Pine Pollen Polysaccharides’ and Sulfated Polysaccharides’ Effects on UC Mice through Modulation of Cell Tight Junctions and RIPK3-Dependent Necroptosis Pathways
Abstract
:1. Introduction
2. Results
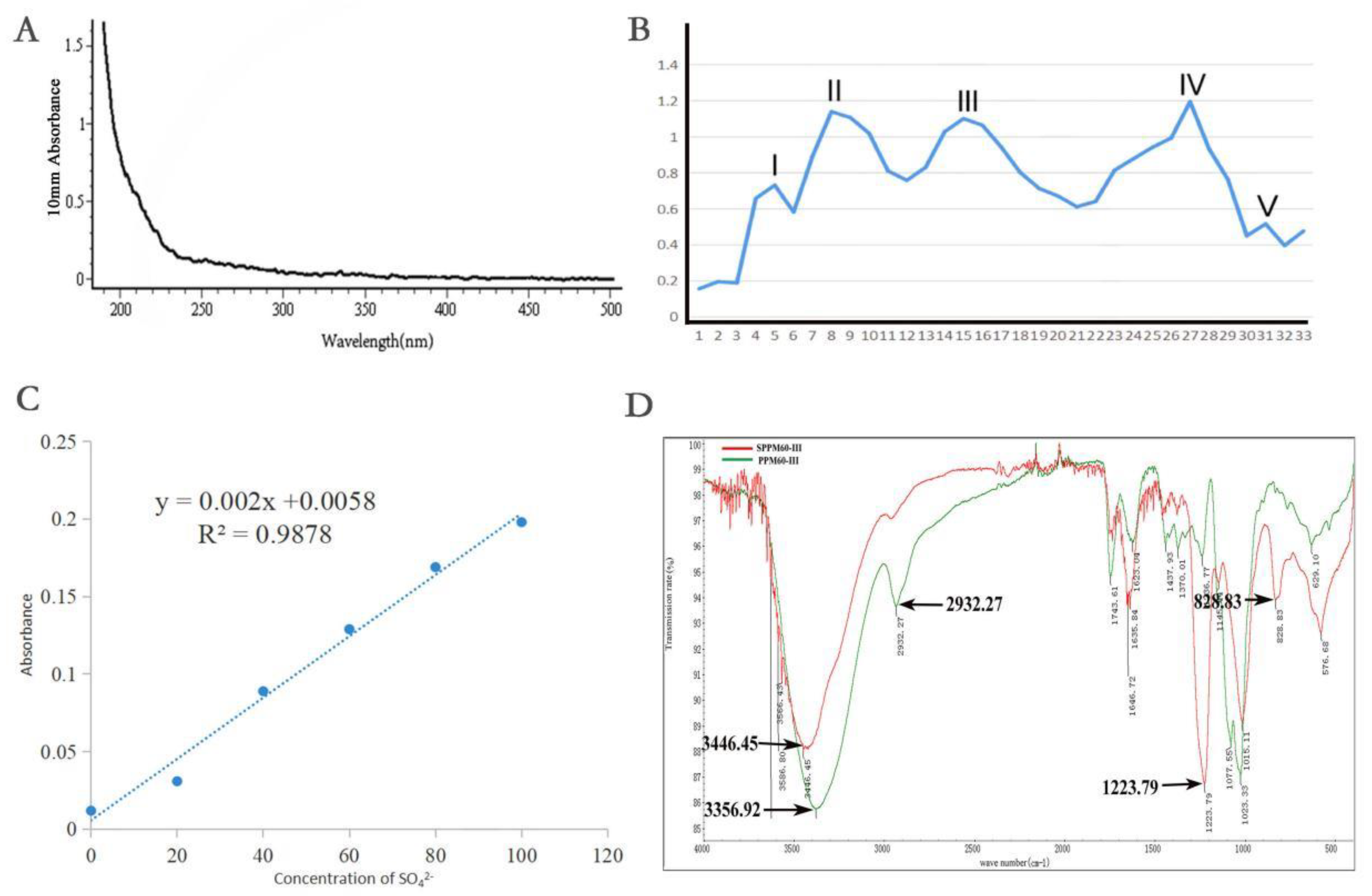
2.1. Extraction, Separation, and Sulfation of Polysaccharides from Pollen of Pinus yunnanensis
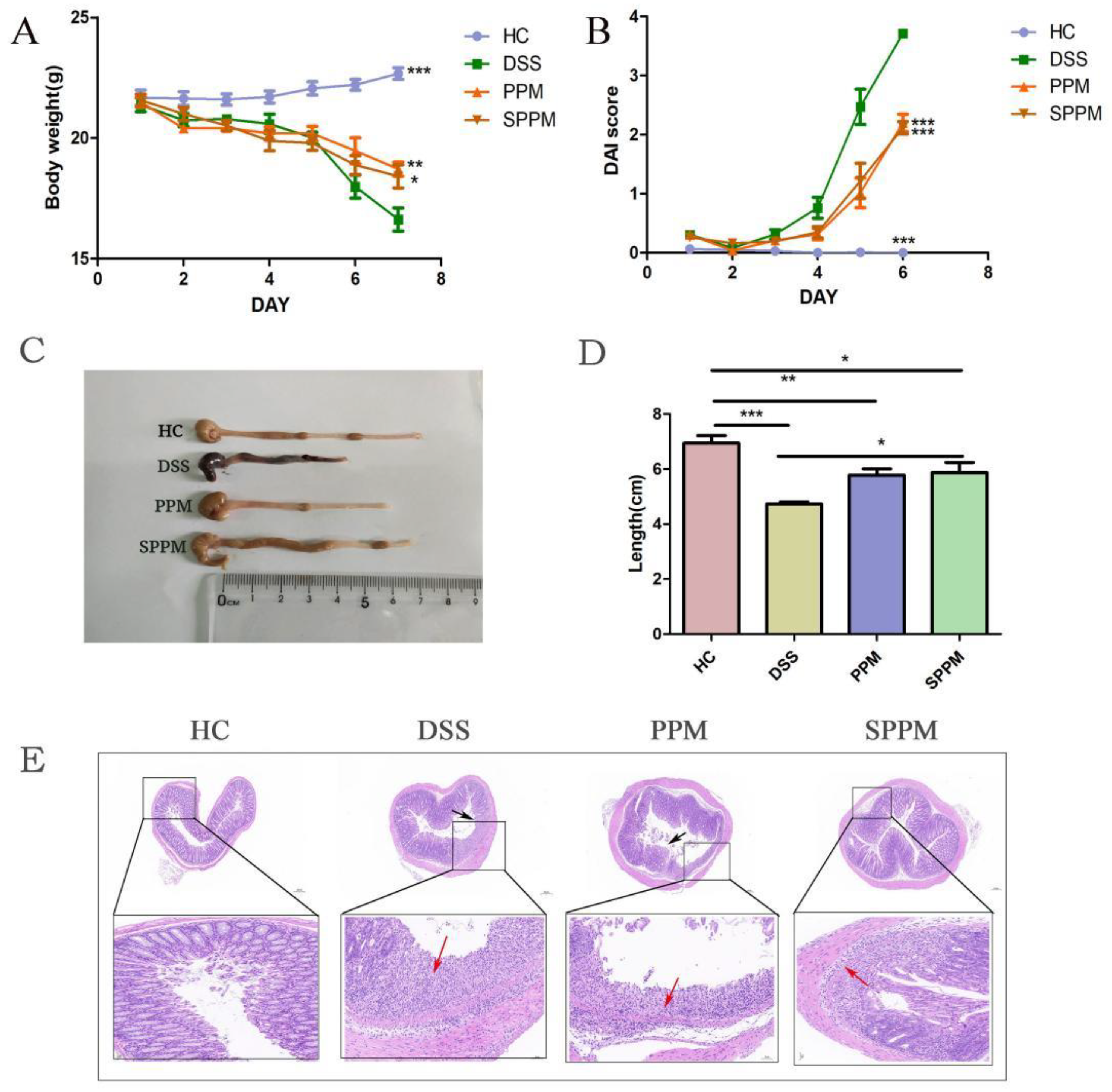
2.2. Effects of PPM60-III and SPPM60-III on UC Mice
2.3. Effects of PPM60-III and SPPM60-III on Inflammatory Factors and Related Enzymes in UC Mice
2.4. Effect of PPM6-III and SPPM6-III on Tight Junctions of Colonic Epithelium in UC Mice
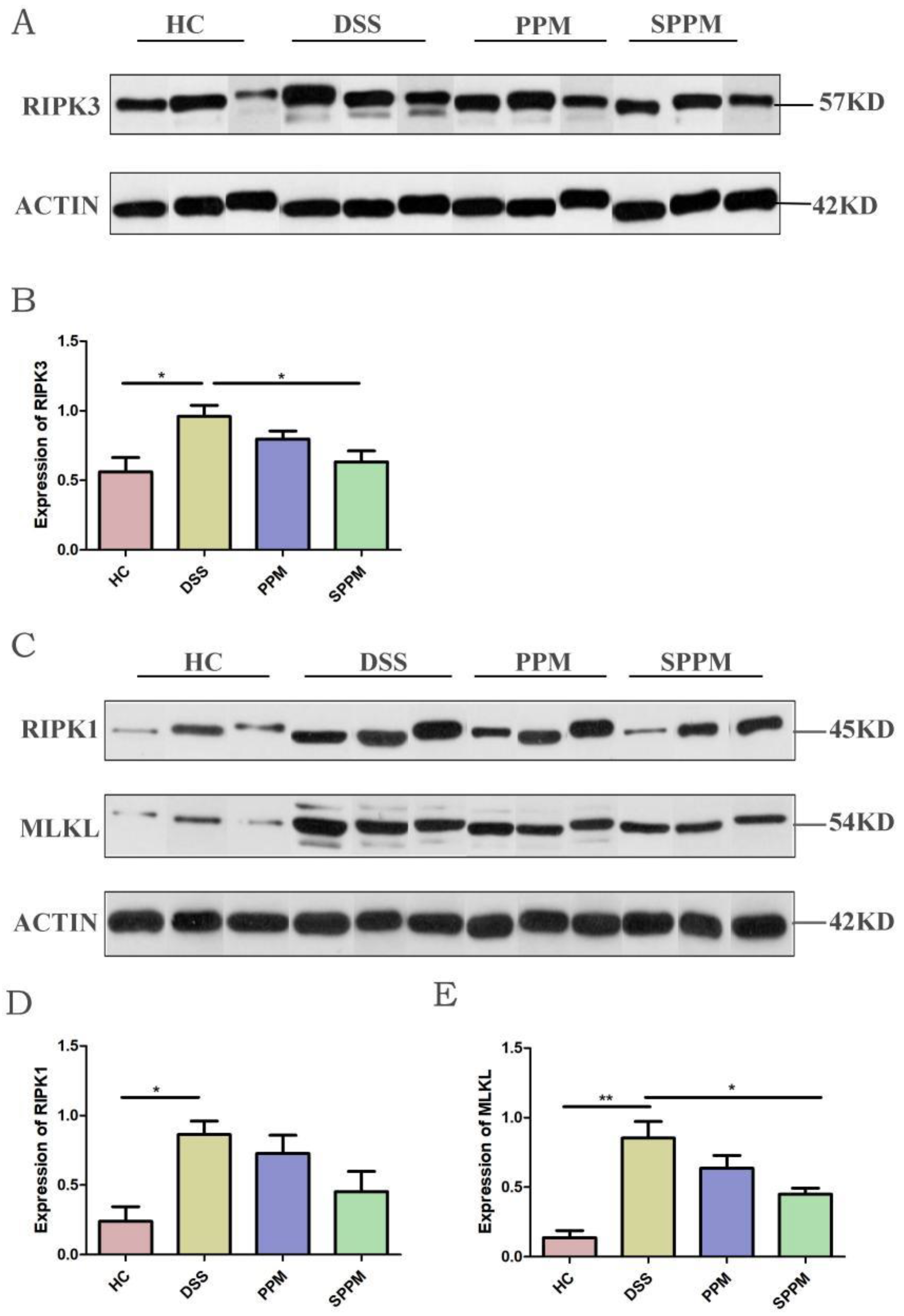
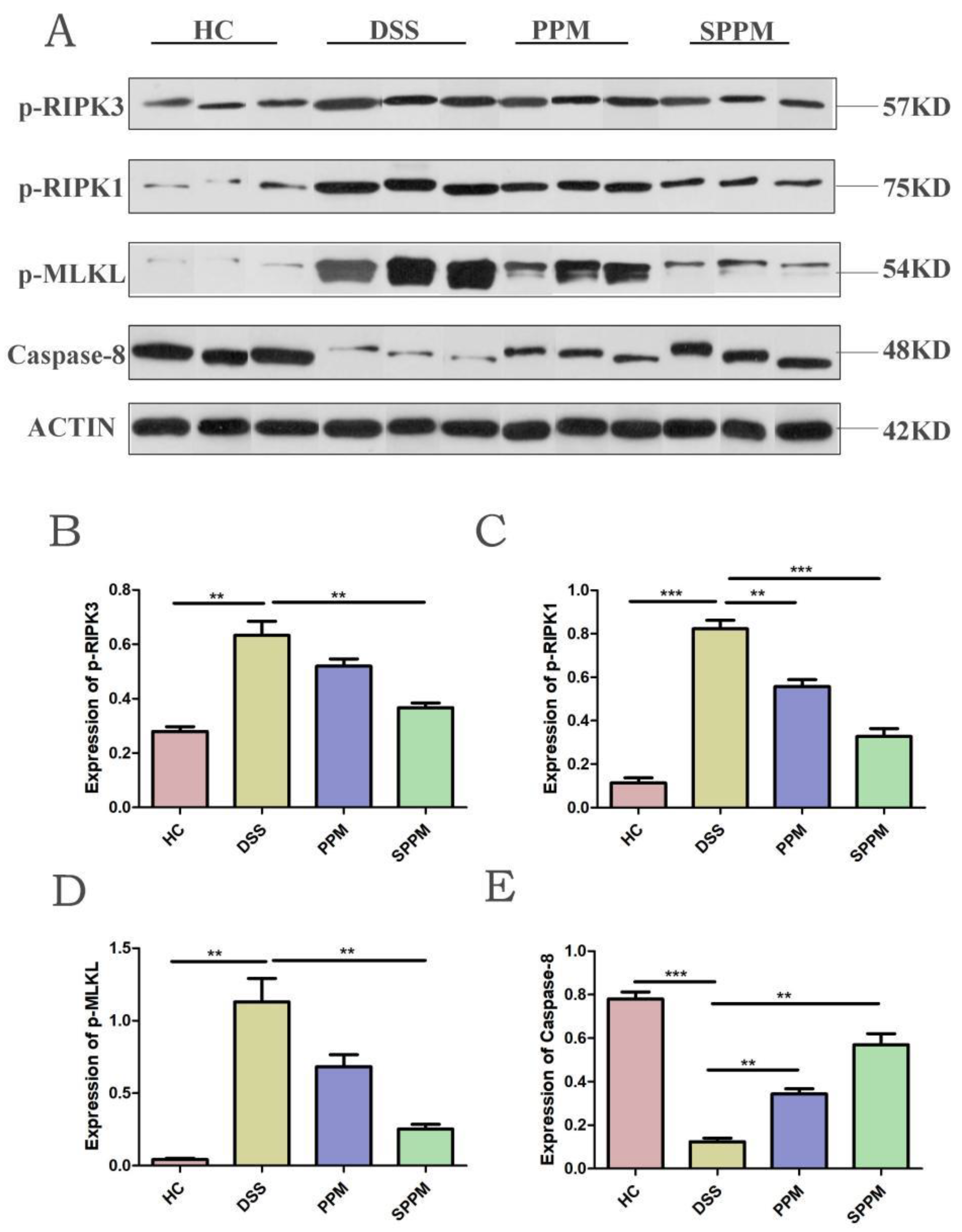
2.5. Effects of PPM60-III and SPPM60-III on UC Mice by Regulating the RIPK3 Pathway
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction, Separation, and Purity Detection of Polysaccharides from Pine Pollen of Pinus yunnanensis
4.3. Sulfation of Polysaccharides from Pine Pollen
4.4. Animals and Model
4.5. Assessment of Disease Activity Index (DAI)
4.6. Colon Length Analysis
4.7. H&E Staining
4.8. Electron Microscope Analysis
4.9. Expression of Inflammatory Cytokines by ELISA
4.10. Western Blotting
4.11. Immunofluorescence Staining
4.12. RNA Preparation and Quantitative Real-Time PCR Analysis
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qiu, J.; Chen, L.; Yi, X.; Li, M.Z. The complete chloroplast genome of Pinus yunnanensis Franchet (Pinaceae). Mitochondrial DNA B Resour. 2019, 4, 2600–2601. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.B.; Liang, N.; Bu, F.L.; Lai, B.Y.; Zhang, Y.P.; Cao, H.J.; Fei, Y.T.; Nicola, R.; Liu, J.P. The potential effects and use of Chinese herbal medicine pine pollen (Pinus pollen): A bibliometric analysis of pharmacological and clinical studies. World J. Tradit. Chin. Med. 2020, 6, 163–170. [Google Scholar] [CrossRef]
- Jin, X.; Cong, T.; Zhao, L.; Ma, L.; Li, R.S.; Zhao, P.; Guo, C.J. The protective effects of Masson pine pollen aqueous extract on CCl4-induced oxidative damage of human hepatic cells. Int. J. Clin. Exp. Med. 2015, 8, 17773–17780. [Google Scholar]
- Lee, K.H.; Kim, A.J.; Choi, E.M. Antioxidant and antiinflammatory activity of pine pollen extract in vitro. Phytother. Res. 2009, 23, 41–48. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, L.; Cheung, P.C. Physicochemical properties and antitumor activities of water-soluble native and sulfated hyperbranched mushroom polysaccharides. Carbohydr. Res. 2006, 341, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.L.; Huang, R.S.; He, X.M.; Chen, N.F.; Han, B.X.; Deng, H. Dendrobium huoshanense polysaccharides prevent inflammatory response of ulcerative colitis rat through inhibiting the NF-κB signaling pathway. Chem. Biodivers. 2021, 18, e2100130. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, Y.; Yu, J.; Jiang, L.; Xiang, Y.Y.; Wang, J.; Du, Y.X.; Cui, X.M.; Ge, F. A neutral polysaccharide from Ophiocordyceps lanpingensis restrains cisplatin-induced nephrotoxicity. Food Sci. Nutr. 2021, 9, 3602–3616. [Google Scholar] [CrossRef]
- Li, C.; Cai, Y.Y.; Yan, Z.X. Brain-derived neurotrophic factor preserves intestinal mucosal barrier function and alters gut microbiota in mice. Kaohsiung J. Med. Sci. 2018, 34, 134–141. [Google Scholar] [CrossRef]
- Wen, J.; Qian, S.; Yang, Q.; Deng, L.; Mo, Y.; Yu, Y.F. Overexpression of netrin-1 increases the expression of tight junction-associated proteins, claudin-5, occludin, and ZO-1, following traumatic brain injury in rats. Exp. Med. 2014, 8, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Xiao, N.; Zeng, L.; Xiao, J.; Huang, J.Z.; Xu, Y.A.; Chen, Y.L.; Ren, Y.H.; Du, B. Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr. Polym. 2020, 250, 116958. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhang, Y.; Li, Q.; Zeng, F.; Wang, K.P. Inhibition of dextran sodium sulfate-induced experimental colitis in mice by Angelica sinensis polysaccharide. J. Med. Food 2020, 23, 584–592. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.Q.; Song, J.; Tan, X.B.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sadi, R.; Ye, D.; Dokladny, K.; Ma, T.Y. Mechanism of IL-1 beta-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008, 180, 5653–5661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, M.; Li, X.; Zhang, H.W.; Wang, L.X.; Wu, X.X.; Zhang, H.B.; Luo, Y. Catalytically inactive RIP1 and RIP3 deficiency protect against acute ischemic stroke by inhibiting necroptosis and neuroinflammation. Cell Death Dis. 2020, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.V.; Weist, B.M.; van Raam, B.J.; Marro, B.S.; Nguyen, L.V.; Srinivas, P.; Bell, B.D.; Luhrs, K.A.; Lane, T.E.; Salvesen, G.S.; et al. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc. Natl. Acad. Sci. USA 2011, 108, 15312–15317. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.C.; Liu, W.L.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflammation 2018, 15, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lei, H.; Hu, X.; Dong, W.G. Hesperetin ameliorates DSS-induced colitis by maintaining the epithelial barrier via blocking RIPK3/MLKL necroptosis signaling. Eur. J. Pharmacol. 2020, 873, 172992. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, X.; Wu, Q.; Lu, Y.F.; Shi, J.S.; Chen, X.P. Dihydrotanshinone I, a natural product, ameliorates DSS-induced experimental ulcerative colitis in mice. Toxicol. Appl. Pharmacol. 2018, 344, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Pabla, B.S.; Schwartz, D.A. Assessing Severity of Disease in Patients with Ulcerative Colitis. Gastroenterol. Clin. North Am. 2020, 49, 671–688. [Google Scholar] [CrossRef]
- Wang, Y.L. Effects of Pine Pollen Polysaccharides and Sulfated Polysaccharides on Colitis and Intestinal Flora in Mice. Master’s Thesis, Shandong Normal University, Jinan, China, 2020. [Google Scholar]
- Xing, L. Sulfated Polysaccharides from Pine Pollen on the Effects of Immune Regulation of Mice Macrophages. Master’s Thesis, Shandong Normal University, Jinan, China, 2015. [Google Scholar]
- Sha, Z.; Shang, H.; Miao, Y.; Huang, J.; Niu, X.Y.; Chen, R.C.; Peng, D.; Wei, K.; Zhu, R.L. Polysaccharides from Pinus massoniana pollen improve intestinal mucosal immunity in chickens. Poult. Sci. 2021, 100, 507–516. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, Y.; Du, Y.; Wu, J.Q.; Li, B.Q.; Li, J.; Yu, J.; Hu, L.P.; Shen, S.; Wang, J.B.; et al. Potentiation of Taishan Pinus massoniana pollen polysaccharide on the immune response and protection elicited by a highly pathogenic porcine reproductive and respiratory syndrome virus glycoprotein 5 subunit in pigs. Mol. Cell. Probes 2016, 30, 83–92. [Google Scholar] [CrossRef]
- Shang, H.; Sha, Z.; Wang, H.; Miao, Y.Q.; Niu, X.Y.; Chen, R.C.; Huang, J.; Huang, H.; Wei, K.; Zhu, R.L. Taishan Pinus massoniana pollen polysaccharide inhibits H9N2 subtype influenza virus infection both in vitro and in vivo. Vet. Microbiol. 2020, 248, 108803. [Google Scholar] [CrossRef]
- Zhou, C.; Yin, S.; Yu, Z.; Feng, Y.X.; Wei, K.; Ma, W.M.; Ge, L.J.; Yan, Z.G.; Zhu, R.L. Preliminary characterization, antioxidant and hepatoprotective activities of polysaccharides from Taishan Pinus massoniana pollen. Molecules 2018, 23, 281. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Liu, Z.; Li, M.; Guo, J.; Chen, L.L.; Ding, L.; Ding, X.; Zhou, T.; Zhang, J. Polysaccharide from Potentilla anserina L ameliorate pulmonary edema induced by hypobaric hypoxia in rats. Biomed. Pharmacother. 2021, 139, 111669. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, H.; Xu, W.; Liu, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. Echinacea purpurea polysaccharide prepared by fractional precipitation prevents alcoholic liver injury in mice by protecting the intestinal barrier and regulating liver-related pathways. Int. J. Biol. Macromol. 2021, 187, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Mani, S.; Malarvizhi, R.; Sali, V.K.; Vasanthi, H.R. Immunomodulatory activity of brown algae Turbinaria ornata derived sulfated polysaccharide on LPS induced systemic inflammation. Phytomedicine 2021, 89, 153615. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cui, W.; Gao, Z.; Zhang, J.J.; Jia, L. Structural characterization and amelioration of sulfated polysaccharides from Ganoderma applanatum residue against CCl4-induced hepatotoxicity. Int. Immunopharmacol. 2021, 96, 107554. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zou, J.; Chen, M.; Zhang, Z.M.; Liu, C.; Jiang, S.; Qian, D.; Duan, J.-A. Protective effects of Lizhong decoction on ulcerative colitis in mice by suppressing inflammation and ameliorating gut barrier. J. Ethnopharmacol. 2020, 259, 112919. [Google Scholar] [CrossRef]
- Li, Y.; Pan, X.; Yin, M.; Li, C.P.; Han, L.R. Preventive effect of lycopene in dextran sulfate sodium-induced ulcerative colitis mice through the regulation of TLR4/TRIF/NF-κB signaling pathway and tight junctions. J. Agric. Food Chem. 2021, 69, 13500–13509. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, X.; Liu, X.; Li, Y.T.; Tan, Q.L.; Dan, Q.; Yuan, T.; Liu, X.B.; Liu, R.H.; Liu, Z.G. Ficus carica polysaccharide attenuates DSS-induced ulcerative colitis in C57BL/6 mice. Food Funct. 2020, 11, 6666–6679. [Google Scholar] [CrossRef]
- Niu, X.; Shang, H.; Chen, S.; Chen, R.C.; Huang, J.; Miao, Y.Q.; Cui, W.P.; Wang, H.; Sha, Z.; Peng, D.; et al. Effects of Pinus massoniana pollen polysaccharides on intestinal microenvironment and colitis in mice. Food Funct. 2021, 12, 252–266. [Google Scholar] [CrossRef]
- Long, W.; Mou, G.E.; Zhang, G.H.; Dong, H.; Wu, H.Y.; Zhou, Z.W.; Sun, Y.M. Dark Tea Extracts Protect Mice from Sepsis. Nat. Prod. Res. Dev. 2018, 30, 582–587. [Google Scholar] [CrossRef]
- Liang, T.Z.; Wang, L.M.; Yu, C.; Wang, X.Y.; Liu, Y.L.; Zhu, H.L. Effects of Lentinula edodes polysaccharides on expression of genes related to jejunal cell death signaling pathway stimulated by lipopolysaccharide in piglets. Chin. J. Anim. Nutr. 2020, 32, 915–924. [Google Scholar]
- Lee, S.H.; Kwon, J.Y.; Moon, J.; Choi, J.W.; Jhun, J.; Jung, K.; Cho, K.H.; Darlami, O.; Lee, H.H.; Jung, E.S.; et al. Inhibition of RIPK3 pathway attenuates intestinal inflammation and cell death of inflammatory bowel disease and suppresses necroptosis in peripheral mononuclear cells of ulcerative colitis patients. Immune Netw. 2020, 20, e16. [Google Scholar] [CrossRef] [PubMed]
- Negroni, A.; Colantoni, E.; Pierdomenico, M.; Palone, F.; Costanzo, M.; Oliva, S.; Tiberti, A.; Cucchiara, S.; Stronati, L. RIP3 and pMLKL promote necroptosis-induced inflammation and alter membrane permeability in intestinal epithelial cells. Dig. Liver Dis. 2017, 49, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Scofield, R.H. Western blotting: An introduction. Methods Mol. Biol. 2015, 1312, 17–30. [Google Scholar] [PubMed]
- Im, K.; Mareninov, S.; Diaz, M.; Yong, W.H. An Introduction to Performing Immunofluorescence Staining. Methods Mol. Biol. 2019, 1897, 299–311. [Google Scholar]
- Green, M.R.; Sambrook, J. Quantification of RNA by Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR). Cold Spring Harb. Protoc. 2018, 2018, prot095042. [Google Scholar] [CrossRef]




| DAI Score | Weight Loss | Stool Consistency | Occult/Gross Bleeding |
|---|---|---|---|
| 0 1 | 0 1~5% | Normal | Normal |
| 2 3 | 5~10% 10~15% | Loose | Guiac (+) |
| 4 | >15% | Diarrhoea | Gross bleeding |
| Forward5′-3′ | Reveser5′-3′ | |
|---|---|---|
| GAPDH | CCTCGTCCCGTAGACAAAATG | TGAGGTCAATGAAGGGGTCGT |
| FADD | CGCGTGAGCAAACGAAAGC | CACACAATGTCAAATGCCACC |
| RIPK1 | AGAAGAAGGGAACTATTCGCTGG | CATCTATCTGGGTCTTTAGCACG |
| RIPK3 | CAGTGGGACTTCGTGTCCG | CAAGCTGTAGGTAGCACATC |
| MLKL | TTAGGCCAGCTCATCTATGAACA | TGCACACGGTTTCCTAGACG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, H.; Wang, Z.; Geng, Y. Pine Pollen Polysaccharides’ and Sulfated Polysaccharides’ Effects on UC Mice through Modulation of Cell Tight Junctions and RIPK3-Dependent Necroptosis Pathways. Molecules 2022, 27, 7682. https://doi.org/10.3390/molecules27227682
Li Z, Wang H, Wang Z, Geng Y. Pine Pollen Polysaccharides’ and Sulfated Polysaccharides’ Effects on UC Mice through Modulation of Cell Tight Junctions and RIPK3-Dependent Necroptosis Pathways. Molecules. 2022; 27(22):7682. https://doi.org/10.3390/molecules27227682
Chicago/Turabian StyleLi, Zhenxiang, Hanyue Wang, Zhanjiang Wang, and Yue Geng. 2022. "Pine Pollen Polysaccharides’ and Sulfated Polysaccharides’ Effects on UC Mice through Modulation of Cell Tight Junctions and RIPK3-Dependent Necroptosis Pathways" Molecules 27, no. 22: 7682. https://doi.org/10.3390/molecules27227682
APA StyleLi, Z., Wang, H., Wang, Z., & Geng, Y. (2022). Pine Pollen Polysaccharides’ and Sulfated Polysaccharides’ Effects on UC Mice through Modulation of Cell Tight Junctions and RIPK3-Dependent Necroptosis Pathways. Molecules, 27(22), 7682. https://doi.org/10.3390/molecules27227682






