, 35, 4169–4175. [Google Scholar] [CrossRef]
- Zeng, M.; Fang, Z. Preparation of sub-micrometer porous membrane from chitosan/polyethylene glycol semi-IPN. J. Membr. Sci. 2004, 245, 95–102. [Google Scholar] [CrossRef]
- Jessop, P.G.; Subramaniam, B. Gas-Expanded Liquids. Chem. Rev. 2007, 107, 2666–2694. [Google Scholar] [CrossRef]
- Solinas, M.; Pfaltz, A.; Cozzi, P.G.; Leitner, W. Enantioselective Hydrogenation of Imines in Ionic Liquid/Carbon Dioxide Media. J. Am. Chem. Soc. 2004, 126, 16142–16147. [Google Scholar] [CrossRef]
- Thran, A.; Kroll, G.; Faupel, F. Correlation Between Fractional Free Volume and Diffusivity of Gas Molecules in Glassy Polymers. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 3344–3358. [Google Scholar] [CrossRef]
- Tığlı, R.S.; Karakeçili, A.; Gümüşderelioğlu, M. In vitro characterization of chitosan scaffolds: Influence of composition and deacetylation degree. J. Mater. Sci. Mater. Med. 2007, 18, 1665–1674. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.Y.; Mao, Z.W.; Zhou, J.; Shen, J.C.; Hu, X.Q.; Han, C.M. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, G.; Tylingo, R.; Szweda, P.; Augustin, E.; Sadowska, M.; Milewski, S. Preparation and characterization of genipin cross-linked porous chitosan–collagen–gelatin scaffolds using chitosan–CO2 solution. Carbohydr. Polym. 2014, 102, 901–911. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2012, 32, 35–41. [Google Scholar] [CrossRef]
- Kittur, F.S.; Kumar, K.R.; Tharanathan, R.N. Functional packaging properties of chitosan films. Z. Lebensm.-Unters.-Forsch. A 1998, 206, 44–47. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou, B.-S.; Williams, T.; Wood, D.; Du, W.-X.; Sedej, I.; Ban, Z.; Rodov, V.; Poverenov, E.; Vinokur, Y.; et al. Vitamin D-fortified chitosan films from mushroom waste. Carbohydr. Polym. 2017, 167, 97–104. [Google Scholar] [CrossRef]
- Peter, M.; Binulal, N.; Soumya, S.; Nair, S.; Furuike, T.; Tamura, H.; Jayakumar, R. Nanocomposite scaffolds of bioactive glass ceramic nanoparticles disseminated chitosan matrix for tissue engineering applications. Carbohydr. Polym. 2010, 79, 284–289. [Google Scholar] [CrossRef]
- Kumar, P. Nano-TiO2 Doped Chitosan Scaffold for the Bone Tissue Engineering Applications. Int. J. Biomater. 2018, 2018, 6576157. [Google Scholar] [CrossRef] [Green Version]
- El-Azzami, L.A.; Grulke, E.A. Dual mode model for mixed gas permeation of CO2, H2, and N2 through a dry chitosan membrane. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 2620–2631. [Google Scholar] [CrossRef]
- Sato, Y.; Takikawa, T.; Takishima, S.; Masuoka, H. Solubilities and diffusion coefficients of carbon dioxide in poly(vinyl acetate) and polystyrene. J. Supercrit. Fluids 2001, 19, 187–198. [Google Scholar] [CrossRef]
- Shieh, J.-J.; Chung, T.S. Gas permeability, diffusivity, and solubility of poly(4-vinylpyridine) film. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2851–2861. [Google Scholar] [CrossRef]
- Primo, A.; Quignard, F. Chitosan as efficient porous support for dispersion of highly active gold nanoparticles: Design of hybrid catalyst for carbon–carbon bond formation. Chem. Commun. 2010, 46, 5593–5595. [Google Scholar] [CrossRef]
- Frindy, S.; el Kadib, A.; Lahcini, M.; Primo, A.; García, H. Copper Nanoparticles Stabilized in a Porous Chitosan Aerogel as a Heterogeneous Catalyst for C−S Cross-coupling. ChemCatChem 2015, 7, 3307–3315. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.Y.; Wang, G.L.; Zhang, M.; Hu, X.Y. Facile synthesis of submicron Cu2O and CuO crystallites from a solid metallorganic molecular precursor. J. Cryst. Growth 2006, 294, 278–282. [Google Scholar] [CrossRef]
- Dhas, N.A.; Raj, C.P.; Gedanken, A. Synthesis, Characterization, and Properties of Metallic Copper Nanoparticles. Chem. Mater. 1998, 10, 1446–1452. [Google Scholar] [CrossRef]
- Rohindra, D.R.; Nand, A.V.; Khurma, J.R. Swelling properties of chitosan hydrogels. South Pac. J. Nat. Appl. Sci. 2004, 22, 32–35. [Google Scholar] [CrossRef]
- Agrawal, A.; Tratnyek, P.G. Reduction of Nitro Aromatic Compounds by Zero-Valent Iron Metal. Environ. Sci. Technol. 1995, 30, 153–160. [Google Scholar] [CrossRef]
- Li, Y.-P.; Cao, H.-B.; Liu, C.-M.; Zhang, Y. Electrochemical reduction of nitrobenzene at carbon nanotube electrode. J. Hazard. Mater. 2007, 148, 158–163. [Google Scholar] [CrossRef]
- Spain, J.C. Biodegradation of Nitroaromatic Compounds. Annu. Rev. Microbiol. 1995, 49, 523–555. [Google Scholar] [CrossRef]
- Wang, A.-J.; Cheng, H.-Y.; Liang, B.; Ren, N.-Q.; Cui, D.; Lin, N.; Kim, B.H.; Rabaey, K. Efficient Reduction of Nitrobenzene to Aniline with a Biocatalyzed Cathode. Environ. Sci. Technol. 2011, 45, 10186–10193. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Printing Processes and Printing Inks, Carbon Black and Some Nitro Compounds. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; No. 65; International Agency for Research on Cancer: Lyon, France, 1996; pp. 381–408. Available online: https://www.ncbi.nlm.nih.gov/books/NBK424275/ (accessed on 2 June 2022).
- EPA 4304T; National Recommended Water Quality Criteria. Environmental Protection Agency Office of Water, Office of Science and Technology: Washington, DC, USA, 2004.
- 1999 GHZB1; Environmental Quality Standard for Surface Water. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 1999. (In Chinese)
- Duan, Z.; Ma, G.; Zhang, W. Preparation of Copper Nanoparticles and Catalytic Properties for the Reduction of Aromatic Nitro Compounds. Bull. Korean Chem. Soc. 2012, 33, 4003–4006. [Google Scholar] [CrossRef] [Green Version]
- Zeynizadeh, B.; Zabihzadeh, M.; Shokri, Z. Cu Nanoparticles: A Highly Efficient Non-Noble Metal Catalyst For Rapid Reduction Of Nitro Compounds To Amines With Nabh4 In Water. J. Iran. Chem. Soc. 2016, 13, 1487–1492. [Google Scholar] [CrossRef]
- Askadskii, A.A.; Matveev, Y.I.; Matveeva, T.P. The generalized equation for evaluation of the equilibrium modulus of elasticity and Mc value for thin and dense networks. Polym. Sci. Ser. A 1988, 12, 2542–2550. [Google Scholar]
- Askadskii, A.A. Methods for Calculating the Physical Properties of Polymers. Rev. J. Chem. 2015, 5, 83–142. [Google Scholar]
- Matseevich, T.A.; Kovriga, O.V.; Askadskii, A.A. Theoretical analysis of the influence of chemical composition and mixing ration of polymer-solvent nanoparticles on the glass transition temperature. Plast. Massy 2016, 7–8, 48–52. (In Russian) [Google Scholar]


| Sample | Zone (mm) | |
|---|---|---|
| B. subtilis ATCC 6633 | C. albicans ATCC 2091 | |
| Cu/CS | 28 | 17 |
| Amoxiclav/clavulonic acid 20/10 µg | 40 | - |
| Amphotericin B 40 µg | - | 15 |
| Gas | Porosity (%) | Density (g/cm3) |
|---|---|---|
| H2 | 41 ± 8 | 0.81 ± 0.05 |
| CO2 | 35 ± 4 | 0.65 ± 0.04 |
| H2 + CO2 | 52 ± 9 | 0.210 ± 0.012 |
| He + CO2 | 51 ± 6 | 0.234 ± 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamer, K.S.; Pigaleva, M.A.; Pestrikova, A.A.; Nikolaev, A.Y.; Naumkin, A.V.; Abramchuk, S.S.; Sadykova, V.S.; Kuvarina, A.E.; Talanova, V.N.; Gallyamov, M.O. Water Saturated with Pressurized CO2 as a Tool to Create Various 3D Morphologies of Composites Based on Chitosan and Copper Nanoparticles. Molecules 2022, 27, 7261. https://doi.org/10.3390/molecules27217261
Stamer KS, Pigaleva MA, Pestrikova AA, Nikolaev AY, Naumkin AV, Abramchuk SS, Sadykova VS, Kuvarina AE, Talanova VN, Gallyamov MO. Water Saturated with Pressurized CO2 as a Tool to Create Various 3D Morphologies of Composites Based on Chitosan and Copper Nanoparticles. Molecules. 2022; 27(21):7261. https://doi.org/10.3390/molecules27217261
Chicago/Turabian StyleStamer, Katerina S., Marina A. Pigaleva, Anastasiya A. Pestrikova, Alexander Y. Nikolaev, Alexander V. Naumkin, Sergei S. Abramchuk, Vera S. Sadykova, Anastasia E. Kuvarina, Valeriya N. Talanova, and Marat O. Gallyamov. 2022. "Water Saturated with Pressurized CO2 as a Tool to Create Various 3D Morphologies of Composites Based on Chitosan and Copper Nanoparticles" Molecules 27, no. 21: 7261. https://doi.org/10.3390/molecules27217261
APA StyleStamer, K. S., Pigaleva, M. A., Pestrikova, A. A., Nikolaev, A. Y., Naumkin, A. V., Abramchuk, S. S., Sadykova, V. S., Kuvarina, A. E., Talanova, V. N., & Gallyamov, M. O. (2022). Water Saturated with Pressurized CO2 as a Tool to Create Various 3D Morphologies of Composites Based on Chitosan and Copper Nanoparticles. Molecules, 27(21), 7261. https://doi.org/10.3390/molecules27217261







