Synthesis, Characterization, and X-ray Crystallography, of the First Cyclohexadienyl Trifluoromethyl Metal Complex (η5-C6H7)Fe(CO)2CF3
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taw, F.L.; Mueller, A.E.; Janicke, A.H.; Cantat, M.T.; Scott, B.L.; Hay, P.J. Hughes, R.P.; Kiplinger, J.L. Titanium(IV) Trifluoromethyl Complexes: New Perspectives on Bonding from Organometallic Fluorocarbon Chemistry. Organometallics 2012, 31, 1484–1499. [Google Scholar] [CrossRef]
- Alonso, C.N.; Martínez De Marigorta, E.; Rubiales, G.; Palacios, F. Carbon Trifluoromethylation Reactions of Hydrocarbon Derivatives and Heteroarenes. Chem. Rev. 2015, 115, 1847–1935. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A.; Chambers, R.D.; Prakash, G.K.S. Synthetic Fluorine Chemistry; John Wiley: Hoboken, NJ, USA, 1992. [Google Scholar]
- Douvris, C. Synthesis and characterization of the first η6-arene trifluoromethyl transition-metal complex. J. Fluor. Chem. 2021, 245, 109755. [Google Scholar] [CrossRef]
- Abula, A.; Xu, Z.; Zhu, Z.; Peng, C.; Chen, Z.; Zhu, W. Akber Aisa. H. Substitution Effect of the Trifluoromethyl Group on the Bioactivity in Medicinal Chemistry: Statistical Analysis and Energy Calculations. J. Chem. Inf. Model. 2020, 60, 6242–6250. [Google Scholar] [CrossRef]
- Mei, H.; Ibrahim, F.J. Two New Diazonium Bis(perfluoroalkyl)arylsulfonyl Imide Zwitterionic Monomers from Perfluoro(3-oxa-4-pentene)sulfonyl Fluoride for Proton Exchange Membrane Fuel Cells. Fluor. Chem. 2017, 199, 46–51. [Google Scholar] [CrossRef]
- McCormack, P.L. Celecoxib. Drugs 2011, 71, 2457–2489. [Google Scholar] [CrossRef]
- Morrison, J.A. Trifluoromethyl-Containing Transition Metal Complexes. Adv. Organomet. Chem. 1993, 35, 211. [Google Scholar]
- Lampropoulos, C.; Rashad, G.; Douvris, C. Syntheses and Characterization of the First Cycloheptatrienyl Transition-Metal Complexes with a M-CF3 Bond. Molecules 2021, 26, 6838. [Google Scholar] [CrossRef]
- Kwan, A.L.; Morris, R.H. A Plausible Mechanism for the Iridium-Catalyzed Hydrogenation of a Bulky N-Aryl Imine in the (S)-Metolachlor Process. Molecules 2022, 27, 5106. [Google Scholar] [CrossRef]
- Huang, Z.; Dong, G. Catalytic Csingle bondC bond forming transformations via direct β-Csingle bondH functionalization of carbonyl compounds. Tetrahedron Lett. 2014, 55, 5869–5889. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.; Lopez-Serrano, J.; Peloso, R.; Carmona, E. Methyl complexes of the transition metals. Chem. Eur. J. 2016, 22, 6432. [Google Scholar] [CrossRef] [PubMed]
- Douvris, C.; Lampropoulos, C.; Matatov, D.; Wink, D.J.; Kuznetsov, A.E.; Bussan, D. Synthesis and structural characterization of the first stable cycloheptatrienyl metal complexes bearing a CF3 moiety. DFT investigations of structures, energetics, NBO charges, and frontier MOs of W-CF3 and Mo-CF3 with η7-C7H7 and η5-C5H5. Polyhedron 2022, 221, 115875. [Google Scholar] [CrossRef]
- Shapovalov, S.S.; Gordienko, A.V.; Pasynskii, A.A.; Torubaev, Y.V.; Skabitskii, I.V.; Aleksandrov, G.G. (Dicarbonyl)(π-cyclohexadienyl)iron iodide, trichlorostannate, and tris(cymantrenecarboxylato)stannate: Synthesis and molecular structures. Russ. J. Coord. Chem. 2011, 37, 447–451. [Google Scholar] [CrossRef]
- Krause, L.J.; Morrison, J.A. Trifluoromethyl group 2B compounds: bis(trifluoromethyl)cadmium.base. New, more powerful ligand-exchange reagents and low-temperature difluorocarbene sources. J. Am. Chem. Soc. 1981, 103, 2995. [Google Scholar] [CrossRef]
- Hartwig, J.F. Organotransition Metal Chemistry: From Bonding to Catalysis, 1st ed.; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- Loizou, D.C.; Castillo, J.; Oki, A.R.; Hosmane, N.S.; Morrison, J.A. Synthersis of cyclopentadienyldinitrosyl(trifluoromethyl)chromium(0), CpCr(NO)2CF3, and cyclopentadienyldinitrosyl(trifluoromethyl)molybdenum(0), CpMo(NO)2CF3. Crystal Structure of CpMo(NO)2CF3. Organometallics 1992, 11, 4189–4193. [Google Scholar] [CrossRef]
- Anderson, S.; Hill, A.F.; Clark, G.R. Reaction of [Fe[C(:O)NPr-iso2](CO)4]- with trifluoroacetic anhydride: Molecular structure of [Fe(CF3)[.eta.2-C(:O)NPr-iso2](CO)2(PPh3). Organometallics 1992, 11, 1988–1990. [Google Scholar] [CrossRef]
- Trifonova, E.A.; Perekalin, D.S.; Loskutova, N.L.; Nelyubina, Y.V.; Kudinov, A.R. Synthesis of cyclohexadienyl ruthenium complexes by replacement of the naphthalene ligand in [(η5-C6H3Me4)Ru(η6-C10H8)]+. J. Organomet. Chem. 2015, 785, 106–111. [Google Scholar] [CrossRef]
- McDonald, R.N.; Schell, P.L.; McGhee, W.D. Oxidative-addition processes in the reactions of (OC)4Fe.cntdot. with XCY3 molecules. Organometallics 1984, 3, 182–184. [Google Scholar] [CrossRef]
- Sanner, R.D.; Satcher, J.H.; Droege, M.W. Synthesis and characterization of (trifluoromethyl) gold complexes. Organometallics 1989, 8, 1498–1506. [Google Scholar] [CrossRef]
- Tyrra, W.E.J. Oxidative perfluoroorganylation methods in group 12–16 chemistry: The reactions of haloperfluoroorganics and In and InBr, a convenient new route to AgRf (Rf = CF3, C6F5) and reactions of AgRf with group 12–16 elements. Fluor. Chem. 2001, 112, 149. [Google Scholar] [CrossRef]
- Hughes, R.P.; Laritchev, R.B.; Yuan, J.; Golen, J.A.; Rucker, A.N.; Rheingold, A.L. A Simple Route to Difluorocarbene and Perfluoroalkylidene Complexes of Iridium. J. Am. Chem. Soc. 2005, 127, 15020. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Tobita, E.; Ando, M.; Kondo, K. A Convenient Trifluoromethylation of Aromatic Halides with Sodium Trifluoroacetate. Chem. Lett. 1981, 10, 1719. [Google Scholar] [CrossRef] [Green Version]
- Daniels, A.L.; Da Gama, J.G.; Edjoc, R.; Gabidullin, B.M.; Baker, R.T. Synthesis and Reactivity of Mn–CF3 Complexes. Inorganics 2019, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.S.; Burkey, D.J. Spirocyclic and Bicyclic Cyclohexadienyl Complexes from Intramolecular Nucleophilic Addition Reactions in Dicationic Arene Complexes. Organometallics 2003, 22, 1761–1765. [Google Scholar] [CrossRef]
- Shirin, Z.; Pramanik, A.; Ghosh, P.; Mukherjee, R. Stable Cyclohexadienyl Complexes of Ruthenium in a Piano Stool Geometry Containing a Tridentate Nitrogen Donor Ligand. First Structural Characterization of the (η5-Cyanocyclohexadienyl)ruthenium(II) Complex and Spectroelectrochemical Correlation. Inorg. Chem. 1996, 35, 3431–3433. [Google Scholar] [CrossRef]
- Hoffmann, R.; Hofmann, P. The electronic origin of geometrical deformations in cyclohexadienyl and cyclobutenyl transition metal complexes. J. Am. Chem. Soc. 1976, 98, 598–604. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Mitchell, A.S.; Spackman, M.A. Hirshfeld Surfaces: A New Tool for Visualising and Exploring Molecular Crystals. Chem. Eur. J. 1998, 4, 2136–2141. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic Potentials mapped on Hirshfeld surfaces provide direct Insight into intermolecular interactions in Crystals. Cryst. Eng.Commun. 2008, 10, 377–388. [Google Scholar] [CrossRef]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Madura, I.D.; Zachara, J.; Hajmowicz, H.; Synoradzki, L. Interplay of carbonyl–carbonyl, Csingle bondH⋯O and Csingle bondH⋯π interactions in hierarchical supramolecular assembly of tartaric anhydrides – Tartaric acid and its O-acyl derivatives: Part 11. J. Mol. Struct. 2012, 1017, 98–105. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Cryst. Eng. Commun. 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Garnero, M.; Corrales, S.A.; Williams, E.R.; Mowson, A.; Ozarowski, A.; Wernsdorfer, W.; Christou, G.; Lampropoulos, C. Controlled Dimerization of Mn12 Single-Molecule Magnets. Inorg. Chem. 2017, 56, 14755–14758. [Google Scholar] [CrossRef] [PubMed]
- Laos, R.; Lampropoulos, C.; Benner, S.A. The surprising pairing of 2-amino-imidazo[1,2-a][1,3,5]triazin-4-one, a component of an expanded DNA alphabet. Acta Cryst. 2019, C75, 22–28. [Google Scholar]
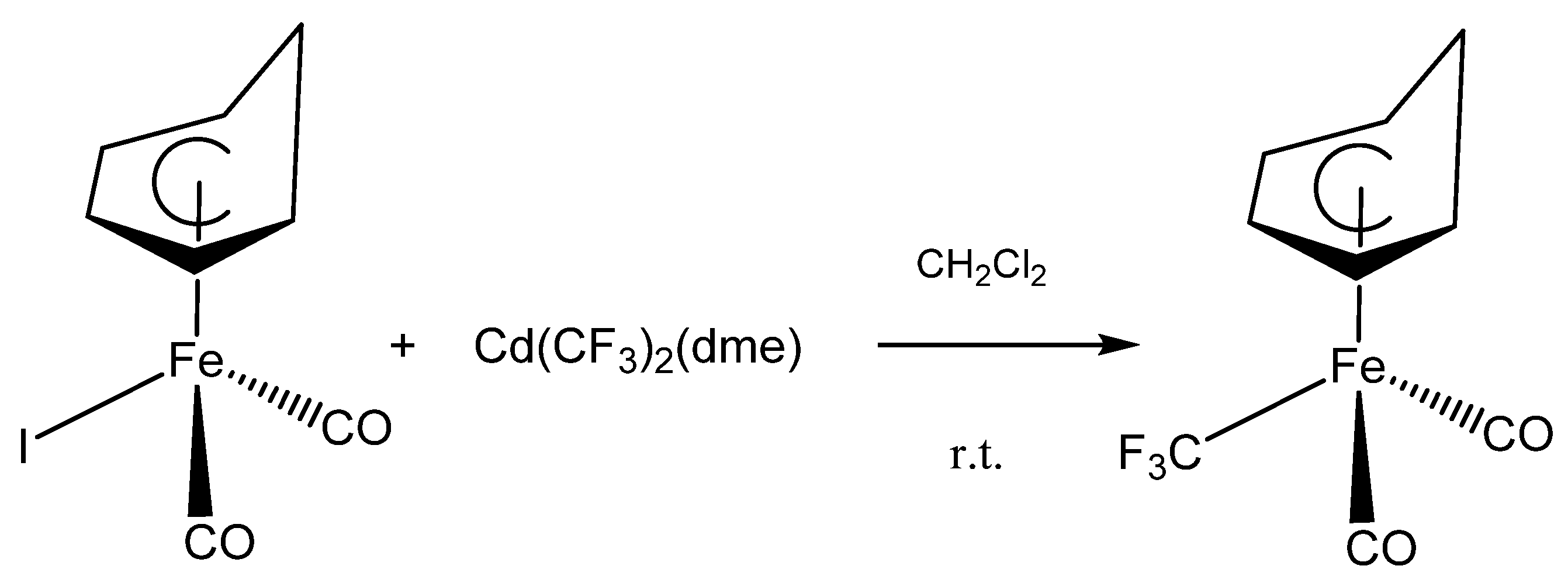


| Contact | Percent of Total Contacts | Contact | Percent of Total Contacts |
|---|---|---|---|
| F–H | 32.8% | F–C | 3.9% |
| O–H | 31.4% | O–O | 1.7% |
| H–H | 14.2% | O–C | 1.3% |
| F–O | 8.2% | C–C | 0% |
| C–H | 6.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douvris, C.; Matatov, D.; Bussan, D.; Lampropoulos, C.; Wink, D.J. Synthesis, Characterization, and X-ray Crystallography, of the First Cyclohexadienyl Trifluoromethyl Metal Complex (η5-C6H7)Fe(CO)2CF3. Molecules 2022, 27, 7595. https://doi.org/10.3390/molecules27217595
Douvris C, Matatov D, Bussan D, Lampropoulos C, Wink DJ. Synthesis, Characterization, and X-ray Crystallography, of the First Cyclohexadienyl Trifluoromethyl Metal Complex (η5-C6H7)Fe(CO)2CF3. Molecules. 2022; 27(21):7595. https://doi.org/10.3390/molecules27217595
Chicago/Turabian StyleDouvris, Chris, David Matatov, Derek Bussan, Christos Lampropoulos, and Donald J. Wink. 2022. "Synthesis, Characterization, and X-ray Crystallography, of the First Cyclohexadienyl Trifluoromethyl Metal Complex (η5-C6H7)Fe(CO)2CF3" Molecules 27, no. 21: 7595. https://doi.org/10.3390/molecules27217595







