Comparative Metabolomic Profiling of the Metabolic Differences of Δ9-Tetrahydrocannabinol and Cannabidiol
Abstract
:1. Introduction
2. Results
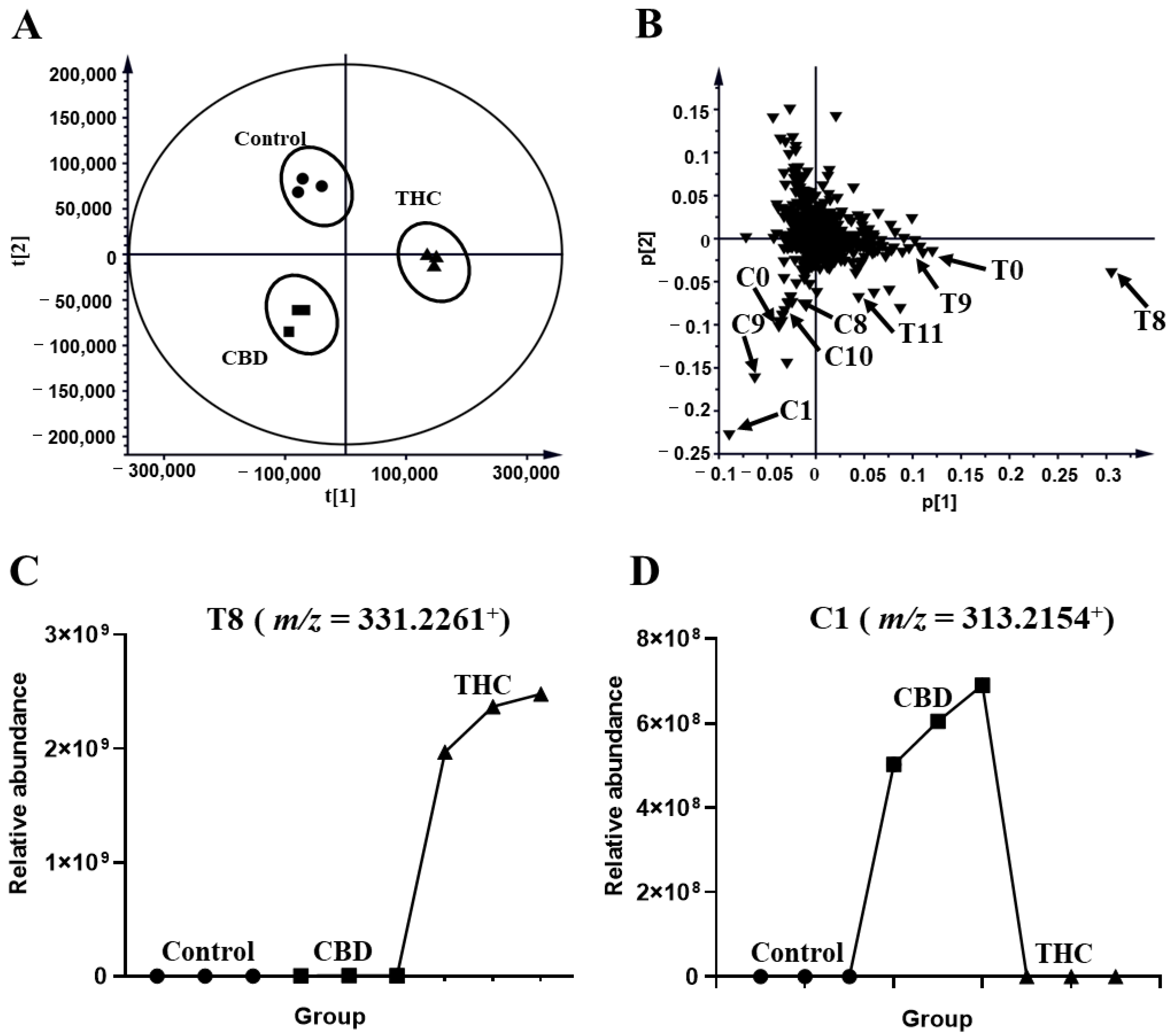
2.1. Metabolomic Profiling of HLM and MLM Treated with THC or CBD
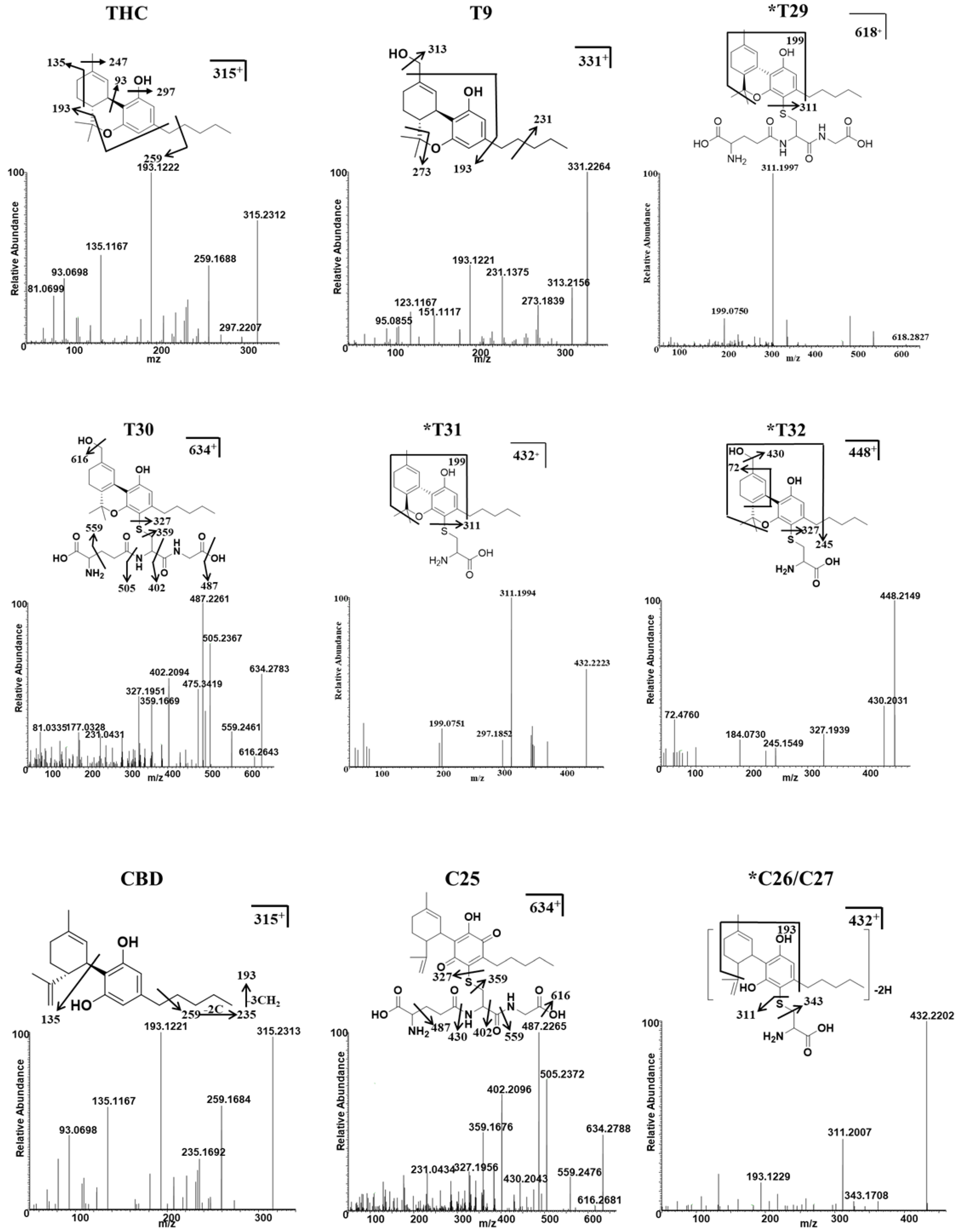
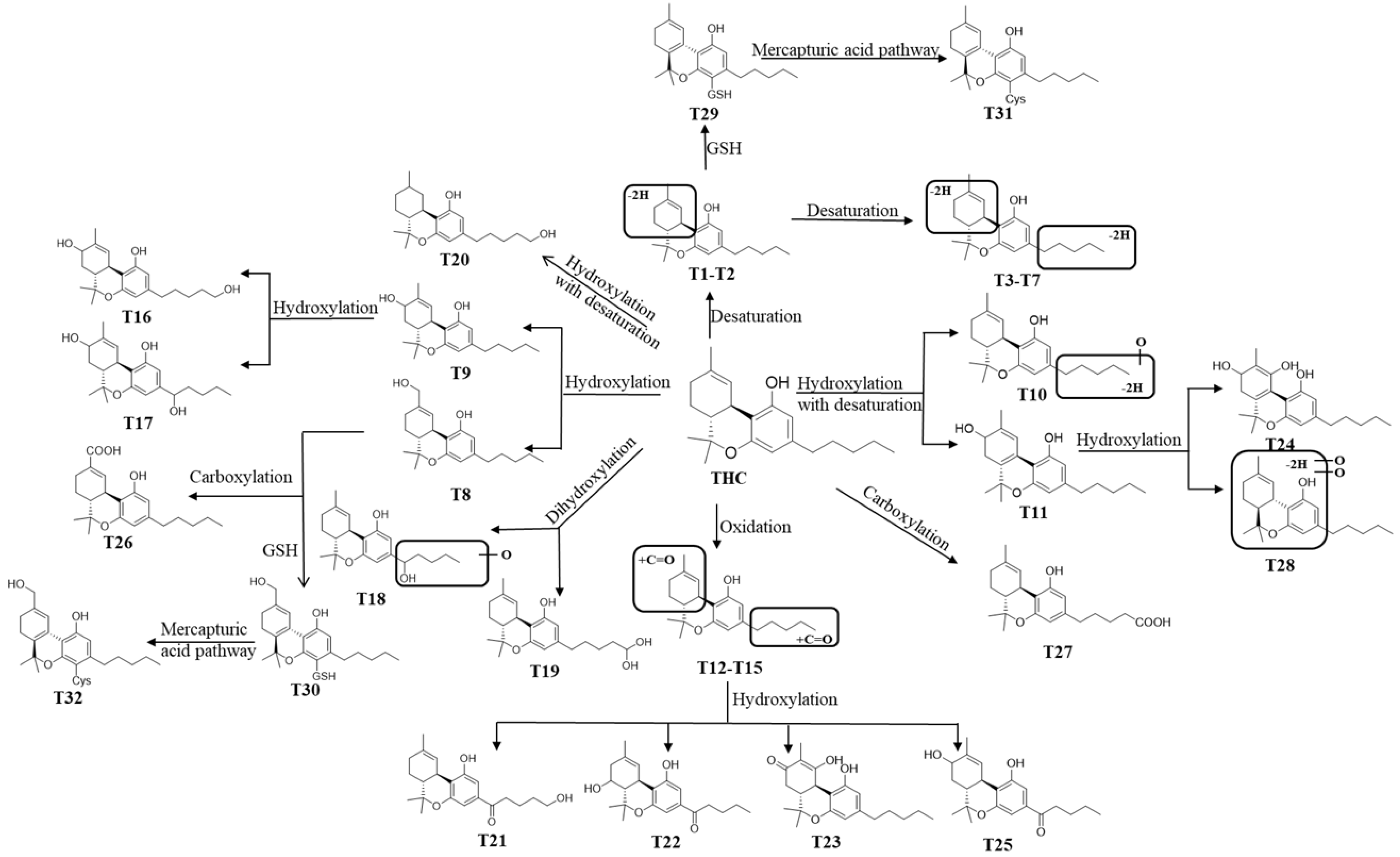
2.2. THC and CBD Metabolites in HLM and MLM Profiles
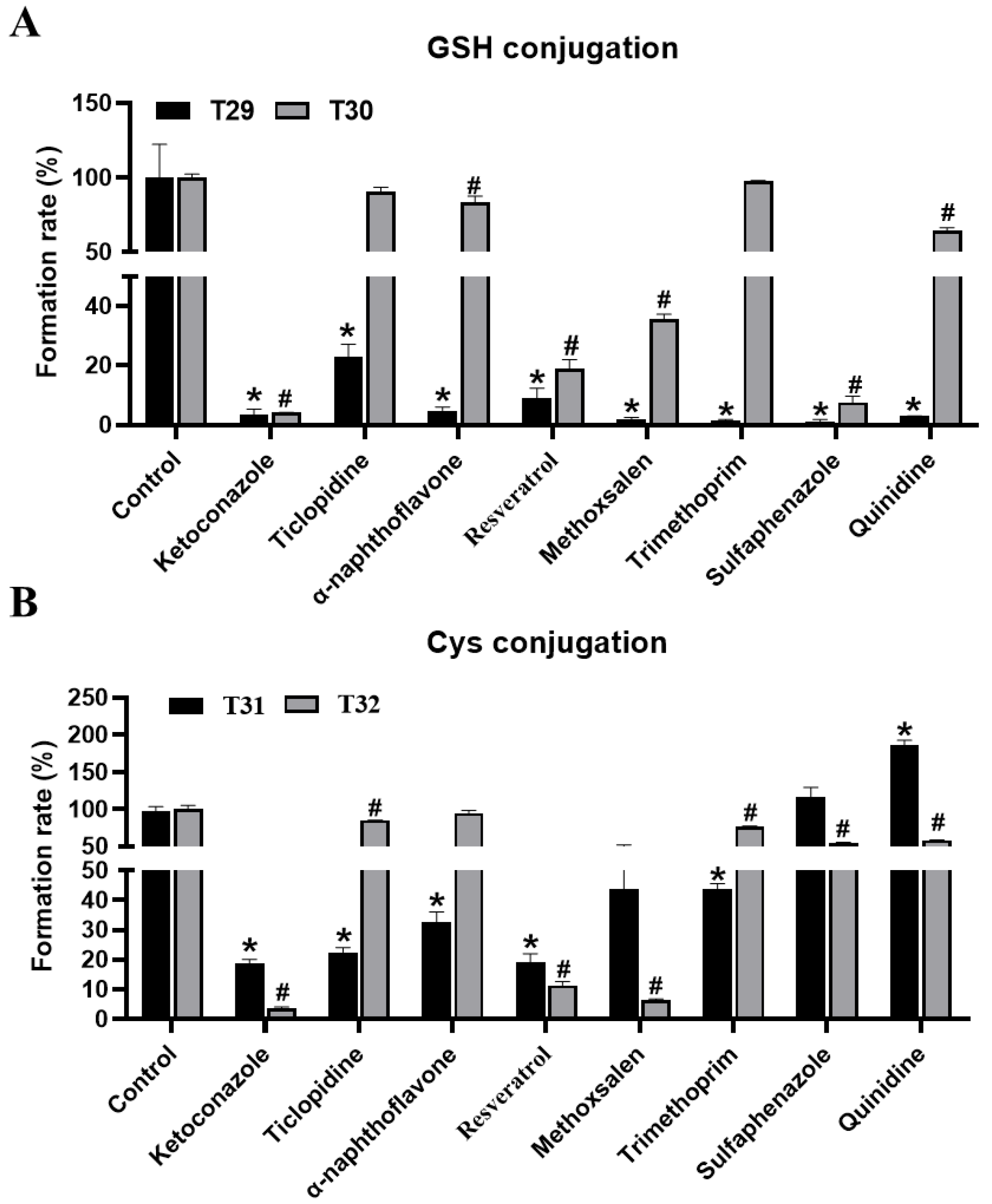
2.3. Identification of GSH and Cys Conjugates of THC and CBD
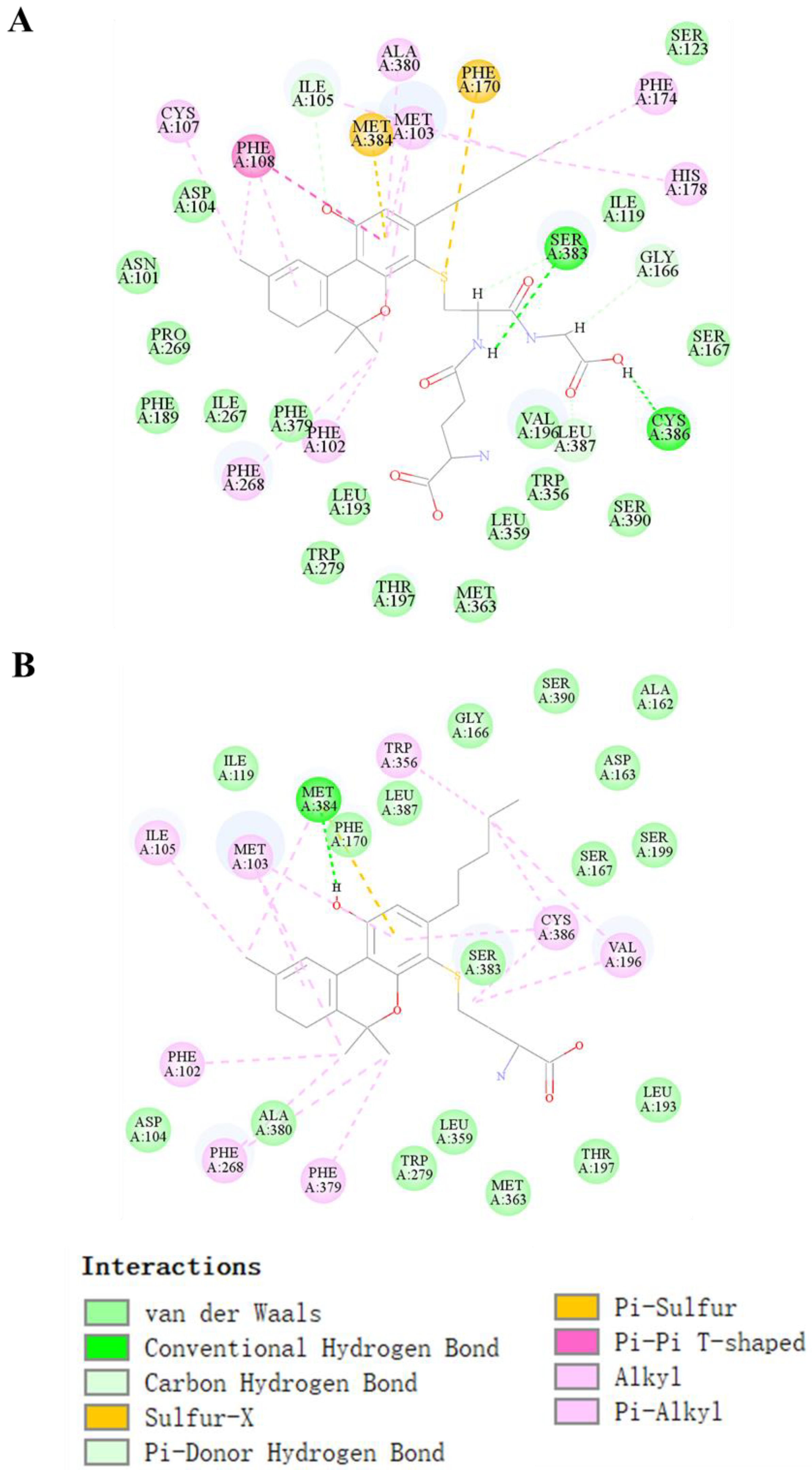
2.4. Molecular Docking Prediction of THC and CBD Metabolites
2.5. CYPs Involved in the Formation of THC and CBD Metabolites
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Metabolism of THC and CBD in the MLM and HLM Systems
4.3. Trapping Reactive Metabolites using GSH and Cys
4.4. Metabolism of THC and CBD in Human Recombinant CYP450s
4.5. UHPLC Q-Exactive MS Analysis
4.6. Data Processing, Feature Selection, and Metabolite Annotation
4.7. Molecular Docking Prediction Concerning How the CB1 Receptor Interacts with Metabolites
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular pharmacology of phytocannabinoids. Prog. Chem. Org. Nat. Prod. 2017, 103, 61–101. [Google Scholar] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.D.; Chen, X.L.; Xiao, X.R.; Wang, Y.K.; Liu, F.; Zhao, Q.; Li, X.; Yang, X.W.; Li, F. Comparative metabolism of tripolide and triptonide using metabolomics. Food Chem. Toxicol. 2018, 115, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Y.K.; Yang, X.N.; Xiao, X.R.; Zhang, T.; Yang, X.W.; Qin, H.B.; Li, F. Metabolic activation of myristicin and its role in cellular toxicity. J. Agric. Food Chem. 2019, 67, 4328–4336. [Google Scholar] [CrossRef]
- Wang, Y.K.; Yang, X.N.; Zhu, X.; Xiao, X.R.; Yang, X.W.; Qin, H.B.; Gonzalez, F.J.; Li, F. Role of metabolic activation in elemicin-induced cellular toxicity. J. Agric. Food Chem. 2019, 67, 8243–8252. [Google Scholar] [CrossRef]
- Chen, C.; Kim, S. LC-MS-based metabolomics of xenobiotic-induced toxicities. Comput. Struct. Biotechnol. J. 2013, 4, e201301008. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.F.; Zhao, Q.; Cheng, Y.; Yan, D.M.; Zhu, W.F.; Li, F. Metabolomics reveals the role of isopentenyl group in coumarins metabolism. Biomed. Chromatogr. BMC 2021, e5239. [Google Scholar] [CrossRef]
- Rotermann, M. What has changed since cannabis was legalized? Health Rep. 2020, 31, 11–20. [Google Scholar]
- Berman, P.; Sulimani, L.; Gelfand, A.; Amsalem, K.; Lewitus, G.M.; Meiri, D. Cannabinoidomics - An analytical approach to understand the effect of medical Cannabis treatment on the endocannabinoid metabolome. Talanta 2020, 219, 121336. [Google Scholar] [CrossRef]
- Golombek, P.; Müller, M.; Barthlott, I.; Sproll, C.; Lachenmeier, D.W. Conversion of cannabidiol (CBD) into psychotropic cannabinoids including tetrahydrocannabinol (THC): A controversy in the scientific literature. Toxics 2020, 8, 41. [Google Scholar] [CrossRef]
- Huestis, M.A.; Henningfield, J.E.; Cone, E.J. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J. Anal. Toxicol. 1992, 16, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.M.; Torrens, A.; Lallai, V.; Castillo, E.; Manca, L.; Martinez, M.X.; Justeson, D.N.; Fowler, C.D.; Piomelli, D.; Mahler, S.V. Pharmacokinetic and pharmacodynamic properties of aerosolized (“vaped”) THC in adolescent male and female rats. Psychopharmacology 2021, 238, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Laskar, A.; Younus, H. Aldehyde toxicity and metabolism: The role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab. Rev. 2019, 51, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, R.A.; Möller, C.; DeCaprio, A.P. LC-MS/MS based detection and characterization of covalent glutathione modifications formed by reactive drug of abuse metabolites. Xenobiot. Fate Foreign Compd. Biol. Syst. 2019, 49, 778–790. [Google Scholar] [CrossRef]
- Farina, M.; Aschner, M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129285. [Google Scholar] [CrossRef]
- Bornheim, L.M.; Grillo, M.P. Characterization of cytochrome P450 3A inactivation by cannabidiol: Possible involvement of cannabidiol-hydroxyquinone as a P450 inactivator. Chem. Res. Toxicol. 1998, 11, 1209–1216. [Google Scholar] [CrossRef]
- Olney, J.W. Role of excitotoxins in developmental neuropathology. APMIS Suppl. 1993, 40, 103–112. [Google Scholar]
- Stott, C.; White, L.; Wright, S.; Wilbraham, D.; Guy, G. A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. SpringerPlus 2013, 2, 236. [Google Scholar] [CrossRef] [Green Version]
- Stout, S.M.; Cimino, N.M. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metab. Rev. 2014, 46, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.H.; Chen, J.; Liu, L.; Messick, K.; Ly, J. Rifampin-mediated induction of Tamoxifen metabolism in a humanized PXR-CAR-CYP3A4/3A7-CYP2D6 mouse model. Drug Metab. Dispos. Biol. Fate Chem. 2016, 44, 1736–1741. [Google Scholar] [CrossRef] [Green Version]
- Helmig, S.; Seelinger, J.U.; Döhrel, J.; Schneider, J. RNA expressions of AHR, ARNT and CYP1B1 are influenced by AHR Arg554Lys polymorphism. Mol. Genet. Metab. 2011, 104, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Galaj, E.; Xi, Z.X. Possible receptor mechanisms underlying cannabidiol effects on addictive-like behaviors in experimental animals. Int. J. Mol. Sci. 2020, 22, 134. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef]
- Qin, X.; Hakenjos, J.M.; MacKenzie, K.R.; Barzi, M.; Chavan, H.; Nyshadham, P.; Wang, J.; Jung, S.Y.; Guner, J.Z.; Chen, S.; et al. Metabolism of a selective serotonin and norepinephrine reuptake inhibitor duloxetine in liver microsomes and mice. Drug Metab. Dispos. Biol. Fate Chem. 2022, 50, 128–139. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, S.; Wang, Z.; Wang, Y.; Liu, T.; Li, S.; Wang, C.; Wang, H.; Tu, P. Identification of metabolites of deoxyschizandrin in rats by UPLC–Q-TOF-MS/MS based on multiple mass defect filter data acquisition and multiple data processing techniques. J. Chromatogr. B 2014, 949, 115–126. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef]
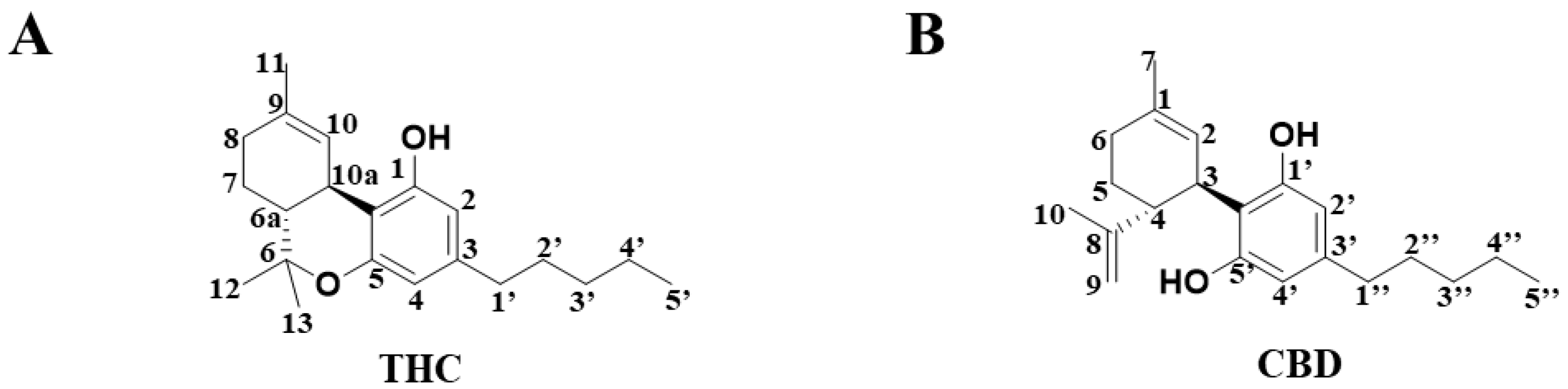

| Number | RT (min) | Clog P | Observed m/z [M + H] | Delta ppm | MS/MS Fragments | Predicted Molecular Formula | Identification | Source | NAPDH Independent |
|---|---|---|---|---|---|---|---|---|---|
| T0 | 10.32 | 7.24 | 315.2308 | −4.98 | 93, 193, 259, 297, 315 | C21H30O2 | THC | HLM, MLM | - |
| *T1 | 9.89 | 6.95 | 313.2161 | −1.94 | 95, 175, 193, 257, 271, 313 | C21H28O2 | THC-2H | HLM, MLM | - |
| *T2 | 10.2 | 7.16 | 313.2163 | −1.24 | 95, 193, 217, 271, 295, 313 | C21H28O2 | THC-2H | HLM, MLM | - |
| *T3 | 4.65 | 6.51 | 311.2009 | −0.64 | 95, 185, 213, 255, 269, 311 | C21H26O2 | THC-4H | HLM, MLM | - |
| *T4 | 4.83 | 6.65 | 311.1996 | −4.88 | 95, 191, 255, 269, 293, 311 | C21H26O2 | THC-4H | HLM, MLM | NADPH |
| *T5 | 5.35 | 6.86 | 311.1997 | −4.56 | 93, 217, 241, 269, 296, 311 | C21H26O2 | THC-4H | HLM, MLM | NADPH |
| *T6 | 6.68 | - | 311.1996 | −4.98 | 93, 193, 217, 223, 265, 311 | C21H26O2 | THC-4H | HLM, MLM | NAPDH |
| T7 | 9.48 | 7.39 | 311.2000 | −3.53 | 195, 223, 293, 311 | C21H26O2 | THC-4H | HLM, MLM | - |
| T8 | 6.81 | 5.35 | 331.2261 | −3.50 | 95, 193, 257, 271, 313 | C21H30O3 | THC+O | HLM, MLM | NAPDH |
| T9 | 8.38 | 5.45 | 331.2261 | −3.62 | 95, 193, 231, 273, 313 | C21H30O3 | THC+O | HLM, MLM | NAPDH |
| T10 | 4.51 | - | 329.2115 | −0.72 | 193, 217, 245, 301, 329 | C21H28O3 | THC+O-2H | HLM, MLM | NAPDH |
| T11 | 4.64 | 5.27 | 329.2100 | −5.10 | 213, 261, 311, 329 | C21H28O3 | THC+O-2H | HLM, MLM | - |
| T12 | 4.81 | 5.3 | 329.2107 | −3.03 | 95, 191, 255, 311, 329 | C21H28O3 | THC+O-2H | HLM, MLM | NAPDH |
| T13 | 4.98 | 5.49 | 329.2100 | −4.92 | 95, 191, 255, 311, 329 | C21H28O3 | THC+O-2H | HLM | NAPDH |
| T14 | 5.09 | 5.64 | 329.2114 | −1.03 | 95, 191, 213, 255, 311, 329 | C21H28O3 | THC+O-2H | HLM | NAPDH |
| T15 | 7.47 | 6.16 | 329.2100 | −4.92 | 217, 231, 245, 287, 311, 329 | C21H28O3 | THC+O-2H | MLM | NAPDH |
| T16 | 5.50 | 3.64 | 349.2370 | −2.57 | 193, 259, 313, 349 | C21H32O4 | THC+2H+2O | MLM | NAPDH |
| T17 | 5.35 | 3.59 | 347.2216 | −1.84 | 191, 273, 311, 329, 347 | C21H30O4 | THC+2O | HLM | NAPDH |
| T18 | 5.4 | - | 347.2215 | −2.10 | 191, 273, 311, 329, 347 | C21H30O4 | THC+2O | MLM | NAPDH |
| T19 | 6.00 | 4.42 | 347.2216 | −1.75 | 193, 206, 233, 273, 311, 329, 347 | C21H30O4 | THC+2O | MLM | NAPDH |
| T20 | 6.39 | 5.73 | 333.2414 | −4.80 | 217, 217, 261, 289, 315, 333 | C21H32O3 | THC+O+2H | MLM | - |
| T21 | 4.06 | 4.17 | 345.2053 | −3.88 | 191, 233, 243, 285, 345 | C21H28O4 | THC+2O-2H | MLM | NAPDH |
| T22 | 4.48 | 4.41 | 345.2052 | −3.96 | 191, 261, 285, 309, 327, 345 | C21H28O4 | THC+2O-2H | HLM, MLM | NAPDH |
| T23 | 5.06 | 5.04 | 345.2053 | −3.79 | 229, 271, 285, 327, 345 | C21H28O4 | THC+2O-2H | HLM, MLM | NAPDH |
| T24 | 4.72 | 4.83 | 345.2053 | −3.79 | 253, 271, 327, 345 | C21H28O4 | THC+2O-2H | MLM | - |
| T25 | 4.31 | 4.27 | 345.2053 | −3.70 | 253, 261, 267, 309, 345 | C21H28O4 | THC+2O-2H | HLM, MLM | NAPDH |
| T26 | 5.23 | 5.7 | 345.2054 | −3.44 | 229, 271, 285, 327, 345 | C21H28O4 | THC+COOH | HLM, MLM | NAPDH |
| T27 | 4.97 | 5.29 | 345.2054 | −3.62 | 95, 207, 271, 309, 327, 345 | C21H28O4 | THC+COOH | HLM, MLM | NAPDH |
| T28 | 4.58 | - | 345.2058 | −2.31 | 253, 271, 327, 345 | C21H28O4 | THC+2O-2H | HLM | NAPDH |
| *T29 | 4.53 | 3.96 | 618.2827 | −3.39 | 129, 199, 311, 618 | C31H43N3O8S | THC+GSH-2H | HLM/ MLM+ GSH | - |
| T30 | 4.48 | 2.18 | 634.2780 | −2.83 | 327, 359, 487, 616, 634 | C31H43N3O9S | THC+GSH+O-2H | HLM/ MLM+ GSH | - |
| *T31 | 4.87 | 4.65 | 432.2223 | 3.47 | 199, 297, 311, 432 | C24H33NO4S | THC+CYS-2H | HLM/ MLM+ Cys | - |
| *T32 | 4.83 | 2.86 | 448.2141 | −3.57 | 245, 327, 399, 448 | C24H33NO5S | THC+Cys+O-2H | HLM/ MLM+ Cys | - |
| CYP1A1 | CYP1A2 | CYP1B1 | CYP2A6 | CYP2B6 | CYP2C19 | CYP2C8 | CYP2C9 | CYP2D6 | CYP2E1 | CYP3A4 | CYP3A5 | CYP4A11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | - | - | - | 10.41 | 11.16 | - | - | - | - | 7.99 | 27.39 | 36.62 | 6.44 |
| T2 | - | 7.35 | 9.29 | 13.48 | 12.39 | - | 9.60 | - | 7.91 | 10.50 | 14.20 | 7.58 | 7.70 |
| T3 | - | - | - | - | - | - | - | - | - | - | - | 100.00 | - |
| T4 | - | - | - | - | - | 52.58 | - | - | 43.90 | - | 3.52 | - | - |
| T5 | - | 3.99 | - | - | - | 81.65 | - | - | - | - | 14.36 | - | - |
| T6 | - | 24.06 | - | 26.89 | - | - | - | - | - | - | 36.22 | 12.83 | - |
| T7 | 4.77 | 5.62 | 6.91 | 9.76 | 11.93 | 3.00 | 9.24 | 7.40 | 7.06 | 11.17 | 8.89 | 8.19 | 6.06 |
| T8 | 2.76 | 0.08 | 0.18 | - | 0.79 | 27.12 | 4.12 | 61.98 | 2.42 | - | 0.26 | 0.27 | 0.02 |
| T9 | - | 1.20 | 1.22 | 1.35 | 1.41 | - | 1.25 | - | - | 1.19 | 54.52 | 36.74 | 1.13 |
| T11 | 23.87 | - | - | - | - | - | 2.83 | - | 6.39 | - | 7.87 | 59.04 | - |
| T12 | - | 3.82 | - | - | - | - | 7.49 | - | 65.29 | - | 23.40 | - | - |
| T13 | - | - | - | - | - | - | - | - | - | - | 100.00 | - | - |
| T14 | 93.09 | - | - | - | - | - | - | - | 3.55 | - | 3.36 | - | - |
| T15 | 6.70 | 7.71 | 8.02 | 8.93 | 9.20 | 7.29 | 8.74 | 8.30 | 6.79 | 8.00 | 7.31 | 6.36 | 6.66 |
| T16 | 7.32 | 8.80 | 9.61 | 10.62 | 10.61 | 6.61 | 10.49 | 8.79 | 6.26 | 8.36 | 12.55 | - | - |
| T17 | - | 3.62 | 0.88 | - | - | 8.29 | - | - | - | - | 28.31 | 58.90 | - |
| T18 | 10.34 | - | - | - | - | 84.09 | - | - | - | - | 5.57 | - | - |
| T19 | 3.81 | 4.61 | 4.19 | 4.76 | 5.79 | 3.85 | 4.89 | 4.60 | 3.01 | 3.94 | 15.44 | 37.72 | 3.39 |
| T20 | 3.65 | 8.23 | 9.72 | 5.45 | 8.52 | 6.69 | 9.82 | 10.85 | 2.36 | 4.25 | 6.66 | 13.35 | 10.45 |
| T22 | 29.04 | 1.54 | 8.11 | - | - | 24.44 | 2.88 | - | 23.59 | - | 2.96 | 7.43 | - |
| T23 | 39.72 | 3.87 | 0.92 | - | - | 36.47 | - | 0.59 | - | - | 18.43 | - | - |
| T24 | 30.16 | 2.04 | - | - | - | 27.04 | 8.41 | 0.84 | - | - | 9.22 | 22.28 | - |
| T25 | - | - | 5.56 | - | - | - | - | - | - | - | 20.83 | 73.61 | - |
| T26 | - | - | 0.87 | - | - | 54.76 | 10.43 | 0.57 | - | - | 13.84 | 19.55 | - |
| T27 | 25.62 | 0.62 | 1.58 | - | - | 10.81 | 3.88 | 0.45 | - | - | 12.84 | 44.20 | - |
| T28 | - | 1.87 | 4.78 | - | - | 22.19 | 6.52 | - | 59.93 | - | 4.70 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, Q.; Zhang, T.; Dai, M.; Li, B.; Pu, Q.; Zhao, M.; Cheng, Y.; Yan, D.; Zhao, Q.; Wu, Z.E.; et al. Comparative Metabolomic Profiling of the Metabolic Differences of Δ9-Tetrahydrocannabinol and Cannabidiol. Molecules 2022, 27, 7573. https://doi.org/10.3390/molecules27217573
Rao Q, Zhang T, Dai M, Li B, Pu Q, Zhao M, Cheng Y, Yan D, Zhao Q, Wu ZE, et al. Comparative Metabolomic Profiling of the Metabolic Differences of Δ9-Tetrahydrocannabinol and Cannabidiol. Molecules. 2022; 27(21):7573. https://doi.org/10.3390/molecules27217573
Chicago/Turabian StyleRao, Qianru, Ting Zhang, Manyun Dai, Bin Li, Qianlun Pu, Min Zhao, Yan Cheng, Dongmei Yan, Qi Zhao, Zhanxuan E. Wu, and et al. 2022. "Comparative Metabolomic Profiling of the Metabolic Differences of Δ9-Tetrahydrocannabinol and Cannabidiol" Molecules 27, no. 21: 7573. https://doi.org/10.3390/molecules27217573





