Modification of the Nutritional Quality and Oxidative Stability of Lupin (Lupinus mutabilis Sweet) and Sacha Inchi (Plukenetia volubilis L.) Oil Blends
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization
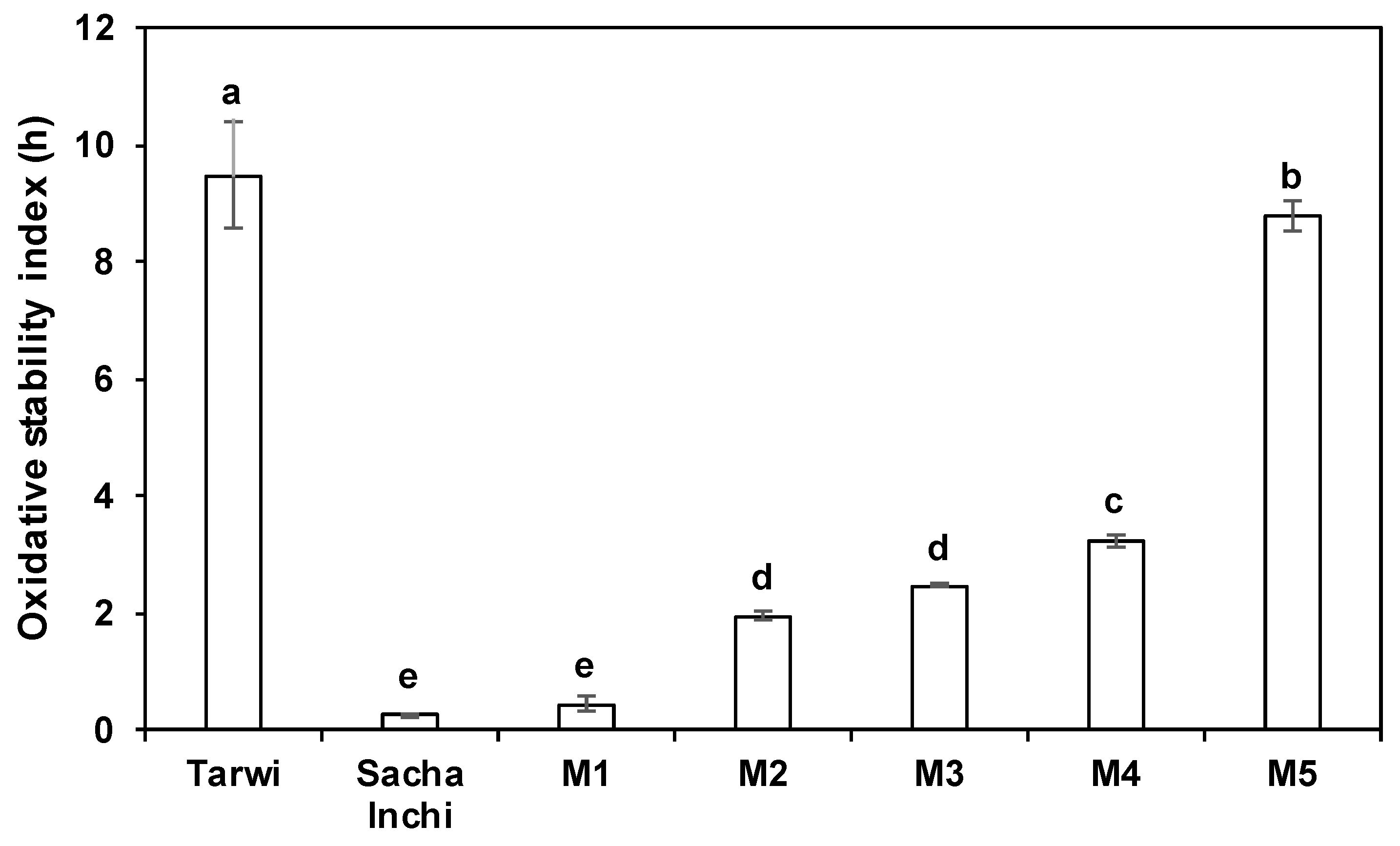
2.2. Oxidative Stability Index
2.3. Thermodynamic Study and Shelf Life
3. Materials and Methods
3.1. Materials
3.2. Oils Extraction and Blends Preparation
3.3. Chemical Analyses
3.4. Oxidative Stability Index (OSI)
Thermodynamic Analysis and Shelf Life
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarno, M.; Iuliano, M.; Viscusi, G.; Zarli, A.; Ciambelli, P. A Nickel/Palladium/Ruthenium-Graphene based nanocatalyst for selective catalytic hydrogenation of vegetable oils. Ind. Crops Prod. 2021, 170, 113815. [Google Scholar] [CrossRef]
- Dijkstra, A.J. Interesterification, chemical or enzymatic catalysis. Lipid Technol. 2015, 27, 134–136. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Madhujith, T. Current trends in applications of enzymatic interesterification of fats and oils: A review. LWT-Food Sci. Technol. 2020, 132, 109880. [Google Scholar] [CrossRef]
- Kellens, M.; Gibon, V.; Hendrix, M.; De Greyt, W. Palm oil fractionation. Eur. J. Lipid Sci. Technol. 2007, 109, 336–349. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Vegetable oil blending: A review of physicochemical, nutritional and health effects. Trends Food Sci. Technol. 2016, 57, 52–58. [Google Scholar] [CrossRef]
- Rodríguez, G.; Villanueva, E.; Cortez, D.; Sanchez, E.; Aguirre, E.; Hidalgo, A. Oxidative stability of chia (Salvia hispanica L.) and sesame (Sesamum indicum L.) oil blends. J. Am. Oil Chem. Soc. 2020, 97, 729–735. [Google Scholar] [CrossRef]
- Roiaini, M.; Ardiannie, T.; Norhayati, H. Physicochemical properties of canola oil, olive oil and palm olein blends. Int. Food Res. J. 2015, 22, 1227–1233. [Google Scholar]
- Li, Y.; Ma, W.J.; Qi, B.K.; Rokayya, S.; Li, D.; Wang, J.; Feng, H.X.; Sui, X.N.; Jiang, L.Z. Blending of soybean oil with selected vegetable oils: Impact on oxidative stability and radical scavenging activity. Asian Pac. J. Cancer Prev. 2014, 15, 2583–2589. [Google Scholar] [CrossRef]
- Rudzinska, M.; Hassanein, M.M.; Abdele-Razek, A.G.; Ratusz, K. Blends of rapeseed oil with black cumin and rice bran oils for increasing the oxidative stability. J. Food Sci. Technol. 2016, 53, 1055–1062. [Google Scholar] [CrossRef]
- Wang, S.N.; Sui, X.N.; Wang, Z.J.; Qi, B.K.; Jiang, L.Z.; Li, Y.; Wang, R.; Wei, X. Improvement in thermal stability of soybean oil by blending with camellia oil during deep fat frying. Eur. J. Lipid Sci. Technol. 2016, 118, 524–531. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Domínguez, G.; Campos, D. Comparison of the physico-chemical and phytochemical characteristics of the oil of two Plukenetia species. Food Chem. 2015, 173, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Fanali, C.; Dugo, L.; Cacciola, F.; Beccaria, M.; Grasso, S.; Dachà, M.; Dugo, P.; Mondello, L. Chemical characterisation of sacha inchi (Plukenetia volubilis L.) oil. J. Agric. Food Chem. 2011, 59, 13043–13049. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Tanwar, B.; Sihag, M.; Sharma, V. Sacha inchi (Plukenetia volubilis L.): An emerging source of nutrients, omega-3 fatty acid and phytochemicals. Food Chem. 2022, 373 Pt B, 131459. [Google Scholar] [CrossRef]
- Rodríguez, G.; Squeo, G.; Estivi, L.; Quezada Berru, S.; Buleje, D.; Caponio, F.; Brandolini, A.; Hidalgo, A. Changes in stability, tocopherols, fatty acids and antioxidant capacity of sacha inchi (Plukenetia volubilis) oil during French fries deep-frying. Food Chem. 2021, 340, 127942. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, R.; Zuloeta, G.; Pedreschi, R.; Mignolet, E.; Larondelle, Y.; Campos, D. Sacha Inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopheroles, phytosteroles, phenolic compounds and antioxidant capacity. Food Chem. 2013, 141, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Briceño Berru, L.; Glorio-Paulet, P.; Basso, C.; Scarafoni, A.; Camarena, F.; Hidalgo, A.; Brandolini, A. Chemical composition, tocopherol and carotenoid content of seeds from different Andean lupin (Lupinus mutabilis) ecotypes. Plant Foods Hum. Nutr. 2021, 76, 98–104. [Google Scholar] [CrossRef]
- Carvajal-Larenas, F.E.; Linnemann, A.R.; Nout, M.J.R.; Koziol, M.; Van Boekel, M.A.J.S. Lupinus mutabilis: Composition, uses, toxicology, and debittering. Crit. Rev. Food Sci. Nutr. 2015, 56, 1454–1487. [Google Scholar] [CrossRef]
- Curti, C.A.; Curti, R.N.; Bonini, N.; Ramón, A.N. Changes in the fatty acid composition in bitter Lupinus species depend on the debittering process. Food Chem. 2018, 263, 151–154. [Google Scholar] [CrossRef]
- Calder, P.C. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Huerta-Yépez, S.; Tirado-Rodríguez, A.B.; Hankinsona, O. Role of diets rich in omega-3 and omega-6 in the development of cancer. Boletín Médico Del Hosp. Infant. De México 2016, 73, 446–456. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Subash-Babu, P. Effects of increasing ratios of dietary omega-6/omega-3 fatty acids on human monocyte immunomodulation linked with atherosclerosis. J. Funct. Foods 2018, 41, 258–267. [Google Scholar] [CrossRef]
- Shetty, S.S.; Kumari, N.S.; Shetty, P.K. ω-6/ω-3 fatty acid ratio as an essential predictive biomarker in the management of type 2 diabetes mellitus. Nutrition 2020, 79, 110968. [Google Scholar] [CrossRef]
- Anwar, S.; Kausar, M.A.; Parveen, K.; Siddiqui, W.A.; Zahra, A.; Ali, A.; Badraoui, R.; Jamal, A.; Akhtar, N.; Saeed, M.A. vegetable oil blend administration mitigates the hyperglycemia-induced redox imbalance, renal histopathology, and function in diabetic nephropathy. J. King Saud Univ. - Sci. 2022, 34, 102018. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.; Liu, Z.; Perez, J.M. Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim. Acta 2000, 364, 87–97. [Google Scholar] [CrossRef]
- Presti, G.; Giuliano, S.; Gulotta, E.; Monfreda, M. Legal blends between olive oil and other vegetable oils: Quantification of olive oil and identification of “virgin olive oils”, “refined olive oils” and “olive pomace oils”. Talanta Open 2021, 3, 100039. [Google Scholar] [CrossRef]
- Pascual-Chagman, G.; Santa-Cruz-Olivos, J.; Hidalgo, A.; Benavente, F.; Pérez-Camino, M.C.; Sotelo, A.; Paucar-Menacho, L.M.; Encina-Zelada, C.R. Lupinus mutabilis oil obtained by expeller press: Yield, physicochemical characterisation, antioxidant capacity, fatty acids and oxidative stability analyses. Sci. Agropecu. 2021, 12, 219–227. [Google Scholar] [CrossRef]
- Villacrés, E.; Quelal, M.B.; Jácome, X.; Cueva, G.; Rosell, C.M. Effect of debittering and solid-state fermentation processes on the nutritional content of lupine (Lupinus mutabilis Sweet). Int. J. Food Sci. Technol. 2020, 55, 2589–2598. [Google Scholar] [CrossRef]
- Zampelas, A.; Paschos, G.; Rallidis, L.; Yiannakouris, N. Linoleic acid to alpha-linolenic acid ratio. World Rev. Nutr. Diet 2003, 92, 92–108. [Google Scholar]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Sheppard, K.W.; Cheatham, C.L. Omega-6/omega-3 fatty acid intake of children and older adults in the US: Dietary intake in comparison to current dietary recommendations and the Healthy Eating Index. Lipids Health Dis. 2018, 17, 43. [Google Scholar] [CrossRef]
- Codex Stan 210-1999; Codex Alimentarius. Standard for Named Vegetables Oils. Food and Agriculture Organization of the United Nations: Rome, Italy, 2015.
- Brandolini, A.; Glorio-Paulet, P.; Estivi, L.; Locatelli, N.; Cordova-Ramos, J.S.; Hidalgo, A. Tocopherols, carotenoids and phenolics changes during Andean lupin (Lupinus mutabilis Sweet) seeds processing. J. Food Compos. Anal. 2022, 106, 104335. [Google Scholar] [CrossRef]
- Aranda-Ventura, J.; Villacrés-Vallejo, J.; Rios-Isern, F. Composición química, características físico-químicas, trazas metálicas y evaluación genotóxica del aceite de Plukenetia volubilis L. (sacha inchi). Rev. Peru. de Med. Integr. 2019, 4, 4–14. [Google Scholar] [CrossRef]
- Schmidt, Š.; Pokorný, J. Potential application of oilseeds as sources of antioxidants for lipids—A review. Czech J. Food Sci. 2005, 23, 93–102. [Google Scholar] [CrossRef]
- Martínez, M.L.; Curti, M.I.; Roccia, P.; Llabot, J.M.; Penci, M.C.; Bodoira, R.M.; Ribotta, P.D. Oxidative stability of walnut (Juglans regia L.) and chia (Salvia hispanica L.) oils microencapsulated by spray drying. Powder Technol. 2015, 270A, 271–277. [Google Scholar] [CrossRef]
- Obranović, M.; Škevin, D.; Kraljić, K.; Pospišil, M.; Neđeral, S.; Blekić, M.; Putnik, P. Influence of climate, variety and production process on tocopherols, plastochromanol-8 and pigments in flaxseed oil. Food Technol. Biotechnol. 2015, 53, 496–504. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A. Nutritional properties of einkorn wheat (Triticum monococcum L.). J. Sci. Food Agric. 2014, 94, 601–612. [Google Scholar] [CrossRef]
- Salvatierra-Pajuelo, Y.M.; Azorza-Richarte, M.E.; Paucar-Menacho, L.M. Optimización de las características nutricionales, texturales y sensoriales de cookies enriquecidas con chía (Salvia hispánica) y aceite extraído de tarwi (Lupinus mutabilis). Sci. Agropecu. 2019, 10, 7–17. [Google Scholar] [CrossRef]
- Rodríguez, G.; Villanueva, E.; Glorio, P.; Baquerizo, M. Oxidative stability and estimate of the shelf life of sacha inchi (Plukenetia volubilis L.) oil. Sci. Agropecu. 2015, 6, 155–163. [Google Scholar] [CrossRef]
- García-Moreno, P.; Pérez-Gálvez, R.; Guadix, A.; Guadix, E.M. Influence of the parameters of the Rancimat test on the determination of the oxidative stability index of cod liver oil. LWT-Food Sci. Technol. 2013, 51, 303–308. [Google Scholar] [CrossRef]
- Heidarpour, M.; Farhoosh, R. A preliminary Rancimat based kinetic approach of detecting olive oil adulteration. LWT-Food Sci. Technol. 2018, 90, 77–82. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Comparison of the oxidative stability of linseed (Linum usitatissimum L.) oil by pressure differential scanning calorimetry and Rancimat measurements. J. Food Sci. Technol. 2016, 53, 3986–3995. [Google Scholar] [CrossRef] [PubMed]
- Varas Condori, M.; Pascual Chagman, G.; Barriga Sanchez, M.E.; Villegas Vilchez, L.F.; Ursetta, S.; Guevara, A.; Hidalgo, A. Effect of tomato (Solanum lycopersicum L.) lycopene-rich extract on the kinetics of rancidity and shelf-life of linseed (Linum usitatissimum L.) oil. Food Chem. 2020, 302, 125327. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Hoseini-Yazdi, S.-Z. Evolution of oxidative values during kinetic studies on olive oil oxidation in the Rancimat test. J. Am. Oil Chem. Soc. 2014, 91, 281–293. [Google Scholar] [CrossRef]
- Villanueva, E.; Rodríguez, G.; Aguirre, E.; Castro, V. Influencia de antioxidantes en la estabilidad oxidativa del aceite de chia (Salvia hispanica L.) por Rancimat. Sci. Agropecu. 2017, 8, 19–27. [Google Scholar] [CrossRef]
- Villanueva, E.; Castillo, D.; Rodríguez, G. Influence of the Rancimat parameters on the determination of oxidative stability index of Sesamum indicum L. oil. Sci. Agropecu. 2013, 4, 173–180. [Google Scholar] [CrossRef]
- Córdova-Ramos, J.S.; Glorio-Paulet, P.; Camarena, F.; Brandolini, A.; Hidalgo, A. Andean lupin (Lupinus mutabilis Sweet): Processing effects on chemical composition, heat damage, and in vitro protein digestibility. Cereal Chem. 2020, 97, 827–835. [Google Scholar] [CrossRef]
- IUPAC—International Union of Pure and Applied Chemistry. IUPAC—International Union of Pure and Applied Chemistry. IUPAC Standard Methods 2.302, 2.432, 2.507. In Standard Methods for the Analysis of Oils, Fats and Derivates; Blackwell Scientific: Oxford, UK, 1987. [Google Scholar]
- AOCS—American Oil Chemists´ Society. Methods Cd 3d-63, Cd 8-53, Cd 12b-92 and Cd 18-90. In Official Methods and Recommended Practices of the AOCS, 5th ed.; AOCS: Champaign, IL, USA, 1998. [Google Scholar]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Farhoosh, R. The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J. Am. Oil Chem. Soc. 2007, 84, 205–209. [Google Scholar] [CrossRef]
| Tarwi | Sacha Inchi | Blends | |||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | |||
| C16:0 | 10.9 ± 0.01 a | 4.8 ± 0.09 g | 5.2 ± 0.02 f | 6.1 ± 0.10 e | 6.8 ± 0.01 d | 8.8 ± 0.06 c | 10.2 ± 0.07 b |
| C18:0 | 6.3 ± 0.27 a | 3.9 ± 0.04 g | 4.0 ± 0.01 f | 4.1 ± 0.04 e | 4.5 ± 0.08 d | 5.1 ± 0.09 c | 5.5 ± 0.09 b |
| C18:1 (ω-9) | 54.2 ± 1.03 a | 9.4 ± 0.03 g | 14.8 ± 0.01 f | 19.4 ± 0.05 e | 27.4 ± 0.13 d | 35.9 ± 0.86 c | 42.9 ± 0.10 b |
| C18:2 (ω-6) | 25.7 ± 0.50 d | 34.7 ± 0.52 a | 33.0 ± 0.02 a,b | 30.6 ± 0.34 b | 28.2 ± 0.62 c | 27.5 ± 0.09 c | 26.2 ± 0.01 d |
| C18:3 (ω-3) | 2.8 ± 0.45 g | 47.2 ± 0.59 a | 43.0 ± 0.04 b | 39.8 ± 0.15 c | 33.1 ± 0.65 d | 22.7 ± 1.10 e | 13.7 ± 0.03 f |
| MUFA/PUFA | 1.90 ± 0.001 a | 0.11 ± 0.02 g | 0.19 ± 0.001 f | 0.28 ± 0.001 e | 0.45 ± 0.002 d | 0.72 ± 0.003 c | 1.07 ± 0.002 b |
| PUFA/SFA | 1.7 ± 0.01 g | 9.5 ± 0.10 a | 8.3 ± 0.05 b | 6.9 ± 0.01 c | 5.4 ± 0.05 d | 3.6 ± 0.11 e | 2.5 ± 0.01 f |
| ω-3/ω-6 | 1:9.14 f | 1:0.7 a | 1:0.8 b | 1:0.8 b | 1:0.9 c | 1:1.2 d | 1:1.9 e |
| PV | 1.8 ± 0.01 e | 2.0 ± 0.02 a | 2.0 ± 0.02 b | 1.9 ± 0.02 c | 1.9 ± 0.01 c | 1.9 ± 0.01 c,d | 1.9 ± 0.01 d,e |
| p-AV | 1.1 ± 0.01 g | 1.4 ± 0.01 a | 1.4 ± 0.02 b | 1.3 ± 0.02 c | 1.3 ± 0.01 d | 1.2 ± 0.02 e | 1.2 ± 0.01 f |
| Acidity | 0.9 ± 0.01 | 1.1 ± 0.03 | 1.0 ± 0.01 | 1.0 ± 0.01 | 1.0 ± 0.02 | 1.0 ± 0.02 | 0.9 ± 0.01 |
| ToTox | 4.8 ± 0.02 e | 5.5 ± 0.03 a | 5.3 ± 0.05 b | 5.1 ± 0.04 c | 5.1 ± 0.02 c | 5.0 ± 0.03 d | 4.9 ± 0.02 d |
| α–tocopherol | 1.60 ± 0.13 c | 3.26 ± 0.19 a | 3.08 ± 0.21 a | 2.77 ± 0.07 a | 2.21 ± 0.27 b | 2.12 ± 0.13 b,c | 2.08 ± 0.28 b,c |
| γ-tocopherol | 288.2 ± 1.0 f | 1296.5 ± 94.2 a | 1157.7 ± 16.2 b | 998.7 ± 32.3 c | 817.5 ± 35.2 d | 663.6 ± 74.8 e | 511.6 ± 2.9 e |
| δ-tocopherol | 4.55 ± 0.11 g | 794.4 ± 45.5 a | 673.3 ± 1.6 b | 544.3 ± 44.8 c | 412.8 ± 15.5 d | 283.0 ± 51.2 e | 174.4 ± 3.6 f |
| Total tocopherol | 294.4 ± 0.7 g | 2094.1 ± 139.5 a | 1834.0 ± 17.6 b | 1545.8 ± 77.2 c | 1232.5 ± 51.0 d | 948.7 ± 126.1 e | 688.1 ± 1.0 f |
| Lutein | 1.44 ± 0.03 a | nd e | 0.29 ± 0.02 c,d | 0.47 ± 0.05 c | 0.86 ± 0.12 b | 0.94 ± 0.13 b | 1.10 ± 0.29 b |
| Temperature (°C) | Tarwi | Sacha Inchi | M3 |
|---|---|---|---|
| 80 | 17.48 ± 0.45 a | ||
| 90 | 3.54 ± 0.12 b | ||
| 100 | 1.57 ± 0.08 c | 18.28 ± 0.06 a | |
| 110 | 19.84 ± 0.01 a | 0.50 ± 0.01 d | 4.70 ± 0.19 b |
| 120 | 9.48 ± 0.91 b | 0.20 ± 0.01 d | 2.46 ± 0.02 c |
| 130 | 3.58 ± 0.08 c | 1.21 ± 0.03 d | |
| 140 | 1.76 ± 0.03 d |
| Tarwi | Sacha Inchi | M3 | |
|---|---|---|---|
| ∆H++ (kJ/mol) | 104.95 ± 1.07 b | 112.01 ± 1.65 a | 107.04 ± 0.27 b |
| ∆S++ (J/mol) | −1.84 ± 2.54 a | −50.85 ± 4.26 c | −17.43± 0.79 b |
| ∆G++ (kJ/mol) | 105.65 ± 2.03 c | 131.47 ± 3.13 a | 113.77 ± 0.47 b |
| Ea (kJ/mol) | 108.24 ± 1.07 b | 115.13 ± 1.64 a | 110.24 ± 0.27 b |
| Log OSI = α(T) + β | OSI25 | Q10 | |||
|---|---|---|---|---|---|
| α | β | R2 | |||
| Tarwi | −0.036 ± 0.000 | 5.237 ± 0.065 | 0.995 | 2.57 ± 0.32 a | 2.26 ± 0.35 a |
| Sacha inchi | −0.051 ± 0.001 | 5.253 ± 0.047 | 0.980 | 1.11 ± 0.08 b | 2.62 ± 0.43 a |
| M3 | −0.038 ± 0.000 | 4.992 ± 0.007 | 0.966 | 1.26 ± 0.01 b | 2.61 ± 0.91 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, G.; Aguirre, E.; Córdova-Chang, A.; Muñoz-Saenz, J.C.; Baquerizo, M.; Brandolini, A.; Villanueva, E.; Hidalgo, A. Modification of the Nutritional Quality and Oxidative Stability of Lupin (Lupinus mutabilis Sweet) and Sacha Inchi (Plukenetia volubilis L.) Oil Blends. Molecules 2022, 27, 7315. https://doi.org/10.3390/molecules27217315
Rodríguez G, Aguirre E, Córdova-Chang A, Muñoz-Saenz JC, Baquerizo M, Brandolini A, Villanueva E, Hidalgo A. Modification of the Nutritional Quality and Oxidative Stability of Lupin (Lupinus mutabilis Sweet) and Sacha Inchi (Plukenetia volubilis L.) Oil Blends. Molecules. 2022; 27(21):7315. https://doi.org/10.3390/molecules27217315
Chicago/Turabian StyleRodríguez, Gilbert, Elza Aguirre, Any Córdova-Chang, Jenny C. Muñoz-Saenz, Mery Baquerizo, Andrea Brandolini, Eudes Villanueva, and Alyssa Hidalgo. 2022. "Modification of the Nutritional Quality and Oxidative Stability of Lupin (Lupinus mutabilis Sweet) and Sacha Inchi (Plukenetia volubilis L.) Oil Blends" Molecules 27, no. 21: 7315. https://doi.org/10.3390/molecules27217315
APA StyleRodríguez, G., Aguirre, E., Córdova-Chang, A., Muñoz-Saenz, J. C., Baquerizo, M., Brandolini, A., Villanueva, E., & Hidalgo, A. (2022). Modification of the Nutritional Quality and Oxidative Stability of Lupin (Lupinus mutabilis Sweet) and Sacha Inchi (Plukenetia volubilis L.) Oil Blends. Molecules, 27(21), 7315. https://doi.org/10.3390/molecules27217315








