Research Advances of Bioactive Sesquiterpenoids Isolated from Marine-Derived Aspergillus sp.
Abstract
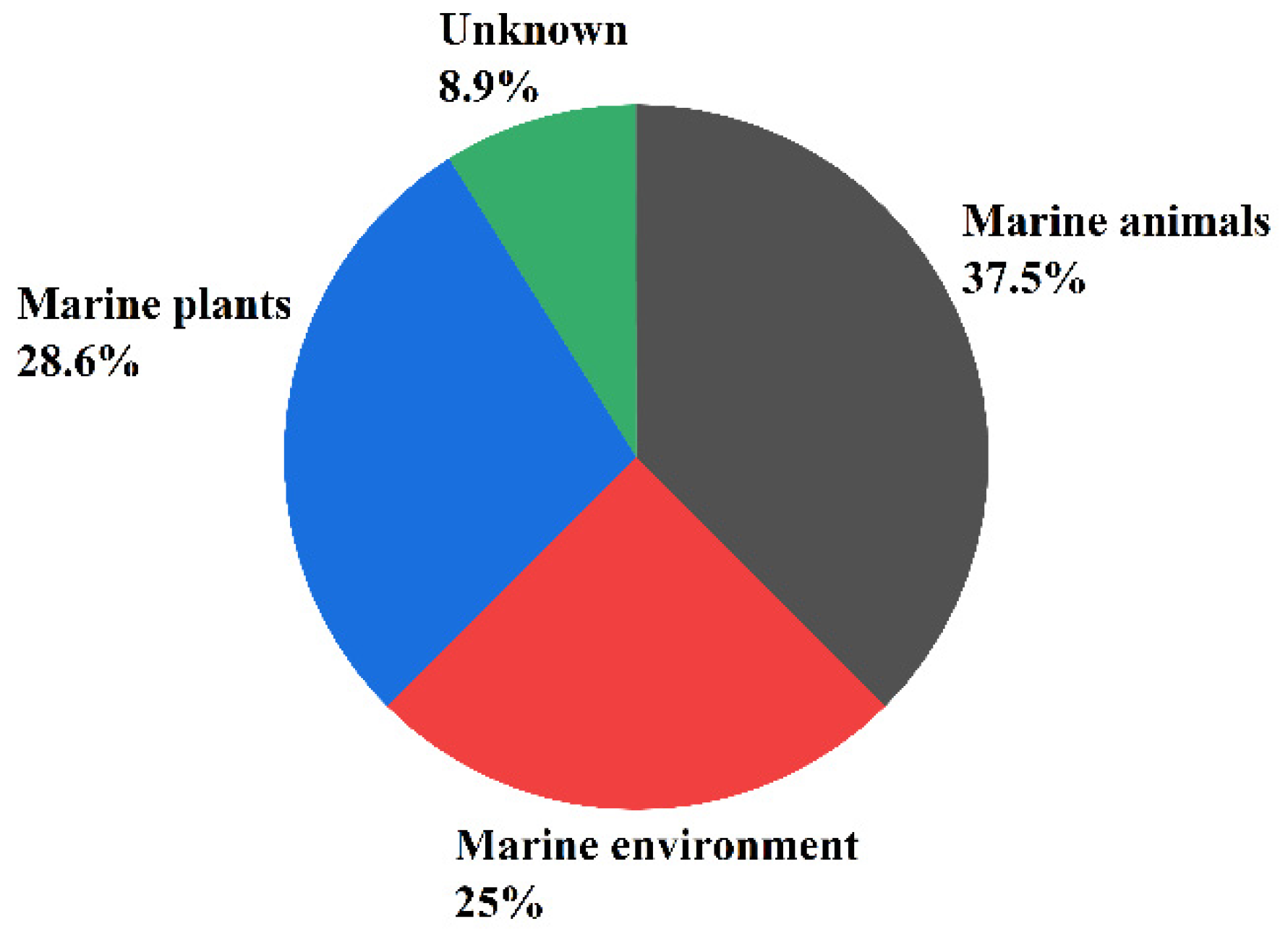
:1. Introduction
2. Characteristics of Sesquiterpenoids from Marine Aspergillus sp.
3. Bioactivity of Sesquiterpenoids from Aspergillus sp.
3.1. Antibacterial Activity
3.2. Antitumor Activity
3.3. Anti-Inflammatory Activity
3.4. Enzymatic Inhibitory Activity
3.5. Other Activities
4. Chemical Synthesis and Biosynthesis of Sesquiterpenoids from Marine Aspergillus sp.
4.1. Chemically Induced Synthesis
4.2. Biosynthetic Pathways
5. Potency of Sesquiterpenoids from Marine Aspergillus sp.
6. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.N.; Meng, L.H.; Wang, B.G. Progress in research on bioactive secondary metabolites from deep-sea derived microorganisms. Mar. Drugs 2020, 18, 614. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Cai, R.L.; Liu, Z.M.; Cui, H.; She, Z.G. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef]
- Spiteller, P. Chemical ecology of fungi. Nat. Prod. Rep. 2015, 32, 971–993. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Proksch, P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011, 49, 1–12. [Google Scholar] [CrossRef]
- Liu, B.; Tang, Z.J.; Chen, N.; Xu, Y.; Ji, Y.B. Research progress of butyrolactones isolated from marine-derived Aspergillus sp. Chin. J. Mar. Drugs 2021, 40, 59–70. [Google Scholar]
- Numata, A.; Takahashi, C.; Matsushita, T.; Miyamoto, T.; Kawai, K.; Usami, Y.; Matsumura, E.; Inoue, M.; Ohishi, H. Fumiquinazolines, novel metabolites of a fungus isolated from a saltfish. Tetrahedron Lett. 1992, 33, 1621–1624. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Liu, H.S.; Zhu, W.M. New natural products from the marine-derived Aspergillus fungi-a review. Acta Microbiol. Sin. 2016, 56, 331–362. [Google Scholar]
- Ebel, R. Terpenes from marine-derived fungi. Mar. Drugs 2010, 8, 2340–2368. [Google Scholar] [CrossRef] [Green Version]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Singab, A.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J. Natural products from marine fungi. Mar. Drugs 2020, 18, 230. [Google Scholar] [CrossRef]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2012, 29, 1334–1366. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Yi, J.; Chang, Y.B.; Sun, C.P.; Ma, X.C. Recent studies on terpenoids in Aspergillus fungi: Chemical diversity, biosynthesis, and bioactivity. Phytochemistry 2022, 193, 113011. [Google Scholar] [CrossRef]
- Li, D.; Xu, Y.; Shao, C.L.; Yang, R.Y.; Zheng, C.J.; Chen, Y.Y.; Fu, X.M.; Qian, P.Y.; She, Z.G.; de Voogd, N.J.; et al. Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Mar. Drugs 2012, 10, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.F.; Lin, X.P.; Qin, C.; Liao, S.; Wan, J.T.; Zhang, T.Y.; Liu, J.; Fredimoses, M.; Chen, H.; Yang, B.; et al. Antimicrobial and antiviral sesquiterpenoids from sponge-associated fungus, Aspergillus sydowii ZSDS1-F6. J. Antibiot. 2014, 67, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhu, H.Y.; Xu, L.C.; Wang, S.P.; Liu, S.; Liu, G.D.; Luo, W.H.; Cao, G.Y.; Zhang, Z.X. Antimicrobial and cytotoxic phenolic bisabolane sesquiterpenoids from the fungus Aspergillus flavipes 297. Fitoterapia 2021, 155, 105038. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.; Li, X.M.; Yin, X.L.; Wang, B.G. Antimicrobial bisabolane-type sesquiterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Nat. Prod. Res. 2021, 35, 4265–4271. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, J.L.; Li, C.; Mu, X.G.; Liu, X.L.; Wang, L.; Zhao, Y.C.; Zhang, P.; Li, X.D.; Zhang, X.X. Antimicrobial secondary metabolites from the seawater-derived fungus Aspergillus sydowii SW9. Molecules 2019, 24, 4596. [Google Scholar] [CrossRef] [Green Version]
- Li, X.D.; Li, X.M.; Yin, X.L.; Li, X.; Wang, B.G. Antimicrobial sesquiterpenoid derivatives and monoterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Mar. Drugs 2019, 17, 563. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.Y.; Wang, C.Y.; Liu, Q.A.; Shao, C.L.; She, Z.G.; Lin, Y.C. Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a gorgonian Dichotella gemmacea. Mar. Drugs 2010, 8, 941–949. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Yu, J.H.; Zhu, K.K.; Wang, Y.Y.; Cheng, Z.Q.; Jiang, C.S.; Dai, J.G.; Wu, J.; Zhang, H. Phenolic bisabolane sesquiterpenoids from a Thai mangrove endophytic fungus, Aspergillus sp. xy02. Fitoterapia 2018, 27, 322–327. [Google Scholar] [CrossRef]
- Liu, S.; Dai, H.; Konuklugil, B.; Orfali, R.S.; Lin, W.H.; Kalscheuer, R.; Liu, Z.; Proksch, P. Phenolic bisabolanes from the sponge-derived fungus Aspergillus sp. Phytochem Lett. 2016, 18, 187–191. [Google Scholar] [CrossRef]
- Liu, X.H.; Miao, F.P.; Li, X.D.; Yin, X.L.; Ji, N.Y. A new sesquiterpene from an endophytic Aspergillus versicolor strain. Nat. Prod. Commun. 2012, 7, 819–820. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.T.; Liu, X.H.; Yan, B.F.; Miao, F.P.; Yin, X.L.; Li, W.Z.; Ji, N.Y. Terpenoids from the marine-derived fungus Aspergillus sp. RR-YLW-12, associated with the Red alga Rhodomela confervoides. J. Nat. Prod. 2021, 84, 1763–1771. [Google Scholar] [CrossRef]
- Zheng, C.J.; Shao, C.L.; Wang, K.L.; Zhao, D.L.; Wang, Y.N. Secondary metabolites and their bioactivities of a soft coral-derived fungus Aspergillus versicolor(ZJ-2008015). Chin. J. Mar. Drugs 2012, 31, 7–13. [Google Scholar]
- Cohen, E.; Koch, L.; Thu, K.M.; Rahamim, Y.; Aluma, Y.; Ilan, M.; Yarden, O.; Carmeli, S. Novel terpenoids of the fungus Aspergillus insuetus isolated from the Mediterranean sponge Psammocinia sp. collected along the coast of Israel. Bioorg. Med. Chem. 2011, 19, 6587–6593. [Google Scholar] [CrossRef]
- Orfali, R.; Perveen, S.; Khan, M.F.; Ahmed, A.F.; Wadaan, M.A.; Al-Taweel, A.M.; Alqahtani, A.S.; Nasr, F.A.; Tabassum, S.; Luciano, P.; et al. Antiproliferative illudalane sesquiterpenes from the marine sediment ascomycete Aspergillus oryzae. Mar. Drugs 2021, 19, 333. [Google Scholar] [CrossRef]
- Liu, Y.F.; Yue, Y.F.; Feng, L.X.; Zhu, H.J.; Cao, F. Asperienes A-D, bioactive sesquiterpenes from the marine-derived fungus Aspergillus flavus. Mar. Drugs 2019, 17, 550. [Google Scholar] [CrossRef] [Green Version]
- Yurchenko, A.N.; Trinh, P.T.H.; Girich, E.V.; Smetanina, O.F.; Rasin, A.B.; Popov, R.S.; Dyshlovoy, S.A.; von Amsberg, G.; Menchinskaya, E.S.; Van, T.T.T.; et al. Biologically active metabolites from the marine sediment-derived fungus Aspergillus flocculosus. Mar. Drugs 2019, 17, 579. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Lin, X.P.; Zhou, X.F.; Wan, J.T.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.Y.; Tu, Z.C.; et al. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1F17. Med. Chem. Comm. 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Anbuchezhian, R.; Sun, W.; Shao, C.L.; Zhang, F.L.; Yin, Y.; Yu, Z.S.; Li, Z.Y.; Wang, C.Y. Cytotoxic nitrobenzoyloxy-substituted sesquiterpenes from sponge derived endozoic fungus Aspergillus insulicola MD10-2. Curr. Pharm. Biotechnol. 2016, 17, 271–274. [Google Scholar] [CrossRef]
- Tan, Y.H.; Yang, B.; Lin, X.P.; Luo, X.W.; Pang, X.Y.; Tang, L.; Liu, Y.H.; Li, X.J.; Zhou, X.F. Nitrobenzoyl sesquiterpenoids with cytotoxic activities from a marine-derived Aspergillus ochraceus fungus. J. Nat. Prod. 2018, 81, 92–97. [Google Scholar] [CrossRef]
- Sun, C.X.; Liu, X.Y.; Sun, N.; Zhang, X.M.; Shah, M.; Zhang, G.J.; Che, Q.; Zhu, T.J.; Li, J.; Li, D.H. Cytotoxic nitrobenzoyl sesquiterpenoids from an antarctica sponge-derived Aspergillus insulicola. J. Nat. Prod. 2022, 85, 987–996. [Google Scholar] [CrossRef]
- Liu, H.B.; Edrada-Ebel, R.; Ebel, R.; Wang, Y.; Schulz, B.; Draeger, S.; Muller, W.E.G.; Wray, V.; Lin, W.H.; Proksch, P. Drimane sesquiterpenoids from the fungus Aspergillus ustus isolated from the marine sponge Suberites domuncula. J. Nat. Prod. 2009, 72, 1585–1588. [Google Scholar] [CrossRef]
- Zhou, H.N.; Zhu, T.J.; Cai, S.X.; Gu, Q.Q.; Li, D.H. Drimane sesquiterpenoids from the mangrove-derived fungus Aspergillus ustus. Chem. Pharm. Bull. 2011, 59, 762–766. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.L.; Shao, C.L.; Chen, J.F.; Guo, Z.Y.; Fu, X.M.; Chen, M.; Chen, Y.Y.; Li, R.; de Voogd, N.J.; She, Z.G.; et al. New bisabolane sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from the sponge Xestospongia testudinaria. Bioorg. Med. Chem. Lett. 2012, 22, 1326–1329. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Su, M.; Hwang, G.J.; Hong, J.; Jung, J.H. New metabolites from the sponge-derived fungus Aspergillus sydowii J05B-7F-4. Nat. Prod. Res. 2017, 31, 1682–1686. [Google Scholar] [CrossRef]
- Deng, C.M.; Huang, C.H.; Wu, Q.L.; Pang, J.Y.; Lin, Y.C. A new sesquiterpene from the mangrove endophytic fungus Aspergillus terreus (No. GX7-3B). Nat. Prod. Res. 2013, 27, 1882–1887. [Google Scholar] [CrossRef]
- Liu, D.S.; Huang, Y.L.; Li, C.M.; Ma, L.Y.; Pan, X.H.; Ferreira, D.; Liu, W.Z. A new sesquiterpenoid derivative from the coastal saline soil fungus Aspergillus fumigatus. Rec. Nat. Prod. 2016, 10, 708–713. [Google Scholar]
- Kitano, M.; Yamada, T.; Amagata, T.; Minoura, K.; Tanaka, R.; Numata, A. Novel pyridino-alpha-pyrone sesquiterpene type pileotin produced by a sea urchin-derived Aspergillus sp. Tetrahedron Lett. 2012, 53, 4192–4194. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Wang, Y.; Miao, C.D.; Liu, P.P.; Hong, K.; Zhu, W.M. Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102. J. Nat. Prod. 2009, 72, 1761–1767. [Google Scholar] [CrossRef]
- Proksch, P.; Ebel, R.; Edrada, R.; Riebe, F.; Liu, H.; Diesel, A.; Bayer, M.; Li, X.; Lin, W.H.; Grebenyuk, V.; et al. Sponge-associated fungi and their bioactive compounds: The Suberites case. Bot. Mar. 2008, 51, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, D.H.; Li, Z.L.; Sun, Y.J.; Hua, H.M.; Liu, T.; Bai, J. Terpenoids from the marine-derived fungus Aspergillus fumigatus YK-7. Molecules 2015, 21, 31. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.C.; Liu, G.D.; Chen, Y.; Liu, S.; Luo, W.H.; Hu, P.F.; Huang, C.M.; Ji, X.; Wang, S.P.; Cao, G.Y. Cytotoxic drimane-type sesquiterpenoids from the fungus Aspergillus flavipes 297. Rec. Nat. Prod. 2021, 16, 488–492. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.N.; Li, T.M.; Zhang, Z.R.; Ding, M.; Long, Y.H.; She, Z.G. 3-Arylisoindolinone and sesquiterpene derivatives from the mangrove endophytic fungi Aspergillus versicolor SYSU-SKS025. Fitoterapia 2018, 124, 177–181. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Qi, S.; Zhan, Y.; Zhang, N.W.; Wu, A.A.; Gui, F.; Guo, K.; Yang, Y.R.; Cao, S.G.; Hu, Z.Y.; et al. Aspertetranones A-D, putative meroterpenoids from the marine algal-associated fungus Aspergillus sp. ZL0-1b14. J. Nat. Prod. 2015, 78, 2405–2410. [Google Scholar] [CrossRef]
- Wu, Z.D.; Li, D.Y.; Zeng, F.R.; Tong, Q.Y.; Zheng, Y.Y.; Liu, J.J.; Zhou, Q.; Li, X.N.; Chen, C.M.; Lai, Y.J.; et al. Brasilane sesquiterpenoids and dihydrobenzofuran derivatives from Aspergillus terreus [CFCC 81836]. Phytochemistry 2018, 156, 159–166. [Google Scholar] [CrossRef]
- Chung, Y.M.; Wei, C.K.; Chuang, D.W.; El-Shazly, M.; Hsieh, C.T.; Asai, T.; Oshima, Y.; Hsieh, T.J.; Hwang, T.L.; Wu, Y.C.; et al. An epigenetic modifier enhances the production of anti-diabetic and anti-inflammatory sesquiterpenoids from Aspergillus sydowii. Bioorgan. Med. Chem. 2013, 21, 3866–3872. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, M.J.; Tang, J.Q.; Li, X.F. Eremophilane sesquiterpenes from a deep marine-derived fungus, Aspergillus sp. SCSIOW2, cultivated in the presence of epigenetic modifying agents. Molecules 2016, 21, 473. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.X.; Zhang, W.X.; Hao, L.L.; Qin, X.Y.; Yang, R.Y.; Li, J.; Huang, X.S. A new sesquiterpene from mangrove endophytic fungus Aspergillus sp. GXNU-MA1. Nat. Prod. Res. 2022, 36, 1857–1863. [Google Scholar] [CrossRef]
- Niu, S.W.; Yang, L.H.; Zhang, G.Y.; Chen, T.T.; Hong, B.H.; Pei, S.X.; Shao, Z.Z. Phenolic bisabolane and cuparene sesquiterpenoids with anti-inflammatory activities from the deep-sea-derived Aspergillus sydowii MCCC 3A00324 fungus. Bioorg. Chem. 2020, 105, 104420. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Deng, W.D.; Zhang, Y.Y.; Ke, M.H.; Zou, B.H.; Luo, X.W.; Su, J.B.; Wang, Y.Y.; Xu, J.L.; Nandakumar, K.S.; et al. A marine fungus-derived nitrobenzoyl sesquiterpenoid suppresses receptor activator of NF-κB ligand-induced osteoclastogenesis and inflammatory bone destruction. Br. J. Pharmacol. 2020, 177, 4242–4260. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.N.; Chen, Y.; Huang, X.S.; Pan, Y.H.; Liu, Z.M.; Yan, T.; Cao, W.H.; She, Z.G. α-Glucosidase inhibitors: Diphenyl ethers and phenolic bisabolane sesquiterpenoids from the mangrove endophytic fungus Aspergillus flavus QQSG-3. Mar. Drugs 2018, 16, 307. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Wei, X.; Hu, J.S.; Wang, S.Y.; Liu, B.X.; Xie, Z.Y.; Rong, L.; Li, X.H.; Zhang, C.X. Researches on the subergane-type sesquiterpenes from the soft coral-derived fungus Aspergillus sp. EGF15-0-3. Chin. J. Org. Chem. 2020, 40, 1275–1280. [Google Scholar] [CrossRef]
- Li, L.; Li, X.M.; Li, H.L.; Belma, K.; Li, X.; Wang, B.G. Chemical constituents of Aspergillus ustus TK-5, an endophytic fungus derived from the ascidian Herdmania momus. Mar. Sci. 2018, 42, 130–137. [Google Scholar]
- Dai, Q.; Zhang, F.L.; Feng, T. Sesquiterpenoids specially produced by fungi: Structures, biological activities, chemical and biosynthesis (2015–2020). J. Fungi 2021, 7, 1026. [Google Scholar] [CrossRef]
- Fu, J.; Li, F.H.; Li, C.K.; Li, B.M.; Chen, R.Y.; Kang, J. Reviews on natural monocyclic sesquiterpenoids and their bioactivities. China J. Chin. Mater. Med. 2019, 44, 3672–3683. [Google Scholar]
- Zhang, Y.H.; Xu, Y.; Wang, C.Y.; Cao, F. Alkaloids and sesquiterpenoids from the marine-derived fungus Aspergillus versicolor. Chem. Nat. Compd. 2020, 56, 971–973. [Google Scholar] [CrossRef]
- Liu, N.Z.; Peng, S.; Yang, J.; Cong, Z.W.; Lin, X.P.; Liao, S.R.; Yang, B.; Zhou, X.F.; Zhou, X.J.; Liu, Y.H.; et al. Structurally diverse sesquiterpenoids and polyketides from a sponge-associated fungus Aspergillus sydowii SCSIO41301. Fitoterapia 2019, 135, 27–32. [Google Scholar] [CrossRef]
- Pang, X.Y.; Lin, X.P.; Zhou, X.F.; Yang, B.; Tian, X.P.; Wang, J.F.; Xu, S.H.; Liu, Y.H. New quinoline alkaloid and bisabolane-type sesquiterpenoid derivatives from the deep-sea-derived fungus Aspergillus sp. SCSIO06786. Fitoterapia 2020, 140, 104406. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Kaewpet, M.; Phongpaichit, S.; Hutadilok-Towatana, N.; Preedanon, S.; Sakayaroj, J. Sesquiterpene and xanthone derivatives from the sea fan-derived fungus Aspergillus sydowii PSU-F154. J. Nat. Prod. 2011, 74, 1663–1667. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Denisenko, V.A.; Ermakova, S.P.; Slinkina, N.N.; Dmitrenok, P.S.; Kim, N.Y. Secondary metabolites from a marine-derived fungus Aspergillus carneus Blochwitz. Phytochemistry 2012, 80, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Weigel, L.M.; Donlan, R.M.; Shin, D.H.; Jensen, B.; Clark, N.C.; McDougal, L.K.; Zhu, W.M.; Musser, K.A.; Thompson, J.; Kohlerschinidt, D.; et al. High-level vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob. Agents Chemother. 2007, 51, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Shen, Y.C.; Chen, Y.J.; Sheu, J.H.; Duh, C.Y. Bioactive sesquiterpenes from a Taiwanese marine sponge Parahigginsia sp. J. Nat. Prod. 1999, 62, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Mcenroe, F.J.; Fenical, W. Structures and synthesis of some new antibacterial sesquiterpenoids from the gorgonian coral Pseudopterogorgia rigida. Tetrahedron 1978, 34, 1661–1664. [Google Scholar] [CrossRef]
- Mulhaupt, T.; Kaspar, H.; Otto, S.; Reichert, M.; Bringmann, G.; Lindel, T. Isolation, structural elucidation, and synthesis of curcutetraol. Eur. J. Org. Chem. 2005, 2005, 334–341. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temraz, A. Novel illudalane sesquiterpenes from Encephalartos villosus Lehm. antimicrobial activity. Nat. Prod. Res. 2016, 30, 2791–2797. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, H.; Seo, Y.H.; Yun, J.; Lee, J.; Kwon, H.C.; Guo, Y.Q.; Kang, J.S.; Kim, J.J.; Lee, D. Cytotoxic drimane sesquiterpenoids isolated from Perenniporia maackiae. J. Nat. Prod. 2018, 81, 1444–1450. [Google Scholar] [CrossRef]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef] [Green Version]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orhan, I.E. Enzyme inhibitors as the attractive targets for the treatment of various diseases. Curr. Med. Chem. 2019, 26, 3206–3207. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Ullah, B.; Ali, M.; Rauf, A.; Khan, H.; Uriarte, E.; Sobarzo-Sanchez, E. Potent in vitro α-glucosidase inhibition of secondary metabolites derived from dryopteris cycadina. Molecules 2019, 24, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Ma, S. Recent advances in synthetic α-glucosidase inhibitors. Chem. Med. Chem. 2017, 12, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef]
- Bono, G.F.; Simao-Silva, D.P.; Batistela, M.S.; Josviak, N.D.; Dias, P.F.R.; Nascimento, G.A.; Souza, R.L.R.; Piovezan, M.R.; Souza, R.K.M.; Furtado-Alle, L. Butyrylcholinesterase: K variant, plasma activity, molecular forms and rivastigmine treatment in Alzheimer’s disease in a Southern Brazilian population. Neurochem. Int. 2015, 81, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Air, G.M. Influenza neuraminidase. Influenza Other Resp. 2012, 6, 245–256. [Google Scholar] [CrossRef]
- Hu, X.Y.; Li, X.M.; Meng, L.H.; Wang, B.G. Antioxidant bisabolane-type sesquiterpenoids from algal-derived fungus Aspergillus sydowii EN-434. J. Oceanol. Limnol. 2020, 38, 1532–1536. [Google Scholar] [CrossRef]
- An, C.L.; Kong, F.D.; Ma, Q.Y.; Xie, Q.Y.; Yuan, J.Z.; Zhou, L.M.; Dai, H.F.; Yu, Z.F.; Zhao, Y.X. Chemical constituents of the marine-derived fungus Aspergillus sp. SCS-KFD66. Mar. Drugs 2018, 16, 468. [Google Scholar] [CrossRef] [Green Version]
- Zhong, M.J.; Kang, H.H.; Ma, L.Y.; Liu, D.S.; Liu, W.Z. Study on the secondary metabolites from Aspergillus pseudoglaucus derived from offshore mud in Dandong. Chin. J. Mar. Drugs 2021, 40, 16–22. [Google Scholar]
- Liu, X.H.; Miao, F.P.; Qiao, M.F.; Cichewicz, R.H.; Ji, N.Y. Terretonin, ophiobolin, and drimane terpenes with absolute configurations from an algicolous Aspergillus ustus. RSC Adv. 2013, 3, 588–595. [Google Scholar] [CrossRef]
- Penesyan, A.; Kjelleberg, S.; Egan, S. Development of novel drugs from marine surface associated microorganisms. Mar. Drugs 2010, 8, 438–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Slot, J.C. Fungal gene cluster diversity and evolution. Adv. Genet. 2017, 100, 141–178. [Google Scholar]
- Lind, A.L.; Wisecaver, J.H.; Lameiras, C.; Wiemann, P.; Palmer, J.M.; Keller, N.P.; Rodrigues, F.; Goldman, G.H.; Rokas, A. Drivers of genetic diversity in secondary metabolic gene clusters within a fungal species. PLoS Biol. 2017, 15, e2003583. [Google Scholar] [CrossRef] [Green Version]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Li, X.Y.; Awakawa, T.; Mori, T.; Ling, M.Q.; Hu, D.; Wu, B.; Abe, I. Heterodimeric non-heme iron enzymes in fungal meroterpenoid biosynthesis. J. Am. Chem. Soc. 2021, 143, 21425–21432. [Google Scholar] [CrossRef]
- Guo, Z.; Zou, Z.M. Discovery of new secondary metabolites by epigenetic regulation and NMR comparison from the plant endophytic fungus monosporascus eutypoides. Molecules 2020, 25, 4192. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, Y.F.; Cao, F.; Wang, C.Y. Bisabolane-type sesquiterpenoids from a gorgonian-derived Aspergillus sp fungus induced by DNA methyltransferase inhibitor. Chem. Nat. Compd. 2016, 52, 1129–1132. [Google Scholar] [CrossRef]
- Wu, J.S.; Yao, G.S.; Shi, X.H.; Rehman, S.U.; Xu, Y.; Fu, X.M.; Zhang, X.L.; Liu, Y.; Wang, C.Y. Epigenetic agents trigger the production of bioactive nucleoside derivatives and bisabolane sesquiterpenes from the marine-derived fungus Aspergillus versicolor. Front. Microbiol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adekenov, S.M. Sesquiterpene lactones with unusual structure. Their biogenesis and biological activity. Fitoterapia 2017, 121, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Ingavat, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Asperaculin A, a sesquiterpenoid from a marine-derived fungus, Aspergillus aculeatus. J. Nat. Prod. 2011, 74, 1650–1652. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, N.; Furukawa, J.; Kobayashi, H.; Iwasaki, S.; Nozoe, S. Cyclobutyl cation rearrangements of 6-protoilluden-8α-ol, 7-protoilluden-6-ol and related compounds. Chem. Pharm. Bull. 1987, 35, 2678–2685. [Google Scholar] [CrossRef] [Green Version]
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 2021, 371, 926–931. [Google Scholar] [CrossRef]
- Taglialatela-Scafati, O. New hopes for drugs against COVID-19 come from the sea. Mar. Drugs 2021, 19, 104. [Google Scholar] [CrossRef]









| Compound Name/Chemical Class | Marine Source | Type of Strains | Activity (MIC) | Reference |
|---|---|---|---|---|
| Compounds 1,3 and 5 | Marine-sponge-derived fungus Aspergillus | not reported | not reported | [14] 2012 |
| Compound 2 | S. albus, M. tetragenus | 1.25–5 µM | ||
| Compound 4 | S. albus, B. subtilis | 2.5–5 µM | ||
| Compound 6 | Marine-sponge-derived fungus Aspergillus sydowii ZSDS1-F6 | A. hydrophila and K. pneumonia | 4.3 and 21.4 µM | [15] 2014 |
| Compound 7 | K. pneumonia | 10.7 µM | ||
| Compound 8 | E. faecalis | 18.8 µM | ||
| Flavilane A(9) | Fresh-seawater-derived fungus Aspergillus flavipes 297 | Pathogenic bacteria | 2–64 µM | [16] 2021 |
| Compound 10 | Pathogenic bacteria and Pathogenic fungus V. mari | |||
| Compounds 11,12 | Deep sea sediment fungus Aspergillus versicolor SD-330 | A. hydrophilia, E. coli, E. tarda, and V. harveyi | 2–8 µM | [17] 2021 |
| Compound 13 | E. coli | 1 µM | ||
| Compound 14 | Seawater-derived fungus Aspergillus sydowii SW9 | E. coli and S. pneumonise | 2–4 µM | [18] 2019 |
| Compounds 15,16 | Deep sea sediment fungus Aspergillus versicolor SD-330 | E. coli, E. trada, V. harveyi, and V. parahaemolyticus | 8 µM | [19] 2019 |
| Compound 17 | E. coli, Aeromonas hydrophilia, E. tarda, V. anguillarum, and V. harveyi | 1–4 µM | ||
| Compound 18 | not reported | not reported | ||
| Compounds 19–21 | Marine-gorgonian-derived fungus Aspergillus | S. aureus | Inhibition zones were 5–11 mm at 100µg/mL | [20] 2010 |
| Compounds 22–28 | Mangrove endophytic fungus Aspergillus xy02 | S. aureus | 31.5–41.9 µM | [21] 2018 |
| Asperchondols A, B(29, 30) | Marine-sponge-derived fungus Aspergillus | S. aureus | 25–50 µM | [22] 2016 |
| Albican-11,14-diol (31) | Marine-alga-derived fungus Aspergillus versicolor | E. coli and S. aureus | Inhibition zones were 7–10.3 mm at 30 μg/disk | [23] 2012 |
| Compounds 32–35 | Marine-alga-derived fungus Aspergillus RR-YLW-12 | V. harveyi, V. splendidus, V. parahaemolytics, and V. anguillarum | not reported | [24] 2021 |
| Compounds 36–38 | Marine-coral-derived fungus Aspergillus versicolor ZJ-2008015 | S. aureus and S. albus | 2.6–6.4 µM | [25] 2012 |
| Compounds 39,40 | Marine-sponge-derived fungus Aspergillus insuetus OY-207 | N. crassa | 140–242 µM | [26] 2011 |
| Compound Name/Chemical Class | Marine Source | Cell lines | Activity (IC50/EC50/ED50/inhibition rate) | Reference |
| Asperorlactone (41) | Marine sediment fungus Aspergillus oryzae | A549, HepG2, and MCF-7 | <100 µM | [27] 2021 |
| Echinolactone D (42) | not reported | |||
| Asperienes A-D (43–46) | Marine fungus Aspergillus flavus CF13–11 | A549, HeLa, MGC-803, and MCF-7 | 1.4–8.3 µM | [28] 2019 |
| Compounds 47,48 | Marine sediment fungus Aspergillus flocculosus | Neuro-2a and 22Rv1 | 3–31.5 µM | [29] 2019 |
| Compounds 49,50 | Marine fungus Aspergillus ochraceus Jcma1F17 | H1975, U937, K562, BGC-823, MOLT-4, MCF-7 A549, HeLa, HL60, and Huh-7 | 1.95–6.35 µM | [30] 2014 |
| Insulicolide A (51) | Marine-sponge-derived fungus Aspergillus insulicola MD10-2 | H-460 | 6.9 µM | [31] 2016 |
| Compounds 52, 53 | Marine fungus Aspergillus ochraceus Jcma1F17 | 786-O, ACHN, and OS-RC-2 | 2.3–11 µM | [32] 2018 |
| Compound 54 | 0.89–1.5 µM | |||
| Compounds 57, 58 | Marine-sponge-derived fungus Aspergillus insulicola | AsPC-1 and PANC-1 | 2.3–4.6 µM | [33] 2022 |
| Compounds 59, 61 | Marine-sponge-derived fungus Aspergillus ustus | L5178Y | 0.6–5.3 µM | [34] 2009 |
| Compound 60 | L5178Y, PC12, and HeLa | 0.6–7.2 µM | ||
| Compound 62 | Mangrove endophytic fungus Aspergillus ustus | P388 | 8.7 µM | [35] 2011 |
| Compounds 63, 65 | Marine-sponge-derived fungus Aspergillus | HePG-2 and Caski | 2.91–12.4 µM | [36] 2012 |
| Compound 64 | not reported | |||
| β-D-glucopyranosylaspergillusene A (66) | Marine-sponge-derived fungus Aspergillus sydowii J05B7F-4 | HePG-2, HCT116, and KB | 50–70 µM | [37] 2017 |
| Compound 67 | Mangrove endophytic fungus Aspergillus terreus GX3-3B | MCF-7, HL-60 | 3.43–4.49 µM | [38] 2013 |
| Compound 68 | MCF-7 | 2.79 µM | ||
| Compound 70 | HL-60 | 0.6 µM | ||
| Aspergiketone (71) | Coastal saline soil fungus Aspergillus fumigatus | HL-60 and A549 | 12.4–22.1 µM | [39] 2016 |
| Oxalicine B (72) | Sea-urchin-derived fungus Aspergillus fumigatus | P388 | 55.9 µM | [40] 2012 |
| Compound 73 | Marine fungus Aspergillus ustus 094102 | HL-60 and A549 | 20.6–30 µM | [41] 2009 |
| Compounds 74, 75 | 9–10.5 µM | |||
| Compound 76 | Marine-sponge-derived fungus Aspergillus ustus | L5178Y | 1.9 µM | [42] 2008 |
| Compound 77 | Marine sediment fungus Aspergillus fumigatus YK-7 | U937 | 84.9 µM | [43] 2015 |
| Asperflavinoid A (78) | Marine fungus Aspergillus flavipes 297 | HepG2 and MKN-45 | 26.8–38.5 µM | [44] 2021 |
| Compound Name/Chemical Class | Marine Source | Target Enzyme | Activity (IC50/inhibitory rate) | Reference |
| 7-Deoxy-7,14-didehydrosydonol (79) | Mangrove endophytic fungus Aspergillus versicolor SYSU-SKS025 | inhibit NO production in RAW 264.7 macrophages | 12.5 µM | [45] 2018 |
| Compounds 80–82 | Marine-algal-derived fungus Aspergillus ZL0-1B14 | inhibit LPS-stimulated RAW264.7 macrophages | not reported | [46] 2015 |
| Compound 83 | inhibit LPS-stimulated RAW264.7 macrophages and exhibited an inhibitory effect against IL-6 production | 69% at 40 µM | ||
| Compound 84,85 | Marine fungus Aspergillus terreus | inhibitory activity of NO production | 37.3% and 47.7% at 40 μM | [47] 2018 |
| Compound 86,87,89 | Marine sediment fungus Aspergillus sydowii | not reported | not reported | [48] 2013 |
| Compounds 88,90 | inhibition against fMLP/CB-induced superoxide anion generation by human neutrophils and inhibitory activity against the release of elastase induced by fMLP/CB | 5.23–16.39 µM | ||
| Compound 91–94 | Marine sediment fungus Aspergillus SCSIOW2 | inhibitory activity of NO production | not reported | [49] 2016 |
| Compounds 95–99 | Mangrove endophytic fungus Aspergillus GXNU-MA1 | inhibitory activity of NO production | 16.15–27.08 µM | [50] 2022 |
| Compounds 100,102–107 | Marine sediment fungus Aspergillus sydowii MCCC3A00324 | against NO secretion in LPS-activited BV-2 microglia cells | 32.6%-45.4% at 10 µM | [51] 2020 |
| Compound 101 | against NO secretion in LPS-activited BV-2 microglia cells and anti-inflammatory effect | 45% at 10 µM | ||
| inhibiting NF-κB activation pathway | not reported | |||
| Compound 108 | Marine fungus Aspergillus ochraceus | suppressed the RANKL-induced osteoclats formation and bone resorption by targeting NF-κB | not reported | [52] 2020 |
| Compound Name/Chemical Class | Marine Source | Target Enzyme | Activity/(IC50) | Reference |
| 7-Deoxy-7,14-didehydrosydonol (79) | Mangrove endophytic fungus Aspergillus versicolor SYSU-SKS025 | inhibitory effect on α-glucosidase | 7.5 µM | [45] 2018 |
| Compounds 109–112 | Mangrove endophytic fungus Aspergillus flavus QQSG-3 | inhibitory effect on α-glucosidase | 1.5–4.5 µM | [53] 2018 |
| Compound 113 | Marine-coral-derived fungus Aspergillus EGF15-0-3 | inhibit ChE | not reported | [54] 2020 |
| 2-deoxy-2β-hydroxysubergorgic (114) | ||||
| Compounds 115–118 | Marine-ascidian-derived fungus Aspergillus ustus TK-5 | inhibitory activity against neuraminidase | 28.4–37.3 µM | [55] 2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Wang, H.; Yan, M.; Sai, C.; Zhang, Z. Research Advances of Bioactive Sesquiterpenoids Isolated from Marine-Derived Aspergillus sp. Molecules 2022, 27, 7376. https://doi.org/10.3390/molecules27217376
Sun L, Wang H, Yan M, Sai C, Zhang Z. Research Advances of Bioactive Sesquiterpenoids Isolated from Marine-Derived Aspergillus sp. Molecules. 2022; 27(21):7376. https://doi.org/10.3390/molecules27217376
Chicago/Turabian StyleSun, Lixiang, Huannan Wang, Maocai Yan, Chunmei Sai, and Zhen Zhang. 2022. "Research Advances of Bioactive Sesquiterpenoids Isolated from Marine-Derived Aspergillus sp." Molecules 27, no. 21: 7376. https://doi.org/10.3390/molecules27217376





