Prodigiosin from Serratia Marcescens in Cockroach Inhibits the Proliferation of Hepatocellular Carcinoma Cells through Endoplasmic Reticulum Stress-Induced Apoptosis
Abstract
:1. Introduction
2. Results
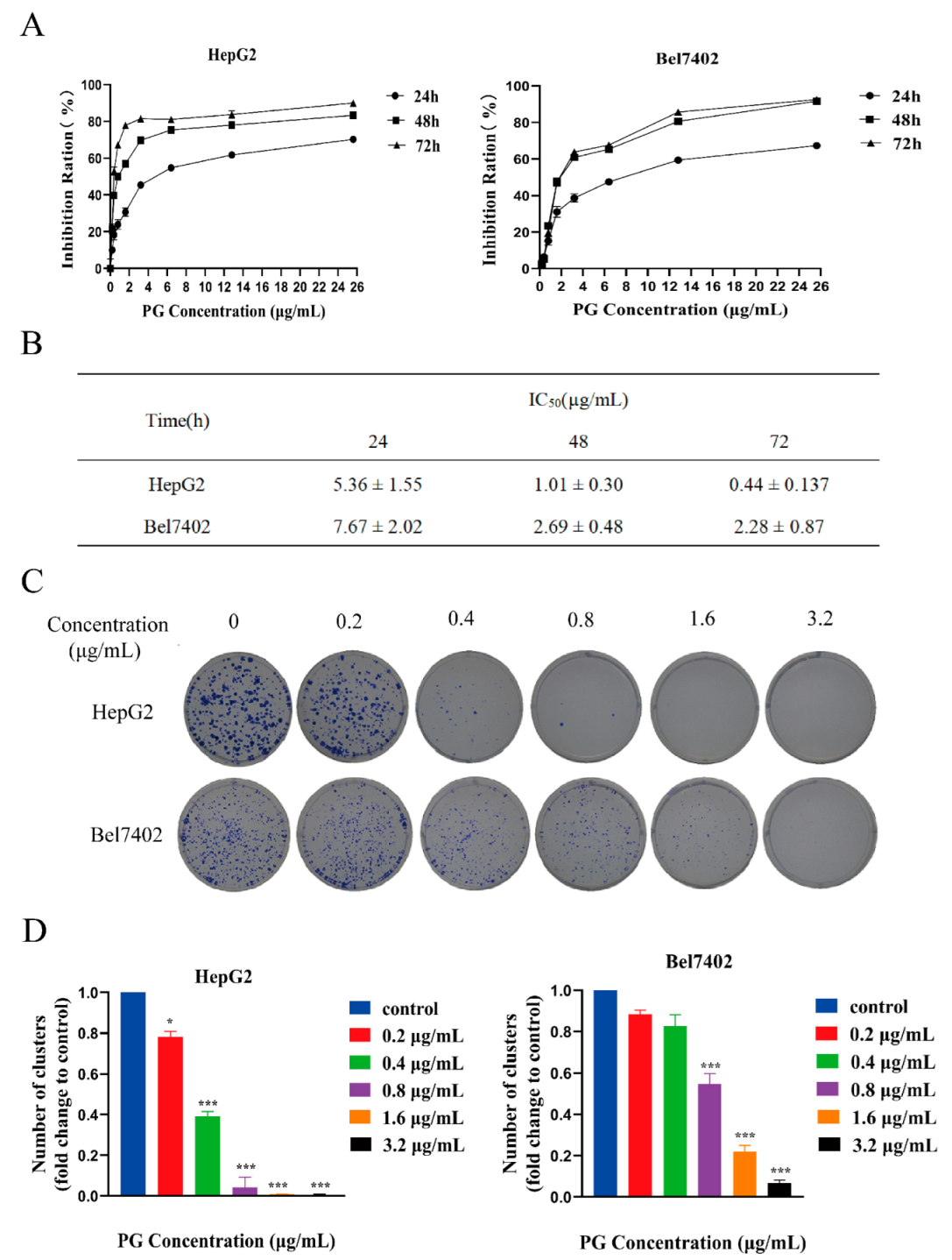
2.1. PG Inhibits the Proliferation and Viability but Promotes the Apoptosis of Hepatocellular Carcinoma Cells
2.2. The Effect of Inhibiting ERS on Apoptosis of HCC Induced by PG
2.3. Effects of PG on ERS in HepG2 and Bel7402 Cells
2.4. PG Reduced the Proliferation of HCC In Vivo
2.5. The Apoptosis-Inducing Effect of PG on Transplanted Tumors in Nude Mice
3. Discussion
4. Materials and Methods
4.1. Cell lines and Laboratory Animals
4.2. Antibodies and Reagents
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. Cell Cycle and Apoptosis Assays Using Flow Cytometry
4.6. TUNEL Assay
4.7. Immunoblot Analysis
4.8. The Localization of PG in Cells Using a Laser Confocal Fluorescence Microscopy
4.9. The measurement of Ca2+ Concentration in Cells Using Flow Cytometry
4.10. Real-Time PCR Analysis
4.11. In Vivo Tumor Model
4.12. Assessment of Liver and Kidney Function
4.13. Hematoxylin–Eosin Staining
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pascual, S.; Herrera, I.; Irurzun, J. New advances in hepatocellular carcinoma. World J. Hepatol. 2016, 8, 421–438. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Huntzicker, E.G.; Hötzel, K.; Choy, L.; Che, L.; Ross, J.; Pau, G.; Sharma, N.; Siebel, C.W.; Chen, X.; French, D.M. Differential effects of targeting Notch receptors in a mouse model of liver. Hepatology 2015, 61, 942–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, W.; Mossmann, D.; Kleemann, J.; Mock, K.; Meisinger, C.; Brummer, T.; Herr, R.; Brabletz, S.; Stemmler, M.P.; Brabletz, T. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat. Commun. 2016, 7, 10498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.; Conrad, C.; Xia, F.; Park, J.S.; Payer, B.; Yin, Y.; Lauwers, G.Y.; Thasler, W.; Lee, J.T.; Avruch, J.; et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular. Cancer Cell 2009, 16, 425–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, R.K.; Chae, S.W.; Kim, H.R.; Chae, H.J. Endoplasmic reticulum stress and cancer. J. Cancer Prev. 2014, 19, 75–88. [Google Scholar] [CrossRef]
- Chevet, E.; Hetz, C.; Samali, A. Endoplasmic reticulum stress-activated cell reprogramming in oncogenesis. Cancer Rev. 2015, 5, 586–597. [Google Scholar] [CrossRef] [Green Version]
- Rutkowski, D.T.; Hegde, R.S. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J. Cell Biol. 2010, 189, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C. The unfolded protein response: Controlling cellfate decisions under ER stressand beyond. Nat. Rev. Mol. Cell. Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Lin, P.B.; Shen, J.; Ou, P.Y.; Liu, L.Y.; Chen, Z.Y.; Chu, F.J.; Wang, J.; Jin, X.B. Prodigiosin isolated from Serratia marcescens in the Periplaneta americana gut and its apoptosis inducing activity in HeLa cells. Oncol. Rep. 2019, 41, 3377–3385. [Google Scholar] [CrossRef]
- Yenkejeh, R.A.; Sam, M.R.; Esmaeillou, M. Targeting survivin with prodigiosin isolated from cell wall of Serratia marcescens induces apoptosis in hepatocellular carcinoma cells. Hum. Exp. Toxicol. 2017, 36, 402–411. [Google Scholar] [CrossRef]
- Lazic, J.; Skaro Bogojevic, S.; Vojnovic, S.; Aleksic, I.; Milivojevic, D.; Kretzschmar, M.; Gulder, T.; Petkovic, M.; Nikodinovic-Runic, J. Synthesis, Anticancer Potential and Comprehensive Toxicity Studies of Novel Brominated Derivatives of Bacterial Biopigment Prodigiosin from Serratia marcescens ATCC 27117. Molecules 2022, 27, 3729. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lim, S.; Yoon, K.H.; Lee, J.I.; Mitchell, R.J.; Orcid, I.D. Biotechnological, Activities and Applications of Bacterial Pigments Violacein and Prodigiosin. J. Biol. Eng. 2021, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qiu, S.; He, J.; Peng, Y.; Xu, H.; Feng, Z.; Huang, H.; Du, Y.; Zhou, Y.; Nie, Y. Prodigiosin impairs autophagosome-lysosome fusion that sensitizes colorectal cancer cells to 5-fluorouracil-induced cell death. Cancer Lett. 2020, 481, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Orcid, I.D.; Weng, C.F. PG-priming Enhances doxorubicin influx to trigger necrotic and autophagic cell death in oral squamous cell carcinoma. J. Clin. Med. 2018, 7, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopleva, M.; Watt, J.; Contractor, R.; Tsao, T.; Harris, D.; Estrov, Z.; Bornmann, W.; Kantarjian, H.; Viallet, J.; Samudio, I.; et al. Mechanisms of Antileukemic Activity of the Novel Bcl-2 Homology Domain-3 Mimetic GX15-070 (Obatoclax). Cancer Res. 2008, 68, 3413–3420. [Google Scholar] [CrossRef] [Green Version]
- Schimmer, A.D.; Raza, A.; Carter, T.H.; Claxton, D.; Erba, H.; DeAngelo, D.J.; Tallman, M.S.; Goard, C.; Borthakur, G. A multicenter phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PLoS ONE 2014, 9, e108694. [Google Scholar] [CrossRef]
- Langer, C.J.; Albert, I.; Ross, H.J.; Kovacs, P.; Blakely, L.J.; Pajkos, G.; Somfay, A.; Zatloukal, P.; Kazarnowicz, A.; Moezi, M.M.; et al. Randomized phase II study of carboplatin and etoposide with or without obatoclax mesylate in extensive-stage small cell lung cancer. Lung Cancer 2014, 85, 420–428. [Google Scholar] [CrossRef]
- Oki, Y.; Copeland, A.; Hagemeister, F.; Fayad, L.E.; Fanale, M.; Romaguera, J.; Younes, A. Experience with obatoclax mesylate (GX15-070), a small molecule pan–Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood 2012, 119, 2171–2172. [Google Scholar] [CrossRef] [Green Version]
- Arellano, M.L.; Borthakur, G.; Berger, M.; Luer, J.; Raza, A. A Phase II, Multicenter, Open-Label Study of Obatoclax Mesylate in Patients with Previously Untreated Myelodysplastic Syndromes With Anemia or Thrombocytopenia. Clin. Lymphoma Myeloma Leuk. 2014, 14, 534–539. [Google Scholar] [CrossRef]
- Chow, C.Y.; Wang, X.; Riccardi, D.; Wolfner, M.F.; Clark, A.G. The genetic architecture of the genome-wide transcriptional response to ER stress in the mouse. PLoS Genet. 2015, 11, e1004924. [Google Scholar] [CrossRef] [PubMed]
- Hasenjäger, A.; Gillissen, B.; Müller, A.; Normand, G.; Hemmati, P.G.; Schuler, M.; Dörken, B.; Daniel, P.T. Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-xL in a strictly caspase-3-dependent manner in human carcinoma cells. Oncogene 2004, 23, 4523–4535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, A.; Espona-Fiedler, M.; Soto-Cerrato, V.; Quesada, R.; Pérez-Tomás, R.; Guallar, V. Molecular interactions of prodiginines with the BH3 domain of anti-apoptotic Bcl-2 family members. PLoS ONE 2013, 8, e5756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nature Cell. Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nature Cell. Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Obayemi, J.D.; Salifu, A.A.; Eluu, S.C.; Uzonwanne, V.O.; Jusu, S.M.; Nwazojie, C.C.; Onyekanne, C.E.; Ojelabi, O.; Payne, L.; Moore, C.M.; et al. LHRH-Conjugated Drugs as Targeted Therapeutic Agents for the Specific Targeting and Localized Treatment of Triple Negative Breast Cancer. Sci. Rep. 2020, 10, 8212. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Y.; Wang, Z.; Wang, Y.P.; Zheng, H. Inhibiting autophagy increases epirubicin’s cytotoxicity in breast cancer cells. Cancer Sci. 2016, 11, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ming, H.; Chen, R.; Ju, J.M.; Peng, W.D.; Zhang, G.X.; Liu, C.F. CIH-induced neurocognitive impairments are associated with hippocampal Ca(2+) overload, apoptosis, and dephosphorylation of ERK1/2 and CREB that are mediated by overactivation of NMDARs. Brain Res. 2015, 1625, 64–72. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, H.; Zhu, L.; Wang, J.; Luo, X.; Liu, W.; Ma, Y. Prodigiosin from Serratia Marcescens in Cockroach Inhibits the Proliferation of Hepatocellular Carcinoma Cells through Endoplasmic Reticulum Stress-Induced Apoptosis. Molecules 2022, 27, 7281. https://doi.org/10.3390/molecules27217281
Wang J, Liu H, Zhu L, Wang J, Luo X, Liu W, Ma Y. Prodigiosin from Serratia Marcescens in Cockroach Inhibits the Proliferation of Hepatocellular Carcinoma Cells through Endoplasmic Reticulum Stress-Induced Apoptosis. Molecules. 2022; 27(21):7281. https://doi.org/10.3390/molecules27217281
Chicago/Turabian StyleWang, Jie, Hancong Liu, Liuchong Zhu, Jingyi Wang, Xiongming Luo, Wenbin Liu, and Yan Ma. 2022. "Prodigiosin from Serratia Marcescens in Cockroach Inhibits the Proliferation of Hepatocellular Carcinoma Cells through Endoplasmic Reticulum Stress-Induced Apoptosis" Molecules 27, no. 21: 7281. https://doi.org/10.3390/molecules27217281
APA StyleWang, J., Liu, H., Zhu, L., Wang, J., Luo, X., Liu, W., & Ma, Y. (2022). Prodigiosin from Serratia Marcescens in Cockroach Inhibits the Proliferation of Hepatocellular Carcinoma Cells through Endoplasmic Reticulum Stress-Induced Apoptosis. Molecules, 27(21), 7281. https://doi.org/10.3390/molecules27217281




