Abstract
The aim of this study is to inactivate Enterococcus faecalis ATCC 29212 present in dairy wastewater effluent using microwave (MW) waves and/or ultrasound waves (US). The ultrasonic bath treatment (35 kHz) had no significant effect on the reduction of the survival rate (predominant declumping effect). At 650 W of microwave treatment, the total destruction was completed at 75 s, while at 350 W a 3 log reduction was achieved. The Weibull model was fitted to the survival curves to describe the inactivation kinetics, and the effect of the combined microwave-ultrasound treatments was evaluated. The scaling parameter α that was estimated from the inactivation kinetics for the microwaves combined with the ultrasound waves in pre-treatment was found to be lower than the scaling parameters obtained in post-treatment, which were in turn lower than those estimated for microwaves or ultrasound waves alone. The use of the ultrasound waves in pre-treatment was more effective than in post-treatment; a total reduction was achieved using a combination of US (30 min) followed by MW (650 W) with α = 28.3 s, while 4.0 log was obtained by reversing all processes with α = 34.5 s. The results from the protein assays indicate that the bacterial wall was damaged and that holes were formed from which protein leakage occurred.
1. Introduction
The food industry, in particular the dairy sector, is one of the most important sources of wastewater, with a propensity to increase the volume generated. The production of such wastewater is one of the main issues in environmental sustainability. These effluents are subject to microbiological and chemical requirements and can have a significant ecotoxicological impact on aquatic life. In Algeria, a large proportion of wastewater from the food industry (85%) is discharged directly into municipal sewers without any treatment [1].
Considering the high content of organic matter, nutrients such as proteins, and carbohydrates, in addition to the higher concentrations of suspended solids, the Biological Oxygen Demand (BOD) as well as the Chemical Oxygen Demand (COD), wastewater streams, along with substantial pH variability, can contain a multitude of microbiological and chemical pollutants [2,3]. A wide range of microbial profiles have been identified in food effluents, including Cryptosporidium parvum, Giardia sp., Escherichia coli, Clostridium perfringens, Enterococcus faecalis, Salmonella sp., etc. [4,5,6,7,8]. It has been shown that the predominant species in samples from environmental sources (compost, wastewater, sediment, and swimming pool water) are E. faecalis (39%) and E. faecium (29%), followed by E. durans/E. hirae, E. casseliflavus/E. gallinarum, and E. raffinosus, with a different prevalence of species depending on the source [9]. These enterococci are an indication of the presence of enteric pathogens [10]. Indeed, disinfection is an important step in wastewater treatment for reducing the pollution levels of the receiving waters and thereby protecting public health.
Treatment technologies commonly used for wastewater include electrochemical treatment, anaerobic treatment, ultrafiltration, chlorination, UV-C irradiation, heat treatment, radiation treatment, and various combinations thereof [11,12,13], such as atmospheric cold plasma [14] and partial denitrification combined with Anammox [15]. Nevertheless, these approaches have drawbacks, i.e., the amount of energy consumed [16], bacterial reactivation [17], the slow metabolic rates of methanogens, a high susceptibility to oxygen concentrations, the complexity of handling biowaste in the case of anaerobic treatment [12], and the influence of environmental factors on the bactericidal effect of cold atmospheric plasma [18]. Several authors have highlighted the use of microwave technology, a green chemistry application with a high sterilization capacity that can effectively inactivate bacteria and enzymes in wastewater [19,20,21,22]. It is a well-known heating and drying process used both for domestic and industrial purposes. Treatment or pretreatment using microwaves provides an increase in the destruction of pathogenic bacteria in the medium, which results in the volume heating of the product and initiates thermal pretreatment [23].
Although the microwave technique has some advantages, some disadvantages remain, and these essentially concern a large consumption of electrical energy being converted into heat. Indeed, given the high specific thermal capacity of water, the decision to use microwaves for wastewater treatment not only implies high energy consumption but also a high cost in operating the system, factors which limit their use. The conventional microwave ovens available on the market provide a high level of disinfection but are not sufficient for use in wastewater sterilization [24].
As a result, another method of disinfection has been the focus of many studies, namely, the use of ultrasound. The effects of ultrasound (US) can be physical (cavitation, mechanical effects, and micromechanical shocks) or chemical, owing to the formation of free radicals (OH- and H- resulting from sonochemical reactions) produced by the decomposition of water within oscillating bubbles [25,26]. Several experiments have explored the use of ultrasound (US) for wastewater disinfection [27,28]. However, in order to achieve a high degree of logarithmic reduction in the microorganisms from the process of ultrasonic irradiation, it is necessary to use a high intensity or a prolonged amount of time. These are the economically limiting factors concerning the application of the large-scale implementation of such disinfection technology. The combining of microwave and ultrasonic methods to reduce the energy needed for successful bacterial destruction is now a practical choice for industry. Leonelli and Mason [29] describe the need to shift towards the optimization of green processes and technologies for the production of microwave and ultrasound reactors on an industrial scale. Therefore, the combination of ultrasound with microwave can induce synergistic effects in terms of efficiency in microbial inactivation, as well as in energy saving [30].
Indeed, the application of this combined technique to the inactivation process for E. faecalis (a pathogenic opportunistic bacterium [31] which is considered the most thermoresistant of the vegetative forms [32]) in wastewater is the main purpose of this study, for which we have set the following objectives: (i) to experimentally evaluate the survival of Enterococcus faecalis ATCC 29212 cells in wastewater dairy effluent treated by microwave and/or ultrasound, (ii) to fit the Weibull model to describe and compare the inactivation kinetics, (iii) to assess the combined effects of microwave-ultrasound treatment, and (iv) to use extracellular protein assays to study cell membrane integrity.
2. Results and Discussion
2.1. Ultrasound Effect
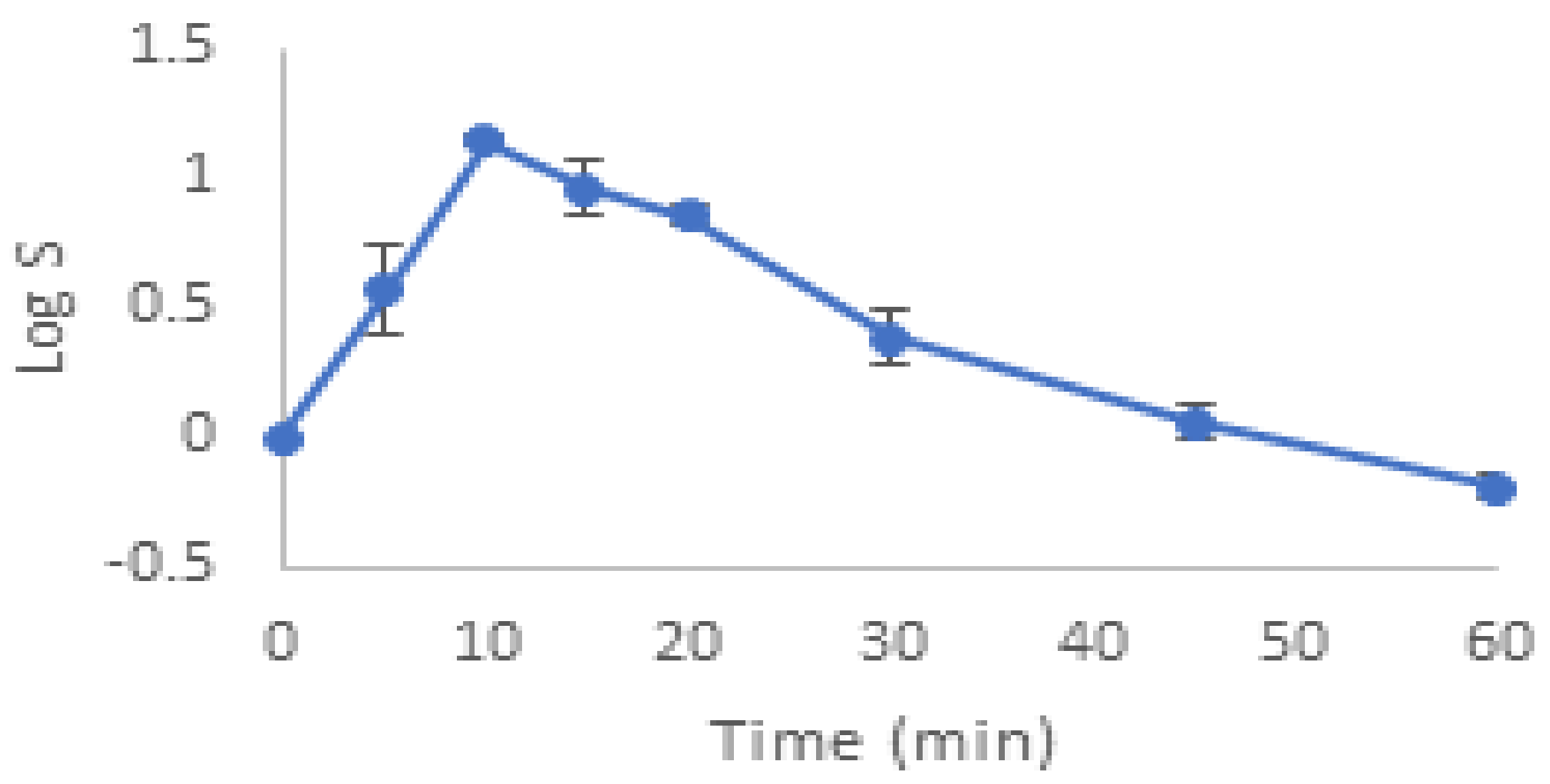
The degradation of E. faecalis ATCC 29121 by ultrasonic bath at a frequency of 35 kHz is shown in Figure 1.
Figure 1.
Destruction of Enterococcus faecalis ATCC 29121 by ultrasonic bath at a frequency of 35 KHz.
The results indicate that there was no significant effect on the viability of the bacterium, with the exception of a small drop in the sonication at 15 min.
On the other hand, this frequency produced an increase of 1.14 log within the first 10 min, followed by a steady decrease. However, the amount remained higher than the initial concentration even after the 60th minute of sonication, which could also indicate that the declumping effect at this frequency (producing a higher number of CFUs) masked the actual deactivation. The CFU measurement represents cell viability after sonication, although it is important to note that a CFU may be a single cell or a group of cells. Therefore, if ultrasound waves trigger the cells, more CFUs will form.
Obioma [33] revealed that Enterococcus faecalis (Gram-positive bacterium with a resistant peptidoglycan cell wall) present in drinking water showed a declumping effect after 2 min of sonication at 20 kHz for a probe. This phenomenon was also observed by Joyce, Phull, Lorimer and Mason [25], who noticed this effect on Bacillus subtilis at a frequency of 20 kHz for the probe and 38 kHz in the bath.
Cell death is due to the high pressure and temperature caused by bubble collapse and the shared forces that destroy the bacterial cell membrane [34].
The exposure of high mechanical pressure waves to liquids creates an acoustic current and a subsequent acoustic cavitation that causes the formation, growth, and implosive collapse of micro and nanobubbles in the liquid. These bubbles have a large specific surface area that increases gas diffusion and generates intense localized heating (approximately 5000 °C) and high pressure (1000 ATM) [35,36].
Ultrasonic cavitation affects the inner membrane (cytoplasmic membrane) of the bacteria, and the lipoprotein layer is disrupted, torn, and shredded [37]. E. faecalis showed resistance to ultrasonic waves, since it has a thick cell wall. It is well-known that the cell wall of Gram-positive bacteria is thicker than that of Gram-negative bacteria, and this thickness mainly affects the effectiveness of the microbial inactivation [38]. A 4 log reduction in E.coli and E. faecalis cells present in drinking water was obtained at 9 min, as reported by Gholami, et al. [39], and no declumping effect was observed at 20 kHz. Amabilis-Sosa, Vázquez-López, Rojas, Roé-Sosa and Moeller-Chávez [28] studied the effect of ultrasound on bacterial inactivation in municipal wastewater (MWW); the results showed that, after 15 min of sonication (20 kHz, 35% amplitude and 600 W/l), the bacterial density was reduced by 1.85 Log10 MPN/100 mL for E. coli and by 3.16 Log10 CFU/mL for B. subtilis. After 30 min, no amount of CFU/mL for B. subtilis was observed in the municipal wastewater, and after 45 min, the reduction in total and faecal coliforms was nearly 6.45 Log10 MPN/100 mL.
Various authors have reported on the lethal effects of several bacteria: Microcystis aeruginosa, Circinalis anabaena, and Chlorella sp. [40]; Legionella pneumophila [41], Pseudomonas aeruginosa, Staphylococcus aureus [42], and Escherichia coli [43,44]. In addition, some papers have described the application of ultrasound disinfection technologies [45,46].
2.2. Modeling and Kinetic Parameter Estimation
Several mathematical models are suitable for fitting concave downward survival curves [47]; a mathematical model based on the Weibull distribution was chosen for its simplicity and flexibility [48,49].
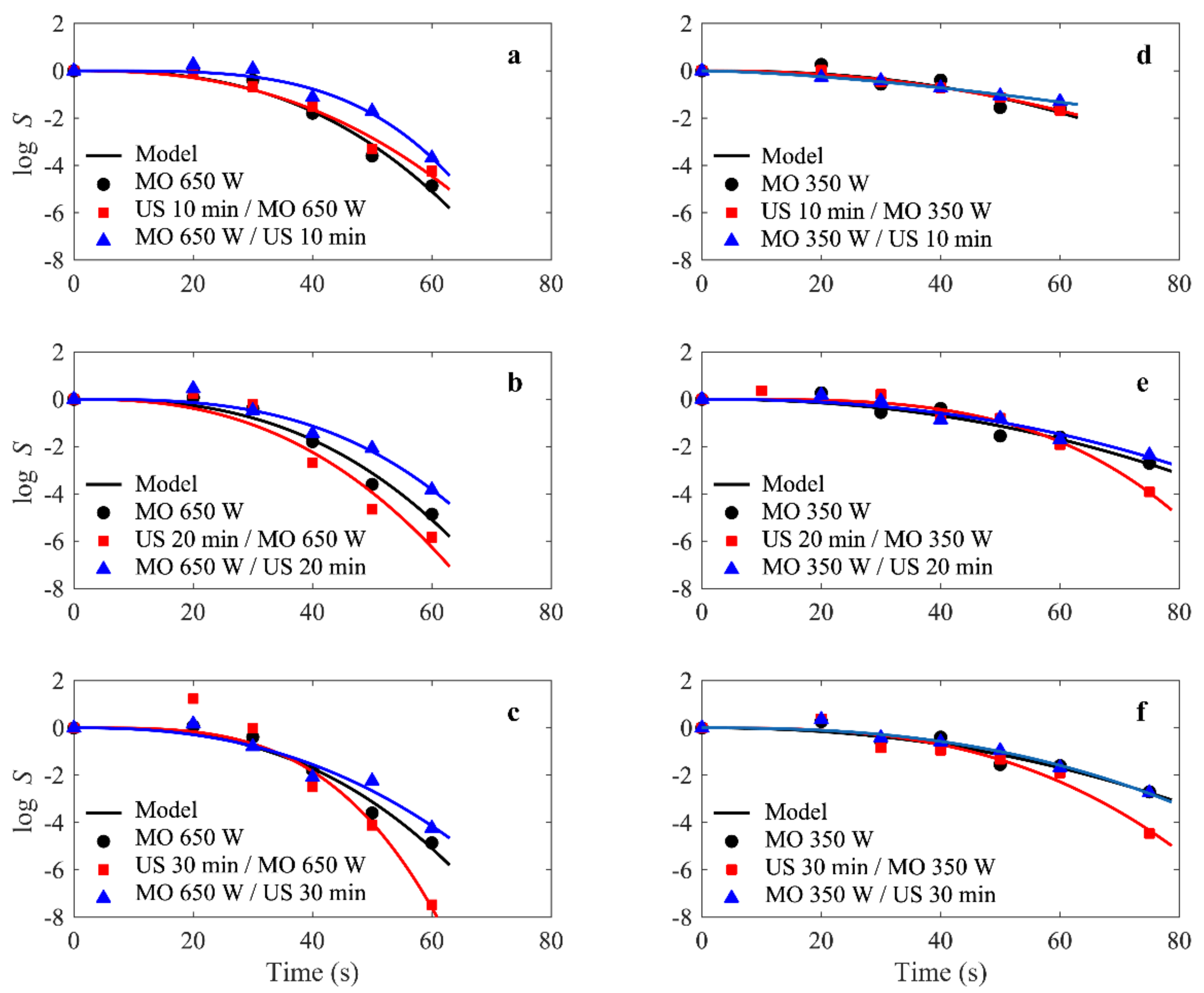
In all cases tested in the present study, the modified Weibull model was a very reasonable choice and was well-fitted to the experimental survival data for E. faecalis ATCC 29121, as illustrated in Figure 2 (shows the experimental and simulated survival rate S(t) for E. faecalis ATCC 29121 under combined microwave-ultrasonic treatments).
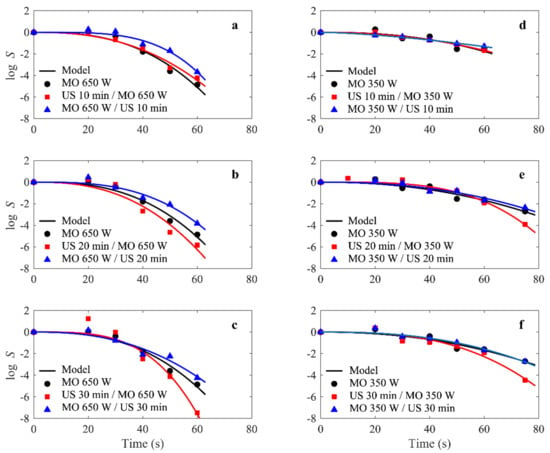
Figure 2.
(a–f) E.faecalis survival rate S(t) curves under combined microwave (350 and 650 W) and ultrasonic (42 KHz) treatments. Solid lines represent the fit of the modified Weibull model to the experimental S(t) data, represented by symbols. The dashed lines are curves added as a visual guide to highlight an increase in survival rate.
The fit quality was determined by both R2 and RMSE values, which ranged from 0.927 to 0.995 and 0.04 to 0.842, respectively (Table 1). This does not mean that other models were not applicable; in fact, other models could also work well. However, the purpose of this paper was not to compare models, but to study the dependence of the Weibull parameters on the power levels and exposure times for microwave and ultrasound treatments.

Table 1.
Estimation of the scale parameter (α) and shape parameter (β) for the fit of survival rate S(t) for different treatments.
2.3. Microwave Effect
The results show that in the first 10 s at a power of 650 W, there was an increase in the microbial load compared to the initial load, which reached 8.99 ± 0.26 log. This can be explained by the declumping effect; the reduction started from the 10 s mark and reached total destruction after 75 s (Figure 2a–c). On the other hand, a power level of 350 W produced an immediate increase in the CFU with respect to the first second, which reached 8.57 log (followed by a steady decrease). However, the CFU level remained unchanged above the initial concentration, even after 20 s of microwave irradiation, indicating that only the declumping effect can justify this phenomenon. After 75 s, the destruction did not exceed 3 log. Thus, E. faecalis ATCC 29,121 was more resistant to lower power than to higher power (Figure 2d–f).
These results suggest that the major effect of a high-powered microwave in the first few seconds is the decommissioning of bacterial agglomerates with little deactivation present. They may also indicate that the downgrading effect at low power levels hides the actual deactivation process. In order to model the results obtained, the inactivation was taken into consideration from the 20th second of microwave treatment. The same results were observed by Benjamin, et al. [50], who studied the effect of microwaves on Enterococcus faecalis, Staphylococcus aureus, and Escherichia coli in water. Their findings revealed that E. faecalis appeared to be the most thermally stable of all the bacteria tested (Table 2).

Table 2.
Ultrasound and microwave irradiation in microbial decontamination technologies for wastewater.
Considering the influence of growth conditions on the shape of survival curves, and in order to quantify the resistance of E. faecalis ATCC 29212, the scale parameter (α) of each survival curve was re-estimated with a fixed value β (β = 2.6) for each growth condition. Based on the regression results for the Weibull model, it was found that the α parameter generally showed a downward trend and that the β parameter tended to increase in value with power levels.
When the survival curves for these two parameters in Figure 2 were compared, it became obvious that the curve with the lowest β value had a more pronounced drag, and the α value for the microwave at 350 W was much higher than at 650 W of power.
An increase in microwave power resulted in a decrease in the inactivation rate: at 650 W, the estimated scale parameter α (32.2) was the lowest, while at 350 W (50.0), it was the highest, as shown in Table 1.
At MW power levels of 300 W and above (frequency of 2.45 GHz), the disinfection of water was performed when it was heated from 45 to 100 °C. Therefore, the treatment time depended on the MW heating power and the volume of the sample [57]. Ara, et al. [58], reported that the high-level disinfection of enterococci and salmonella is possible in 9.5 min at a temperature of 72 °C reached using microwaves. In addition, microwave irradiation reduced the bacterial content of the sewage sludge prior to anaerobic digestion [59]. Hollywood, et al. [60] reported that a reduction from 3 to 4 log after 7 to 11 min has been obtained from ground beef using a power level of 650 W and suggested that the use of microwave treatment on this strain has a thermal effect and that there was no other factor affecting the inactivation of this strain. Enterococcus faecalis was treated at a charge of 1011 CFU/mL in a dextrose broth at a power level of 650 W. After 5 min of treatment, survivors were still present, whereas a dosage of 10 min at 650 W was sufficient to destroy all of them. Altogether, the effect of bacterial inactivation from microwave treatment was much more pronounced in a liquid medium than in a solid medium, and as observed by other authors, microbial inactivation was faster when accompanied by an increase in microwave power [61,62].
2.4. Combination Effect
It cannot be completely excluded that the inherent inhomogeneity of the dairy wastewater effluent medium, which is caused by milk fat, may pose experimental problems in terms of the regulation of the concentration of this fat in the medium and the agglutination of microorganisms.
The concentration of this fat affects the inactivation of the microorganism, as it has a protective effect on bacterial cells, preventing interactions between bacteria and reactive species (microwave and ultrasound)[14]. Variations in the inactivation of E. faecalis ATCC 29212 per microwave and ultrasound treatments were highly dependent on the ultrasound exposure time/power level and the microwave exposure time, as well as on the process chronology (the use of the ultrasound either in pre-treatment or in post-treatment). In all treatments, β is greater than one, which determines the value of its concavity parameter downward, meaning that the remaining cells become more and more sensitive to heat. In other words, this indicates that cumulative damage is occurring, making it increasingly difficult for cells to survive.
The values of α decreased from 45.1 s for US (10 min)/MW (350 W) to 43.1 s for US (30 min)/MW (350 W), and from 57.3 ± 6 s for MW (350 W)/US (10 min) to 50.5 ± 2.8 s for MW (350 W)/US (30 min). In addition, there is a decrease in the value of α at a power level of 650 W, i.e., 33.6, 29.6 ± 2.4, and 28.3 for US 10 min/MW (650 W), US 20 min/MW (650 W), and US 30 min/MW (650 W), respectively. This can be explained by the fact that the more the bacteria are exposed to higher power levels, the stronger the bacterial inactivation effect. These results suggest that the combination of microwaves and ultrasound waves may be useful in improving the inactivation process for Enterococcus faecalis.
US pretreatment can increase the efficiency of the microwave inactivation of E. faecalis ATCC 29212 in DE. Microwave treatment at 350 W for 75 s combined with ultrasound pre-treatment for 20 and 30 min resulted in a reduction of 3.8 and 4.3 log, respectively, as compared to a reduction of only 2.2 and 3.6 log when sonication was performed as a post-treatment. The same is true for 650 W, where it can be seen that microwave treatment for 60 s followed by ultrasound treatment for 30 min resulted in a 5.0 log destruction, while the total population reduction was achieved when ultrasound was used as pre-treatment.
No study on the subject of this particular technology (the ultrasonic-microwave combination) has been performed; however, studies involving combinations of microwave and ultrasound technology along with other methods of wastewater disinfection have been (Table 2). Blume and Neis [53] evaluated the scientific and economic potential of using an US application as a pre-treatment step in combination with UV to optimize the disinfection process for wastewater; they showed that for 30 s of UV treatment (14 W with 3 W emitted at 254 nm) followed by 5 s of ultrasound treatment at 50 or 310 W/L, the microbial reduction levels obtained were 3.3 log and 3.7 log units, respectively. A synergistic effect was obtained in the elimination of enterococci by US/ozone. Chen, Tang, Wang, Yuan, Wang, Ali and Hu [54], obtained a value of only 1.11 log for enterococcus present in the wastewater after treatment with ultrasound waves at 200 W for 30 min, followed by ozone at a concentration of 4.2 mg O3/L; this is still low compared to our results.
However, combined microwave-ultrasound treatment is less important than microwave treatment alone at a power of 650 W; for the following time values: MW (650 W)/US 10 min, MW (650 W)/US 20 min, and MW (650 W)/US 30 min, α = 37.8 s, α = 36.3 s, and α = 34.5 s, respectively. Based on the data obtained from this study, under the same experimental conditions, the ultrasound pre-treatment application had a better effect on the inactivation of Enterococcus faecalis ATCC 29,212 than the microwave treatment followed by the US. These results are consistent with the work of Wang, et al. [63].
Since E. faecalis ATCC 29,212 has shown a high resistance to microwave treatments and since ultrasound treatments weaken the bacterial wall and contribute to the extraction of intracellular compounds, it could be presumed that the heat generated by microwaves in the liquid and the fragility of the bacterial wall thus accelerate the inactivation and death mechanisms of microbial cells. The results obtained show that a significant reduction in the bacterial population was obtained with a reduced treatment time.
2.5. Protein Assays
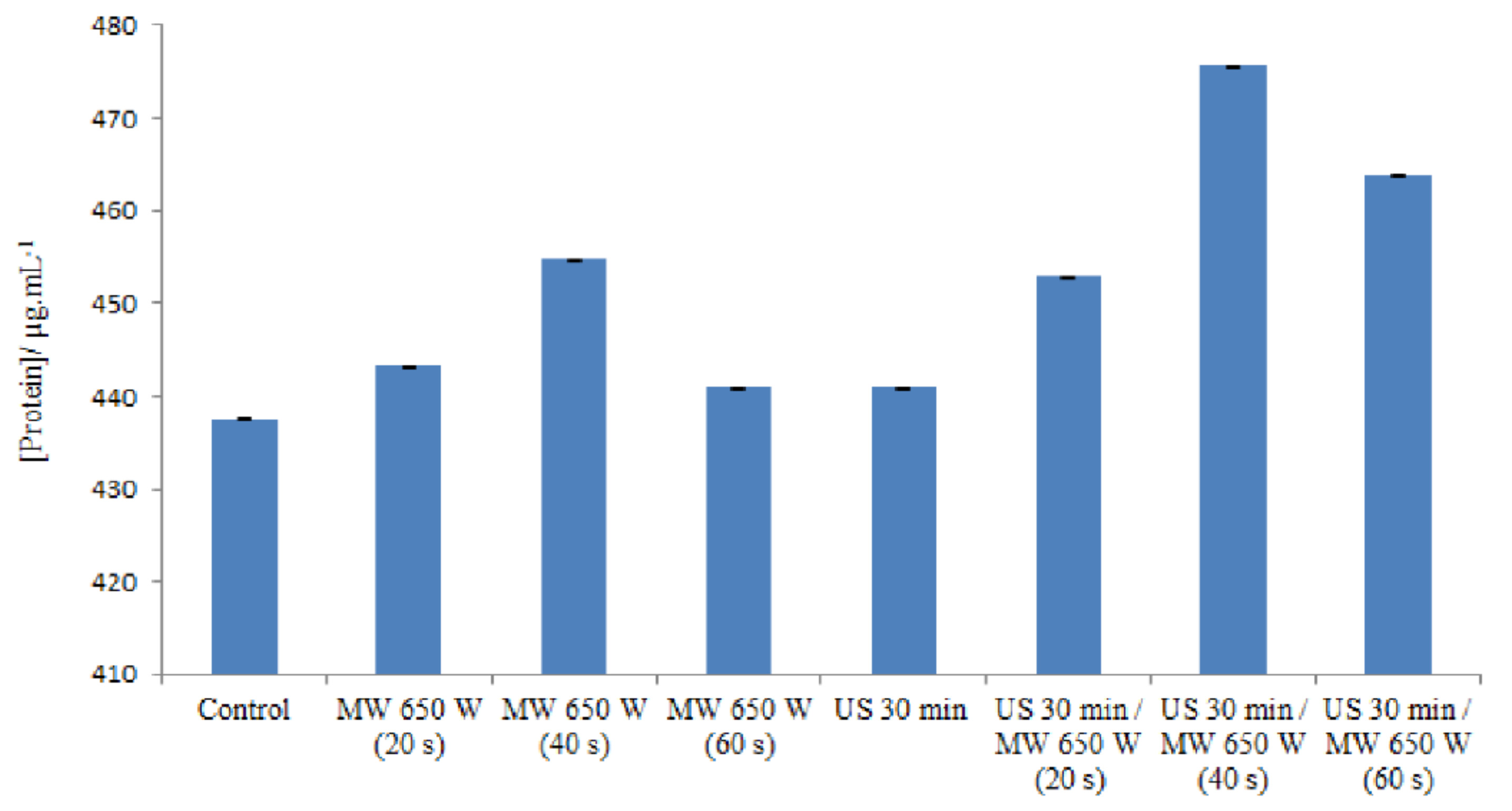
The release of intracellular proteins was measured in order to study the cell membrane damage caused by microwave and ultrasound irradiation. Figure 3 indicates the various amounts of proteins in different processes.
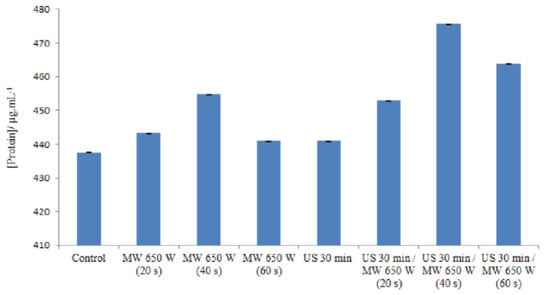
Figure 3.
Different concentrations of proteins in different processes in DE.
Microwave heating at 650 W/20 s did not result in a large difference in the amount of protein released from the cells (443.30 μg/mL) compared to the control (437.6 μg/mL). However, when the treatment time was increased to 40 s, substantial differences in the amount of protein released were observed at a value of 454.8 μg/mL. The protein content increased, and these results indicate that most of the microwave-heated cells may be ghost cells with released intracellular material [64]. Indeed, in response to temperature increases as well as chemical or physical stresses, prokaryotic cells synthesize specific proteins involved in cell protection. These stress proteins include a large family called HSPs (which stands for Heat Shock Proteins). Some HSPs are constitutively expressed in cells under normal culture conditions. The induction of a high expression of these proteins occurs when cells are subjected to stress. These proteins are considered “molecular chaperones”: they are thought to be involved in protein folding [65].
A low level of protein leakage was observed when E. faecalis ATCC 29212 cells were irradiated for 60 s, a time sufficient for a 3.31 log reduction in the number of viable cells.
On the one hand, some authors propose mechanisms to explain the existence of a stress response under non-thermal conditions. Depending on the power of the microwaves, the effect could be different. Microwave fields could alter the correct folding of some proteins, but not enough to induce a stress response. Misfolded proteins are therefore not protected by the HSP system [66]. On the other hand, according to the principle of the Bradford method, which is used in this study for the determination of proteins, there is a binding (complexation) of Coomassie Blue G-250 with the basic amino acids (arginine, histidine, and lysine) and the hydrophobic residues of amino acids present in the protein(s). The combination of these two explanations leads us to suggest a hypothesis which posits that there would be a decrease in the amount of proteins in the medium when it is at 650 W for 60 s; indeed, after microwave treatment and the refolding of the proteins as explained above, the basic amino acids will not be accessible. Instead, they will be “hidden” in the three-dimensional structure of the proteins with respect to Coomassie blue. This decreases the speed of the basic amino acid–Coomassie blue couple; consequently, the absorbance will be reduced. Nevertheless, according to Woo, et al. [67], who studied the leakage of Bacillus subtilis proteins treated using microwaves at 20, 40, and 60 °C, similar results were found and, according to them, the reason for this decrease is still unknown. By using slightly less intense ultrasound waves (35 kHz), it is possible to increase the permeability of the membrane to macromolecules. In this case, the membrane is not broken, only slightly damaged [68], hence an explanation for the low amount of proteins released in the medium. The combined effect from the ultrasound and microwave treatments leads to an increase in the amount of proteins released into the medium; the ultrasound waves weaken the membrane and the microwaves complement the effect of the ultrasound waves by reacting on the membrane.
3. Materials and Methods
3.1. Preparation of Model Effluent
Model dairy effluent (DE) with a pH value of 6.0 was prepared, as reported by Daverey and Pakshirajan [69], containing 2 g/L semi-skimmed milk powder (Régilait, France), 0.2% (w/v) milk fat (Ghee, nature foods, Portugal), 0.01% (w/v) sodium hydroxide (Sigma), and sterile distilled water. The milk powder consisted of 31% protein, 45% carbohydrates, and 14% fat content, with 9% saturated fatty acids and 0.92% salt.
3.2. Bacterial Strain and Culture Conditions
Experiments were performed with Enterococcus faecalis ATCC 29212 collected from the Pasteur Institute (Algiers, Algeria). The strain was maintained on tryptone soy agar (TSA; Conda Pronadisa, Spain) at 4 °C until use. Young cultures were prepared by suspending colonies in Tryptone Soy Broth (TSB, Conda Pronadisa, Spain) and were incubated at 37 °C for 18 h. Bacterial cells were then recovered by the process of centrifugation (4000× g for 15 min at 4 °C) [70,71].
The inoculation of the E. faecalis ATCC 29212 strain was adjusted to a final concentration of 1.5 × 108 colony forming units (UFC/mL) in the dairy effluent, as verified by a spectrophotometer (OD600 = 0.08–0.13) [72].
3.3. Microwave and Ultrasound Treatment Procedure
The ultrasound treatment was performed by the sonication of 40 mL of inoculated DE in an ultrasonic bath (35 kHz) for times ranging between 5 and 60 min. The sample temperature was held at 30 ± 2 °C by gradually adding ice to the ultrasonic bath. Irradiation was conducted in a household microwave (Whirlpool Talent MT263, Malaysia) with a rotating plate (diameter 280 mm) at a speed of 2.46 rpm. The equipment emits a nominal power of 90, 160, 350, 500, 650, 750 and 850 W at 2450 MHz. Inoculated sterile wastewater dairy effluent samples of 40 mL were placed in the microwave cavity and irradiated at 350 and 650 W for different exposure times, ranging between 10 and 75 s. Once treatment was completed, the sample was quickly removed from the cavity and immediately cooled in an ice-water bath. The ultrasound effect as pre-or post-treatment on the microwave inactivation kinetics of E. faecalis ATCC 29212 was then investigated.
The combined treatments applied between microwave and ultrasound were: 10, 20 and 30 min for pre- or post-ultrasound treatment and 350 W or 650 W for microwave inactivation kinetics. The experimental procedure was the same as described previously.
3.4. Enumeration of Survival Cells
Aliquots (1 mL) of treated and untreated samples were serially diluted in sterile physiological water and spread-plated onto Muller–Hinton medium. The surviving bacterial count was determined after 24 h of incubation at 37 °C. All experiments were performed in triplicate.
3.5. Modeling Inactivation Kinetics and Statistical Methods
The survival rate at time t, denoted S, was calculated as the ratio change for viable E. faecalis ATCC 29212 at any time N(t) (CFU/mL) compared to the initial number of microorganisms N(0) (CFU/mL) as a control:
The diminution of the log-survival rate data for E. faecalis ATCC 29212 obtained in thermo-sonication and microwave treatments was described by the modified Weibull model [48]:
where is the time of the first decimal reduction and can be called the scale parameter, and β is the so-called shape parameter. In order to compare the time of the first decimal reduction for the obtained inactivation kinetics, the Equation (1) was fitted for the second time by setting β equal to the mean of the previously estimated value for β.
Modified Weibull model parameters were estimated using nonlinear regression with a curve fitting toolbox (MATLAB 6.5, The Math-Works Inc., Natick, MA, USA). The root mean squared error (RMSE) between all the experimental and predicted data, the adjusted coefficients of determination (R2), and confidence intervals (calculated with 95% probability) were used as goodness-of-fit indicators for the estimated parameters.
3.6. Protein Determination
The protein quantities were estimated according to the method of Bradford [73] with the Coomassie G250 brilliant blue reagent. Both micro and macro assay methods were used.
4. Conclusions
In conclusion, the study demonstrated the proof-of-principle for food wastewater safe treatment effluents using ultrasound waves (in pre-treatment) coupled with microwaves for decontamination, with useful efficiency over short treatment periods. The coupling effectiveness varies with the experimental parameters chosen, i.e., the ultrasound exposure time and the power of the microwave. The coupling of ultrasound and microwave treatments was proven to be a promising technological application, as the main indicator micro-organisms in dairy wastewater effluents were reduced and fully inactivated.
The time values specified for treatment, US 30 min/MW 650 W at 60 s, were sufficient to effectively eliminate heat-resistant bacteria in dairy effluents, while greater resistance to inactivation was presented to microwave only or ultrasound only processing. Therefore, this problem was solved either by extending the duration of the microwave treatment alone, according to the power level, or by combining ultrasound and microwave treatments and, more specifically, by pre-treating with ultrasound, which effectively eliminated the total bacterial load present in the dairy effluents.
Author Contributions
Conceptualization, O.-N.K. and A.B.; methodology, A.B.; data curation, O.-N.K.; funding acquisition, O.-N.K.; investigation, O.-N.K., Y.S., A.A. and K.B.; writing—original draft, O.-N.K.; writing—review and editing, O.-N.K., A.B., Y.S., K.B., A.A., K.K. and F.D.; formal analysis, O.-N.K.; validation, O.-N.K.; project administration, K.M.; supervision, K.M.; co-supervision, P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education, Algeria. The authors would also like to thank ALIES and the Natural Bioactives lab from CBIOS laboratory (Bio.Natural @CBIOS) of the University of Lisboa for fellowship grant. This project was financed by the Foundation for Science and Technology (FCT, Portugal) by grants UIDB/04567/2020 and UIDP/04567/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Algeria’s Ministry of Higher Education and Scientific Research, the University of Lisboa, and Natural bioactives lab from CBIOS and ALIES-COFAC for fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Ayeche, R. Treatment by coagulation-flocculation of dairy wastewater with the residual lime of National Algerian Industrial Gases Company (NIGC-Annaba). Energy procedia 2012, 18, 147–156. [Google Scholar] [CrossRef]
- Henderson, J.C. Wastewater Effluent Transport and Contamination: A Model for Groundwater Contamination in the Central West Bank; Drexel University: Philadelphia, PA, USA, 2019. [Google Scholar]
- Hu, H.; Li, X.; Wu, S.; Yang, C. Sustainable livestock wastewater treatment via phytoremediation: Current status and future perspectives. Bioresource Technology 2020, 315, 123809. [Google Scholar] [CrossRef]
- Chapman, P.M. Whole effluent toxicity testing—usefulness, level of protection, and risk assessment. Environ. Toxicol. Chem. Int. J. 2000, 19, 3–13. [Google Scholar] [CrossRef]
- Di, H.; Cameron, K.; Silva, R.; Russell, J.; Barnett, J. A lysimeter study of the fate of 15N-labelled nitrogen in cow urine with or without farm dairy effluent in a grazed dairy pasture soil under flood irrigation. New Zealand J. Agric. Res. 2002, 45, 235–244. [Google Scholar] [CrossRef]
- Ibekwe, A.; Grieve, C. Detection and quantification of Escherichia coli O157: H7 in environmental samples by real-time PCR. J. Appl. Microbiol. 2003, 94, 421–431. [Google Scholar] [CrossRef]
- Chapman, P.M. Determining when contamination is pollution—weight of evidence determinations for sediments and effluents. Environ. Int. 2007, 33, 492–501. [Google Scholar] [CrossRef]
- Dungan, R.S.; Leytem, A.B. The characterization of microorganisms in dairy wastewater storage ponds. J. Environ. Qual. 2013, 42, 1583–1588. [Google Scholar] [CrossRef][Green Version]
- Pinto, B.; Pierotti, R.; Canale, G.; Reali, D. Characterization of ‘faecal streptococci’ as indicators of faecal pollution and distribution in the environment. Lett. Appl. Microbiol. 1999, 29, 258–263. [Google Scholar] [CrossRef]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; Zhan, X.; Gardiner, G.E. Inactivation of enteric indicator bacteria and system stability during dry co-digestion of food waste and pig manure. Sci. Total Environ. 2018, 612, 293–302. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic treatment of dairy wastewaters: A review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Patel, S.K.; Das, D.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Lee, J.-K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative hydrogen production using various biomass-based materials as feedstock. Renew. Sustain. Energy Rev. 2018, 92, 284–306. [Google Scholar] [CrossRef]
- Patange, A.; Boehm, D.; Giltrap, M.; Lu, P.; Cullen, P.; Bourke, P. Assessment of the disinfection capacity and eco-toxicological impact of atmospheric cold plasma for treatment of food industry effluents. Sci. Total Environ. 2018, 631, 298–307. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Dai, Y.; Su, X.; Xiao, Y.; Wu, D.; Sun, F.; Mei, R.; Chen, J.; Lin, H. Effective partial denitrification of biological effluent of landfill leachate for Anammox process: Start-up, influencing factors and stable operation. Sci. Total Environ. 2022, 807, 150975. [Google Scholar] [CrossRef]
- Ghernaout, D. Disinfection via Electrocoagulation Process: Implied Mechanisms and Future Tendencies. Microbiology 2019, 15, 79–90. [Google Scholar]
- Kebbi, Y.; Muhammad, A.I.; Sant’Ana, A.S.; do Prado-Silva, L.; Liu, D.; Ding, T. Recent advances on the application of UV-LED technology for microbial inactivation: Progress and mechanism. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3501–3527. [Google Scholar] [CrossRef]
- Lazra, Y.; Dubrovin, I.; Multanen, V.; Bormashenko, E.; Bormashenko, Y.; Cahan, R. Effects of atmospheric plasma corona discharges on soil bacteria viability. Microorganisms 2020, 8, 704. [Google Scholar] [CrossRef]
- Tonuci, L.; Paschoalatto, C.; Pisani Jr, R. Microwave inactivation of Escherichia coli in healthcare waste. Waste Manag. 2008, 28, 840–848. [Google Scholar] [CrossRef]
- Vialkova, E.; Zemlyanova, M.; Danilov, O. Energy efficiency in municipal waste treatment. Proc. MATEC Web Conf. 2018, 170, 04020. [Google Scholar] [CrossRef][Green Version]
- Rao, B.; Su, X.; Lu, X.; Wan, Y.; Huang, G.; Zhang, Y.; Xu, P.; Qiu, S.; Zhang, J. Ultrahigh pressure filtration dewatering of municipal sludge based on microwave pretreatment. J. Environ. Manag. 2019, 247, 588–595. [Google Scholar] [CrossRef]
- Kuglarz, M.; Karakashev, D.; Angelidaki, I. Microwave and thermal pretreatment as methods for increasing the biogas potential of secondary sludge from municipal wastewater treatment plants. Bioresour. Technol. 2013, 134, 290–297. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.-L. Microwave irradiation: A sustainable way for sludge treatment and resource recovery. Renew. Sustain. Energy Rev. 2013, 18, 288–305. [Google Scholar] [CrossRef]
- Najdovski, L.; Dragaš, A.Z.; Kotnik, V. The killing activity of microwaves on some non-sporogenic and sporogenic medically important bacterial strains. J. Hosp. Infect. 1991, 19, 239–247. [Google Scholar] [CrossRef]
- Joyce, E.; Phull, S.; Lorimer, J.; Mason, T. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason. Sonochemistry 2003, 10, 315–318. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Vukušić, T.; Stulić, V.; Mrvčić, J.; Milošević, S.; Šimunek, M.; Herceg, Z. The effect of high power ultrasound and cold gas-phase plasma treatments on selected yeast in pure culture. Food Bioprocess Technol. 2015, 8, 791–800. [Google Scholar] [CrossRef]
- Drakopoulou, S.; Terzakis, S.; Fountoulakis, M.; Mantzavinos, D.; Manios, T. Ultrasound-induced inactivation of gram-negative and gram-positive bacteria in secondary treated municipal wastewater. Ultrason. Sonochemistry 2009, 16, 629–634. [Google Scholar] [CrossRef]
- Amabilis-Sosa, L.E.; Vázquez-López, M.; Rojas, J.L.G.; Roé-Sosa, A.; Moeller-Chávez, G.E. Efficient bacteria inactivation by ultrasound in municipal wastewater. Environments 2018, 5, 47. [Google Scholar] [CrossRef]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Rostami, S.; Behruzian, M.; Samani, B.H.; Lorigooini, Z.; Hosseinabadi, T.; Zareiforoush, H.; Behruzian, A. Study of Combined Ultrasound-microwave Effect on Chemical Compositions and E. coli Count of Rose Aromatic Water. Iran. J. Pharm. Res. IJPR 2018, 17, 146. [Google Scholar]
- García-Granja, P.E.; López, J.; Vilacosta, I.; Ortiz-Bautista, C.; Sevilla, T.; Olmos, C.; Sarriá, C.; Ferrera, C.; Gómez, I.; San Román, J.A. Polymicrobial infective endocarditis: Clinical features and prognosis. Medicine 2015, 94. [Google Scholar] [CrossRef]
- Mulak, V.; BECEL, P.; TAILLIEZ, R. Bactériologie des produits de la mer: Caractérisation des flores bactériennes après traitement thermique. Sci. Des Aliment. 1992, 12, 415–428. [Google Scholar]
- Obioma, A. Impact of low frequency ultrasound on pathogens in polluted potable water. Sch. J. App. Med. Sci. 2015, 3, 1978–1984. [Google Scholar]
- Doosti, M.; Kargar, R.; Sayadi, M. Water treatment using ultrasonic assistance: A review. Proc. Int. Acad. Ecol. Environ. Sci. 2012, 2, 96. [Google Scholar]
- Mason, T.; Joyce, E.; Phull, S.; Lorimer, J. Potential uses of ultrasound in the biological decontamination of water. Ultrason. Sonochemistry 2003, 10, 319–323. [Google Scholar] [CrossRef]
- Mahvi, A.; Maleki, A.; Rezaee, R.; Safari, M. Reduction of humic substances in water by application of ultrasound waves and ultraviolet irradiation. J. Environ. Health Sci. Eng. 2009, 6, 233–240. [Google Scholar]
- Broekman, S.; Pohlmann, O.; Beardwood, E.; de Meulenaer, E.C. Ultrasonic treatment for microbiological control of water systems. Ultrason. Sonochemistry 2010, 17, 1041–1048. [Google Scholar] [CrossRef]
- Butz, P.; Tauscher, B. Emerging technologies: Chemical aspects. Food Res. Int. 2002, 35, 279–284. [Google Scholar] [CrossRef]
- Gholami, M.; Mirzaei, R.; Mohammadi, R.; Zarghampour, Z.; Afshari, A. Destruction of Escherichia coli and Enterococcus faecalis using low frequency ultrasound technology: A response surface methodology. Health Scope 2014, 3, 1–9. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Nguyen, T.; Roddick, F.A. Impact of sonication at 20 kHz on Microcystis aeruginosa, Anabaena circinalis and Chlorella sp. Water Res. 2012, 46, 1473–1481. [Google Scholar] [CrossRef]
- Declerck, P. Biofilms: The environmental playground of Legionella pneumophila. Environ. Microbiol. 2010, 12, 557–566. [Google Scholar] [CrossRef]
- Kalantar, E.; Maleki, A.; Khosravi, M.; Mahmodi, S. Evaluation of ultrasoundwaves effect on antibiotic resistance pseudomonas aeruginosa and staphylococcus aureus isolated from hospital and their comparison with standard species. Iran. J. Health Environ. 2010, 3, 319–326. [Google Scholar]
- Kwak, T.Y.; Kim, N.H.; Rhee, M.S. Response surface methodology-based optimization of decontamination conditions for Escherichia coli O157: H7 and Salmonella Typhimurium on fresh-cut celery using thermoultrasound and calcium propionate. Int. J. Food Microbiol. 2011, 150, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zhou, B.; Liang, W.; Feng, H.; Martin, S.E. Inactivation of Escherichia coli cells with sonication, manosonication, thermosonication, and manothermosonication: Microbial responses and kinetics modeling. J. Food Eng. 2009, 93, 354–364. [Google Scholar] [CrossRef]
- Gómez-López, M.; Bayo, J.; García-Cascales, M.; Angosto, J. Decision support in disinfection technologies for treated wastewater reuse. J. Clean. Prod. 2009, 17, 1504–1511. [Google Scholar] [CrossRef]
- Toor, R.; Mohseni, M. UV-H2O2 based AOP and its integration with biological activated carbon treatment for DBP reduction in drinking water. Chemosphere 2007, 66, 2087–2095. [Google Scholar] [CrossRef]
- Ortuño, C.; Duong, T.; Balaban, M.; Benedito, J. Combined high hydrostatic pressure and carbon dioxide inactivation of pectin methylesterase, polyphenol oxidase and peroxidase in feijoa puree. J. Supercrit. Fluids 2013, 82, 56–62. [Google Scholar] [CrossRef]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguérinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef]
- Peleg, M.; Cole, M.B. Reinterpretation of microbial survival curves. Crit. Rev. Food Sci. Nutr. 1998, 38, 353–380. [Google Scholar] [CrossRef]
- Benjamin, E.; Reznik, A.; Williams, A. Mathematical models for conventional and microwave thermal deactivation of Enterococcus faecalis, Staphylococcus aureus and Escherichia coli. Cell. Mol. Biol. 2007, 53, 42–48. [Google Scholar] [CrossRef]
- Dehghani, M.H. Effectiveness of ultrasound on the destruction of E. coli. Am. J. Environ. Sci. 2005, 1, 187–189. [Google Scholar] [CrossRef][Green Version]
- Naddeo, V.; Meriç, S.; Kassinos, D.; Belgiorno, V.; Guida, M. Fate of pharmaceuticals in contaminated urban wastewater effluent under ultrasonic irradiation. Water Res. 2009, 43, 4019–4027. [Google Scholar] [CrossRef] [PubMed]
- Blume, T.; Neis, U. Improved wastewater disinfection by ultrasonic pre-treatment. Ultrason. Sonochemistry 2004, 11, 333–336. [Google Scholar] [CrossRef]
- Chen, X.; Tang, R.; Wang, Y.; Yuan, S.; Wang, W.; Ali, I.M.; Hu, Z.-H. Effect of ultrasonic and ozone pretreatment on the fate of enteric indicator bacteria and antibiotic resistance genes, and anaerobic digestion of dairy wastewater. Bioresour. Technol. 2021, 320, 124356. [Google Scholar] [CrossRef]
- Mawioo, P.M.; Rweyemamu, A.; Garcia, H.A.; Hooijmans, C.M.; Brdjanovic, D. Evaluation of a microwave based reactor for the treatment of blackwater sludge. Sci. Total Environ. 2016, 548, 72–81. [Google Scholar] [CrossRef]
- Pino-Jelcic, S.A.; Hong, S.M.; Park, J.K. Enhanced anaerobic biodegradability and inactivation of fecal coliforms and Salmonella spp. in wastewater sludge by using microwaves. Water Environ. Res. 2006, 78, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Vialkova, E.; Obukhova, M.; Belova, L. Microwave irradiation in technologies of wastewater and wastewater sludge treatment: A review. Water 2021, 13, 1784. [Google Scholar] [CrossRef]
- Ara, E.; Sartaj, M.; Kennedy, K. Effect of microwave pre-treatment of thickened waste activated sludge on biogas production from co-digestion of organic fraction of municipal solid waste, thickened waste activated sludge and municipal sludge. Waste Manag. Res. 2014, 32, 1200–1209. [Google Scholar] [CrossRef]
- Hong, S.M.; Park, J.K.; Teeradej, N.; Lee, Y.; Cho, Y.; Park, C. Pretreatment of sludge with microwaves for pathogen destruction and improved anaerobic digestion performance. Water Environ. Res. 2006, 78, 76–83. [Google Scholar] [CrossRef]
- Hollywood, N.; Varabioff, Y.; Mitchell, G. The effect of microwave and conventional cooking on the temperature profiles and microbial flora of minced beef. Int. J. Food Microbiol. 1991, 14, 67–75. [Google Scholar] [CrossRef]
- Benlloch-Tinoco, M.; Martínez-Navarrete, N.; Rodrigo, D. Impact of temperature on lethality of kiwifruit puree pasteurization by thermal and microwave processing. Food control 2014, 35, 22–25. [Google Scholar] [CrossRef]
- Valero, A.; Cejudo, M.; García-Gimeno, R. Inactivation kinetics for Salmonella Enteritidis in potato omelet using microwave heating treatments. Food Control 2014, 43, 175–182. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Liao, X.; Hu, X. Effects of microwave and ultrasonic wave treatment on inactivation of Alicyclobacillus. Int. J. Food Sci. 2010, 45, 459–465. [Google Scholar] [CrossRef]
- Kim, H.-S.; Chang, S.W.; Baek, S.-H.; Han, S.H.; Lee, Y.; Zhu, Q.; Kum, K.-Y. Antimicrobial effect of alexidine and chlorhexidine against Enterococcus faecalis infection. Int. J. Oral Sci. 2013, 5, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Cleary, S.F.; Cao, G.; Liu, L.M.; Egle, P.M.; Shelton, K.R. Stress proteins are not induced in mammalian cells exposed to radiofrequency or microwave radiation. Bioelectromagnetics 1997, 18, 499–505. [Google Scholar] [CrossRef]
- Laurence, J.A.; French, P.W.; Lindner, R.A.; Mckenzie, D.R. Biological effects of electromagnetic fields—Mechanisms for the effects of pulsed microwave radiation on protein conformation. J. Theor. Biol. 2000, 206, 291–298. [Google Scholar] [CrossRef]
- Woo, I.-S.; Rhee, I.-K.; Park, H.-D. Differential damage in bacterial cells by microwave radiation on the basis of cell wall structure. Appl. Environ. Microbiol. 2000, 66, 2243–2247. [Google Scholar] [CrossRef]
- Peuker. Ullmann’s Encyclopedia of Industrial Chemistry. Sonochemistry 2006, 17, 363–376. [Google Scholar]
- Daverey, A.; Pakshirajan, K. Pretreatment of synthetic dairy wastewater using the sophorolipid-producing yeast Candida bombicola. Appl. Biochem. Biotechnol. 2011, 163, 720–728. [Google Scholar] [CrossRef]
- Muñoz-Cuevas, M.; Guevara, L.; Aznar, A.; Martínez, A.; Periago, P.M.; Fernández, P.S. Characterisation of the resistance and the growth variability of Listeria monocytogenes after high hydrostatic pressure treatments. Food Control 2013, 29, 409–415. [Google Scholar] [CrossRef]
- Cabassi, C.S.; Falanga, G.; Romani, A. Disinfectant and antimicrobial compositions, in particular for the veterinary field. U.S. Patent Application 15/318,570, 11 May 2017. [Google Scholar]
- Pourhajibagher, M.; Chiniforush, N.; Shahabi, S.; Ghorbanzadeh, R.; Bahador, A. Sub-lethal doses of photodynamic therapy affect biofilm formation ability and metabolic activity of Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 2016, 15, 159–166. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).