Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients
Abstract
1. Introduction
2. Phenolic Compounds
2.1. Antioxidant Activity
2.2. Antimicrobial Activity
3. Phenolic Compounds in Food Packaging
3.1. Flavonoids and Tannins
3.2. Phenolic Acids—Hydroxybenzoic and Hydroxycinnamic Acids
3.3. Lignans
3.4. Stillbenes
3.5. Lignins
3.6. Coumarins
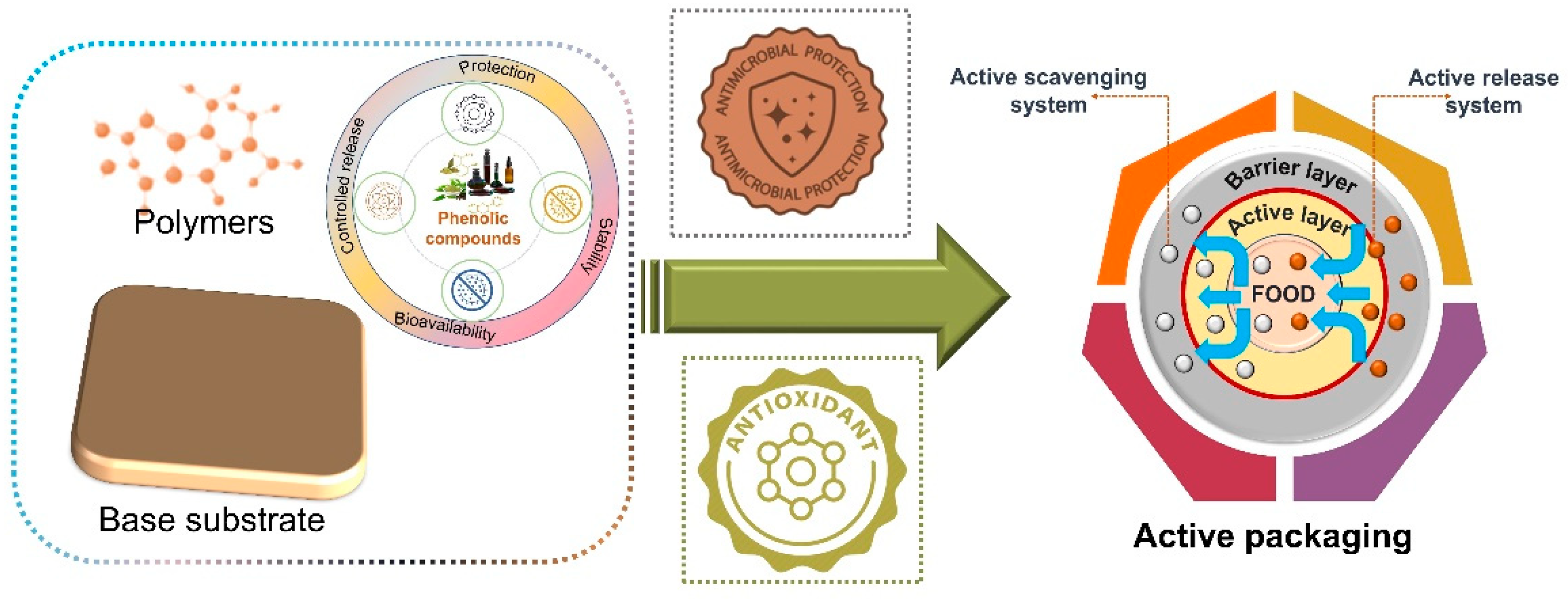
4. Application of Phenolic Compounds in Packaging Films/Coatings
4.1. Active Packaging
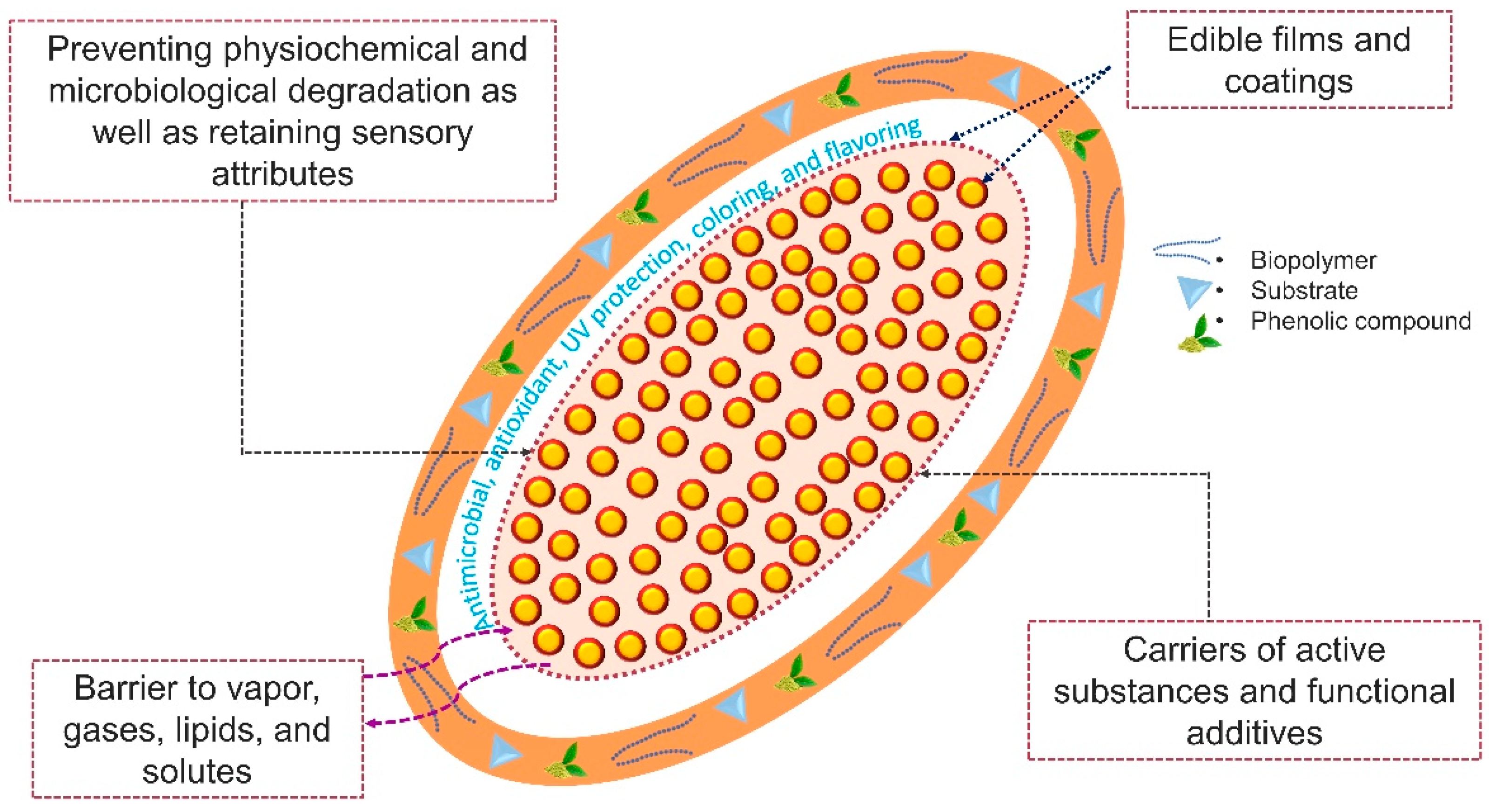
4.2. Edible Films and Coatings
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Singh, A.K.; Ramakanth, D.; Kumar, A.; Lee, Y.S.; Gaikwad, K.K. Active packaging technologies for clean label food products: A review. J. Food Meas. Charact. 2021, 15, 4314–4324. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EU Guidance to the Commission Regulation (EC) No 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come into the Contact with Food (Version 1.0); Publications Office of the European Union: Luxembourg, 2009. [Google Scholar]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Wicochea-Rodríguez, J.D.; Chalier, P.; Ruiz, T.; Gastaldi, E. Active food packaging based on biopolymers and aroma compounds: How to design and control the release. Front. Chem. 2019, 7, 398. [Google Scholar] [CrossRef]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Fernando, A.L.; Silva, A.S. Active edible packaging. Encyclopedia 2021, 1, 30. [Google Scholar] [CrossRef]
- Salgado, P.R.; Ortiz, C.M.; Musso, Y.S.; Di Giorgio, L.; Mauri, A.N. Edible films and coatings containing bioactives. Curr. Opin. Food Sci. 2015, 5, 86–92. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and incorporation of functional ingredients in edible films and coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2021, 20, 875–900. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.; Verma, A.K.; Barba, F.J.; Singh, V.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Brand, W.; van Kesteren, P.C.; Swart, E.; Oomen, A.G. Overview of potential adverse health effects of oral exposure to nanocellulose. Nanotoxicology 2022, 16, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, F.K.; Daliri, E.B.-M.; Elahi, F.; Chelliah, R.; Lee, B.-H.; Oh, D.-H. New insights on the use of polyphenols as natural preservatives and their emerging safety concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-derived natural antioxidants in meat and meat products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- Mark, R.; Lyu, X.; Lee, J.J.; Parra-Saldívar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Faustino, H.; Gil, N.; Baptista, C.; Duarte, A.P. Antioxidant activity of lignin phenolic compounds extracted from kraft and sulphite black liquors. Molecules 2010, 15, 9308–9322. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, C.; Dole, P.; Cathala, B.; Avérous, L.; Boquillon, N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Saltmarsh, M. Food Additives and Why They Are Used. In Saltmarsh’s Essential Guide to Food Additives, 5th ed.; The Royal Society of Chemistry: London, UK, 2020; pp. 1–9. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Karama, M.; Hadjichralambous, C.; Sechi, P.; Grispoldi, L. Is EU regulation on the use of antioxidants in meat preparation and in meat products still cutting edge? Eur. Food Res. Technol. 2020, 246, 661–668. [Google Scholar] [CrossRef]
- Barouh, N.; Bourlieu-Lacanal, C.; Figueroa-Espinoza, M.C.; Durand, E.; Villeneuve, P. Tocopherols as antioxidants in lipid-based systems: The combination of chemical and physicochemical interactions determines their efficiency. Compr. Rev. Food Sci. Food Saf. 2022, 21, 642–688. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Núñez, M.A.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovačević, D.B.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Wu, X.; Liu, W.; Jing, X.; Liu, Y.; Yan, J.; Liu, S.; Qin, W. Combination of calcium lactate impregnation with UV-C irradiation maintains quality and improves antioxidant capacity of fresh-cut kiwifruit slices. Food Chem. X 2022, 14, 100329. [Google Scholar] [CrossRef]
- Vargas-Torrico, M.F.; von Borries-Medrano, E.; Aguilar-Méndez, M.A. Development of gelatin/carboxymethylcellulose active films containing Hass avocado peel extract and their application as a packaging for the preservation of berries. Int. J. Biol. Macromol. 2022, 206, 1012–1025. [Google Scholar] [CrossRef]
- de Gonzalez, M.N.; Hafley, B.; Boleman, R.; Miller, R.; Rhee, K.; Keeton, J. Antioxidant properties of plum concentrates and powder in precooked roast beef to reduce lipid oxidation. Meat Sci. 2008, 80, 997–1004. [Google Scholar] [CrossRef]
- Sergio, L.; Spremulli, L.; Gatto, M.; Pieralice, M.; Linsalata, V.; La Sala, G.; Di Venere, D. Postharvest performance of intermediate moisture peaches and prunes as affected by packaging and storage conditions. Acta Hortic. 2015, 1071, 739–746. [Google Scholar] [CrossRef]
- Amin, Q.; Hafiza, A.; Wani, T. Antioxidant studies of pre-treated packaged prunes prepared from plum (Prunus domestica) cv. STANLEY. Asian J. Hortic. 2013, 8, 250–255. [Google Scholar]
- Licciardello, F.; Wittenauer, J.; Saengerlaub, S.; Reinelt, M.; Stramm, C. Rapid assessment of the effectiveness of antioxidant active packaging—Study with grape pomace and olive leaf extracts. Food Packag. Shelf Life 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; Velasco, J.I.; de Oliveira, R.A. Active edible films based on arrowroot starch with microparticles of blackberry pulp obtained by freeze-drying for food packaging. Polymers 2019, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J. Active and intelligent films made from starchy sources/blackberry pulp. J. Polym. Environ. 2018, 26, 2374–2391. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Guazi, J.S.; Conti-Silva, A.C.; Nicoletti, V.R. Active packaging for postharvest storage of cherry tomatoes: Different strategies for application of microencapsulated essential oil. Food Packag. Shelf Life 2021, 29, 100723. [Google Scholar] [CrossRef]
- Contini, C.; Álvarez, R.; O’sullivan, M.; Dowling, D.P.; Gargan, S.Ó.; Monahan, F.J. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Dowling, D.P.; Monahan, F.J. Development of active packaging containing natural antioxidants. Procedia Food Sci. 2011, 1, 224–228. [Google Scholar] [CrossRef][Green Version]
- Contini, C.; Valzacchi, S.; O’Sullivan, M.; Simoneau, C.; Dowling, D.P.; Monahan, F.J. Overall migration and kinetics of release of antioxidant compounds from citrus extract-based active packaging. J. Agric. Food Chem. 2013, 61, 12155–12163. [Google Scholar] [CrossRef]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.; Kumar, A. Essential oils as natural food antimicrobial agents: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef]
- Irkin, R.; Esmer, O.K. Novel food packaging systems with natural antimicrobial agents. J. Food Sci. Technol. 2015, 52, 6095–6111. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ullah, F.; Majeed, I.; Ayaz, A.; Hussain Munis, M.F. Organometallic assembling of chitosan-Iron oxide nanoparticles with their antifungal evaluation against Rhizopus oryzae. Appl. Organomet. Chem. 2019, 33, e5190. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ayaz, A.; Habib, S.; Bahadur, S.; Hussain, S.; Muhammad, S.; Ullah, F. Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal. Agric. Biotechnol. 2020, 28, 101729. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Shahabi, N.; Ghorbani, M. Development of Antimicrobial Active Food Packaging Film Based on Gelatin/Dialdehyde Quince Seed Gum Incorporated with Apple Peel Polyphenols. Food Bioprocess Technol. 2022, 15, 693–705. [Google Scholar] [CrossRef]
- Mauro, M.; Pinto, P.; Settanni, L.; Puccio, V.; Vazzana, M.; Hornsby, B.L.; Fabbrizio, A.; Di Stefano, V.; Barone, G.; Arizza, V. Chitosan Film Functionalized with Grape Seed Oil—Preliminary Evaluation of Antimicrobial Activity. Sustainability 2022, 14, 5410. [Google Scholar] [CrossRef]
- Aydogdu, A.; Radke, C.J.; Bezci, S.; Kirtil, E. Characterization of curcumin incorporated guar gum/orange oil antimicrobial emulsion films. Int. J. Biol. Macromol. 2020, 148, 110–120. [Google Scholar] [CrossRef]
- Thivya, P.; Bhosale, Y.; Anandakumar, S.; Hema, V.; Sinija, V. Study on the characteristics of gluten/alginate-cellulose/onion waste extracts composite film and its food packaging application. Food Chem. 2022, 390, 133221. [Google Scholar] [CrossRef]
- Das, S.K.; Vishakha, K.; Das, S.; Chakraborty, D.; Ganguli, A. Carboxymethyl cellulose and cardamom oil in a nanoemulsion edible coating inhibit the growth of foodborne pathogens and extend the shelf life of tomatoes. Biocatal. Agric. Biotechnol. 2022, 42, 102369. [Google Scholar] [CrossRef]
- Hosseini, M.; Razavi, S.; Mousavi, M. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2009, 33, 727–743. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef]
- Taherimehr, M.; YousefniaPasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5344. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Júnior, M.R.M.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Huang, X.; Ji, Y.; Guo, L.; Xu, Q.; Jin, L.; Fu, Y.; Wang, Y. Incorporating tannin onto regenerated cellulose film towards sustainable active packaging. Ind. Crops Prod. 2022, 180, 114710. [Google Scholar] [CrossRef]
- Cano, A.; Contreras, C.; Chiralt, A.; González-Martínez, C. Using tannins as active compounds to develop antioxidant and antimicrobial chitosan and cellulose based films. Carbohydr. Polym. Technol. Appl. 2021, 2, 100156. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of gallic acid loaded Hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef]
- Landete, J. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Cao, Y.; Warner, R.D.; Fang, Z. Effect of chitosan/nisin/gallic acid coating on preservation of pork loin in high oxygen modified atmosphere packaging. Food Control 2019, 101, 9–16. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, D.; Warner, R.D.; Ha, M. Effect of gallic acid/chitosan coating on fresh pork quality in modified atmosphere packaging. Food Chem. 2018, 260, 90–96. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.; Coelho, J.; Domingues, M.R.; Daina, S.; Sadocco, P.; Santos, S.A.; Freire, C.S. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120–128. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H.; Su, W.-W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Benbettaieb, N.; Nyagaya, J.; Seuvre, A.-M.; Debeaufort, F.D.R. Antioxidant activity and release kinetics of caffeic and p-coumaric acids from hydrocolloid-based active films for healthy packaged food. J. Agric. Food Chem. 2018, 66, 6906–6916. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Silva, F.; Figueiras, A.; Gallardo, E.; Nerín, C.; Domingues, F.C. Strategies to improve the solubility and stability of stilbene antioxidants: A comparative study between cyclodextrins and bile acids. Food Chem. 2014, 145, 115–125. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Antioxidant polyethylene films based on a resveratrol containing clay of interest in food packaging applications. Food Packag. Shelf Life 2015, 6, 30–41. [Google Scholar] [CrossRef]
- Silva, Â.; Duarte, A.; Sousa, S.; Ramos, A.; Domingues, F.C. Characterization and antimicrobial activity of cellulose derivatives films incorporated with a resveratrol inclusion complex. LWT 2016, 73, 481–489. [Google Scholar] [CrossRef]
- Aminzare, M.; Moniri, R.; Hassanzad Azar, H.; Mehrasbi, M.R. Evaluation of antioxidant and antibacterial interactions between resveratrol and eugenol in carboxymethyl cellulose biodegradable film. Food Sci. Nutr. 2022, 10, 155–168. [Google Scholar] [CrossRef]
- Zadeh, E.M.; O’Keefe, S.F.; Kim, Y.-T. Utilization of lignin in biopolymeric packaging films. ACS Omega 2018, 3, 7388–7398. [Google Scholar] [CrossRef]
- Vinardell, M.; Ugartondo, V.; Mitjans, M. Potential applications of antioxidant lignins from different sources. Ind. Crops Prod. 2008, 27, 220–223. [Google Scholar] [CrossRef]
- Beaucamp, A.; Wang, Y.; Culebras, M.; Collins, M.N. Carbon fibres from renewable resources: The role of the lignin molecular structure in its blendability with biobased poly (ethylene terephthalate). Green Chem. 2019, 21, 5063–5072. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.; Kenny, J.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly (lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef]
- Abraham, K.; Wöhrlin, F.; Lindtner, O.; Heinemeyer, G.; Lampen, A. Toxicology and risk assessment of coumarin: Focus on human data. Mol. Nutr. Food Res. 2010, 54, 228–239. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Chambin, O.; Assifaoui, A.; Al-Assaf, S.; Karbowiak, T.; Debeaufort, F. Release of coumarin incorporated into chitosan-gelatin irradiated films. Food Hydrocoll. 2016, 56, 266–276. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 2011, 44, 550–556. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Bahmid, N.A.; Taha, A.; Khalifa, I.; Khan, S.; Rostamabadi, H.; Jafari, S.M. Recent advances in food applications of phenolic-loaded micro/nanodelivery systems. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Kuai, L.; Liu, F.; Chiou, B.-S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Junior, C.A.C. Green strategies for active food packagings: A systematic review on active properties of graphene-based nanomaterials and biodegradable polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Jokar, M.; Nafchi, A.M. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J. Food Sci. Technol. 2016, 53, 1608–1619. [Google Scholar] [CrossRef]
- Kõrge, K.; Bajić, M.; Likozar, B.; Novak, U. Active chitosan–chestnut extract films used for packaging and storage of fresh pasta. Int. J. Food Sci. Technol. 2020, 55, 3043–3052. [Google Scholar] [CrossRef]
- Shukor, U.; Nordin, N.; Tawakkal, I.; Talib, R.; Othman, S. Utilization of jackfruit peel waste for the production of biodegradable and active antimicrobial packaging films. In Biopolymers and Biocomposites from Agro-Waste for Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 171–192. [Google Scholar] [CrossRef]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant starch-based films with encapsulated eugenol. Application to sunflower oil preservation. LWT 2019, 113, 108290. [Google Scholar] [CrossRef]
- Sanches, M.A.R.; Camelo-Silva, C.; da Silva Carvalho, C.; de Mello, J.R.; Barroso, N.G.; da Silva Barros, E.L.; Silva, P.P.; Pertuzatti, P.B. Active packaging with starch, red cabbage extract and sweet whey: Characterization and application in meat. LWT 2021, 135, 110275. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A.M. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Hosseini, H.; Hamgini, E.Y.; Jafari, S.M.; Bolourian, S. Improving the oxidative stability of sunflower seed kernels by edible biopolymeric coatings loaded with rosemary extract. J. Stored Prod. Res. 2020, 89, 101729. [Google Scholar] [CrossRef]
- Kumar, N.; Ojha, A.; Upadhyay, A.; Singh, R.; Kumar, S. Effect of active chitosan-pullulan composite edible coating enrich with pomegranate peel extract on the storage quality of green bell pepper. LWT 2021, 138, 110435. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of edible pectin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3, 4-dihydroxyphenylglycol on beef meat during refrigerated storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Panda, P.K.; Sadeghi, K.; Lee, S.; Chung, C.; Park, Y.; Park, J.; Seo, J. Facile thermal and hydrolytic conversion of tannic acid: Enhancement of antimicrobial activity and biocompatibility for biomedical applications. Mater. Chem. Phys. 2022, 285, 126141. [Google Scholar] [CrossRef]
- Li, P.; Sirviö, J.A.; Haapala, A.; Khakalo, A.; Liimatainen, H. Anti-oxidative and UV-absorbing biohybrid film of cellulose nanofibrils and tannin extract. Food Hydrocoll. 2019, 92, 208–217. [Google Scholar] [CrossRef]
- Olejar, K.J.; Ray, S.; Ricci, A.; Kilmartin, P.A. Superior antioxidant polymer films created through the incorporation of grape tannins in ethyl cellulose. Cellulose 2014, 21, 4545–4556. [Google Scholar] [CrossRef]
- Missio, A.L.; Mattos, B.D.; Ferreira, D.D.F.; Magalhães, W.L.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; De Leon-Zapata, M.A.; Alvarez-Perez, O.B.; Torres-León, C.; Nieto-Oropeza, D.E.; Ventura-Sobrevilla, J.M.; Aguilar, M.A.; Ruelas-Chacón, X.; Rojas, R.; Ramos-Aguiñaga, M.E. Basic and applied concepts of edible packaging for foods. In Food Packaging and Preservation; Academic Press: Cambridge, MA, USA, 2018; pp. 1–61. [Google Scholar] [CrossRef]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovations in Food Packaging; Academic Press: Cambridge, UK, 2005; pp. 403–433. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part A 2014, 31, 374–395. [Google Scholar] [CrossRef]
- Farhoosh, R.; Golmovahhed, G.A.; Khodaparast, M.H. Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.). Food Chem. 2007, 100, 231–236. [Google Scholar] [CrossRef]
- Perumalla, A.; Hettiarachchy, N.S. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Duguma, H.T. Potential applications and limitations of edible coatings for maintaining tomato quality and shelf life. Int. J. Food Sci. Technol. 2022, 57, 1353–1366. [Google Scholar] [CrossRef]
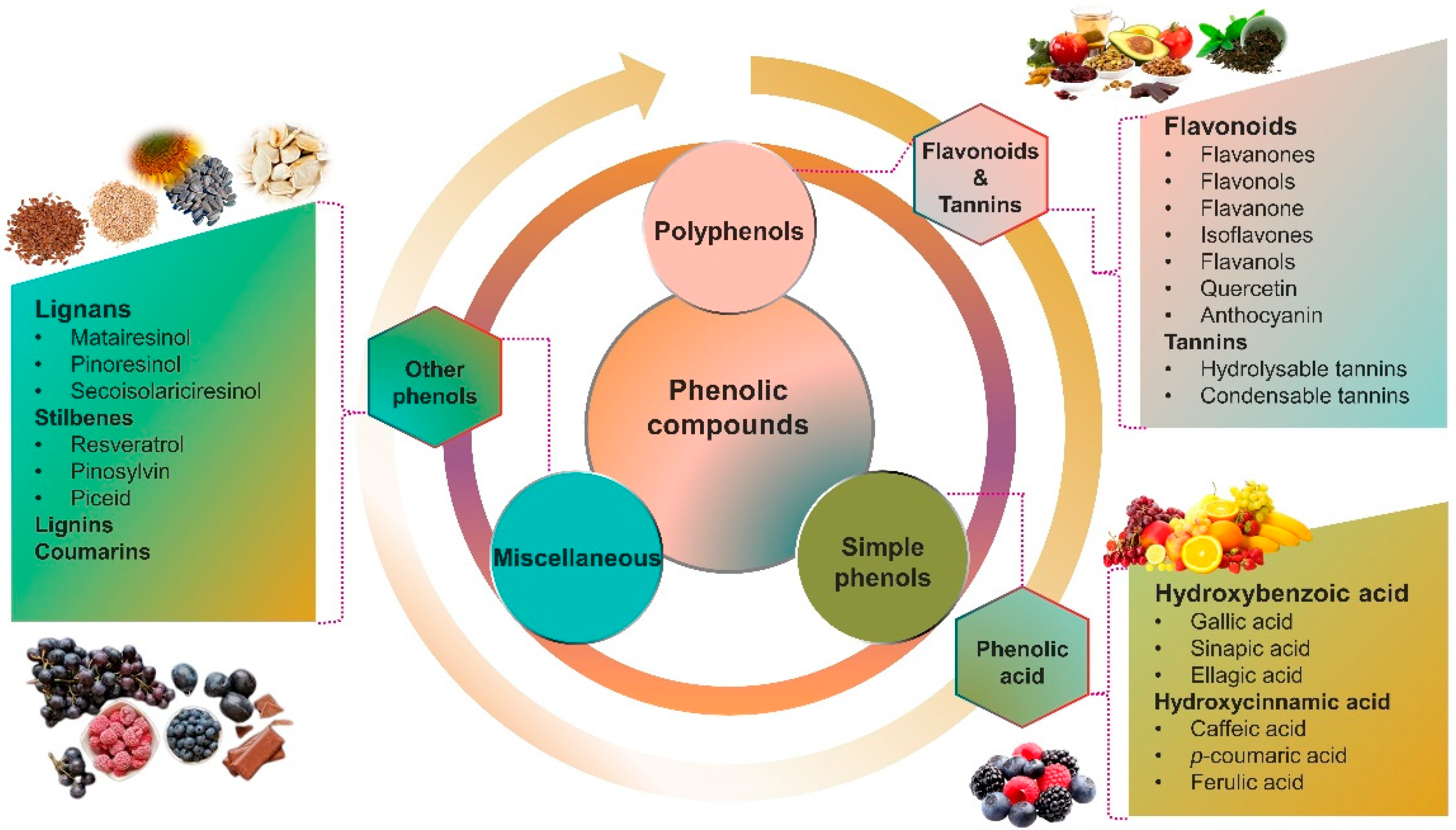
| Phenolic Compounds | Basic Skeleton | Main Sources | Characteristics | References |
|---|---|---|---|---|
| Flavonoids (flavanones, flavonols, flavanone, isoflavones, flavanols, quercetin, anthocyanin) | C6–C3–C6 | Wide range of sources (berries, herbs, cacao, grapes, green and black tea, citrus fruits, spinach, soybeans, olives, cherries, and red wine) | Antioxidant, antimicrobial, anti-infective, and antifungal activities | [3,18] |
| Tannins (hydrolysable tannins, condensable tannins) | (C6–C1)n | Tea, coffee, chocolate, berries, apples, and wine | Antimicrobial, antifungal, and antioxidant capabilities in addition to UV absorption | [19] |
| Phenolic acids (hydroxybenzoic acid (gallic acid, sinapic acid, ellagic acid) and hydroxycinnamic acid (caffeic acid, p-coumaric acid, ferulic acid) | C6–C1 and C6–C3 | Berries, persimmon, apple juice, grapes, mustard, oranges, rye, coffee, mushrooms, propolis, tea, and wine | Antioxidant, antimicrobial, and anti-infection activities | [20] |
| Lignans (matairesinol, pinoresinol, secoisolariciresinol) | C6–C3 | Oilseeds such as flaxseed, sesame, legumes, whole grains, and berries | Antioxidants and antimicrobial properties | [21] |
| Stillbenes (resveratrol, pinosylvin, piceid) | C6–C2–C6 | Grapes, pine, almond, peanuts, sorghum, berries, and wine | Antioxidant, and anti-infective, activities | [18,20] |
| Lignins | (C3–C6)n | Spruce, jute, cotton, hemp, pine, and birch | Antioxidants and antimicrobial properties | [22,23] |
| Coumarins | C6–C3 | Cinnamon, cloves, tonka bean, celery, and apricots | Antioxidants and antimicrobial properties | [20] |
| Packaging Type | Polymers | Phenolic Compounds | Structure | Sources | Characteristics | Applications | Reference |
|---|---|---|---|---|---|---|---|
| Active film | Chitosan | Catechin | 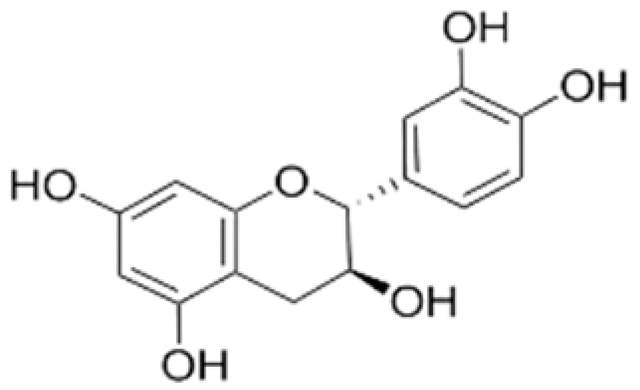 | Kombucha tea | Retardation of lipid oxidation inhibited microbial growth of E. coli and S. aureus, enhanced the antioxidant activity, and increased the protective effect of the film against ultraviolet. | Minced beef | [87] |
| Active film | Polyvinyl alcohol | Gallic acid |  | Apple pomace | Exhibited antioxidant capacity in delaying lipid oxidation. | Soybean oil | [88] |
| Active film | Chitosan | Tannin | 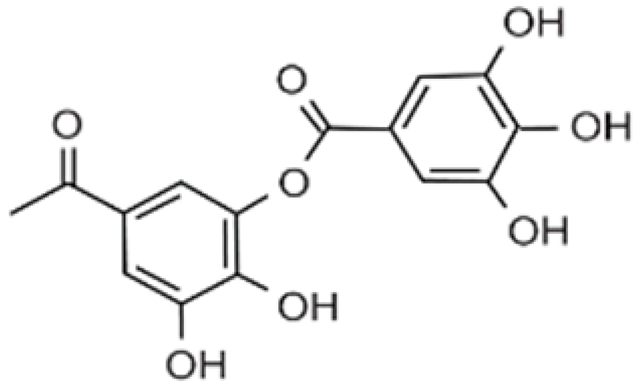 | Chestnut extract | Prevented microbial growth on the surface of pasta for 60-day storage. | Fresh pasta | [89] |
| Active film | Tapioca starch | Thymol | 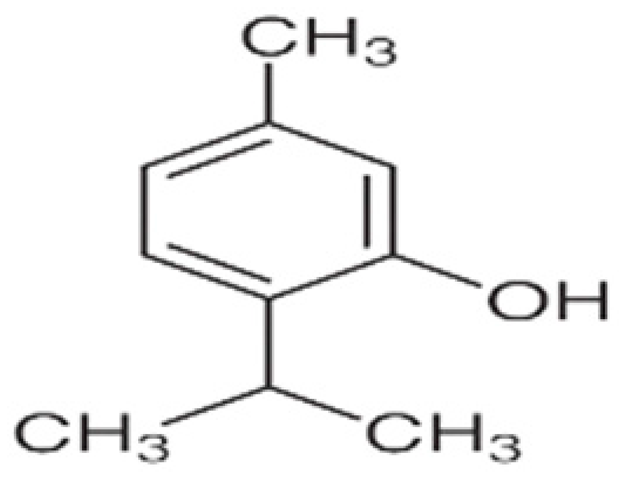 | Jackfruit peel | Inhibitory effect against E. coli and S. aureus, improvement in water vapor permeability, flexibility, and reduction in water solubility. | Cherry tomato | [90] |
| Active film | Corn starch | Eugenol | 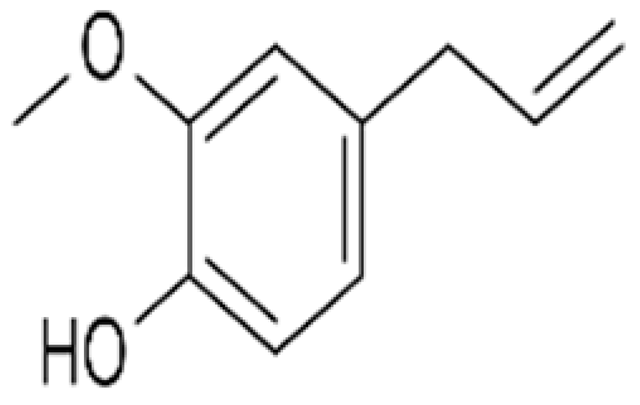 | Clove and cinnamon oil | Effective in preventing sunflower oil oxidation in accelerated storage conditions. | Sunflower oil | [91] |
| Active film | Corn starch | Anthocyanin | 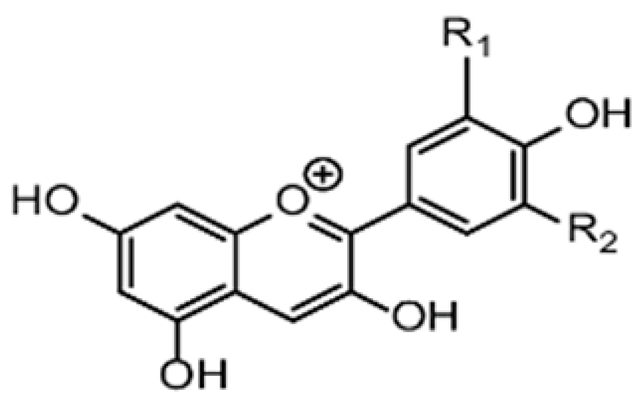 | Red cabbage | Increased antioxidant activity with improved water vapor permeability and mechanical strength. | Ground beef | [92] |
| Edible coating | Chitosan | Catechin, Epicatechin gallate | 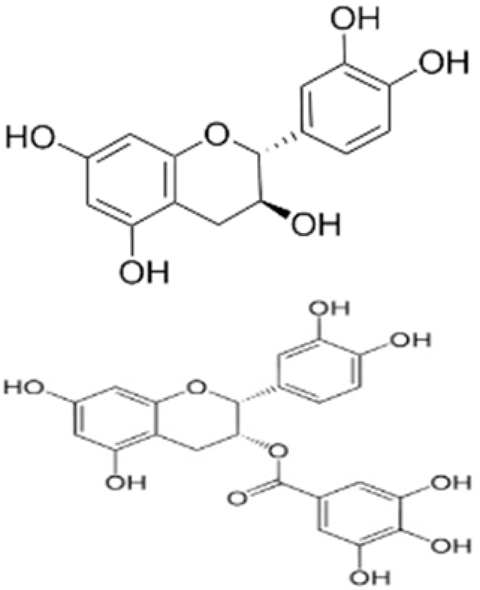 | Green tea extract | Effective in lowering oxidation activity, fungal development, and preserving the sensory qualities. | Walnut kernels | [93] |
| Edible coating | Carboxymethyl cellulose and Whey protein concentrate | Gallic acid | 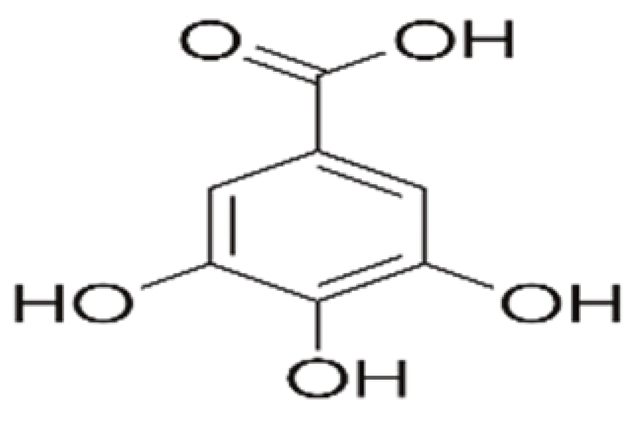 | Rosemary extract | Desirable color and oxidative stability with formulated coating. | Sunflower seeds | [94] |
| Edible active film | Pectin-gelatin blending | Hydroxytyrosol (HT), 3,4-dihydroxyphenylglycol (DHPG) | 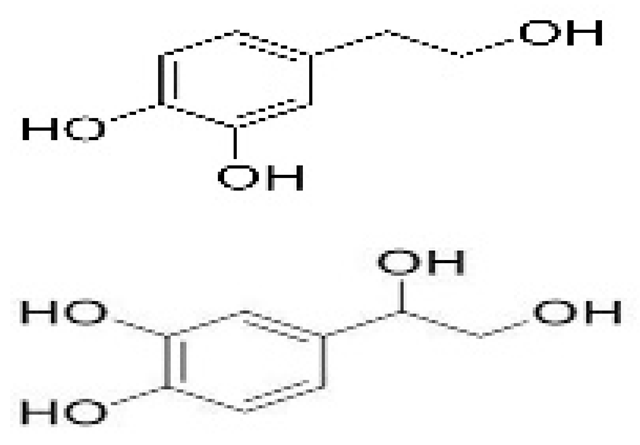 | Olive oil extract | Exhibited antioxidant activity and delayed lipid oxidation of beef meat. | Beef meat | [95] |
| Edible coating | Chitosan/Pullulan | gallic acid, ellagic acid, and other phenolic compounds | 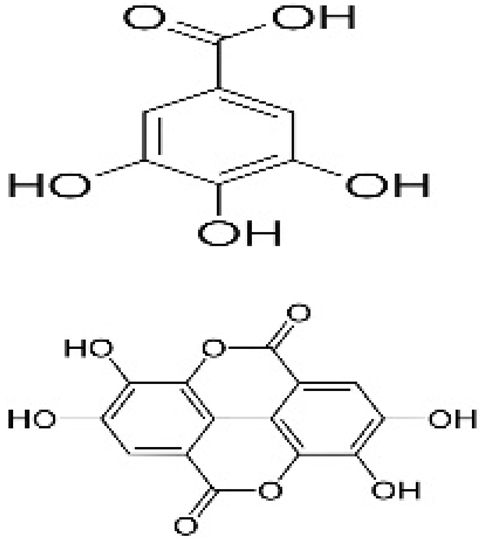 | Pomegranate peel | Shelf-life extension and physiochemical quality maintenance over a storage period of 18 days. | Bell pepper | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.K.; Kim, J.Y.; Lee, Y.S. Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients. Molecules 2022, 27, 7513. https://doi.org/10.3390/molecules27217513
Singh AK, Kim JY, Lee YS. Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients. Molecules. 2022; 27(21):7513. https://doi.org/10.3390/molecules27217513
Chicago/Turabian StyleSingh, Ajit Kumar, Jae Young Kim, and Youn Suk Lee. 2022. "Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients" Molecules 27, no. 21: 7513. https://doi.org/10.3390/molecules27217513
APA StyleSingh, A. K., Kim, J. Y., & Lee, Y. S. (2022). Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients. Molecules, 27(21), 7513. https://doi.org/10.3390/molecules27217513








