Assessment of Cucurbita spp. Peel Extracts as Potential Sources of Active Substances for Skin Care and Dermatology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Active Compounds of Cosmetic Significance Present in Cucurbita sp. Peel Extracts
2.2. Antioxidant Properties of the Cucurbita sp. Peel Extracts
2.3. The Influence of Cucurbita spp. Peel Extracts on the Tyrosinase Activity
2.4. In Vitro Cytotoxicity
2.5. In Vitro Sun Protection Factor (SPF) of Pumpkin Peel Extracts
2.6. Corelation of the Pumpkin Peel Extract’s Activity with the Content of the Compounds
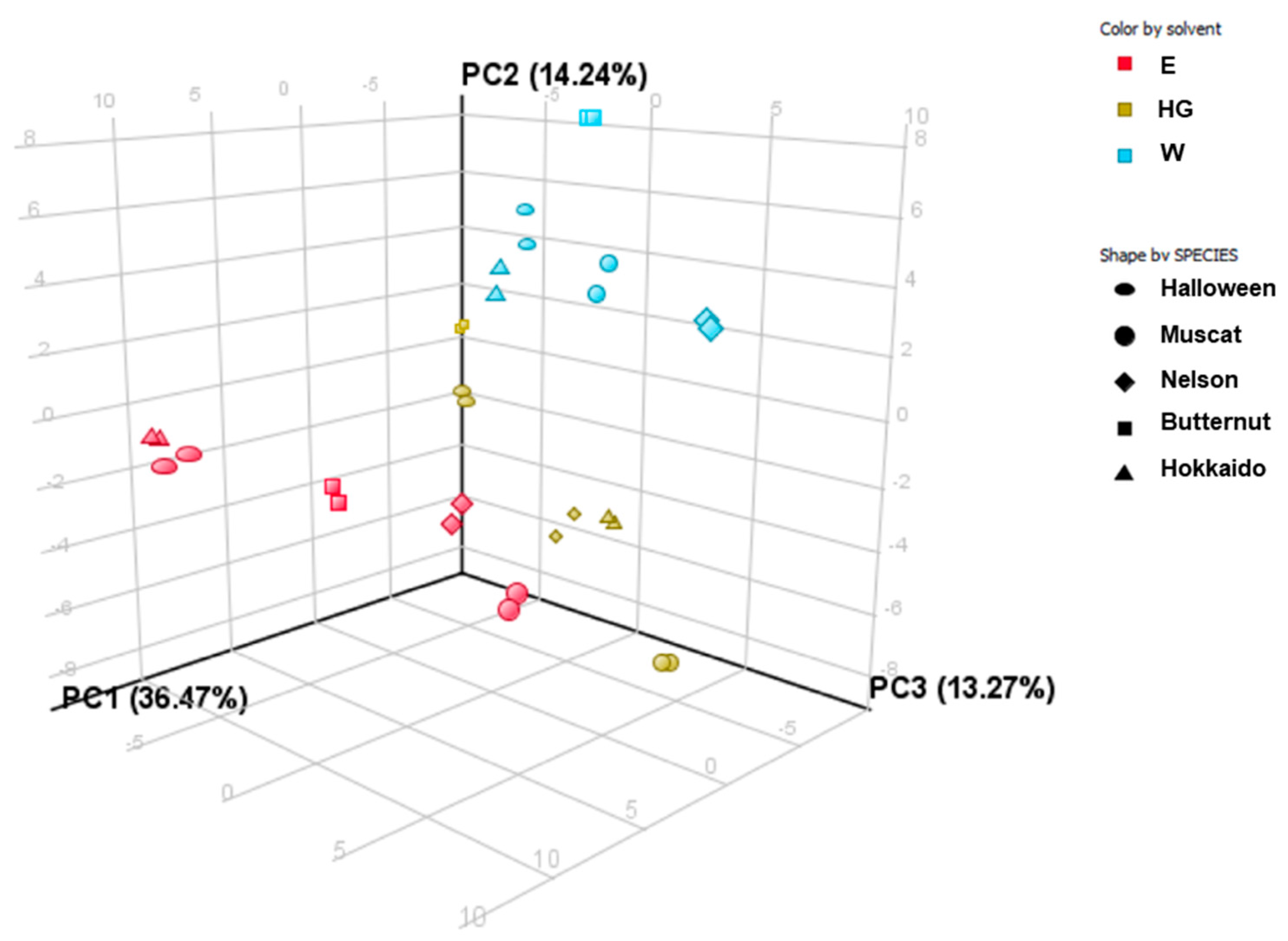
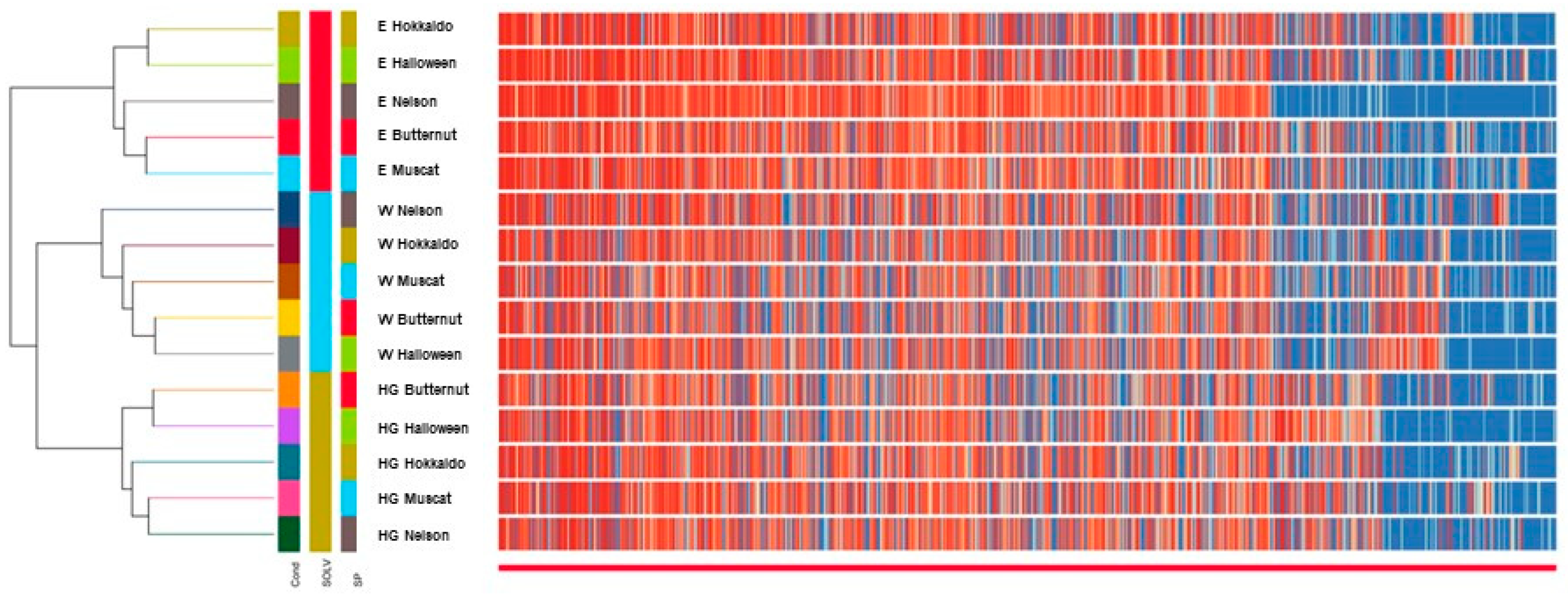
2.7. PCA and Clustering Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Extraction Procedure
3.3. Analysis of the Total Phenolics and Flavonoids
3.4. The HPLC-ESI-Q-TOF-MS Analysis of the Extracts
3.5. DPPH and ABTS Radical Scavenging Assays
3.6. Tyrosinase Activity Assay
3.7. In Vitro Cytotoxicity
3.8. Determination of the In Vitro Sun Protection Factor (SPF)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Barbulova, A.; Colucci, G.; Apone, F. New Trends in Cosmetics: By-Products of Plant Origin and Their Potential Use as Cosmetic Active Ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowe, C. Review on potential use of fruit and vegetables by-products as a valuable source of natural food additives. Food Sci. Qual. Manag. 2015, 45, 47–61. [Google Scholar]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products–A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Ueda, J.M.; Pedrosa, M.C.; Heleno, S.A.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Food Additives from Fruit and Vegetable By-Products and Bio-Residues: A Comprehensive Review Focused on Sustainability. Sustainability 2022, 14, 5212. [Google Scholar] [CrossRef]
- Pietzsch, N.; Ribeiro, J.L.D.; de Medeiros, J.F. Benefits, challenges and critical factors of success for Zero Waste: A systematic literature review. Waste Manag. 2017, 67, 324–353. [Google Scholar] [CrossRef]
- Amberg, N.; Fogarassy, C. Green Consumer Behavior in the Cosmetics Market. Resources 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D.; et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef] [Green Version]
- Cosmetic Ingredient Database COSMILE. Available online: https://cosmile-info.eu/ (accessed on 14 October 2022).
- Kulczyński, B.; Gramza-Michałowska, A. The Profile of Secondary Metabolites and Other Bioactive Compounds in Cucurbita pepo L. and Cucurbita moschata Pumpkin Cultivars. Molecules 2019, 24, 2945. [Google Scholar] [CrossRef] [Green Version]
- Kulczyński, B.; Gramza-Michałowska, A. The Profile of Carotenoids and Other Bioactive Molecules in Various Pumpkin Fruits (Cucurbita maxima Duchesne) Cultivars. Molecules 2019, 24, 3212. [Google Scholar] [CrossRef] [PubMed]
- Kostecka-Gugała, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and Health-Beneficial Nutrients in Fruits of Eighteen Cucurbita Cultivars: Analysis of Diversity and Dietary Implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Bhat, R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Shaygan, S.; Fakhri, S.; Bahrami, G.; Rashidi, K.; Farzaei, M.H. Wound-Healing Potential of Cucurbita moschata Duchesne Fruit Peel Extract in a Rat Model of Excision Wound Repair. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 6697174. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Farzaei, M.H.; Abdolghaffari, A.H.; Rahimi, R.; Samadi, N.; Heidari, M.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M.; et al. Evaluation of phytochemicals, antioxidant and burn wound healing activities of Cucurbita moschata Duchesne fruit peel. Iran. J. Basic Med. Sci. 2017, 20, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Ribeiro, M.M.d.S.; dos Santos, L.C.; de Novais, N.S.; Viganó, J.; Veggi, P.C. An evaluative review on Stryphnodendron adstringens extract composition: Current and future perspectives on extraction and application. Ind. Crop. Prod. 2022, 187, 115325. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielińska, M.; Bułkowska, K.; Witońska, I. Valorization of distillery stillage by polyphenol recovery using microwave-assisted, ultrasound-assisted and conventional extractions. J. Environ. Manag. 2022, 322, 116150. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Achilonu, M.C.; Nwafor, I.C.; Umesiobi, D.O.; Sedibe, M.M. Biochemical proximates of pumpkin (Cucurbitaeae spp.) and their beneficial effects on the general well-being of poultry species. J. Anim. Physiol. Anim. Nutr. 2017, 102, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Grzybek, M.; Kukula-Koch, W.; Strachecka, A.; Jaworska, A.; Phiri, A.M.; Paleolog, J.; Tomczuk, K. Evaluation of Anthelmintic Activity and Composition of Pumpkin (Cucurbita pepo L.) Seed Extracts—In Vitro and in Vivo Studies. Int. J. Mol. Sci. 2016, 17, 1456. [Google Scholar] [CrossRef] [PubMed]
- Gilder, J.; Cronshaw, J. Adenosine triphosphatase in the phloem of Cucurbita. Planta 1973, 110, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Oh, S.; Lee, H.; Kim, C.; Song, K. Isolation of Anticomplementary Substances from Cucurbita Moschata Duch. J. Food Sci. 2002, 67, 1348–1351. [Google Scholar] [CrossRef]
- Koch, W.; Kukuła-Koch, W.; Czop, M.; Helon, P.; Gumbarewicz, E. The Role of Extracting Solvents in the Recovery of Polyphenols from Green Tea and Its Antiradical Activity Supported by Principal Component Analysis. Molecules 2020, 25, 2173. [Google Scholar] [CrossRef]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Zeljković, S. Influence of Extraction Solvent on the Phenolic Profile and Bioactivity of Two Achillea Species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Delonga, K.; Levaj, B.; Djakovic, S.; Pospisil, J. Phenolic Profiles of Raw Apricots, Pumpkins, and Their Purees in the Evaluation of Apricot Nectar and Jam Authenticity. J. Agric. Food Chem. 2005, 53, 4836–4842. [Google Scholar] [CrossRef]
- Diaz, I.; Namkoong, J.; Wu, J.Q.; Giancola, G. Amino acid complex (AAComplex) benefits in cosmetic products: In vitro and in vivo clinical studies. J. Cosmet. Dermatol. 2021, 21, 3046–3052. [Google Scholar] [CrossRef]
- Avato, P.; Tava, A. Rare fatty acids and lipids in plant oilseeds: Occurrence and bioactivity. Phytochem. Rev. 2021, 21, 401–428. [Google Scholar] [CrossRef]
- Weeraphan, T.; Savarajara, A. Production of oil with increasing palmitoleic acid content by Cyberlindnera subsufficiens NG8.2 from oil palm empty fruit bunch via co-fermentation with cassava starch. Agric. Nat. Resour. 2021, 55, 958–967. [Google Scholar]
- Sroczyk, E.A.; Berniak, K.; Jaszczur, M.; Stachewicz, U. Topical electrospun patches loaded with oil for effective gamma linoleic acid transport and skin hydration towards atopic dermatitis skincare. Chem. Eng. J. 2022, 429, 132256. [Google Scholar] [CrossRef]
- Agarwal, S.; Arya, D.; Khan, S. Comparative fatty acid and trace elemental analysis identified the best raw material of jojoba (Simmondsia chinensis) for commercial applications. Ann. Agric. Sci. 2018, 63, 37–45. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Chao, E.; Tian, J.; Fan, L.; Zhang, T. Drying methods influence the physicochemical and functional properties of seed-used pumpkin. Food Chem. 2021, 369, 130937. [Google Scholar] [CrossRef]
- Kikuchi, T.; Ueda, S.; Kanazawa, J.; Naoe, H.; Yamada, T.; Tanaka, R. Three New Triterpene Esters from Pumpkin (Cucurbita maxima) Seeds. Molecules 2014, 19, 4802–4813. [Google Scholar] [CrossRef] [Green Version]
- Endo, K.; Mizutani, T.; Okano, Y.; Masaki, H. A red pumpkin seed extract reduces melanosome transfer to keratinocytes by activation of Nrf2 signaling. J. Cosmet. Dermatol. 2018, 18, 827–834. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Rathinavelu, A.; Levy, A.; Sivanesan, D.; Murugan, D.; Jornadal, J.; Quinonez, Y.; Jaffe, M.; Gossell-Williams, M. Cytotoxic effect of pumpkin (curcurbita pepo) seed extracts in lncap prostate cancer cells is mediated through apoptosis. Curr. Top. Nutraceuticals Res. 2013, 11, 137–143. [Google Scholar]
- Elkholy, Y.M.; Helal, M.H.; Hamza, A.S.; Masoud, M.S.; Badr, S.E.A. The potential cytotoxicity and antimicrobial activities for rind and seeds oil extracts of pumpkin (Cucurbita pepo L.). J. Agric. Chem. Biotechnol. 2009, 34, 19–38. [Google Scholar] [CrossRef]
- Piccolella, S.; Bianco, A.; Crescente, G.; Santillo, A.; Chieffi Baccari, G.; Pacifico, S. Recovering Cucurbita pepo cv. ‘Lungo Fiorentino’ Wastes: UHPLC-HRMS/MS metabolic profile, the basis for establishing their nutra- and cosmeceutical valorisa-tion. Molecules 2019, 15, 1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timares, L.; Katiyar, S.K.; Elmets, C.A. DNA Damage, Apoptosis and Langerhans Cells—Activators of UV-induced Immune Tolerance. Photochem. Photobiol. 2008, 84, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [Green Version]
- Dutra, E.A.; Oliveira, D.A.; Kedor-Hackmann, E.R.M.; Santoro, M.I. Determination of sun protection factor (SPF) of sun-screens by ultraviolet spectrophotometry. Braz. J. Pharm. Sci. 2004, 40, 381–385. [Google Scholar]
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The efficacy and safety of sunscreen use for the prevention of skin cancer. Can. Med. Assoc. J. 2020, 192, E1802–E1808. [Google Scholar] [CrossRef]
- Mansur, J.S.; Breder, M.N.; Mansur, M.C.; Azulay, R.D. Determination of sun protection factor by spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Lacatusu, I.; Arsenie, L.V.; Badea, G.; Popa, O.; Oprea, O.; Badea, N. New cosmetic formulations with broad photoprotective and antioxidative activities designed by amaranth and pumpkin seed oils nanocarriers. Ind. Crop. Prod. 2018, 123, 424–433. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Matejić, J.; Džamić, A.; Mihajilov-Krstev, T.; Ranđelović, V.; Krivošej, Z.; Marin, P. Total phenolic content, flavonoid concentration, antioxidant and antimicrobial activity of methanol extracts from three Seseli L. taxa. Open Life Sci. 2012, 7, 1116–1122. [Google Scholar] [CrossRef]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea biebersteinii Afan. Extract. Molecules 2021, 26, 964. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Ishikawa, S.; Tomoda, H. Inhibition of tyrosinase activity and melanine pigmentation by 2-hydroxytyrosol. Acta Pharm. Sin. B 2014, 4, 141–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]


| Species/Variety | Extract | Total Phenolics (mg GAE/g dw) | Flavonoids (mg QE/g dw) | Protein (mg BSA/g dw) |
|---|---|---|---|---|
| C. maxima/Halloween | W | 12.116 ± 0.233 a | 5.032 ± 0.910 a | 29.892 ± 3.513 a |
| HG | 13.920 ± 0.153 b | 3.967 ± 0.269 ab | 25.841 ± 4.719 a | |
| E | 17.599 ± 0.124 c | 3.114 ± 0.558 b | 7.871 ± 0.535 b | |
| C. maxima/Hokkaido | W | 17.336 ± 0.249 a | 7.108 ± 0.120 a | 47.470 ± 5.277 a |
| HG | 12.593 ± 0.493 b | 2.967 ± 0.081 b | 22.128 ± 4.421 b | |
| E | 4.623 ± 0.082 c | 3.629 ± 0.258 c | 15.588 ± 0.925 b | |
| C. moschata/Butternut | W | 11.005 ± 0.179 a | 5.032 ± 0.123 a | 18.618 ± 0.385 a |
| HG | 9.879 ± 0.737 b | 4.542 ± 0.099 b | 10.722 ± 0.578 b | |
| E | 6.575 ± 0.109 c | 3.381 ± 0.055 c | 5.136 ± 0.545 c | |
| C. moschata/Nelson | W | 16.871 ± 0.382 a | 4.294 ± 0.254 a | 68.695 ± 7.790 a |
| HG | 10.646 ± 0.386 b | 3.036 ± 0.123 b | 16.692 ± 4.022 b | |
| E | 16.599 ± 0.247 a | 1.738 ± 0.079 c | 44.427 ± 7.319 c | |
| C. moschata/Muscat | W | 14.700 ± 0.935 a | 2.598 ± 0.127 a | 76.143 ± 4.261 a |
| HG | 11.619 ± 0.406 b | 2.511 ± 0.222 a | 21.957 ± 3.207 b | |
| E | 7.171 ± 0.189 c | 1.983 ± 0.111 b | 25.681 ± 3.579 b |
| Ionization Mode | RT [min] | Molecular Formula | m/z Experimental | m/z Calculated | Delta [ppm] | DBE | Tentative Compound | Ref. | W | HG | E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [M-H]− | 2.00 | C6H12O6 | 179.0570 | 179.0561 | −4.93 | 1 | Hexahydroxycyclohexane | + | + | + | |
| [M-H]+ | 2.05 | C5H11NO2 | 118.0863 | 118.0863 | −0.38 | 1 | L-valine | [22] | +++ | ++ | nd |
| [M-H]− | 2.06 | C16H18O9 | 353.0858 | 353.0878 | 5.66 | 8 | Chlorogenic acid | [11] | nd | + | nd |
| [M-H]+ | 2.96 | C10H13N5O4 | 268.1043 | 268.1040 | −1.01 | 7 | Adenosine | [23] | nd | ++ | +++ |
| [M-H]+ | 4.61 | C9H11NO2 | 166.0862 | 166.0863 | 0.33 | 5 | L-phenylalanine | [22] | +++ | ++ | +++ |
| [M-H]+ | 12.22 | C9H11NO3 | 182.0808 | 182.0812 | 2.04 | 5 | L-tyrosine | [22] | ++ | nd | nd |
| [M-H]+ | 12.78 | C11H12N2O2 | 205.0986 | 205.0972 | −7.08 | 7 | L-tryptophan | [22] | ++ | nd | +++ |
| [M-H]− | 13.87 | C7H6O3 | 137.0245 | 137.0244 | −0.60 | 5 | p-hydroxybenzoic acid | [24] | + | ++ | + |
| [M-H]− | 18.21 | C9H8O3 | 163.0408 | 163.0350 | −4.46 | 6 | p-coumaric acid | [24] | + | + | + |
| [M-H]− | 21.90 | C15H10O5 | 269.0485 | 269.0455 | −10.93 | 11 | Apigenin | [25] | nd | nd | + |
| [M-H]− | 36.45 | C16H30O2 | 253.2207 | 253.2173 | −13.36 | 2 | Palmitoleic acid | [22] | + | nd | nd |
| [M-H]− | 37.20 | C16H32O2 | 255.2340 | 255.2330 | −4.08 | 1 | Palmitic acid | [22] | ++ | + | + |
| [M-H]− | 37.66 | C18H32O2 | 279.2333 | 279.2330 | −1.24 | 3 | Linoleic acid | [22] | ++ | nd | ++ |
| [M-H]− | 37.88 | C18H34O2 | 281.2495 | 281.2486 | −3.17 | 2 | Oleic acid | [22] | ++ | + | nd |
| Ionization Mode | RT [min] | Molecular Formula | m/z Experimental | m/z Calculated | Delta [ppm] | DBE | Tentative Compound | W | HG | E |
|---|---|---|---|---|---|---|---|---|---|---|
| [M-H]+ | 1.90 | C6H14N4O2 | 175.1192 | 175.1190 | −0.27 | 2 | Arginin | nd | nd | ++ |
| [M-H]+ | 2.09 | C5H11NO2 | 118.0869 | 118.0863 | −5.51 | 1 | L-valine | nd | ++ | + |
| [M-H]+ | 2.22 | C5H9NO2 | 116.0706 | 116.0706 | −9.52 | 2 | Proline | nd | nd | ++ |
| [M-H]+ | 2.88 | C10H13N5O4 | 268.1048 | 268.1040 | −2.88 | 7 | Adenosine | nd | +++ | ++ |
| [M-H]+ | 3.97 | C9H11NO3 | 182.0811 | 182.0812 | 0.38 | 5 | L-tyrosine | nd | ++ | ++ |
| [M-H]+ | 4.60 | C9H11NO2 | 166.0869 | 166.0863 | −3.91 | 5 | L-phenylalanine | nd | ++ | +++ |
| [M-H]+ | 5.29 | C3H7NO2S | 122.0269 | 122.0270 | 1.04 | 1 | Cysteine | ++ | nd | nd |
| [M-H]− | 10.44 | C7H6O3 | 137.0221 | 137.0244 | 16.79 | 5 | p-hydroxybenzoic acid | + | + | + |
| [M-H]+ | 12.62 | C11H12N2O2 | 205.0973 | 205.0972 | −0.71 | 7 | L-tryptophan | nd | nd | +++ |
| [M-H]+ | 12.83 | C20H18NO4 | 337.1305 | 337.1309 | 1.07 | 13 | Berberine | nd | nd | ++ |
| [M-H]− | 16.47 | C9H8O3 | 163.0407 | 163.0401 | −3.85 | 6 | p-coumaric acid | + | + | + |
| [M-H]− | 21.98 | C15H10O5 | 269.0455 | 269.0455 | 0.17 | 11 | Apigenin | nd | nd | + |
| [M-H]− | 22.78 | C15H10O6 | 285.0416 | 285.0405 | −3.98 | 11 | Kaempferol | nd | nd | + |
| [M-H]− | 22.78 | C15H10O6 | 285.0416 | 285.0405 | −3.98 | 11 | Luteolin | nd | nd | + |
| [M-H]− | 25.34 | C18H36O2 | 283.2639 | 283.2643 | 1.25 | 1 | Stearic acid | + | + | nd |
| [M-H]− | 25.39 | C18H34O2 | 281.2516 | 281.2486 | −10.61 | 2 | Oleic acid | nd | + | + |
| [M-H]− | 25.39 | C16H32O2 | 255.2353 | 255.2330 | −9.16 | 1 | Palmitic acid | nd | + | + |
| [M-H]− | 34.59 | C16H30O2 | 253.2185 | 253.2173 | −4.71 | 2 | Palmitoleic acid | + | nd | nd |
| [M-H]− | 37.69 | C18H32O2 | 279.2339 | 279.2330 | −3.38 | 3 | Linoleic acid | + | nd | ++ |
| Ionization Mode | RT [min] | Molecular Formula | m/z Experimental | m/z Calculated | Delta [ppm] | DBE | Tentative Compound | W | HG | E |
|---|---|---|---|---|---|---|---|---|---|---|
| [M-H]+ | 1.69 | C6H14N4O2 | 175.1201 | 175.1190 | −6.59 | 2 | Arginin | + | ++ | ++ |
| [M-H]+ | 2.03 | C5H9NO2 | 116.0708 | 116.0706 | −1.69 | 2 | Proline | nd | + | ++ |
| [M-H]− | 2.03 | C6H12O6 | 179.0570 | 179.0561 | −4.93 | 1 | Hexahydroxycyclohexane | + | + | + |
| [M-H]+ | 2.05 | C5H11NO2 | 118.0866 | 118.0863 | −2.95 | 1 | L-valine | +++ | ++ | ++ |
| [M-H]+ | 2.12 | C5H10N2O2 | 131.0801 | 131.0815 | 10.72 | 2 | Cucurbitine | nd | nd | ++ |
| [M-H]+ | 2.87 | C10H13N5O4 | 268.1051 | 268.1040 | −4.00 | 7 | Adenosine | +++ | +++ | +++ |
| [M-H]+ | 2.92 | C9H11NO3 | 182.0836 | 182.0812 | −13.42 | 5 | L-tyrosine | + | ++ | ++ |
| [M-H]+ | 3.93 | C3H7NO2S | 122.0257 | 122.0270 | −0.14 | 1 | Cysteine | ++ | nd | nd |
| [M-H]+ | 4.50 | C9H11NO2 | 166.0857 | 166.0863 | 3.36 | 5 | L-phenylalanine | +++ | ++ | +++ |
| [M-H]+ | 5.95 | C6H13NO5 | 180.0843 | 180.0866 | 13.12 | 1 | D-Glucopyranosylamine | nd | ++ | nd |
| [M-H]+ | 6.26 | C6H13N3O3 | 176.1037 | 176.1030 | −4.18 | 2 | Citrulline | nd | + | ++ |
| [M-H]− | 13.05 | C9H8O3 | 163.0419 | 163.0350 | −11.17 | 6 | p-coumaric acid | + | nd | + |
| [M-H]+ | 13.08 | C11H12N2O2 | 205.0962 | 205.0972 | 4.67 | 7 | L-tryptophan | ++ | ++ | ++ |
| [M-H]− | 13.82 | C11H12O5 | 223.0641 | 223.0612 | −12.96 | 6 | Sinapinic acid | nd | + | nd |
| [M-H]− | 13.90 | C7H6O3 | 137.0255 | 137.0244 | −7.84 | 5 | p-hydroxybenzoic acid | + | + | + |
| [M-H]− | 21.87 | C15H10O5 | 269.0482 | 269.0455 | −9.82 | 11 | Apigenin | nd | nd | + |
| [M-H]− | 22.60 | C15H10O6 | 285.0435 | 285.0405 | −10.62 | 11 | Kaempferol | nd | nd | + |
| [M-H]− | 22.60 | C15H10O6 | 285.0435 | 285.0405 | −10.62 | 11 | Luteolin | nd | nd | + |
| [M-H]− | 36.47 | C16H30O2 | 253.2152 | 253.2173 | 8.28 | 2 | Palmitoleic acid | + | + | nd |
| [M-H]− | 37.21 | C16H32O2 | 255.2339 | 255.2330 | −3.69 | 1 | Palmitic acid | + | nd | ++ |
| [M-H]− | 37.66 | C18H32O2 | 279.2352 | 279.2330 | −8.73 | 3 | Linoleic acid | + | + | + |
| [M-H]− | 38.07 | C18H34O2 | 281.2503 | 281.2486 | −6.01 | 2 | Oleic acid | ++ | nd | nd |
| Ionization Mode | RT [min] | Molecular Formula | m/z Experimental | m/z Calculated | Delta [ppm] | DBE | Tentative Compound | W | HG | E |
|---|---|---|---|---|---|---|---|---|---|---|
| [M-H]− | 2.07 | C6H12O6 | 179.0565 | 179.0561 | −2.16 | 1 | Hexahydroxycyclohexane | + | + | + |
| [M-H]+ | 2.12 | C5H11NO2 | 118.0868 | 118.0863 | −4.65 | 1 | L-valine | ++ | ++ | ++ |
| [M-H]+ | 2.16 | C5H10N2O2 | 131.0818 | 131.0815 | −2.28 | 2 | Cucurbitine | ++ | nd | ++ |
| [M-H]+ | 2.20 | C6H13N3O3 | 176.1027 | 176.1030 | 1.53 | 2 | Citrulline | nd | nd | nd |
| [M-H]+ | 2.88 | C10H13N5O4 | 268.1046 | 268.1040 | −2.13 | 7 | Adenosine | nd | +++ | ++ |
| [M-H]+ | 2.92 | C3H7NO2S | 122.0274 | 122.0270 | −3.09 | 1 | Cysteine | + | nd | nd |
| [M-H]+ | 3.42 | C6H13NO5 | 180.0880 | 180.0866 | −7.54 | 1 | D-Glucopyranosylamine | + | + | nd |
| [M-H]+ | 4.82 | C9H11NO2 | 166.0868 | 166.0863 | −3.30 | 5 | L-phenylalanine | ++ | ++ | ++ |
| [M-H]+ | 8.15 | C9H11NO3 | 182.0837 | 182.0812 | −13.97 | 5 | L-tyrosine | + | ++ | ++ |
| [M-H]− | 13.17 | C7H6O3 | 137.0253 | 137.0244 | −6.39 | 5 | p-hydroxybenzoic acid | + | + | + |
| [M-H]+ | 14.09 | C20H18NO4 | 337.1281 | 337.1309 | 8.21 | 13 | Berberine | nd | nd | ++ |
| [M-H]− | 18.08 | C9H8O3 | 163.0425 | 163.0350 | −14.83 | 6 | p-coumaric acid | + | + | + |
| [M-H]− | 22.47 | C15H10O5 | 269.0483 | 269.0455 | −10.19 | 11 | Apigenin | nd | nd | + |
| [M-H]− | 31.10 | C18H32O2 | 279.2360 | 279.2330 | −10.87 | 3 | Linoleic acid | nd | + | + |
| [M-H]− | 34.84 | C16H30O2 | 253.2165 | 253.2173 | 3.16 | 2 | Palmitoleic acid | + | nd | + |
| [M-H]− | 37.24 | C16H32O2 | 255.2345 | 255.2330 | −6.03 | 1 | Palmitic acid | + | nd | ++ |
| Ionization Mode | RT [min] | Molecular Formula | m/z Experimental | m/z Calculated | Delta [ppm] | DBE | Tentative Compound | W | HG | E |
|---|---|---|---|---|---|---|---|---|---|---|
| [M-H]− | 2.03 | C6H12O6 | 179.0545 | 179.0561 | 8.95 | 1 | hexahydroxycyclohexane | + | nd | + |
| [M-H]+ | 2.06 | C5H11NO2 | 118.0868 | 118.0863 | −4.65 | 1 | L-valine | ++ | nd | ++ |
| [M-H]− | 2.10 | C16H18O9 | 353.0882 | 353.0878 | −1.11 | 8 | Chlorogenic acid | + | nd | nd |
| [M-H]+ | 2.12 | C5H9NO2 | 116.0705 | 116.0706 | 0.91 | 2 | Proline | nd | + | ++ |
| [M-H]+ | 2.14 | C5H10N2O2 | 131.0806 | 131.0815 | 6.95 | 2 | Cucurbitine | ++ | nd | nd |
| [M-H]+ | 2.20 | C6H13N3O3 | 176.1027 | 176.1030 | 1.53 | 2 | Citrulline | nd | ++ | nd |
| [M-H]+ | 2.92 | C9H11NO3 | 182.0809 | 182.0812 | 1.49 | 5 | L-tyrosine | ++ | ++ | ++ |
| [M-H]+ | 2.88 | C10H13N5O4 | 268.1046 | 268.1040 | −2.13 | 7 | Adenosine | +++ | +++ | +++ |
| [M-H]+ | 4.60 | C9H11NO2 | 166.0865 | 166.0863 | −1.48 | 5 | L-phenylalanine | +++ | ++ | +++ |
| [M-H]+ | 12.72 | C11H12N2O2 | 205.0970 | 205.0972 | 0.76 | 7 | L-tryptophan | ++ | nd | +++ |
| [M-H]− | 13.22 | C7H6O3 | 137.0250 | 137.0244 | −4.22 | 5 | p-hydroxybenzoic acid | + | nd | + |
| [M-H]− | 13.69 | C11H12O5 | 223.0637 | 223.0612 | −11.17 | 6 | Sinapinic acid | + | nd | + |
| [M-H]− | 18.06 | C9H8O3 | 163.0389 | 163.0350 | 7.12 | 6 | p-coumaric acid | + | + | + |
| [M-H]− | 22.65 | C15H10O6 | 285.0414 | 285.0405 | −3.28 | 11 | Luteolin | nd | - | + |
| [M-H]− | 22.73 | C15H10O6 | 285.0404 | 285.0405 | 0.22 | 11 | Kaempferol | nd | - | + |
| [M-H]− | 31.10 | C18H32O2 | 279.2360 | 279.2330 | −10.87 | 3 | Linoleic acid | nd | + | + |
| [M-H]− | 31.10 | C18H36O2 | 283.2677 | 283.2646 | −12.12 | 1 | Stearic acid | nd | + | - |
| [M-H]− | 33.29 | C16H32O2 | 255.2360 | 255.2330 | −11.89 | 1 | Palmitic acid | nd | + | ++ |
| Species/Variety | Extract | ABTS Scavenging (µg TE/g dw) | DPPH Scavenging (µg TE/g dw) |
|---|---|---|---|
| C. maxima/Halloween | W | 3.018 ± 1.085 | 3.272 ± 0.052 a |
| HG | 3.855 ± 0.055 | 2.047 ± 0.149 b | |
| E | 4.323 ± 0.071 | 2.140 ± 0.169 b | |
| C. maxima/Hokkaido | W | 4.382 ± 0.475 | 2.495 ± 0.055 a |
| HG | 3.764 ± 0.048 | 2.701 ± 0.082 a | |
| E | 4.460 ± 0.043 | 1.999 ± 0.065 b | |
| C. moschata/Butternut | W | 4.524 ± 0.231 a | 3.333 ± 0.004 a |
| HG | 3.304 ± 0.011 b | 0.947 ± 0.026 b | |
| E | 3.600 ± 0.153 b | 1.386 ± 0.111 c | |
| C. moschata/Nelson | W | 3.695 ± 0.040 a | 2.207 ± 0.266 a |
| HG | 3.529 ± 0.021 b | 1.444 ± 0.209 b | |
| E | 3.802 ± 0.025 c | 2.549 ± 0.074 a | |
| C. moschata/Muscat | W | 3.898 ± 0.042 a | 2.862 ± 0.283 a |
| HG | 2.470 ± 0.041 b | 2.294 ± 0.103 b | |
| E | 3.552 ± 0.195 a | 2.473 ± 0.079 ab |
| Species/Variety | Extract | ||
|---|---|---|---|
| W | HG | E | |
| C. maxima ‘Halloween’ | 5.83 ± 0.11 a | 3.06 ± 0.13 b | 7.30 ± 0.35 c |
| C. maxima ‘Hokkaido’ | 6.35 ± 0.13 a | 2.61 ± 0.35 b | 1.64 ± 0.09 c |
| C. moschata ‘Butternut’ | 7.59 ± 0.83 a | 0.38 ± 0.04 b | 2.73 ± 0.08 c |
| C. moschata ‘Nelson’ | 7.27 ± 0.44 a | 1.10 ± 0.08 b | 4.15 ± 0.05 c |
| C. moschata ‘Muscat’ | 2.56 ± 0.04 a | 2.07 ± 0.08 b | 1.83 ± 0.30 b |
| TiO2 (1 mg/mL) | - | - | 17.34 ± 0.32 |
| W Extracts | HG Extracts | E Extracts | |
|---|---|---|---|
| Flavonoids (mg QE/g dw) | |||
| ABTS (µg TE/g dw) | 0.345 | 0.289 | 0.425 |
| DPPH (µg TE/g dw) | 0.001 | −0.649 ** | −0.689 ** |
| SPF | 0.363 | −0.341 | −0.232 |
| Cytotoxicity | −0.163 | −0.320 | 0.121 |
| Mushroom tyrosinase | −0.061 | −0.797 *** | −0.100 |
| Murine tyrosinase | 0.553 * | 0.309 | −0.761 *** |
| Total polyphenols (mg GAE/g dw) | |||
| ABTS (µg TE/g dw) | −0.229 | 0.651 ** | −0.039 |
| DPPH (µg TE/g dw) | −0.808 *** | 0.694 ** | 0.430 |
| SPF | −0.047 | 0.932 *** | 0.831 *** |
| Cytotoxicity | 0.400 | −0.035 | −0.482 |
| Mushroom tyrosinase | −0.147 | 0.038 | −0.165 |
| Murine tyrosinase | −0.236 | 0.790 *** | 0.218 |
| Proteins | |||
| ABTS (µg TE/g dw) | −0.426 | 0.49 | −0.029 |
| DPPH (µg TE/g dw) | −0.721 ** | 0.713 ** | 0.850 *** |
| SPF | −0.443 | 0.797 *** | −0.157 |
| Cytotoxicity | 0.516 * | 0.094 | 0.036 |
| Mushroom tyrosinase | −0.016 | 0.371 | 0.229 |
| Murine tyrosinase | −0.550 * | 0.486 | 0.618 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaweł-Bęben, K.; Czech, K.; Strzępek-Gomółka, M.; Czop, M.; Szczepanik, M.; Lichtarska, A.; Kukula-Koch, W. Assessment of Cucurbita spp. Peel Extracts as Potential Sources of Active Substances for Skin Care and Dermatology. Molecules 2022, 27, 7618. https://doi.org/10.3390/molecules27217618
Gaweł-Bęben K, Czech K, Strzępek-Gomółka M, Czop M, Szczepanik M, Lichtarska A, Kukula-Koch W. Assessment of Cucurbita spp. Peel Extracts as Potential Sources of Active Substances for Skin Care and Dermatology. Molecules. 2022; 27(21):7618. https://doi.org/10.3390/molecules27217618
Chicago/Turabian StyleGaweł-Bęben, Katarzyna, Karolina Czech, Marcelina Strzępek-Gomółka, Marcin Czop, Monika Szczepanik, Anna Lichtarska, and Wirginia Kukula-Koch. 2022. "Assessment of Cucurbita spp. Peel Extracts as Potential Sources of Active Substances for Skin Care and Dermatology" Molecules 27, no. 21: 7618. https://doi.org/10.3390/molecules27217618
APA StyleGaweł-Bęben, K., Czech, K., Strzępek-Gomółka, M., Czop, M., Szczepanik, M., Lichtarska, A., & Kukula-Koch, W. (2022). Assessment of Cucurbita spp. Peel Extracts as Potential Sources of Active Substances for Skin Care and Dermatology. Molecules, 27(21), 7618. https://doi.org/10.3390/molecules27217618










