Can Natural Products Targeting EMT Serve as the Future Anticancer Therapeutics?
Abstract
:1. Introduction
2. Cross-Talk between TGF-β and Other Signaling Pathways Mediating EMT
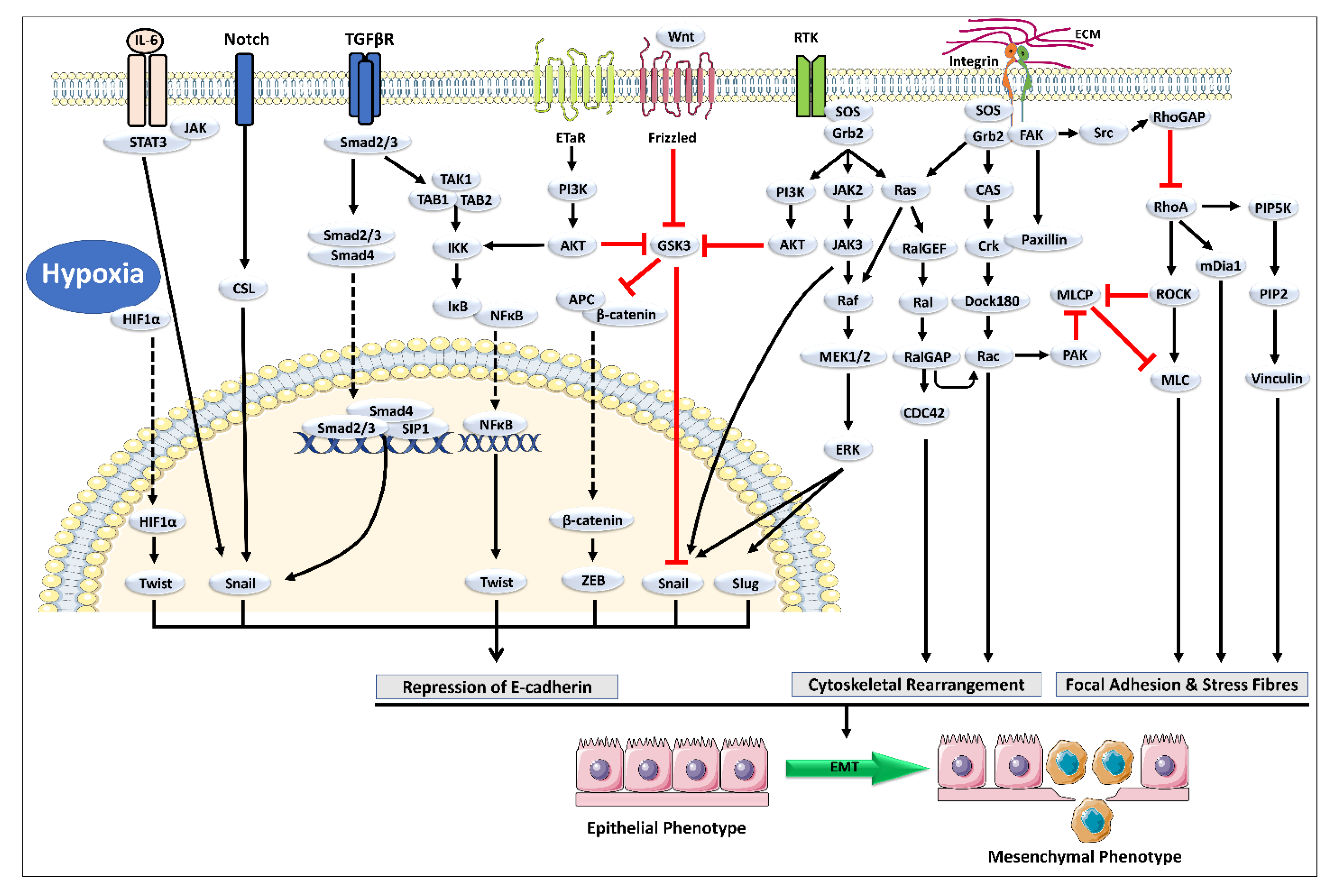
3. Natural Chemical Agents as Potential Leads against Cancer
| Sr. No | Drug Product | Source (Marine Origin) | Mechanism of Action | Indication | FDA Status | Reference |
|---|---|---|---|---|---|---|
| Marine Source | ||||||
| 1 | Eribulin mesylate | Sponge Halichondria okadai | Keeps the cytoskeleton’s growth cycle away from core aggregates tubulin | Metastatic breast cancer | Approved (Spain) | [46] |
| 2 | Brentuximab Vedotin | Sea hare Dollabella Auricularia/ cyanobacteria | Cell cycle arrest from G2 to M phase | Hodgkin lymphoma | Approved (USA.) | [47] |
| 3 | Cytarabine, Ara-C | Sponge Cryptotheca crypta | Inhibition of DNA Synthesis | Acute lymphoblastic leukemia | Approved (USA.) | [46] |
| 4 | Halichondramide (HCA) | Marine sponge Chondrosia corticata | Phosphatase of regenerating liver-3 (PRL-3) and its downstream signaling pathway are suppressed. | Prostate Cancer | Approved | [48] |
| Plant Source | ||||||
| 4 | Ixabepilone | Soragium cellulosum | Cell-cycle arrest and apoptosis-inducer | Hand-foot syndrome | Approved | [49] |
| 5 | Romidepsin | Chromobacterium violaceum | Histone deacetylase inhibitors | Hematological toxicities like anemia | Approved | [50] |
| 6 | Podophyllotoxins | Podophyllum (Berberidaceae) | Inhibit the polymerization of tubulin, arresting the cell cycle in the metaphase | Ovarian cancer, immunosuppressive ability | Approved | [51] |
| 7 | Ligustrazine | Rhizome of Ligusticum wallichii. | Inhibit SK-OV-3 and OVCAR-3 cell viability, proliferation, migration, and invasion. | Ovarian cancer | Approved | [52] |
| Sr. No | NCE. | Source | Mechanism and Outcomes | Method of Validation | Potential Use | Reference |
|---|---|---|---|---|---|---|
| 1 | Oregonin | Alnus sibirica (AS) | Anti-proliferative activity, Inhibition of NF-κB, induction of apoptosis, DNA Methylation | MTT Assay, Western blotting, Flow, methylation-specific PCR, cytometry | Prostate cancer | [53] |
| 2 | Hirsutenone | |||||
| 3 | Hirsutanonol | |||||
| 4 | Chelerythrine chloride | Chelidonium majus and Macleaya cordata | cytotoxicity and anti-proliferative activity | Cell viability assays | NSCLC. | [39] |
| 5 | Thioholgamide | Streptomyces sp. MUSC 136T. | Caspase 3/7 Activation, membrane permeability | MTT assay | Colon, breast, liver, and lung cancers | [54] |
| 6 | 7-deoxy-trans-dihydronarciclasin | Scadoxus pseudocaulus | Apoptosis inducer | Cytotoxicity assay | Follicular lymphoma | [55] |
| 7 | 4-(4-hydroxy-3-methoxyphenyl) curcumin | Anti-proliferative, apoptosis-inducing | MTT assay, Western blotting analysis | Hepatic, colon, chronic myeloid leukemia, and lung cancer | [56,57,58] |
4. Potential NCE to Target EMT
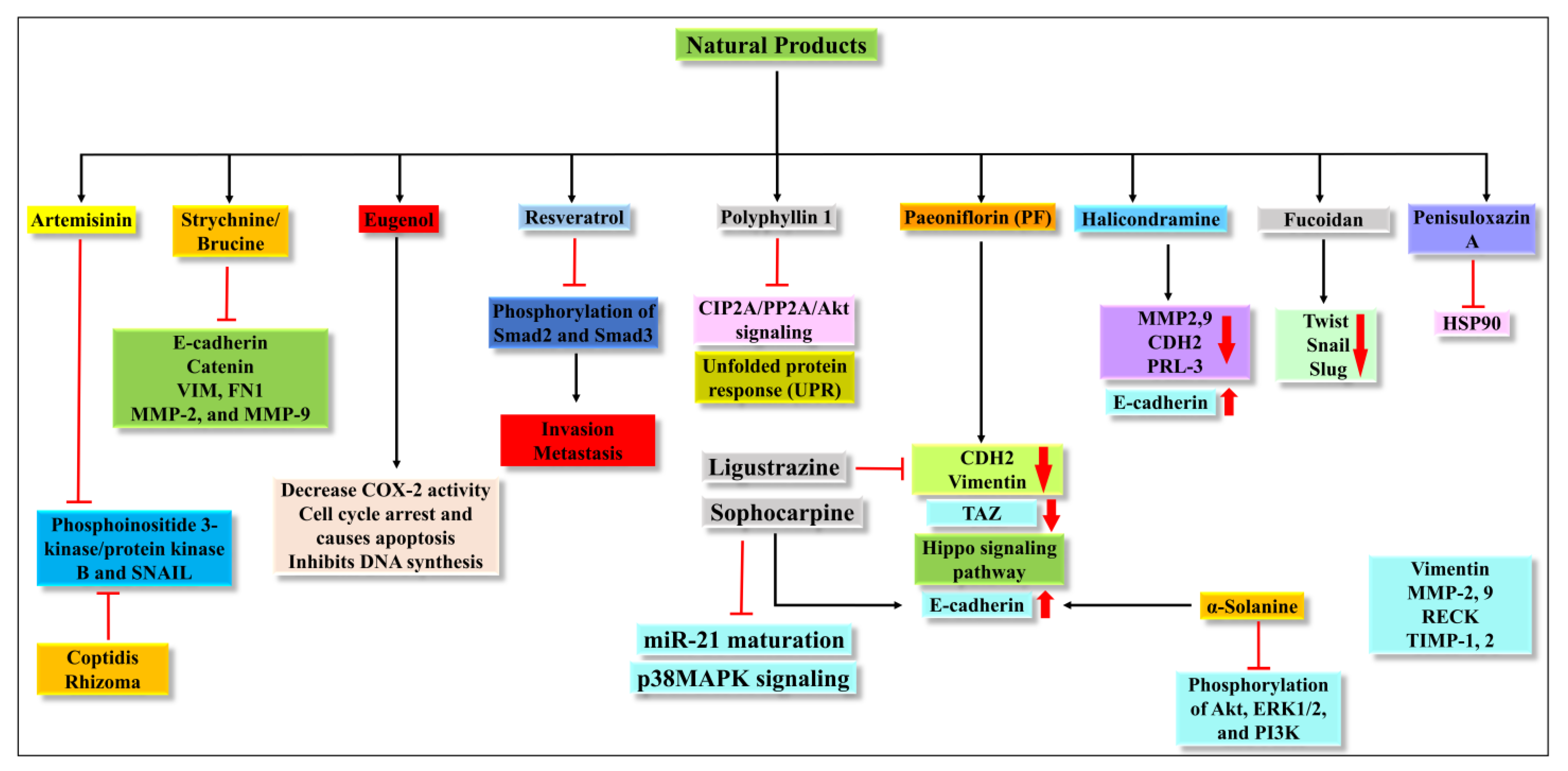
4.1. Artemisinin (ATM)
4.2. Strychnine/Brucine
4.3. Eugenol
4.4. Resveratrol
4.5. Polyphyllin 1
4.6. Paeoniflorin (PF)
4.7. Halicondramine
4.8. Ligustrazine
4.9. Fucoidan
4.10. Penisuloxazin A
4.11. Sophocarpine
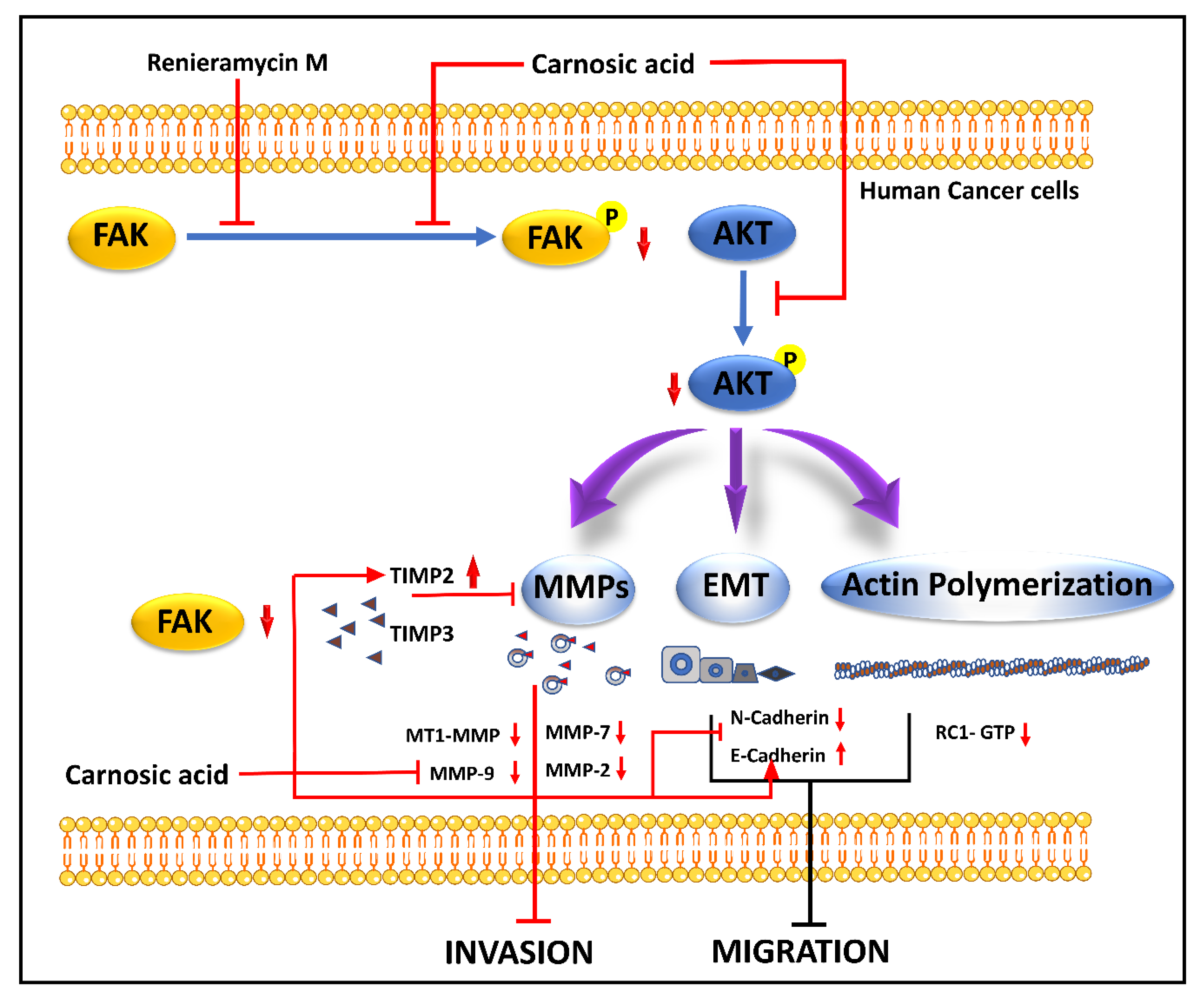
4.12. Renieramycin M
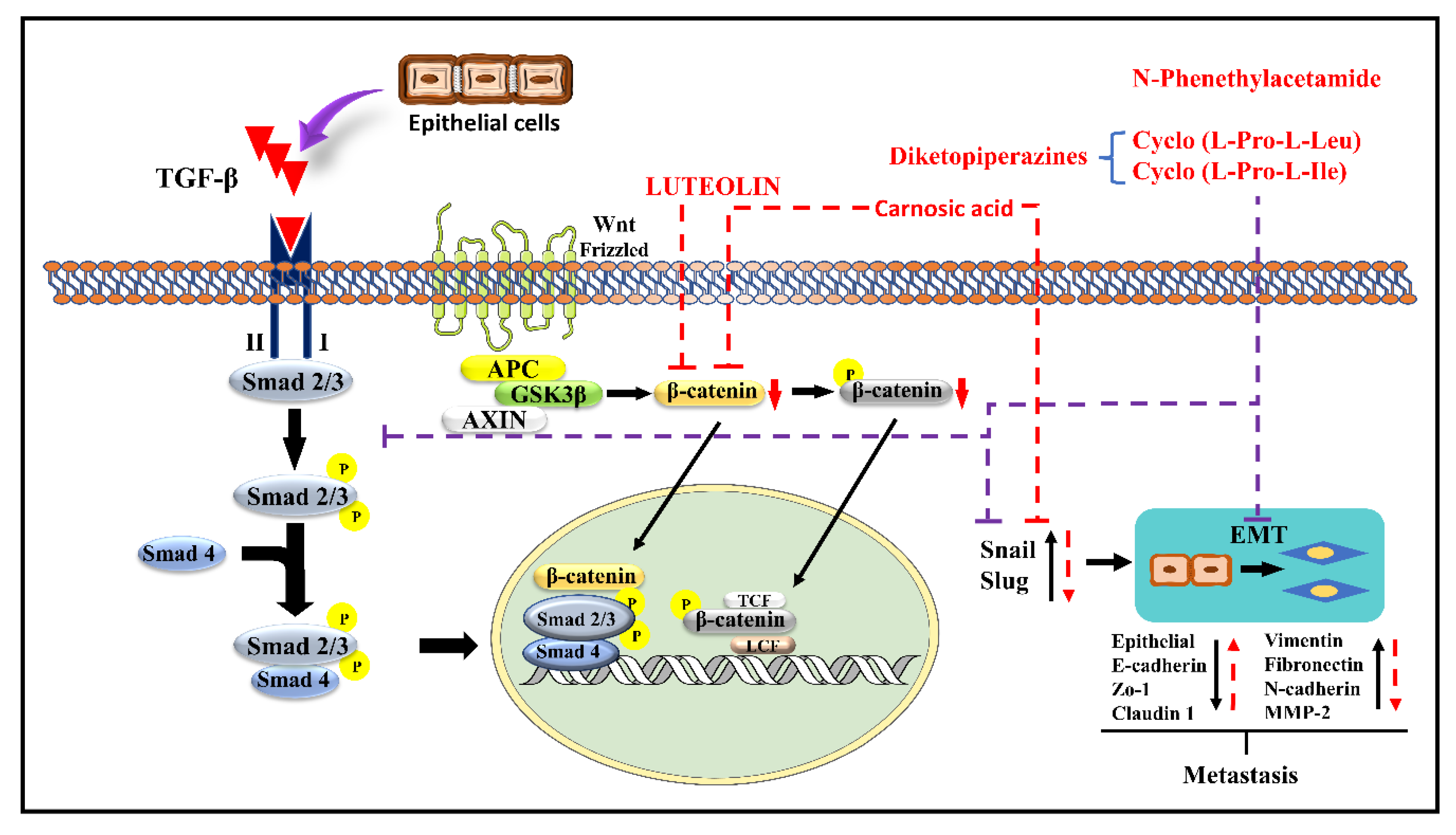
4.13. Luteolin
4.14. Carnosic Acid
4.15. N-Phenethylacetamide
4.16. α-Solanine
4.17. Baicalein, Wogonin (WG), and Oroxylin-A (ORA)
4.18. Coptidis Rhizoma
5. Advantages of Targeting EMT
6. Targeting EMT Process by Molecular Docking (MD)
7. Future Prospective
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Malik, J.A.; Ahmed, S.; Jan, B.; Bender, O.; Al Hagbani, T.; Alqarni, A.; Anwar, S. Drugs repurposed: An advanced step towards the treatment of breast cancer and associated challenges. Biomed. Pharmacother. 2021, 145, 112375. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.K.; Luna, A.; La, K.C.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337.e310. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González-Mateo, G.T. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef] [Green Version]
- Gundamaraju, R.; Lu, W.; Paul, M.K.; Jha, N.K.; Gupta, P.K.; Ojha, S.; Chattopadhyay, I.; Rao, P.V.; Ghavami, S. Autophagy and EMT in cancer and metastasis: Who controls whom? Biochim. Biophys. Acta-Mol. Basis Dis. 2022, 1868, 166431. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef]
- Chiu, K.-J.; Chiou, H.-Y.C.; Huang, C.-H.; Lu, P.-C.; Kuo, H.-R.; Wang, J.-W.; Lin, M.-H. Natural Compounds Targeting Cancer-Associated Fibroblasts against Digestive System Tumor Progression: Therapeutic Insights. Biomedicines 2022, 10, 713. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Tan, Z.W.; Zhu, P.; Tan, N.S. Cancer-associated fibroblasts in tumor microenvironment—Accomplices in tumor malignancy. Cell. Immunol. 2019, 343, 103729. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, M.S.; Nushtaeva, A.A.; Richter, V.A.; Koval, O. Cancer-associated fibroblasts and their role in tumor progression. Vavilov J. Genet. Breed. 2022, 26, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-H.; Hsia, S.-M.; Shieh, T.-M. Lysyl Oxidase and the Tumor Microenvironment. Int. J. Mol. Sci. 2016, 18, 62. [Google Scholar] [CrossRef] [Green Version]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Tune, B.X.J.; Sim, M.S.S.; Poh, C.L.; Mac Guad, R.; Woon, C.K.; Hazarika, I.; Das, A.; Gopinath, S.C.B.; Rajan, M.; Sekar, M.; et al. Matrix Metalloproteinases in Chemoresistance: Regulatory Roles, Molecular Interactions, and Potential Inhibitors. J. Oncol. 2022, 2022, 3249766. [Google Scholar] [CrossRef]
- Mochizuki, S.; Ao, T.; Sugiura, T.; Yonemura, K.; Shiraishi, T.; Kajiwara, Y.; Okamoto, K.; Shinto, E.; Okada, Y.; Ueno, H. Expression and Function of a Disintegrin and Metalloproteinases in Cancer-Associated Fibroblasts of Colorectal Cancer. Digestion 2020, 101, 18–24. [Google Scholar] [CrossRef]
- Glentis, A.; Oertle, P.; Mariani, P.; Chikina, A.; El Marjou, F.; Attieh, Y.; Zaccarini, F.; Lae, M.; Loew, D.; Dingli, F.; et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017, 8, 924. [Google Scholar] [CrossRef]
- Lindsey, S.; Langhans, S.A. Crosstalk of Oncogenic Signaling Pathways during Epithelial–Mesenchymal Transition. Front. Oncol. 2014, 4, 358. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Yu, M.; Trobridge, P.; Wang, Y.; Kanngurn, S.; Morris, S.M.; Knoblaugh, S.; Grady, W.M. Inactivation of TGF-β signaling and loss of PTEN cooperate to induce colon cancer in vivo. Oncogene 2014, 33, 1538–1547. [Google Scholar] [CrossRef] [Green Version]
- Holderfield, M.T.; Hughes, C.C. Crosstalk Between Vascular Endothelial Growth Factor, Notch, and Transforming Growth Factor-β in Vascular Morphogenesis. Circ. Res. 2008, 102, 637–652. [Google Scholar] [CrossRef] [Green Version]
- Niimi, H.; Pardali, K.; Vanlandewijck, M.; Heldin, C.-H.; Moustakas, A. Notch signaling is necessary for epithelial growth arrest by TGF-β. J. Cell Biol. 2007, 176, 695–707. [Google Scholar] [CrossRef] [Green Version]
- Bhaskaran, N.; Souchelnytskyi, S. Systemic analysis of TGFβ proteomics revealed involvement of Plag1/CNK1/RASSF1A/Src network in TGFβ1-dependent activation of Erk1/2 and cell proliferation. Proteomics 2008, 8, 4507–4520. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.-F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M.; Doody, J.; Timokhina, I.; Massague, J. A mechanism of repression of TGFbeta / Smad signaling by oncogenic Ras. Genes Dev. 1999, 13, 804–816. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Luo, C.; Huang, C.; Pu, F.-Q.; Zhu, J.-F.; Zhu, Z.-M. PI3K/Akt/GSK-3β signal pathway is involved in P2X7 receptor-induced proliferation and EMT of colorectal cancer cells. Eur. J. Pharmacol. 2021, 899, 174041. [Google Scholar] [CrossRef]
- Ueda, Y.; Wang, S.; Dumont, N.; Yi, J.Y.; Koh, Y.; Arteaga, C.L. Overexpression of HER2 (erbB2) in Human Breast Epithelial Cells Unmasks Transforming Growth Factor β-induced Cell Motility. J. Biol. Chem. 2004, 279, 24505–24513. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Martin, A.; Cufí, S.; Oliveras-Ferraros, C.; Torres-Garcia, V.Z.; Corominas-Faja, B.; Cuyàs, E.; Bonavia, R.; Visa, J.; Martín-Castillo, B.; Barrajón-Catalán, E.; et al. IGF-1R/epithelial-to-mesenchymal transition (EMT) cross-talk suppresses the erlotinib-sensitizing effect of EGFR exon 19 deletion mutations. Sci. Rep. 2013, 3, srep02560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imodoye, S.O.; Adedokun, K.A.; Muhammed, A.O.; Bello, I.O.; Muhibi, M.A.; Oduola, T.; Oyenike, M.A. Understanding the Complex Milieu of Epithelial-Mesenchymal Transition in Cancer Metastasis: New Insight Into the Roles of Transcription Factors. Front. Oncol. 2021, 11, 4360. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Aware, C.B.; Patil, D.N.; Suryawanshi, S.S.; Mali, P.R.; Rane, M.R.; Gurav, R.G.; Jadhav, J.P. Natural bioactive products as promising therapeutics: A review of natural product-based drug development. S. Afr. J. Bot. 2022. [Google Scholar] [CrossRef]
- Dongare, P.N.; Motule, A.S.; More, M.P.; Patinge, P.A.; Bakal, R. An overview on anti-cancer drugs from marine source. World J. Pharm. Res. 2021, 10, 950–956. [Google Scholar] [CrossRef]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. 2009, 59, 365–378. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine-Sourced Anti-Cancer and Cancer Pain Control Agents in Clinical and Late Preclinical Development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic Potential of Natural Products in Treatment of Cervical Cancer: A Review. Nutrients 2021, 13, 154. [Google Scholar] [CrossRef]
- Heng, W.S.; Cheah, S.-C. Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma. Molecules 2020, 25, 224. [Google Scholar] [CrossRef] [Green Version]
- El-Adl, K.; Sakr, H.M.; Yousef, R.G.; Mehany, A.B.; Metwaly, A.M.; Elhendawy, M.A.; Radwan, M.M.; ElSohly, M.A.; Abulkhair, H.S.; Eissa, I.H. Discovery of new quinoxaline-2(1H)-one-based anticancer agents targeting VEGFR-2 as inhibitors: Design, synthesis, and anti-proliferative evaluation. Bioorganic Chem. 2021, 114, 105105. [Google Scholar] [CrossRef]
- Park, S.-A.; Surh, Y.-J. Modulation of tumor microenvironment by chemopreventive natural products. Ann. New York Acad. Sci. 2017, 1401, 65–74. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef]
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural Product Target Network Reveals Potential for Cancer Combination Therapies. Front. Pharmacol. 2019, 10, 557. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological Effects of Cisplatin Combination with Natural Products in Cancer Chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef]
- Pereira, R.B.; Evdokimov, N.M.; Lefranc, F.; Valentaõ, P.; Kornienko, A.; Pereira, D.M.; Andrade, P.B.; Gomes, N.G.M. Marine-derived anti-cancer agents: Clinical benefits, innovative mechanisms, and new targets. Mar. Drugs 2019, 17, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.F.; Su, J.; Ouyang, S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, G.D.; Jeon, J.-E.; Shin, J.; Lee, S.K. Antimetastatic Effect of Halichondramide, a Trisoxazole Macrolide from the Marine Sponge Chondrosia corticata, on Human Prostate Cancer Cells via Modulation of Epithelial-to-Mesenchymal Transition. Mar. Drugs 2013, 11, 2472–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarici, F.; Aksoy, S. Efficacy and safety of ixabepilone monotherapy and ixabepilone-capecitabine combination in patients with heavily pretreated metastatic breast cancer. Int. J. Hematol. Oncol. 2021, 31, 85–91. [Google Scholar] [CrossRef]
- Mehta-Shah, N.; Lunning, M.A.; Moskowitz, A.J.; Boruchov, A.M.; Ruan, J.; Lynch, P.; Hamlin, P.A.; Leonard, J.; Matasar, M.J.; Myskowski, P.L.; et al. Romidepsin and lenalidomide-based regimens have efficacy in relapsed/refractory lymphoma: Combined analysis of two phase I studies with expansion cohorts. Am. J. Hematol. 2021, 96, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.; Gohar, U.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-Ui-Haq, M.; Toma, S.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, Recent Advances and Future Prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guan, D.; Jiang, M.; Liang, C.; Li, L.; Zhao, N.; Zha, Q.; Zhang, W.; Lu, C.; Zhang, G.; et al. efficacy of leflunomide combined with ligustrazine in the treatment of rheumatoid arthritis: Prediction with network pharmacology and validation in a clinical trial. Chin. Med. 2019, 14, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seonu, S.-Y.; Kim, M.-J.; Yin, J.; Lee, M.-W. Alnus sibirica Compounds Exhibiting Anti-Proliferative, Apoptosis-Inducing, and GSTP1 Demethylating Effects on Prostate Cancer Cells. Molecules 2021, 26, 3830. [Google Scholar] [CrossRef]
- Dahlem, C.; Siow, W.X.; Lopatniuk, M.; Tse, W.K.F.; Kessler, S.M.; Kirsch, S.H.; Hoppstädter, J.; Vollmar, A.M.; Müller, R.; Luzhetskyy, A.; et al. Thioholgamide A, a New Anti-Proliferative Anti-Tumor Agent, Modulates Macrophage Polarization and Metabolism. Cancers 2020, 12, 1288. [Google Scholar] [CrossRef] [PubMed]
- Laure, A.; Pagning, N.; Tamokou, J.; Muhammad, B.T.; Ngnokam, D.; Azefacktapondjou, L.; Ali, M.S.; Hameed, M.W. Potential anti-proliferative effects of chemical constituents and hemisynthetic derivatives from Scadoxus pseudocaulus (Amarillydaceae). Afr. Health Sci. 2020, 20, 469–475. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, C.; Xiao, Z.; Huang, X.; Xu, J. Enhanced anti-hepatoma effect of a novel curcumin analog C086 via solid dispersion technology. Drug Deliv. 2020, 27, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, J.; Chen, R.; Liu, Y.; Lou, L.; Wu, Y.; Huang, L.; Fan, Y.; Gao, P.; Huang, M.; et al. Dual Inhibition of Bcr-Abl and Hsp90 by C086 Potently Inhibits the Proliferation of Imatinib-Resistant CML Cells. Clin. Cancer Res. 2015, 21, 833–843. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Liu, Y.; Chen, Y.; Xu, J. C086, a novel analog of curcumin, induces growth inhibition and down-regulation of NF?B in colon cancer cells and xenograft tumors. Cancer Biol. Ther. 2011, 12, 797–807. [Google Scholar] [CrossRef]
- Anibogwu, R.; De Jesus, K.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, H.; Wang, R.; Liu, D.; Waxman, S.; Zhao, L.; Jing, Y. Dihydroartemisinin and its derivative induce apoptosis in acute myeloid leukemia through Noxa-mediated pathway requiring iron and endoperoxide moiety. Oncotarget 2015, 6, 5582–5596. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, X.; Li, H.; Li, X. Dihydroartemisinin inhibits IL-6-induced epithelial–mesenchymal transition in laryngeal squamous cell carcinoma via the miR-130b-3p/STAT3/β-catenin signaling pathway. J. Int. Med Res. 2021, 49, 030006052110094. [Google Scholar] [CrossRef]
- Nandi, D.; Cheema, P.S.; Singal, A.; Bharti, H.; Nag, A. Artemisinin Mediates Its Tumor-Suppressive Activity in Hepatocellular Carcinoma Through Targeted Inhibition of FoxM1. Front. Oncol. 2021, 11, 751271. [Google Scholar] [CrossRef]
- Lu, L.; Huang, R.; Wu, Y.; Jin, J.-M.; Chen, H.-Z.; Zhang, L.-J.; Luan, X. Brucine: A Review of Phytochemistry, Pharmacology, and Toxicology. Front. Pharmacol. 2020, 11, 377. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Zhang, M.; Ma, F. Brucine suppresses breast cancer metastasis via inhibiting epithelial mesenchymal transition and matrix metalloproteinases expressions. Chin. J. Integr. Med. 2018, 24, 40–46. [Google Scholar] [CrossRef]
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol exerts apoptotic effect and modulates the sensitivity of HeLa cells to cisplatin and radiation. Eur. J. Mol. Clin. Med. 2020, 7, 3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fangjun, L.; Zhijia, Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer 2018, 9, 25–29. [Google Scholar] [CrossRef]
- Petrocelli, G.; Farabegoli, F.; Valerii, M.; Giovannini, C.; Sardo, A.; Spisni, E. Molecules Present in Plant Essential Oils for Prevention and Treatment of Colorectal Cancer (CRC). Molecules 2021, 26, 885. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Murugan, R.S.S.; Priyadarsini, R.V.; Vinothini, G.; Nagini, S. Eugenol induces apoptosis and inhibits invasion and angiogenesis in a rat model of gastric carcinogenesis induced by MNNG. Life Sci. 2010, 86, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Kurnia, P.H.; Bachtiar, E.A.; Ria, Q.F.; Fadhil, F.; Anita, D. Eugenol isolated from Syzygium aromaticum inhibits HeLa cancer cell migration by altering epithelial-mesenchymal transition protein regulators. J. Appl. Pharm. Sci. 2021, 11, 49–53. [Google Scholar] [CrossRef]
- Al-Sharif, I.; Remmal, A.; Aboussekhra, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer 2013, 13, 600. [Google Scholar] [CrossRef]
- Gao, Q.; Yuan, Y.; Gan, H.-Z.; Peng, Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol. Lett. 2015, 9, 2381–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Chen, Y.; Li, Y.; Lyu, X.; Cui, J.; Cheng, Y.; Zheng, T.; Zhao, L.; Zhao, G. Resveratrol Suppresses Epithelial-Mesenchymal Transition in GBM by Regulating Smad-Dependent Signaling. BioMed Res. Int. 2019, 2019, 1321973. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, B.; Rehman, S.U.; Azizullah, A.; Khan, M.F.; Din, S.R.U.; Ahmad, M.; Ali, A.; Tahir, N.; Azam, N.; Gamallat, Y.; et al. Molecular mechanisms of anticancer activities of polyphyllin VII. Chem. Biol. Drug Des. 2021, 97, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-M.; Zhu, M.-L.; Dong, R.-F.; Zhang, C.; Yang, L.; Kong, L.-Y.; Xia, Y.-Z. Polyphyllin I promotes cell death via suppressing UPR-mediated CHOP ubiquitination and degradation in non-small cell lung cancer. Chin. J. Nat. Med. 2021, 19, 255–266. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, K.; Wood, W.G. Paeonia lactiflora Extract Attenuating Cerebral Ischemia and Arterial Intimal Hyperplasia Is Mediated by Paeoniflorin via Modulation of VSMC Migration and Ras MEK/ERK Signaling Pathway. Evid.-Based Complement. Altern. Med. 2013, 2013, 482428. [Google Scholar]
- Wang, X.; Feng, S.; Wang, Y.; Chen, N.; Wang, Z. Phytomedicine Paeoniflorin: A neuroprotective monoterpenoid glycoside with promising anti-depressive properties. Phytomedicine 2021, 90, 153669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2019, 207, 107452. [Google Scholar] [CrossRef]
- Ji, Y.; Dou, Y.-N.; Zhao, Q.-W.; Zhang, J.; Yang, Y.; Wang, T.; Xia, Y.-F.; Dai, Y.; Wei, Z.-F. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol. Sin. 2016, 37, 794–804. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Wang, S.; Song, C.; Hu, Z. Paeoniflorin prevents hypoxia-induced epithelial–mesenchymal transition in human breast cancer cells. OncoTargets Ther. 2016, 9, 2511–2518. [Google Scholar] [CrossRef] [Green Version]
- Niu, K.; Liu, Y.; Zhou, Z.; Wu, X.; Wang, H.; Yan, J. Antitumor Effects of Paeoniflorin on Hippo Signaling Pathway in Gastric Cancer Cells. J. Oncol. 2021, 2021, 4724938. [Google Scholar] [CrossRef]
- Chill, L.; Yosief, T.; Kashman, Y. Halichondramine, a New Tetracyclic Bipiperidine Alkaloid from the Marine Sponge Halichondria sp. J. Nat. Prod. 2002, 65, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Halim, H.; Chunhacha, P.; Suwanborirux, K.; Chanvorachote, P. Anticancer and antimetastatic activities of renieramyein M, a marine tetrahydroisoquinoline alkaloid, in human non-small cell lung cancer cells. Anti-Cancer Res. 2011, 31, 193–201. [Google Scholar]
- Bae, S.Y.; Kim, G.D.; Jeon, J.-E.; Shin, J.; Lee, S.K. Anti-proliferative effect of (19Z)-halichondramide, a novel marine macrolide isolated from the sponge Chondrosia corticata, is associated with G2/M cell cycle arrest and suppression of mTOR signaling in human lung cancer cells. Toxicol. Vitr. 2013, 27, 694–699. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Wang, H.; Liu, X.; Guo, X. Combination treatment of ligustrazine piperazine derivate DLJ14 and adriamycin inhibits progression of resistant breast cancer through inhibition of the EGFR/PI3K/Akt survival pathway and induction of apoptosis. Drug Discov. Ther. 2014, 8, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.-J.; Zhao, J.; Zhuo-Ma, D.; Zhan-Dui, N.; Er-Bu, A.; Tsering, T. Inhibiting tumour metastasis by DQA modified paclitaxel plus ligustrazine micelles in treatment of non-small-cell lung cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3465–3477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ding, S.; Xia, L. Ligustrazine inhibits the proliferation and migration of ovarian cancer cells via regulating miR-211. Biosci. Rep. 2021, 41, BSR20200199. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Lin, T.-Y.; Hwang, P.-A.; Tseng, L.-M.; Chen, R.-H.; Tsao, S.-M.; Hsu, J. Fucoidan induces changes in the epithelial to mesenchymal transition and decreases metastasis by enhancing ubiquitin-dependent TGF receptor degradation in breast cancer. Carcinogenesis 2012, 34, 874–884. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Qi, X.; Du, F.; Zhang, G.; Li, D.; Li, J. PNSA, A Novel C-Terminal Inhibitor of HSP90, Reverses Epithelial–Mesenchymal Transition and Suppresses Metastasis of Breast Cancer Cells In Vitro. Mar. Drugs 2021, 19, 117. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, G.; Li, C.; Zhu, X.; Li, M.; Fu, C.; Li, B. Anti-nociceptive and anti-inflammatory activity of sophocarpine. J. Ethnopharmacol. 2009, 125, 324–329. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Chen, G.; Wu, W.; Zhou, L.; Shi, Y.; Zeng, Q.; Li, Y.; Sun, Y.; Deng, X.; et al. Targeting miR-21 with Sophocarpine Inhibits Tumor Progression and Reverses Epithelial-Mesenchymal Transition in Head and Neck Cancer. Mol. Ther. 2017, 25, 2129–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prateep, A.; Sumkhemthong, S.; Karnsomwan, W.; De-Eknamkul, W.; Chamni, S.; Chanvorachote, P.; Chaotham, C. Avicequinone B sensitizes anoikis in human lung cancer cells. J. Biomed. Sci. 2018, 25, 32. [Google Scholar] [CrossRef] [PubMed]
- Chamni, S.; Sirimangkalakitti, N.; Chanvorachote, P.; Suwanborirux, K.; Saito, N. Chemistry of renieramycins. Part 19: Semi-syntheses of 22-O-amino ester and hydroquinone 5-O-amino ester derivatives of renieramycin M and their cytotoxicity against non-small-cell lung cancer cell lines. Mar. Drugs 2020, 18, 418. [Google Scholar] [CrossRef] [PubMed]
- Oo, Y.; Nealiga, J.Q.L.; Suwanborirux, K.; Chamni, S.; Ecoy, G.A.U.; Pongrakhananon, V.; Chanvorachote, P.; Chaotham, C. 22-O-(N-Boc-l-glycine) ester of renieramycin M inhibits migratory activity and suppresses epithelial–mesenchymal transition in human lung cancer cells. J. Nat. Med. 2021, 75, 949–966. [Google Scholar] [CrossRef]
- Park, S.Y.; Song, H.; Sung, M.-K.; Kang, Y.-H.; Lee, K.W.; Park, J.H.Y. Carnosic Acid Inhibits the Epithelial-Mesenchymal Transition in B16F10 Melanoma Cells: A Possible Mechanism for the Inhibition of Cell Migration. Int. J. Mol. Sci. 2014, 15, 12698–12713. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.G.; Wu, L.T.; Chang, S.H.; Lo, H.H.; Hsieh, S.E.; Li, Y.C. Inhibitory Actions of Berberine on Growth and Arylamine N-Acetyltransferase Activity in Strains of Helicobacter Pylori from Peptic Ulcer Patients. Int. J. Toxicol. 1999, 18, 35–40. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Peng, W.-H.; Tsai, K.-D.; Hsu, S.-L. Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef]
- Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Gong, X.; Li, H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2016, 37, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.A.; Khan, I.A.; Imran, A.; Orhan, I.E.E.; Rizwan, M.; Atif, M.; et al. luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, G.J.; Shin, M.-S.; Moon, J.; Kim, S.; Nam, J.-W.; Kang, K.S.; Choi, H. Chemical Investigation of Diketopiperazines and N-Phenethylacetamide Isolated from Aquimarina sp. MC085 and Their Effect on TGF-β-Induced Epithelial–Mesenchymal Transition. Appl. Sci. 2021, 11, 8866. [Google Scholar] [CrossRef]
- D’Alesio, C.; Bellese, G.; Gagliani, M.C.; Aiello, C.; Grasselli, E.; Marcocci, G.; Bisio, A.; Tavella, S.; Daniele, T.; Cortese, K.; et al. Cooperative antitumor activities of carnosic acid and Trastuzumab in ERBB2+ breast cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 154. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Lin, C.; Kuo, S.; Lai, J.; Wang, Y.; You, H.; Hsu, M.; Chen, C.; Shiu, L. Carnosic acid impedes cell growth and enhances anti-cancer effects of carmustine and lomustine in melanoma. Biosci. Rep. 2018, 38, BSR20180005. [Google Scholar]
- Shen, K.-H.; Liao, A.C.-H.; Hung, J.-H.; Lee, W.-J.; Hu, K.-C.; Lin, P.-T.; Liao, R.-F.; Chen, P.-S. α-Solanine Inhibits Invasion of Human Prostate Cancer Cell by Suppressing Epithelial-Mesenchymal Transition and MMPs Expression. Molecules 2014, 19, 11896–11914. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.-J.; Zhou, W.; Xian, X.-L.; Sun, S.-J.; Ding, P.-J.; Tian, C.-Y.; Tian, F.-L.; Jiang, C.-H.; Fu, T.-T.; Zhao, S.; et al. A Mixture of Baicalein, Wogonin, and Oroxylin-A Inhibits EMT in the A549 Cell Line via the PI3K/AKT-TWIST1-Glycolysis Pathway. Front. Pharmacol. 2022, 12, 4171. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.-J.; Sun, S.-J.; Dai, J.-Y.; Fang, J.-W.; Li, Q.-H.; Yan, C.; Mao, W.-W.; Zhang, Y.-Y. Total flavonoid aglycones extract in Radix scutellariae inhibits lung carcinoma and lung metastasis by affecting cell cycle and DNA synthesis. J. Ethnopharmacol. 2016, 194, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Wang, J.-H.; Lee, J.-S.; Lee, N.-H.; Son, C.-G. Coptidis Rhizoma Suppresses Metastatic Behavior by Inhibiting TGF-β-Mediated Epithelial-Mesenchymal Transition in 5-FU-Resistant HCT116 Cells. Front. Pharmacol. 2022, 13, 2336. [Google Scholar] [CrossRef] [PubMed]
- Juchum, M.; Günther, M.; Laufer, S.A. Fighting cancer drug resistance: Opportunities and challenges for mutation-specific EGFR inhibitors. Drug Resist. Updat. 2015, 20, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Jan, R.; Ahmed, S.; Anwar, S. Breast Cancer Drug Repurposing a Tool for a Challenging Disease. In Drug Repurposing-Molecular Aspects and Therapeutic Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Sommers, C.L.; Heckford, S.E.E.; Skerker, J.M.; Worland, P.; Torri, J.A.; Thompson, E.W.; Byers, S.W.; Gelmann, E.P. Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant human breast cancer cell lines. Cancer Res. 1992, 52, 5190–5197. [Google Scholar]
- Lancet, J.E.E.; Baer, M.R.; Duran, G.E.; List, A.; Fielding, R.; Marcelletti, J.F.; Multani, P.S.S.; Sikic, B.I. A phase I trial of continuous infusion of the multidrug resistance inhibitor zosuquidar with daunorubicin and cytarabine in acute myeloid leukemia. Leuk. Res. 2009, 33, 1055–1061. [Google Scholar] [CrossRef]
- Kühnle, M.; Egger, M.; Müller, C.; Mahringer, A.; Bernhardt, G.; Fricker, G.; König, B.; Buschauer, A. Potent and Selective Inhibitors of Breast Cancer Resistance Protein (ABCG2) Derived from the p-Glycoprotein (ABCB1) Modulator Tariquidar. J. Med. Chem. 2009, 52, 1190–1197. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of Selective Inhibitors of Cancer Stem Cells by High-Throughput Screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liang, C.; Xue, F.; Chen, W.; Zhi, X.; Feng, X.; Bai, X.; Liang, T. Salinomycin decreases doxorubicin resistance in hepatocellular carcinoma cells by inhibiting the β-catenin/TCF complex association via FOXO3a activation. Oncotarget 2015, 6, 10350–10365. [Google Scholar] [CrossRef]
- Hermawan, A.; Wagner, E.; Roidl, A. Consecutive salinomycin treatment reduces doxorubicin resistance of breast tumor cells by diminishing drug efflux pump expression and activity. Oncol. Rep. 2015, 35, 1732–1740. [Google Scholar] [CrossRef] [Green Version]
- Meidhof, S.; Brabletz, S.; Lehmann, W.; Preca, B.; Mock, K.; Ruh, M.; Schüler, J.; Berthold, M.; Weber, A.; Burk, U.; et al. ZEB 1-associated drug resistance in cancer cells is reversed by the class I HDAC inhibitor mocetinostat. EMBO Mol. Med. 2015, 7, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2014, 36, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namba, T.; Kodama, R.; Moritomo, S.; Hoshino, T.; Mizushima, T. Zidovudine, an anti-viral drug, resensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine by inhibition of the Akt-GSK3β-Snail pathway. Cell Death Dis. 2015, 6, e1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomimoto, A.; Endo, H.; Sugiyama, M.; Fujisawa, T.; Hosono, K.; Takahashi, H.; Nakajima, N.; Nagashima, Y.; Wada, K.; Nakagama, H.; et al. metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008, 99, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Jiralerspong, S.; Palla, S.L.; Giordano, S.H.; Meric-Bernstam, F.; Liedtke, C.; Barnett, C.M.; Hsu, L.; Hung, M.-C.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Metformin and Pathologic Complete Responses to Neoadjuvant Chemotherapy in Diabetic Patients With Breast Cancer. J. Clin. Oncol. 2009, 27, 3297–3302. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, H.A.; Iliopoulos, D.; Tsichlis, P.N.; Struhl, K. Metformin Selectively Targets Cancer Stem Cells, and Acts Together with Chemotherapy to Block Tumor Growth and Prolong Remission. Cancer Res. 2009, 69, 7507–7511. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Cheng, X.; Wang, Y.; Han, R.; Li, L.; Xiang, T.; He, L.; Long, H.; Zhu, B.; He, Y. Metformin Inhibits the IL-6-Induced Epithelial-Mesenchymal Transition and Lung Adenocarcinoma Growth and Metastasis. PLoS ONE 2014, 9, e95884. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Zhang, W.; Zheng, G.; Zhang, Z.; Yin, J.; He, Z. Metformin reverses multidrug resistance and epithelial–mesenchymal transition (EMT) via activating AMP-activated protein kinase (AMPK) in human breast cancer cells. Mol. Cell. Biochem. 2013, 386, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-C.; Lee, K.-H.; Lai, I.-L.; Wang, D.; Mo, X.; Kulp, S.K.; Shapiro, C.L.; Chen, C.-S. AMPK Reverses the Mesenchymal Phenotype of Cancer Cells by Targeting the Akt–MDM2–Foxo3a Signaling Axis. Cancer Res. 2014, 74, 4783–4795. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Shim, J.S. Existing drugs and their application in drug discovery targeting cancer stem cells. Arch. Pharmacal Res. 2015, 38, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.-N.; Sim, W.-J.; Racine, V.; Lee, S.-Y.; Goh, B.C.; Thiery, J.P. A Cell-Based Small Molecule Screening Method for Identifying Inhibitors of Epithelial-Mesenchymal Transition in Carcinoma. PLoS ONE 2012, 7, e33183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aref, A.R.R.; Huang, R.Y.-J.; Yu, W.-M.; Chua, K.N.; Sun, W.; Tu, T.-Y.; Bai, J.; Sim, W.-J.; Zervantonakis, I.; Thiery, J.P.; et al. Screening therapeutic EMT blocking agents in a three-dimensional microenvironment. Integr. Biol. 2013, 5, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Tu, T.-Y.; Kim, C.; Thiery, J.P.; Kamm, R.D. Identification of drugs as single agents or in combination to prevent carcinoma dissemination in a microfluidic 3D environment. Oncotarget 2015, 6, 36603–36614. [Google Scholar] [CrossRef] [Green Version]
- Alamri, A.; Rauf, A.; Khalil, A.A.; Alghamdi, A.; Alafnan, A.; Alshammari, A.; Alshammari, F.; Malik, J.A.; Anwar, S. In Silico Screening of Marine Compounds as an Emerging and Promising Approach against Estrogen Receptor Alpha-Positive Breast Cancer. BioMed Res. Int. 2021, 2021, 9734279. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, B.; Hamidpour, S.K.; Alavi-Moghadam, S.; Yavari, H.; Shahbazbadr, A.; Tavirani, M.R.; Gilany, K.; Larijani, B. Molecular Docking as a Therapeutic Approach for Targeting Cancer Stem Cell Metabolic Processes. Front. Pharmacol. 2022, 13, 768556. [Google Scholar] [CrossRef] [PubMed]
- Jagust, P.; De Luxán-Delgado, B.; Parejo-Alonso, B.; Sancho, P. Metabolism-Based Therapeutic Strategies Targeting Cancer Stem Cells. Front. Pharmacol. 2019, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Taylor, W.F.; Jabbarzadeh, E. The use of natural products to target cancer stem cells. Am. J. Cancer Res. 2017, 7, 1588–1605. [Google Scholar] [PubMed]
- Anwar, S.; Saleem, H.; Khurshid, U.; Ansari, S.Y.; Alghamdi, S.; Al-Khulaidi, A.W.A.; Malik, J.A.; Ahemad, N.; Awadh Ali, N.A. Comparative phytochemical composition, oleuropein quantification, antioxidant and cytotoxic properties of Olea europaea L. leaves. Nat. Prod. Res. 2022. [Google Scholar] [CrossRef] [PubMed]

| Type of the Tumor | Study Type | Effective Dose | Mechanism | References |
|---|---|---|---|---|
| Lung cancer | In vitro | 1000 μM | Decrease cycloxygenase-2 activity, which leads to cell cycle arrest in the S phase followed by cell death | [66] |
| Colon cancer | In vitro | 800 μM | Boosts the cytotoxic effects of cisplatin and doxorubicin synergistically. | [67] |
| Gastric cancer | In vitro | Low conc. | Inhibits cancer growth by upregulating preinvasive and angiogenic molecules and favoring apoptosis via the mitochondrial pathway via altering Bcl-2 family proteins. | [68] |
| Cervical cancer | In vitro | 50–200 μM | Prevents the cell cycle and causes apoptosis, and inhibits DNA synthesis. | [69] |
| Breast cancer | In vitro | 2 μM | Suppresses breast cancer-related oncogenes by downregulating E2F1 and its downstream anti-apoptotic target | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, S.; Malik, J.A.; Ahmed, S.; Kameshwar, V.A.; Alanazi, J.; Alamri, A.; Ahemad, N. Can Natural Products Targeting EMT Serve as the Future Anticancer Therapeutics? Molecules 2022, 27, 7668. https://doi.org/10.3390/molecules27227668
Anwar S, Malik JA, Ahmed S, Kameshwar VA, Alanazi J, Alamri A, Ahemad N. Can Natural Products Targeting EMT Serve as the Future Anticancer Therapeutics? Molecules. 2022; 27(22):7668. https://doi.org/10.3390/molecules27227668
Chicago/Turabian StyleAnwar, Sirajudheen, Jonaid Ahmad Malik, Sakeel Ahmed, Verma Abhishek Kameshwar, Jowaher Alanazi, Abdulwahab Alamri, and Nafees Ahemad. 2022. "Can Natural Products Targeting EMT Serve as the Future Anticancer Therapeutics?" Molecules 27, no. 22: 7668. https://doi.org/10.3390/molecules27227668
APA StyleAnwar, S., Malik, J. A., Ahmed, S., Kameshwar, V. A., Alanazi, J., Alamri, A., & Ahemad, N. (2022). Can Natural Products Targeting EMT Serve as the Future Anticancer Therapeutics? Molecules, 27(22), 7668. https://doi.org/10.3390/molecules27227668









