First Evidence of Anti-Steatotic Action of Macrotympanain A1, an Amphibian Skin Peptide from Odorrana macrotympana
Abstract
1. Introduction
2. Results
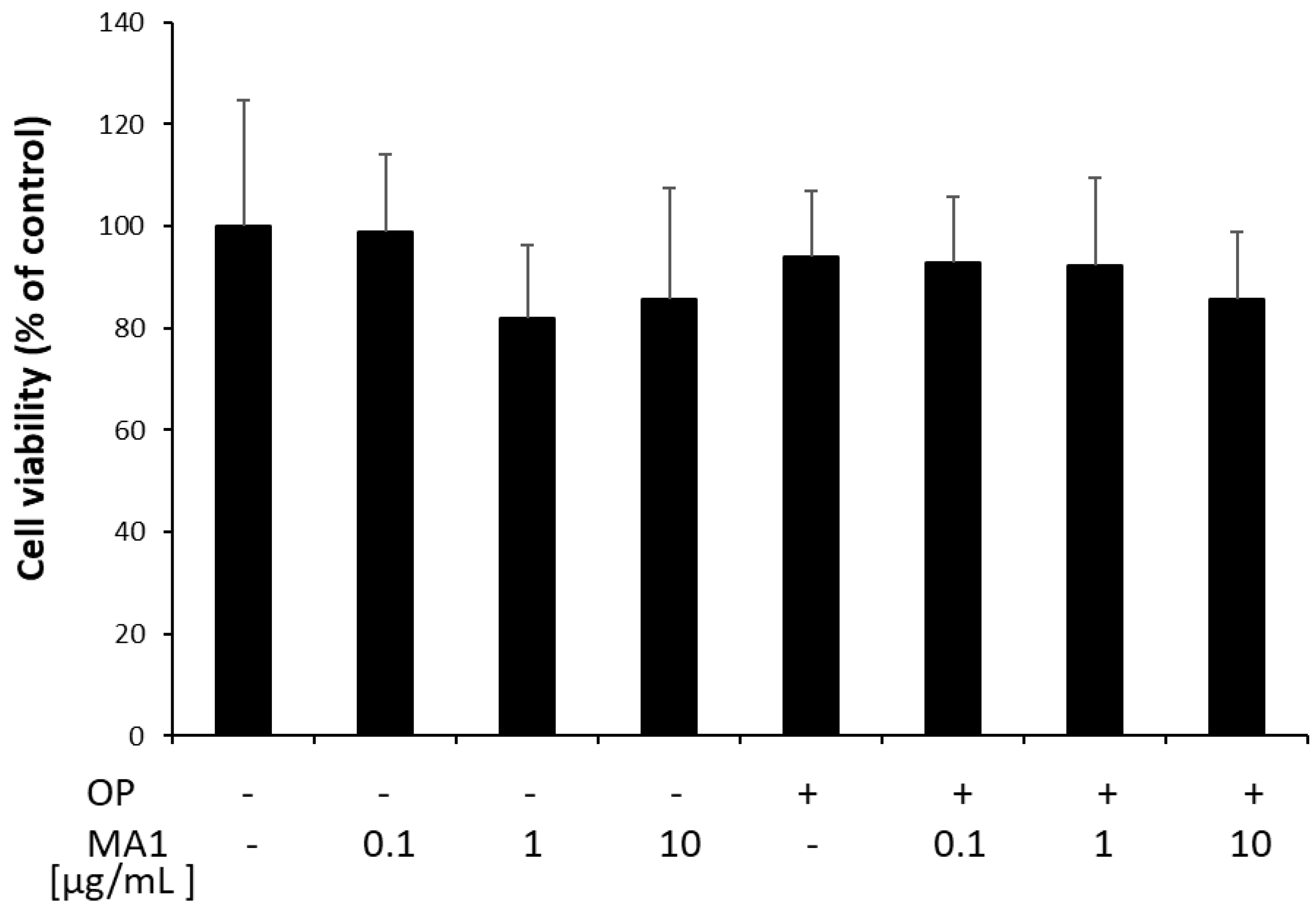
2.1. MA1 Does Not Affect FaO Cell Viability
2.2. MA1 Exerts Anti-Steatotic Effects
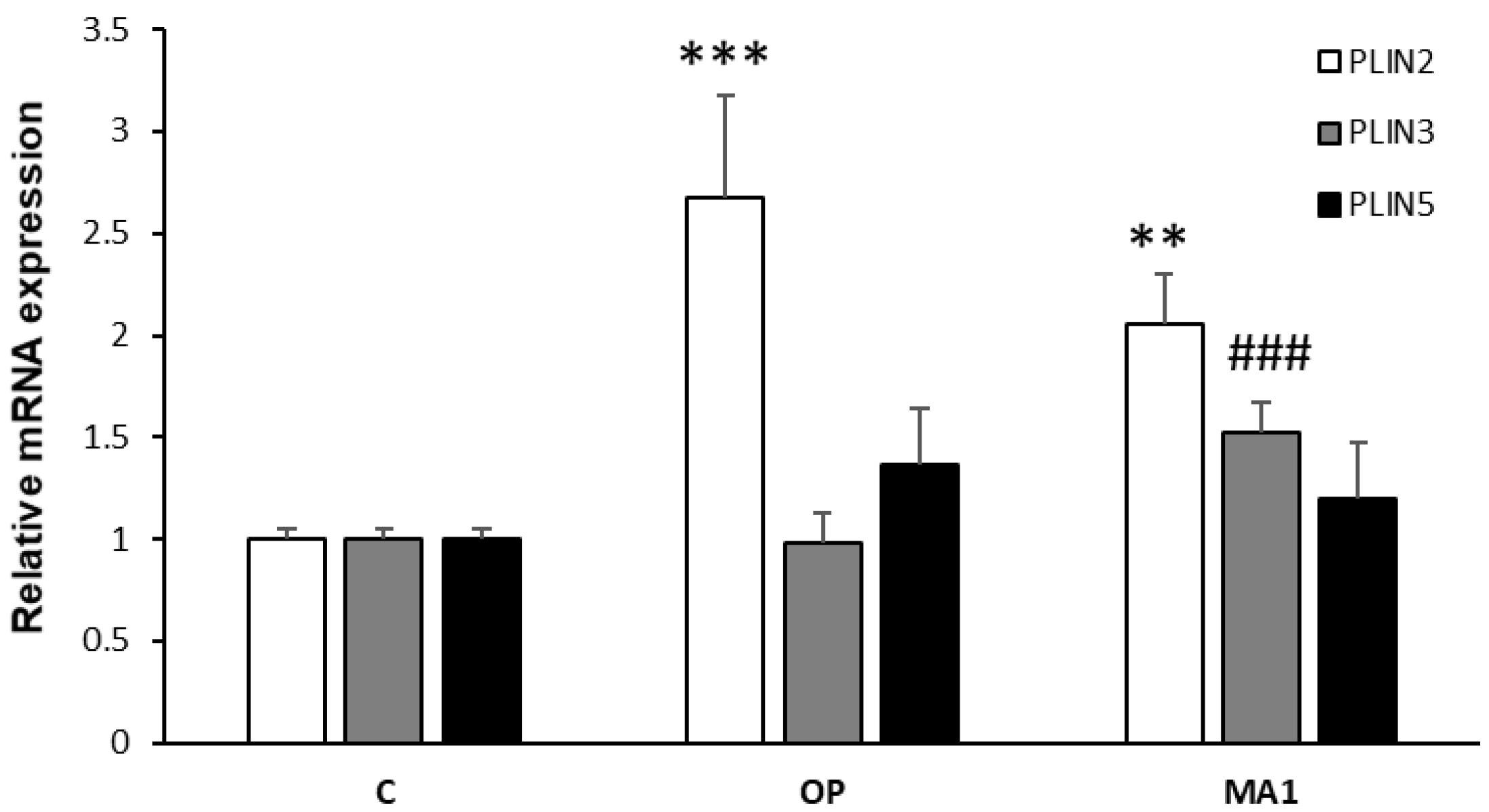
2.3. MA1 Regulates the Hepatic Expression of PLINs
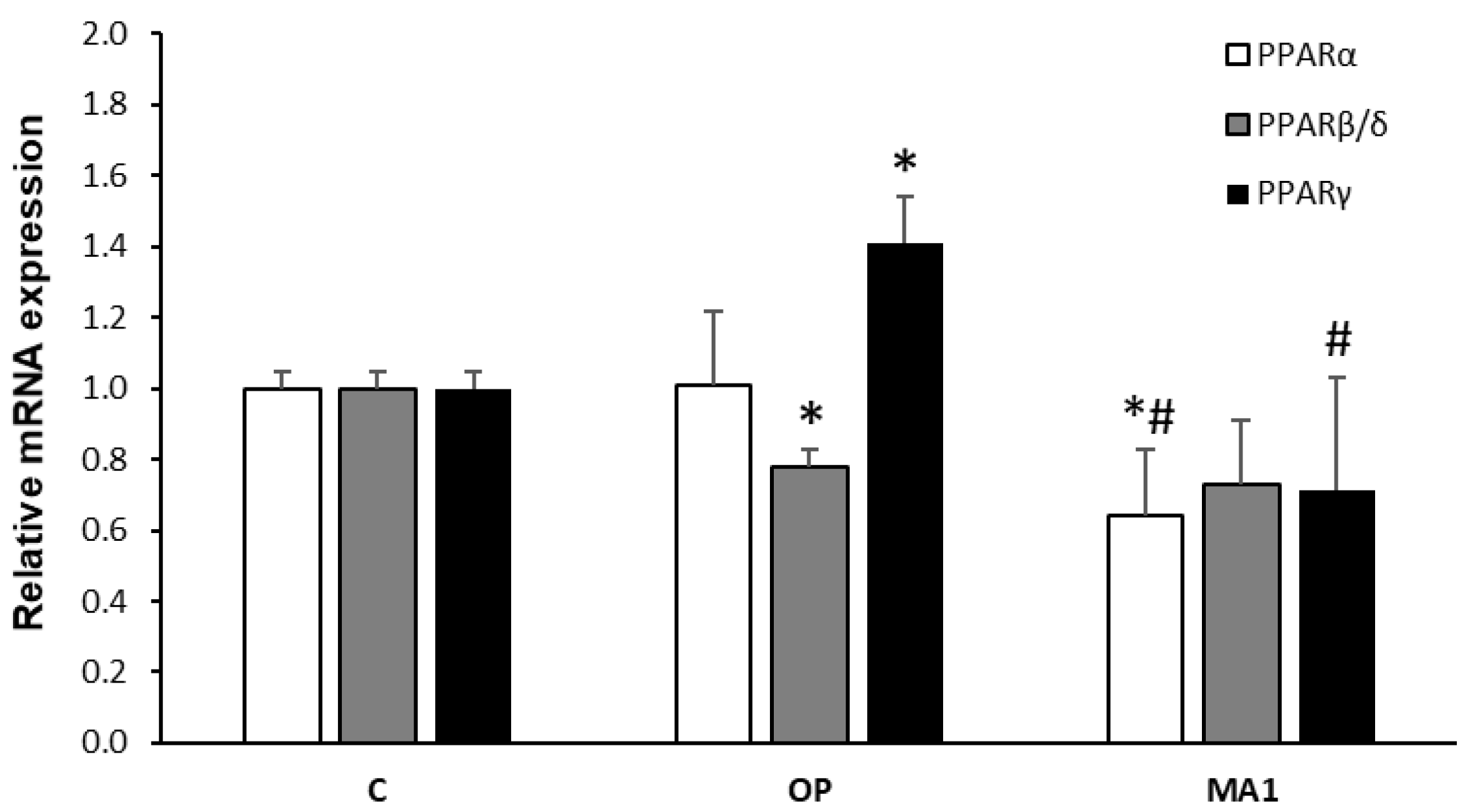
2.4. MA1 Regulates the Hepatic Expression of PPARs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. MA1 Synthesis
4.3. Cell Culture and Treatments
4.4. MTT Assay for Determination of Cell Viability
4.5. Quantification of Triglycerides (TGs)
4.6. Fluorescence Microscopy
4.7. RNA Extraction and Quantitative Real-Time PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Duellman, W.E.; Trueb, L. Biology of Amphibians, 2nd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Demori, I.; Rashed, Z.E.; Corradino, V.; Catalano, A.; Rovegno, L.; Queirolo, L.; Salvidio, S.; Biggi, E.; Zanotti-Russo, M.; Canesi, L.; et al. Peptides for Skin Protection and Healing in Amphibians. Molecules 2019, 24, 347. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.P.; Reinert, L.K.; Harper, L.K.; Woodhams, D.C.; Rollins-Smith, L.A. Immune Defenses against Batracho-chytrium Dendrobatidis, a Fungus Linked to Global Amphibian Declines, in the South African Clawed Frog, Xenopus Laevis. Infect. Immun. 2010, 78, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Rollins-Smith, L.A.; Conlon, J.M. Antimicrobial Peptide Defenses against Chytridiomycosis, an Emerging Infec-tious Disease of Amphibian Populations. Dev. Comp. Immunol. 2005, 29, 589–598. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Ramsey, J.P.; Pask, J.D.; Reinert, L.K.; Woodhams, D.C. Amphibian Immune Defenses against Chytridiomycosis: Impacts of Changing Environments. Integr. Comp. Biol. 2011, 51, 552–562. [Google Scholar] [CrossRef]
- Gammill, W.M.; Scott Fites, J.; Rollins-Smith, L.A. Norepinephrine Depletion of Antimicrobial Peptides from the Skin Glands of Xenopus laevis. Dev. Comp. Immunol. 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Crump, M.L. Why Are Some Species in Decline but Others Not? In Amphibian Declines; Lannoo, M., Ed.; University of California Press: Berkeley, CA, USA, 2005; pp. 7–9. [Google Scholar]
- Xu, X.; Lai, R. The Chemistry and Biological Activities of Peptides from Amphibian Skin Secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Zasloff, M. Peptides from Frog Skin. Annu. Rev. Biochem. 1990, 59, 395–414. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Leprince, J. Peptidomic Analysis in the Discovery of Therapeutically Valuable Peptides in Amphibian Skin Secretions. Expert Rev. Proteom. 2019, 16, 897–908. [Google Scholar] [CrossRef]
- Yang, X.; Lee, W.-H.; Zhang, Y. Extremely Abundant Antimicrobial Peptides Existed in the Skins of Nine Kinds of Chinese Odorous Frogs. J. Proteome Res. 2012, 11, 306–319. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Jiang, J.; Qiao, L.; Lu, Y.; Zhou, K.; Zheng, G.; Zhai, X.; Liu, J. Molecular Phylogeny and Diversi-fication of the Genus Odorrana (Amphibia, Anura, Ranidae) Inferred from Two Mitochondrial Genes. Mol. Phylogenetics Evol. 2013, 69, 1196–1202. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Therapeutic Landscape for NAFLD in 2020. Gastroenterology 2020, 158, 1984–1998.e3. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugiane-si, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Palma, R.; Pronio, A.; Romeo, M.; Scognamiglio, F.; Ventriglia, L.; Ormando, V.M.; Lamazza, A.; Pontone, S.; Fed-erico, A.; Dallio, M. The Role of Insulin Resistance in Fueling NAFLD Pathogenesis: From Molecular Mechanisms to Clinical Implications. J. Clin. Med. 2022, 11, 3649. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Abdel-Wahab, Y.H.; Flatt, P.R. Peptides from Frog Skin with Potential for Develop-ment into Agents for Type 2 Diabetes Therapy. Peptides 2018, 100, 275–281. [Google Scholar] [CrossRef]
- El Rashed, Z.; Lupidi, G.; Grasselli, E.; Canesi, L.; Khalifeh, H.; Demori, I. Antioxidant and Antisteatotic Activities of Fucoidan Fractions from Marine and Terrestrial Sources. Molecules 2021, 26, 4467. [Google Scholar] [CrossRef]
- Grasselli, E.; Canesi, L.; Portincasa, P.; Voci, A.; Vergani, L.; Demori, I. Models of Non-Alcoholic Fatty Liver Disease and Potential Translational Value: The Effects of 3,5-L-Diiodothyronine. Ann. Hepatol. 2017, 16, 707–719. [Google Scholar] [CrossRef]
- Grasselli, E.; Baldini, F.; Vecchione, G.; Oliveira, P.; Sardāo, V.; Voci, A.; Portincasa, P.; Vergani, L. Excess Fructose and Fatty Acids Trigger a Model of Non-alcoholic Fatty Liver Disease Progression in Vitro: Protective Effect of the Flavonoid Silybin. Int. J. Mol. Med. 2019, 44, 705–712. [Google Scholar] [CrossRef]
- Soltaninejad, H.; Zare-Zardini, H.; Ordooei, M.; Ghelmani, Y.; Ghadiri-Anari, A.; Mojahedi, S.; Hamidieh, A.A. Antimicrobial Peptides from Amphibian Innate Immune System as Potent Antidiabetic Agents: A Literature Review and Bioinformatics Analysis. J. Diabetes Res. 2021, 2021, 2894722. [Google Scholar] [CrossRef]
- Srinivasan, D.K.; Ojo, O.O.; Owolabi, B.O.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. [I10W]Tigerinin-1R En-hances Both Insulin Sensitivity and Pancreatic Beta Cell Function and Decreases Adiposity and Plasma Triglycerides in High-Fat Mice. Acta Diabetol. 2016, 53, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Power, G.J.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Jiansheng, H.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H. A Potent, Non-Toxic Insulin-Releasing Peptide Isolated from an Extract of the Skin of the Asian Frog, Hylarana Guntheri (Anura:Ranidae). Regul. Pept. 2008, 151, 153–159. [Google Scholar] [CrossRef]
- El Rashed, Z.; Lupidi, G.; Kanaan, H.; Grasselli, E.; Canesi, L.; Khalifeh, H.; Demori, I. Antioxidant and Antisteatotic Activities of a New Fucoidan Extracted from Ferula Hermonis Roots Harvested on Lebanese Mountains. Molecules 2021, 26, 1161. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, A.R.; Brasaemle, D.L.; McAndrews-Hill, M.; Sztalryd, C.; Londos, C. Adoption of PERILIPIN as a Unifying Nomenclature for the Mammalian PAT-Family of Intracellular Lipid Storage Droplet Proteins. J. Lipid Res. 2010, 51, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Ikura, Y.; Arimoto, J.; Sugioka, K.; Iezzoni, J.C.; Park, S.H.; Naruko, T.; Itabe, H.; Kawada, N.; Caldwell, S.H.; et al. Expression of Perilipin and Adipophilin in Nonalcoholic Fatty Liver Disease; Relevance to Oxidative Injury and Hepatocyte Ballooning. J. Atheroscler. Thromb. 2009, 16, 893–901. [Google Scholar] [CrossRef]
- Souza-Mello, V. Peroxisome Proliferator-Activated Receptors as Targets to Treat Non-Alcoholic Fatty Liver Disease. World J. Hepatol. 2015, 7, 1012. [Google Scholar] [CrossRef]
- Remington, G.J.; Teo, C.; Wilson, V.; Chintoh, A.; Guenette, M.; Ahsan, Z.; Giacca, A.; Hahn, M. Metformin Attenuates Olanzapineinduced Hepatic, but Not Peripheral Insulin Resistance. J. Endocrinol. 2015, 277, 71–81. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Lukic, M.L.; Flatt, P.R. Potential Therapeutic Applications of Multifunctional Host-Defense Peptides from Frog Skin as Anti-Cancer, Anti-Viral, Immunomodulatory, and Anti-Diabetic Agents. Peptides 2014, 57, 67–77. [Google Scholar] [CrossRef]
- Mechkarska, M.; Attoub, S.; Sulaiman, S.; Pantic, J.; Lukic, M.L.; Michael Conlon, J. Anti-Cancer, Immunoregulatory, and Antimicrobial Activities of the Frog Skin Host-Defense Peptides Pseudhymenochirin-1Pb and Pseudh-ymenochirin-2Pa. Regul. Pept. 2014, 194–195, 69–76. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The Biophysics and Cell Biology of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef]
- De la Rosa Rodriguez, M.A.; Kersten, S. Regulation of Lipid Droplet-Associated Proteins by Peroxisome Prolifera-tor-Activated Receptors. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 1212–1220. [Google Scholar] [CrossRef]
- Wahli, W.; Michalik, L. PPARs at the Crossroads of Lipid Signaling and Inflammation. Trends Endocrinol. Metab. 2012, 23, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Lambruschini, C.; Demori, I.; El Rashed, Z.; Rovegno, L.; Canessa, E.; Cortese, K.; Grasselli, E.; Moni, L. Synthesis, Photoisomerization, Antioxidant Activity, and Lipid-Lowering Effect of Ferulic Acid and Feruloyl Amides. Molecules 2020, 26, 89. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Masih-Khan, E.; Lo, M.; van Breemen, C.; McManus, B.M.; Dubé, G.P. Hepatic Over-Expression of Peroxisome Proliferator Activated Receptor Γ2 in the Ob/Ob Mouse Model of Non-insulin Dependent Diabetes Mellitus. Mol. Cell. Biochem. 2001, 224, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Cui, W.; Lopresti, M.; Mashek, M.T.; Najt, C.P.; Hu, H.; Mashek, D.G. Hepatic PLIN5 Signals via SIRT1 to Promote Autophagy and Prevent Inflammation during Fasting. J. Lipid Res. 2020, 61, 338–350. [Google Scholar] [CrossRef]
- Leamy, A.K.; Egnatchik, R.A.; Shiota, M.; Ivanova, P.T.; Myers, D.S.; Brown, H.A.; Young, J.D. Enhanced Synthesis of Saturated Phospholipids Is Associated with ER Stress and Lipotoxicity in Palmitate Treated Hepatic Cells. J. Lipid Res. 2014, 55, 1478–1488. [Google Scholar] [CrossRef]
- Sztalryd, C.; Bell, M.; Lu, X.; Mertz, P.; Hickenbottom, S.; Chang, B.H.-J.; Chan, L.; Kimmel, A.R.; Londos, C. Func-tional Compensation for Adipose Differentiation-Related Protein (ADFP) by Tip47 in an ADFP Null Embryonic Cell Line. J. Biol. Chem. 2006, 281, 34341–34348. [Google Scholar] [CrossRef]
- Minehira, K.; Gual, P. Role of Lipid Droplet Proteins in the Development of NAFLD and Hepatic Insulin Resistance. In Non-Alcoholic Fatty Liver Disease—Molecular Bases, Prevention and Treatment; Valenzuela, R., Ed.; InTech Open: London, UK, 2018; ISBN 978-953-51-3923-2. [Google Scholar]
- Garcia-Macia, M.; Santos-Ledo, A.; Leslie, J.; Paish, H.L.; Collins, A.L.; Scott, R.S.; Watson, A.; Burgoyne, R.A.; White, S.; French, J.; et al. A Mammalian Target of Rapamycin-Perilipin 3 (MTORC1-Plin3) Pathway Is Essential to Activate Lipophagy and Protects Against Hepatosteatosis. Hepatology 2021, 74, 3441–3459. [Google Scholar] [CrossRef]
- Fougerat, A.; Montagner, A.; Loiseau, N.; Guillou, H.; Wahli, W. Peroxisome Proliferator-Activated Receptors and Their Novel Ligands as Candidates for the Treatment of Non-Alcoholic Fatty Liver Disease. Cells 2020, 9, 1638. [Google Scholar] [CrossRef]
- Kersten, S. Integrated Physiology and Systems Biology of PPARα. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Pyper, S.R.; Viswakarma, N.; Yu, S.; Reddy, J.K. PPARα: Energy Combustion, Hypolipidemia, Inflammation and Cancer. Nucl. Recept. Signal 2010, 8, nrs.08002. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, E.; Canesi, L.; Voci, A.; De Matteis, R.; Demori, I.; Fugassa, E.; Vergani, L. Effects of 3,5-Diiodo-L-Thyronine Administration on the Liver of High Fat Diet-Fed Rats. Exp. Biol. Med. 2008, 233, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Redonnet, A.; Groubet, R.; No[euml ]l-Suberville, C.; Bonilla, S.; Martinez, A.; Higueret, P. Exposure to an Obesity-Inducing Diet Early Affects the Pattern of Expression of Peroxisome Proliferator, Retinoic Acid, and Triiodothyronine Nuclear Receptors in the Rat. Metabolism 2001, 50, 1161–1167. [Google Scholar] [CrossRef]
- Patsouris, D.; Reddy, J.K.; Müller, M.; Kersten, S. Peroxisome Proliferator-Activated Receptor α Mediates the Effects of High-Fat Diet on Hepatic Gene Expression. Endocrinology 2006, 147, 1508–1516. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-Regulation of PPAR-γ MRNA Expression in the Liver of Obese Patients: An Additional Reinforcing Lipogenic Mechanism to SREBP-1c Induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Schadinger, S.E.; Bucher, N.L.R.; Schreiber, B.M.; Farmer, S.R. PPARγ2 Regulates Lipogenesis and Lipid Accumu-lation in Steatotic Hepatocytes. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E1195–E1205. [Google Scholar] [CrossRef]
- Cardinali, B.; Lunardi, G.; Millo, E.; Armirotti, A.; Damonte, G.; Profumo, A.; Gori, S.; Iacono, G.; Levaggi, A.; Del Mastro, L. Trastuzumab Quantification in Serum: A New, Rapid, Robust ELISA Assay Based on a Mimetic Peptide That Specifically Recognizes Trastuzumab. Anal. Bioanal. Chem. 2014, 406, 4557–4561. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- D’Alesio, C.; Bellese, G.; Gagliani, M.C.; Lechiara, A.; Dameri, M.; Grasselli, E.; Lanfrancone, L.; Cortese, K.; Castag-nola, P. The Chromodomain Helicase CHD4 Regulates ERBB2 Signaling Pathway and Autophagy in ERBB2+ Breast Cancer Cells. Biol. Open 2019, 8, bio.038323. [Google Scholar] [CrossRef]
- Grasselli, E.; Voci, A.; Demori, I.; Vecchione, G.; Compalati, A.D.; Gallo, G.; Goglia, F.; De Matteis, R.; Silvestri, E.; Vergani, L. Triglyceride Mobilization from Lipid Droplets Sustains the Anti-Steatotic Action of Iodothyronines in Cultured Rat Hepatocytes. Front. Physiol. 2016, 6, 418. [Google Scholar] [CrossRef]
- Grasselli, E.; Voci, A.; Canesi, L.; Goglia, F.; Ravera, S.; Panfoli, I.; Gallo, G.; Vergani, L. Non-receptor-mediated actions are responsible for the lipid-lowering effects of iodothyronines in FaO rat hepatoma cells. J. Endocrinol. 2011, 210, 59–69. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence (5′→3′) | Annealing T (°C) | Product Length (bp) | Accession ID |
|---|---|---|---|---|
| GAPDH Fwd | GACCCCTTCATTGACCTCAAC | 60 | 136 | DQ403053 |
| GAPDH Rev | CGCTCCTGGAAGATGGTGATGGG | |||
| PPARα Fwd | CCCCACTTGAAGCAGATGACC | 60 | 139 | NM_013196 |
| PPARα Rev | CCCTAAGTACTGGTAGTCCGC | |||
| PPARβ/δ Fwd | AATGCCTACCTGAAAAACTTCAAC | 60 | 96 | AJ306400.1 |
| PPARβ/δ Rev | TGCCTGCCACAGCGTCTCAAT | |||
| PPARγ Fwd | CGGAGTCCTCCCAGCTGTTCGCC | 60 | 116 | Y12882 |
| PPARγ Rev | GGCTCATATCTGTCTCCGTCTTC | |||
| PLIN2 Fwd | CCGAGCGTGGTGACGAGGG | 60 | 148 | AAH85861 |
| PLIN2 Rev | GAGGTCACGGTCCTCACTCCC | |||
| PLIN3 Fwd | GGAACTGGTGTCATCAACAG | 60 | 108 | NW_047865.1 |
| PLIN3 Rev | GGTCACATCCACTGCTCCTG | |||
| PLIN5 Fwd | GGATGTCCGGTGATCAGAC | 60 | 96 | XM_576698 |
| PLIN5 Rev | GTGCACGTGGCCCTGACCAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demori, I.; El Rashed, Z.; De Negri Atanasio, G.; Parodi, A.; Millo, E.; Salis, A.; Costa, A.; Rosa, G.; Zanotti Russo, M.; Salvidio, S.; et al. First Evidence of Anti-Steatotic Action of Macrotympanain A1, an Amphibian Skin Peptide from Odorrana macrotympana. Molecules 2022, 27, 7417. https://doi.org/10.3390/molecules27217417
Demori I, El Rashed Z, De Negri Atanasio G, Parodi A, Millo E, Salis A, Costa A, Rosa G, Zanotti Russo M, Salvidio S, et al. First Evidence of Anti-Steatotic Action of Macrotympanain A1, an Amphibian Skin Peptide from Odorrana macrotympana. Molecules. 2022; 27(21):7417. https://doi.org/10.3390/molecules27217417
Chicago/Turabian StyleDemori, Ilaria, Zeinab El Rashed, Giulia De Negri Atanasio, Alice Parodi, Enrico Millo, Annalisa Salis, Andrea Costa, Giacomo Rosa, Matteo Zanotti Russo, Sebastiano Salvidio, and et al. 2022. "First Evidence of Anti-Steatotic Action of Macrotympanain A1, an Amphibian Skin Peptide from Odorrana macrotympana" Molecules 27, no. 21: 7417. https://doi.org/10.3390/molecules27217417
APA StyleDemori, I., El Rashed, Z., De Negri Atanasio, G., Parodi, A., Millo, E., Salis, A., Costa, A., Rosa, G., Zanotti Russo, M., Salvidio, S., Cortese, K., & Grasselli, E. (2022). First Evidence of Anti-Steatotic Action of Macrotympanain A1, an Amphibian Skin Peptide from Odorrana macrotympana. Molecules, 27(21), 7417. https://doi.org/10.3390/molecules27217417













