Organic Small-Molecule Electrodes: Emerging Organic Composite Materials in Supercapacitors for Efficient Energy Storage
Abstract
:1. Introduction
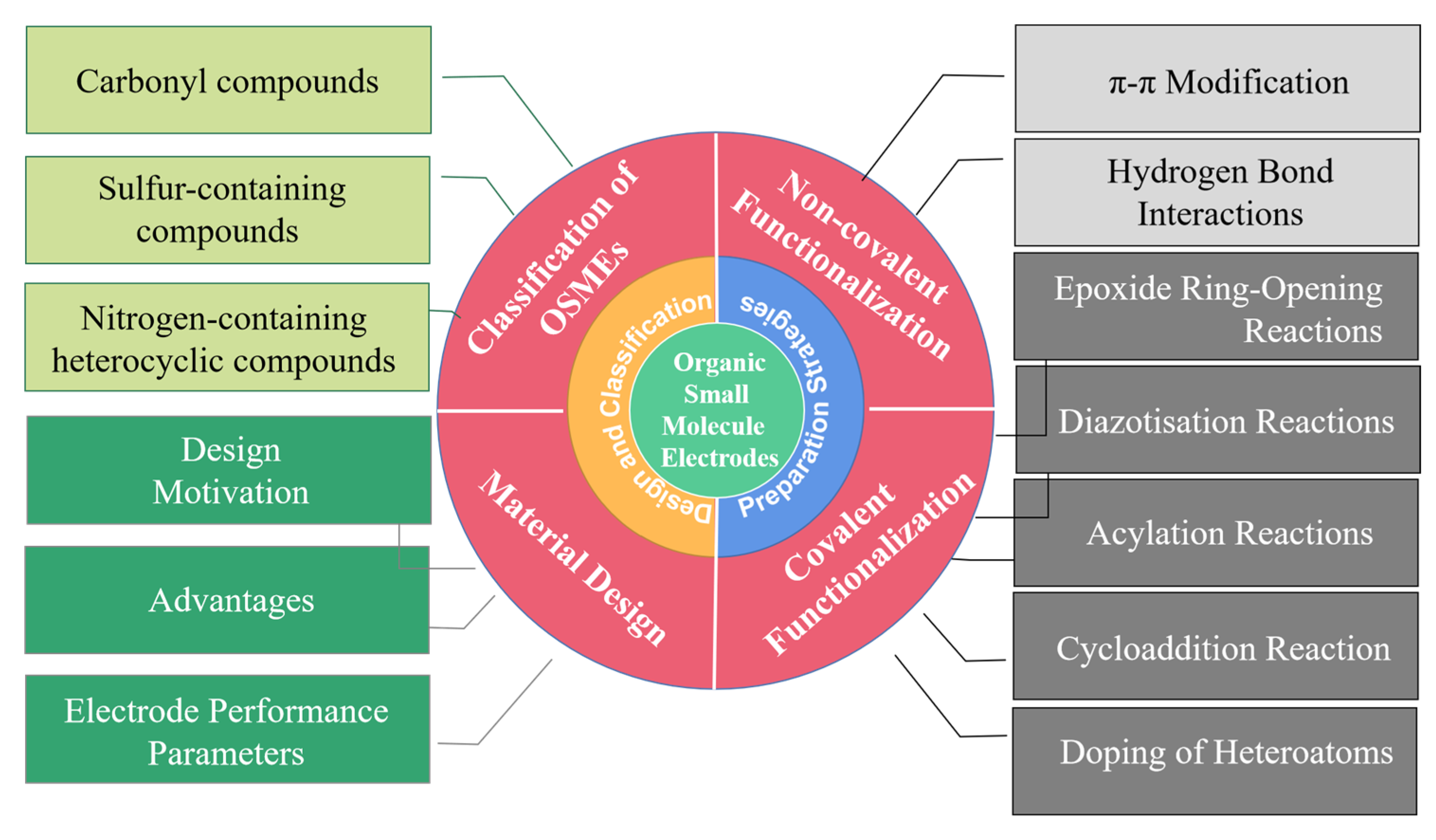
2. Design of Organic Small-Molecule Electrodes
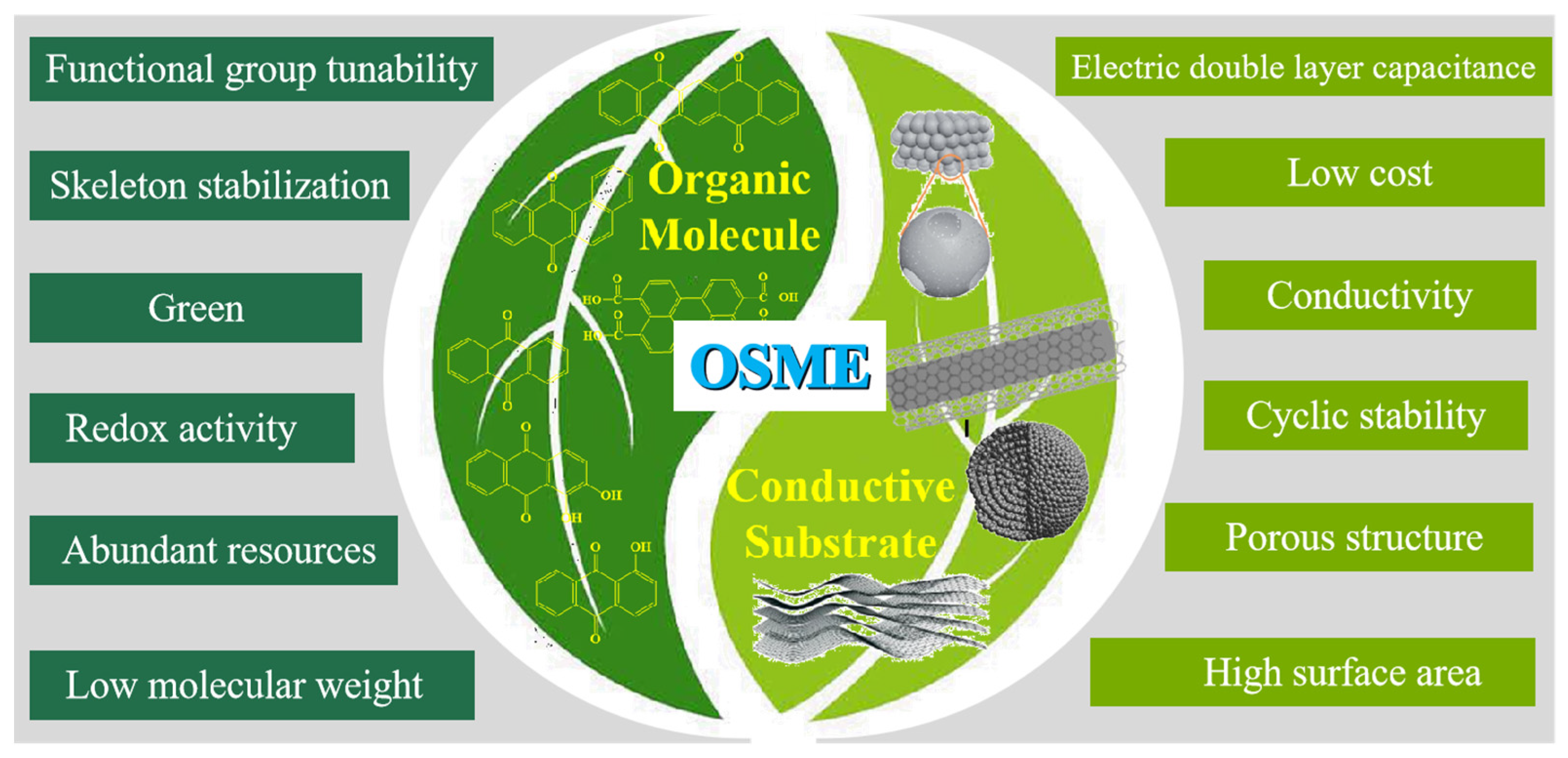
2.1. What Is the Design Motivation of OMEs?
2.2. What Are the Advantages of OMEs?
2.3. How Do the OME’s Electrode Performance Parameters Compare with Other Emerging Materials?
3. Classification of Organic Small-Molecule Electrodes
3.1. Carbonyl Compounds
3.1.1. Quinone Derivatives
3.1.2. Ketones
3.1.3. Amide
3.1.4. Carboxylic Acids and Anhydrides
3.2. Nitrogen-Containing Heterocyclic Compounds
3.3. Sulfur-Containing Compounds
4. Preparation Strategies of Organic Small-Molecule Electrodes
4.1. Covalent Functionalization
4.1.1. Epoxide Ring-Opening Reactions
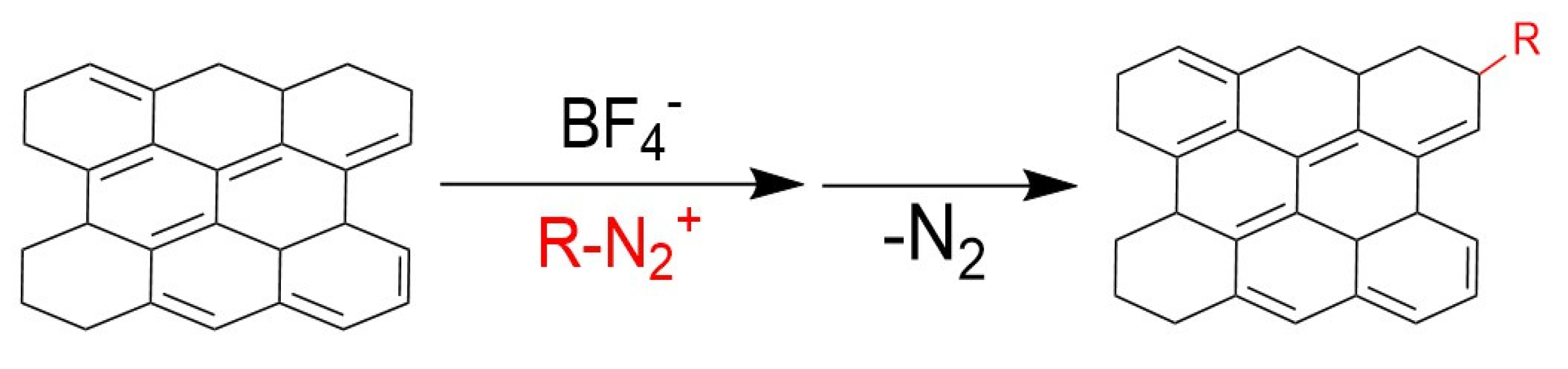
4.1.2. Diazotisation
4.1.3. Acylation Reactions
4.1.4. Cycloaddition Reaction
4.1.5. Doping of Heteroatoms
4.2. Non-Covalent Functionalization
4.2.1. The π–π Interaction
4.2.2. Hydrogen Bond Interactions
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, L.; Chang, D.W.; Baek, J.B.; Lu, W. Carbon nanomaterials for advanced energy conversion and storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and graphene-based materials for energy storage applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yin, Z.; Zhang, H. Three-dimensional graphene materials: Preparation, structures and application in supercapacitors. Energy Environ. Sci. 2014, 7, 1850–1865. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Tan, C.; Yin, Z. 25th Anniversary article: Hybrid nanostructures based on two-dimensional nanomaterials. Adv. Mater. 2014, 26, 2185–2204. [Google Scholar] [CrossRef]
- An, N.; An, Y.; Hu, Z.; Guo, B.; Yang, Y.; Lei, Z. Graphene hydrogels non-covalently functionalized with alizarin: An ideal electrode material for symmetric supercapacitors. J. Mater. Chem. A 2015, 3, 22239–22246. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.Z.; Zhang, J.D.; Lai, W.Y.; Huang, W. High-performance free-standing PEDOT: PSS electrodes for flexible and transparent all-solid-state supercapacitors. J. Mater. Chem. A 2016, 4, 10493–10499. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, B.; Feng, L.; Zheng, J.; You, B.; Chen, J.; Zhao, X.; Zhang, C.; Jiang, S.; He, S. Progress on organic potassium salts involved synthesis of porous carbon nanomaterials: Microstructure engineering for advanced supercapacitors. Nanoscale 2022, 14, 8216–8244. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-derived biochar as thick electrodes for high-rate performance supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Dong, Y.; Ding, Y.; Yang, W.; Han, J.; Jiang, S.; He, S. Nitrogen-doped carbon layer on cellulose derived free-standing carbon paper for high-rate supercapacitors. Appl. Surf. Sci. 2023, 608, 155144. [Google Scholar] [CrossRef]
- Tan, Y.B.; Lee, J.M. Graphene for supercapacitor applications. J. Mater. Chem. A 2013, 1, 14814–14843. [Google Scholar] [CrossRef]
- Dang, C.; Mu, Q.; Xie, X.; Sun, X.; Yang, X.; Zhang, Y.; Maganti, S.; Huang, M.; Jiang, Q.; Seok, I.; et al. Recent progress in cathode catalyst for nonaqueous lithium oxygen batteries: A review. Adv. Compos. Hybrid Mater. 2022, 5, 606–626. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Zhang, J.; Ma, Y.; Hou, C.; Sun, X.; Yang, X.; Zhang, Y.; Kimura, H.; Du, W. Embedding NiS nanoflakes in electrospun carbon fibers containing NiS nanoparticles for hybrid supercapacitors. Chem. Eng. J. 2022, 446, 137262. [Google Scholar] [CrossRef]
- Yang, W.; Peng, D.; Kimura, H.; Zhang, X.; Sun, X.; Pashameah, R.A.; Alzahrani, E.; Wang, B.; Guo, Z.; Du, W. Honeycomb-like nitrogen-doped porous carbon decorated with Co3O4 nanoparticles for superior electrochemical performance pseudo-capacitive lithium storage and supercapacitors. Adv. Compos. Hybrid Mater. 2022, 5, 3146–3157. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Ma, Y.; Zhang, J.; Hou, C.; Sun, X.; Yang, X.; Zhang, Y.; Kimura, H.; Du, W. Morphology controlled hierarchical NiS/carbon hexahedrons derived from nitrilotriacetic acid-assembly strategy for high-performance hybrid supercapacitors. Chem. Eng. J. 2022, 433, 133673. [Google Scholar] [CrossRef]
- Leitner, K.W.; Gollas, B.; Winter, M.; Besenhard, J.O. Combination of redox capacity and double layer capacitance in composite electrodes through immobilization of an organic redox couple on carbon black. Electrochim. Acta 2004, 50, 199–204. [Google Scholar] [CrossRef]
- Kalinathan, K.; DesRoches, D.P.; Liu, X.; Pickup, P.G. Anthraquinone modified carbon fabric supercapacitors with improved energy and power densities. J. Power Sources 2008, 181, 182–185. [Google Scholar] [CrossRef]
- Wang, H.W.; Wu, H.Y.; Chang, Y.Q.; Chen, Y.; Hu, Z. Tert-butylhydroquinone-decorated graphene nanosheets and their enhanced capacitive behaviors. Chin. Sci. Bull. 2011, 56, 2092–2097. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Sun, Y.; Bai, H. High-performance supercapacitor electrodes based on graphene hydrogels modified with 2-aminoanthraquinone moieties. Phys. Chem. Chem. Phys. 2011, 13, 11193–11198. [Google Scholar] [CrossRef]
- Jokar, E.; Shahrokhian, S. Electrochemical functionalization of graphene nanosheets with catechol derivatives as an effective method for preparation of highly performance supercapacitors. Electrochim. Acta 2014, 147, 136–142. [Google Scholar] [CrossRef]
- Jana, M.; Saha, S.; Khanra, P.; Samanta, P.; Koo, H.; Murmu, N.C. Non-covalent functionalization of reduced graphene oxide using sulfanilic acid azocromotrop and its application as a supercapacitor electrode material. J. Mater. Chem. A 2015, 3, 7323–7331. [Google Scholar] [CrossRef]
- Ren, X.; Hu, Z.; Hu, H.; Qiang, R.; Li, L.; Li, Z.; Yang, Y.; Zhang, Z.; Wu, H. Noncovalently-functionalized reduced graphene oxide sheets by water-soluble methyl green for supercapacitor application. Mater. Res. Bull. 2015, 70, 215–221. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, P.; Li, J.; Yang, S.; Wu, K.; Wu, J.; Meng, G.; Liu, Z.; Guo, X. Self-supported flexible supercapacitor based on carbon fibers covalently combined with monoaminophthalocyanine. Chem. Eng. J. 2020, 391, 123535. [Google Scholar] [CrossRef]
- Wang, X.; Hou, L.; Chen, W.; Xie, Y.; Xie, K.; An, N.; Hu, Z. Organic molecule electrode with high capacitive performance originating from efficient collaboration between caffeic acid and graphene & graphene nanomesh hydrogel. Electrochim. Acta 2019, 326, 134953. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Y.; Zhang, Q.; Chen, J. Insights into redox processes and correlated performance of organic carbonyl electrode materials in rechargeable batteries. Adv. Mater. 2022, 34, 2104150. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.K.; Imakita, K.; Imakita, K.; Fujii, M. Microscopic origin of lattice contraction and expansion in undoped rutile TiO2 nanostructures. J. Phys. D Appl. Phys. 2014, 47, 215302. [Google Scholar] [CrossRef] [Green Version]
- Grande, T.; Tolchard, J.R.; Selbach, S.M. Anisotropic thermal and chemical expansion in Sr-substituted LaMnO3+ δ: Implications for chemical strain relaxation. Chem. Mater. 2012, 24, 338–345. [Google Scholar] [CrossRef]
- Han, S.; Park, J.; Lu, W.; Sastry, A.M. Numerical study of grain boundary effect on Li+ effective diffusivity and intercalation-induced stresses in Li-ion battery active materials. J. Power Sources 2013, 240, 155–167. [Google Scholar] [CrossRef]
- Moretti, A.; Passerini, S. Bilayered nanostructured V2O5· nH2O for metal batteries. Adv. Energy Mater. 2016, 6, 1600868. [Google Scholar] [CrossRef]
- Renganathan, S.; Sikha, G.; Santhanagopalan, S.; White, R.E. Theoretical analysis of stresses in a lithium ion cell. J. Electrochem. Soc. 2009, 157, A155. [Google Scholar] [CrossRef]
- Ling, T.; Jaroniec, M.; Qiao, S.Z. Recent progress in engineering the atomic and electronic structure of electrocatalysts via cation exchange reactions. Adv. Mater. 2020, 32, 2001866. [Google Scholar] [CrossRef] [PubMed]
- Brousse, T.; Bélanger, D.; Long, J.W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef] [Green Version]
- Lindström, H.; Södergren, S.; Solbrand, A.; Rensmo, H.; Hjelm, J.; Hagfeldt, A.; Lindquist, S.E. Li+ ion insertion in TiO2 (anatase). 2. Voltammetry on nanoporous films. J. Phys. Chem. B 1997, 101, 7717–7722. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, D.; Liang, P.; Zhang, Z.; Li, Z.; Hu, Z. 2-Amino-3-chloro-1, 4-naphthoquinone covalently anchored on reduced graphene oxide as all-carbon energy storage material. Diam. Relat. Mater. 2022, 125, 108961. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Wang, X.; Zhou, Y.; Zhang, X.; Qiang, L.; Wang, Q.; Hu, Z. Nitrogen-doped hollow carbon spheres functionalized by 9, 10-phenanthrenequinone molecules as a high-performance electrode for asymmetric supercapacitors. New J. Chem. 2019, 43, 6380–6387. [Google Scholar] [CrossRef]
- An, N.; An, Y.; Hu, Z.; Zhang, Y.; Yang, Y.; Lei, Z. Green and all-carbon asymmetric supercapacitor based on polyaniline nanotubes and anthraquinone functionalized porous nitrogen-doped carbon nanotubes with high energy storage performance. RSC Adv. 2015, 5, 63624–63633. [Google Scholar] [CrossRef]
- Guo, B.; Hu, Z.; An, Y.; Jia, P.; Zhang, Y.; Yang, Y.; Li, Z. Nitrogen-doped heterostructure carbon functionalized by electroactive organic small molecules for asymmetric supercapacitors with high energy density. RSC Adv. 2016, 6, 40602–40614. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Shao, H.; Wu, Y.C.; Lin, Z.; Taberna, P.L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhang, Y.; Huang, W.; Zhang, Q. Recent progress in calix [n] quinone (n = 4, 6) and pillar[5]quinone electrodes for secondary rechargeable batteries. Batter. Supercaps 2020, 3, 476–487. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Tie, Z.; Niu, Z. Design strategies for high-performance aqueous Zn/organic batteries. Angew. Chem. Int. Ed. 2020, 59, 21293–21303. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Li, Z.; Jiao, L.; Hou, L.; Ma, F.; He, Y.; Feng, X. Fe3C encapsulated in N-doped carbon shell grown on reduced graphene oxide as a high-performance negative material for electrochemical energy storage. Chem. Eng. J. 2021, 412, 128720. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Gogotsi, Y.; Simon, P. True performance metrics in electrochemical energy storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, Q.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Niu, C.; Meng, J.; Wang, X.; Han, C.; Yan, M.; Zhao, K.; Xu, X.; Ren, W.; Zhao, Y.; Xu, L.; et al. General synthesis of complex nanotubes by gradient electrospinning and controlled pyrolysis. Nat. Commun. 2015, 6, 7402. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Yang, Y.; Hu, Z.; An, Y.; Zhang, Q.; Yang, X.; Wang, X.; Wu, H. Redox-active organic small molecules functionalized nitrogen-doped porous carbon derived from metal-organic framework as electrode materials for supercapacitor. Electrochim. Acta 2017, 223, 74–84. [Google Scholar] [CrossRef]
- Lee, M.; Hong, J.; Seo, D.H.; Nam, D.H.; Nam, D.K.; Kang, K.; Park, C.B. Redox cofactor from biological energy transduction as molecularly tunable energy-storage compound. Angew. Chem. Int. Ed. 2013, 52, 8322–8328. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Zhang, F.; Hu, Z.; Li, Z.; Li, L.; Yang, Y.; Guo, B.; Lei, Z. Non-covalently functionalizing a graphene framework by anthraquinone for high-rate electrochemical energy storage. RSC Adv. 2015, 5, 23942–23951. [Google Scholar] [CrossRef]
- Hou, L.; Kong, C.; Hu, Z.; Yang, Y.; Wu, H.; Li, Z.; Wang, X.; Yan, P.; Feng, X. Non-covalently self-assembled organic small molecules graphene aerogels to enhance supercapacitive performance. Appl. Surf. Sci. 2020, 508, 145192. [Google Scholar] [CrossRef]
- Hou, L.; Kong, C.; Hu, Z.; Wu, B.; Han, Y. Application of multi-active center organic quinone molecular functionalized graphene in fully pseudocapacitive asymmetric supercapacitors. Nanotechnology 2021, 32, 265704. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Zhang, Q.; Wang, X.; An, Y.; Guo, B.; Hu, Z.; Wu, Y. Dissected carbon nanotubes functionalized by 1-hydroxyanthraquinone for high-performance asymmetric supercapacitors. RSC Adv. 2017, 7, 48341–48353. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.; Bai, L.; Hu, C.M.; Wang, X.; Li, W.S.; Zhao, F.G. Graphene-Indanthrone Donor-π-Acceptor Heterojunctions for High-Performance Flexible Supercapacitors. Adv. Energy Mater. 2020, 10, 2000181. [Google Scholar] [CrossRef]
- Anjos, D.M.; McDonough, J.K.; Perre, E.; Brown, G.M.; Overbury, S.H.; Gogotsi, Y.; Presser, V. Pseudocapacitance and performance stability of quinone-coated carbon onions. Nano Energy 2013, 2, 702–712. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Z.; Wu, J.; Yu, Y.; Peng, Z.; Li, B.; Liu, Y.; Kang, H.; Liu, Z. Synthesis of aminopyrene-tetraone-modified reduced graphene oxide as an electrode material for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 4729–4738. [Google Scholar] [CrossRef]
- Xing, R.; Li, R.; Ge, X.; Zhang, Q.; Zhang, B.; Bulin, C.; Sun, H.; Li, Y. Synthesis of 1, 3-dicarbonyl-functionalized reduced graphene oxide/MnO2 composites and their electrochemical properties as supercapacitors. RSC Adv. 2018, 8, 11338–11343. [Google Scholar] [CrossRef]
- Wang, L.; Ye, X.; Zhu, Y.; Jiang, H.; Xia, J.; Yue, Z.; Wan, Z.; Jia, C.; Yao, X. Conjugated molecule functionalized graphene films for energy storage devices with high energy density. Electrochim. Acta 2020, 340, 135804. [Google Scholar] [CrossRef]
- Haghshenas, M.; Mazloum-Ardakani, M.; Amiri-Zirtol, L.; Sabaghian, F. Arginine-functionalized graphene oxide for green and high-performance symmetric supercapacitors. Int. J. Hydrogen Energy 2021, 46, 30219–30229. [Google Scholar] [CrossRef]
- Ma, F.; Wang, X.; Hu, Z.; Hou, L.; Yang, Y.; Li, Z.; He, Y.; Zhu, H. Organic Molecular Electrode with Ultrahigh Rate Capability for Supercapacitors. Energy Fuels 2020, 34, 13079–13088. [Google Scholar] [CrossRef]
- El-Gendy, D.M.; Ghany, N.A.A.; El Sherbini, E.E.; Allam, N.K. Adenine-functionalized spongy graphene for green and high-performance supercapacitors. Sci. Rep. 2017, 7, 43104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, K.; Liu, X.; Ma, W.; Li, S.; Wang, J.; Fan, S. Functionalization of partially reduced graphene oxide hydrogels with 2-Aminopyridine for high-performance symmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 18728–18740. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, C.; Xu, Y.; Kang, H.; Yang, B.; Liu, H.; Li, Z. Self-assembling porous network nanostructure 7-aminoindole decorated reduced graphene oxide for high-performance asymmetric supercapacitor. J. Alloys Compd. 2020, 835, 155412. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Hou, L.; Zhang, X.; Zhou, Y.; Yang, Y.; Hu, Z. Graphene hydrogels functionalized non-covalently by fused heteroaromatic molecule for asymmetric supercapacitor with ultra-long cycle life. Electrochim. Acta 2019, 317, 437–448. [Google Scholar] [CrossRef]
- Li, H.; Hu, X.; Chen, Y.; Li, H.; Bai, G.; Zhou, K. Thiosalicylic Acid Modified Graphene Aerogel as Efficient Electrode Material for Ionic Liquid Electrolyte-Based Supercapacitors. J. Phys. Chem. C 2022, 126, 9304–9312. [Google Scholar] [CrossRef]
- Allen, J.F.; Martin, W. Out of thin air. Nature 2007, 445, 610–612. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; John Wiley Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Yin, X.; Sarkar, S.; Shi, S.; Huang, Q.A.; Zhao, H.; Yan, L.; Zhao, Y.; Zhang, J. Recent progress in advanced organic electrode materials for sodium-ion batteries: Synthesis, mechanisms, challenges and perspectives. Adv. Funct. Mater. 2020, 30, 1908445. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Huang, C.; Zhou, Y.; Fan, B.; Li, Y.; Hu, A.; Tang, Q.; Huang, K. Redox-active p-phenylenediamine functionalized reduced graphene oxide film through covalently grafting for ultrahigh areal capacitance Zn-ion hybrid supercapacitor. J. Power Sources 2021, 488, 229426. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; An, N.; Wang, X.; Yang, Y.; Hu, Z. Covalently functionalized heterostructured carbon by redox-active p-phenylenediamine molecules for high-performance symmetric supercapacitors. New J. Chem. 2019, 43, 1688–1698. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, W.; Zhu, H.; Meng, H.; Wang, C.; Ma, F.; Hu, Z. Graphene covalently functionalized with 2,6-diaminoanthraquinone (DQ) as a high performance electrode material for supercapacitors. New J. Chem. 2020, 44, 16821–16830. [Google Scholar] [CrossRef]
- Alinajafi, H.A.; Ensafi, A.A.; Rezaei, B. Reduced graphene oxide decorated with thionine, excellent nanocomposite material for a powerful electrochemical supercapacitor. Int. J. Hydrogen Energy 2018, 43, 19102–19110. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Alinajafi, H.A.; Rezaei, B. Adenine decorated@ reduced graphene oxide, a new environmental friendly material for supercapacitor application. J. Alloys Compd. 2018, 735, 1010–1016. [Google Scholar] [CrossRef]
- Fruehwald, H.M.; Melino, P.D.; MacLean, B.J.; Zenkina, O.V.; Easton, E.B. Carbon materials functionalized by nitrogenous ligands for dual application in energy storage and production: Fuel cells and supercapacitors. Electrochim. Acta 2022, 414, 140209. [Google Scholar] [CrossRef]
- Hou, L.; Hu, Z.; Wu, H.; Wang, X.; Xie, Y.; Li, S.; Ma, F.; Zhu, C. 2-Amino-3-chloro-1, 4-naphthoquinone-covalent modification of graphene nanosheets for efficient electrochemical energy storage. Dalton Trans. 2019, 48, 9234–9242. [Google Scholar] [CrossRef]
- Ma, F.; Hu, Z.; Jiao, L.; Wang, X.; He, Y.; Yang, Y.; Li, Z. Organic Molecule-Functionalized Reduced Graphene Oxide for All-Carbon Asymmetric Supercapacitor Applications. ACS Appl. Energy Mater. 2021, 4, 5493–5503. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Kang, H.; Yang, B.; Li, Z.; Liu, H. 2,6-Diaminopyridine decorated reduced graphene oxide as integrated electrode with excellent electrochemical properties for aqueous supercapacitors. Electrochim. Acta 2022, 404, 139725. [Google Scholar] [CrossRef]
- Huangfu, C.; Liu, Z.; Zhou, Q.; Wang, L.; Wei, T.; Qiu, Z.; Zhang, S.; Fan, Z. Covalent grafting of p-phenylenediamine molecules onto a “bubble-like” carbon surface for high performance asymmetric supercapacitors. J. Mater. Chem. A 2020, 8, 1767–1778. [Google Scholar] [CrossRef]
- Najafi, M.D.; Kowsari, E.; Naderi, H.R.; Chinnappan, A.; Ramakrishna, S.; Ehsani, A.; Shokravi, A. Functionalization of graphene oxide via chromium complexes coordinated on 5-aminopyridine-2-carboxylic acid as a symmetric supercapacitor electrode materials in energy storage devices. Compos. Sci. Technol. 2021, 211, 108844. [Google Scholar] [CrossRef]
- Mehra, P.; Paul, A. Covalently Functionalized Hydroxyl-Rich Few-Layer Graphene for Solid-State Proton Conduction and Supercapacitor Applications. J. Phys. Chem. C 2022, 126, 6135–6146. [Google Scholar] [CrossRef]
- Sviridova, E.; Li, M.; Barras, A.; Addad, A.; Yusubov, M.S.; Zhdankin, V.V.; Yoshimura, A.; Szunerits, S.; Postnikov, S.; Boukherroub, R. Aryne cycloaddition reaction as a facile and mild modification method for design of electrode materials for high-performance symmetric supercapacitor. Electrochim. Acta 2021, 369, 137667. [Google Scholar] [CrossRef]
- Kumar, A.; Tan, C.S.; Kumar, N.; Singh, P.; Sharma, Y.; Leu, J.; Huang, E.W.; Tan, W.; Wei, K.H.; Tseng, T.Y. Pentafluoropyridine functionalized novel heteroatom-doped with hierarchical porous 3D cross-linked graphene for supercapacitor applications. RSC Adv. 2021, 11, 26892–26907. [Google Scholar] [CrossRef]
- Lv, L.; Hu, Z.; An, N.; Xie, K.; Yang, Y.; Zhang, Z.; Li, Z. A green and sustainable organic molecule electrode prepared by fluorenone for more efficient energy storage. Electrochim. Acta 2021, 377, 138088. [Google Scholar] [CrossRef]
- Jiao, L.; Ma, F.; Wang, X.; Li, Z.; Hu, Z.; Yin, Q. Quinolinediol Molecule Electrode and MXene for Asymmetric Supercapacitors with Efficient Energy Storage. ACS Appl. Energy Mater. 2021, 4, 7811–7820. [Google Scholar] [CrossRef]
- Ma, F.; Hu, Z.; Jiao, L.; Wang, X.; Yang, Y.; Li, Z.; He, Y. Synthesis and application of naphthalene diimide as an organic molecular electrode for asymmetric supercapacitors with high energy storage. Adv. Mater. Interfaces 2021, 8, 2002161. [Google Scholar] [CrossRef]
- Wang, G.F.; Qin, H.; Gao, X.; Cao, Y.; Wang, W.; Wang, F.C.; Wu, H.A.; Cong, H.P.; Yu, S.H. Graphene thin films by noncovalent-interaction-driven assembly of graphene monolayers for flexible supercapacitors. Chem 2018, 4, 896–910. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.J.; Deng, G.L.; Huang, X.; Wang, X.; Xue, J.M.; Chen, X.Y. Highly boosting the supercapacitor performance by polydopamine-induced surface modification of carbon materials and use of hydroquinone as an electrolyte additive. Electrochim. Acta 2020, 339, 135940. [Google Scholar] [CrossRef]
- Eigler, S.; Hirsch, A. Chemistry with graphene and graphene oxide-challenges for synthetic chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef]
- Greenwood, J.; Phan, T.H.; Fujita, Y.; Li, Z.; Ivasenko, O.; Vanderlinden, W.; Gorp, H.V.; Frederickx, W.; Lu, G.; Tahara, K.; et al. Covalent modification of graphene and graphite using diazonium chemistry: Tunable grafting and nanomanipulation. ACS Nano 2015, 9, 5520–5535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturala, J.; Luxa, J.; Pumera, M.; Sofer, Z. Chemistry of graphene derivatives: Synthesis, applications, and perspectives. Chem.-A Eur. J. 2018, 24, 5992–6006. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, X.; Hou, L.; Wang, X.; Zhang, Y.; Zhu, C.; Hu, Z.; He, M. Graphene covalently functionalized by cross-linking reaction of bifunctional pillar organic molecule for high capacitance. J. Energy Storage 2021, 38, 102530. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution properties of graphite and graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Z.; Zhang, X.; Wang, Y.; Tian, J.; Huang, Y.; Ma, Y.; Zhang, X.; Chen, Y. A graphene hybrid material covalently functionalized with porphyrin: Synthesis and optical limiting property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
- Liu, Z.B.; Xu, Y.F.; Zhang, X.Y.; Zhang, X.L.; Chen, Y.S.; Tian, J.G. Porphyrin and fullerene covalently functionalized graphene hybrid materials with large nonlinear optical properties. J. Phys. Chem. B 2009, 113, 9681–9686. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [Green Version]
- Veca, L.M.; Lu, F.; Meziani, M.J.; Cao, L.; Zhang, P.; Qi, G.; Qu, L.; Shrestha, M.; Sun, Y. Polymer functionalization and solubilization of carbon nanosheets. Chem. Commun. 2009, 18, 2565–2567. [Google Scholar] [CrossRef]
- Huisgen, R. Kinetics and mechanism of 1,3-dipolar cycloadditions. Angew. Chem. Int. Ed. Engl. 1963, 2, 633–645. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger, M.; Hirsch, A. Organic functionalization of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Tagmatarchis, N.; Prato, M. Functionalization of carbon nanotubes via 1,3-dipolar cycloadditions. J. Mater. Chem. 2004, 14, 437–439. [Google Scholar] [CrossRef]
- Georgakilas, V.; Bourlinos, A.B.; Zboril, R.; Steriotis, T.A.; Dallas, T.; Stubos, A.K.; Trapalis, K. Organic functionalisation of graphenes. Chem. Commun. 2010, 46, 1766–1768. [Google Scholar] [CrossRef] [PubMed]
- Schon, T.B.; McAllister, B.T.; Li, P.F.; Seferos, D.S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [PubMed]

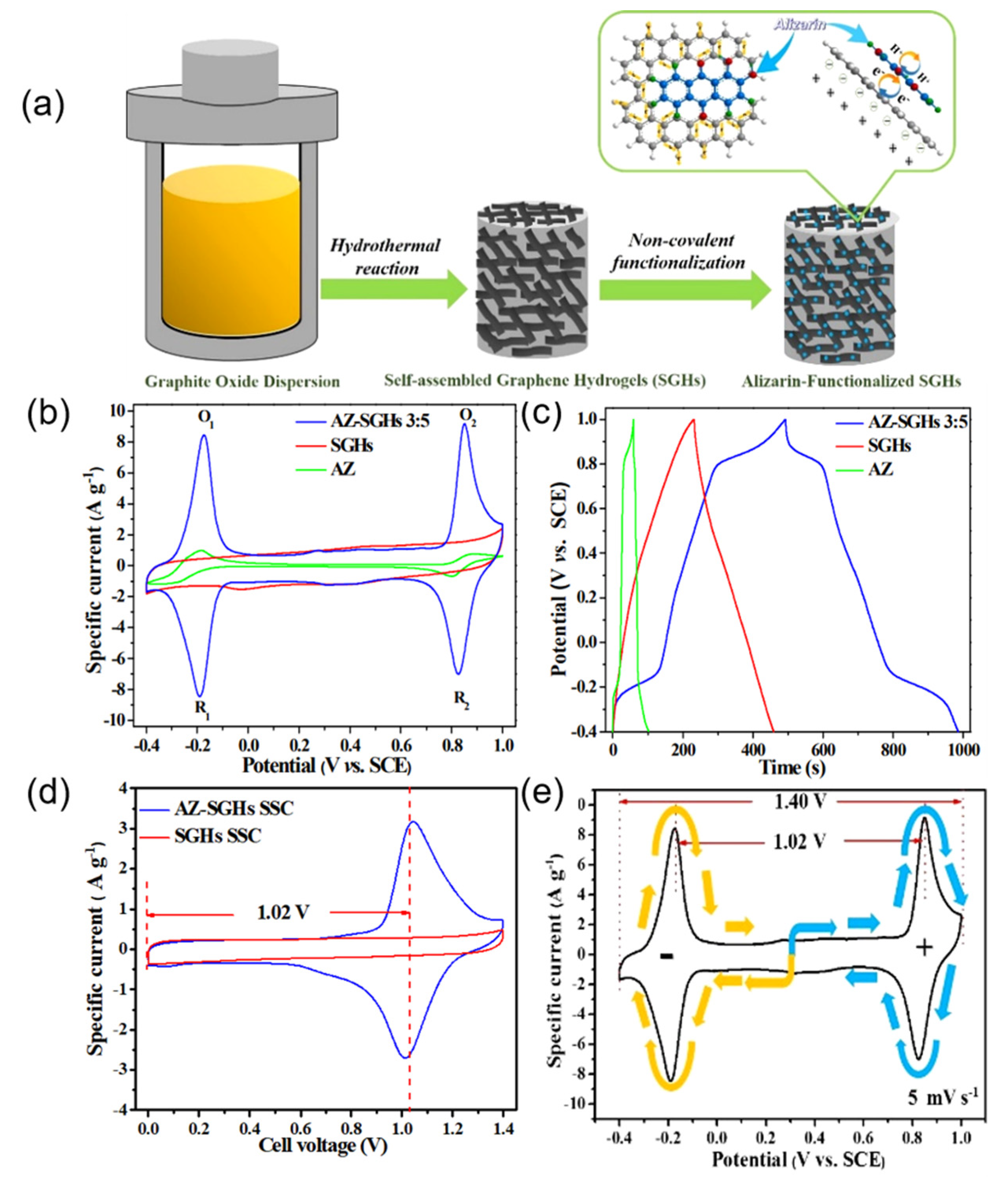
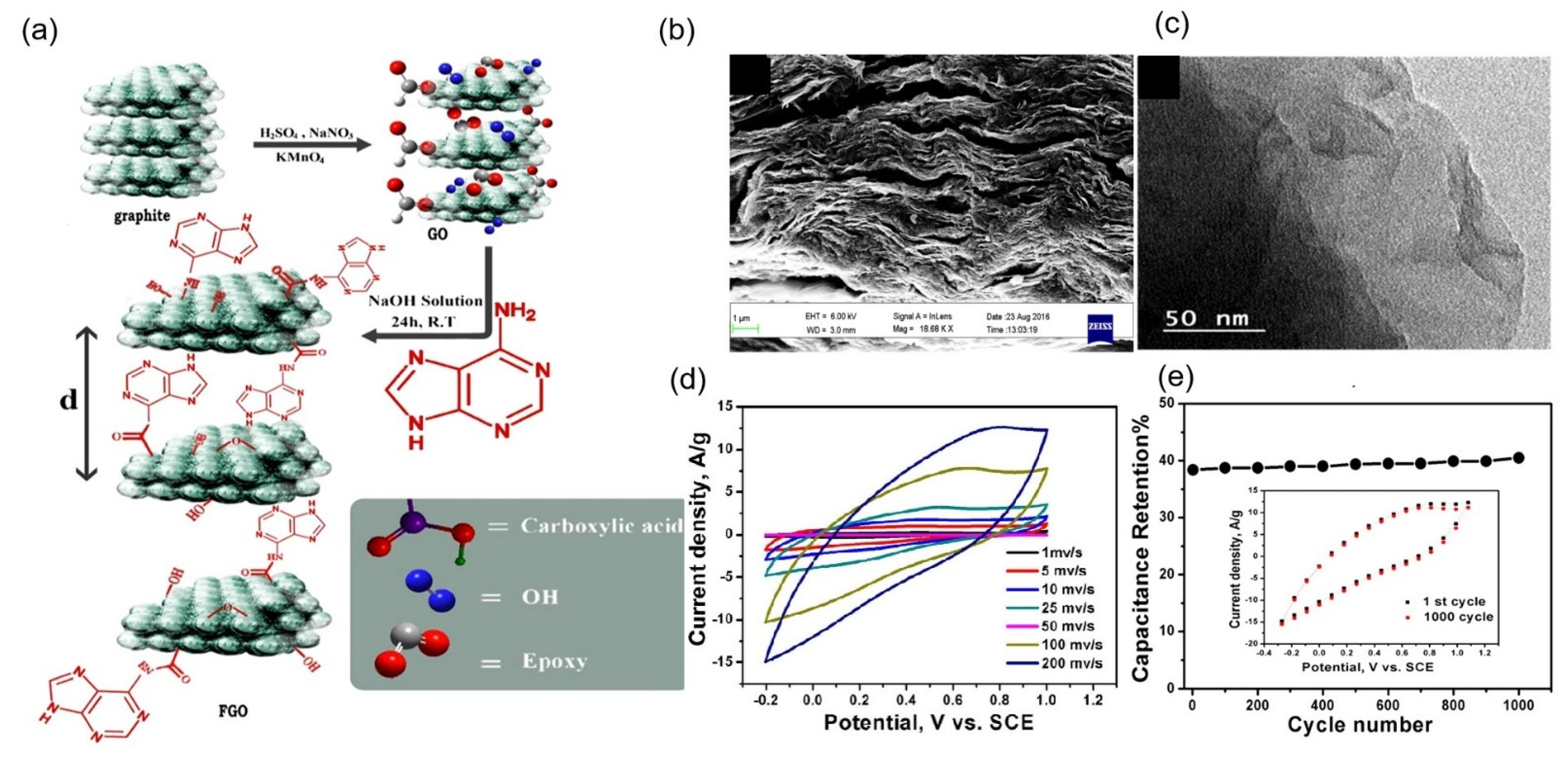

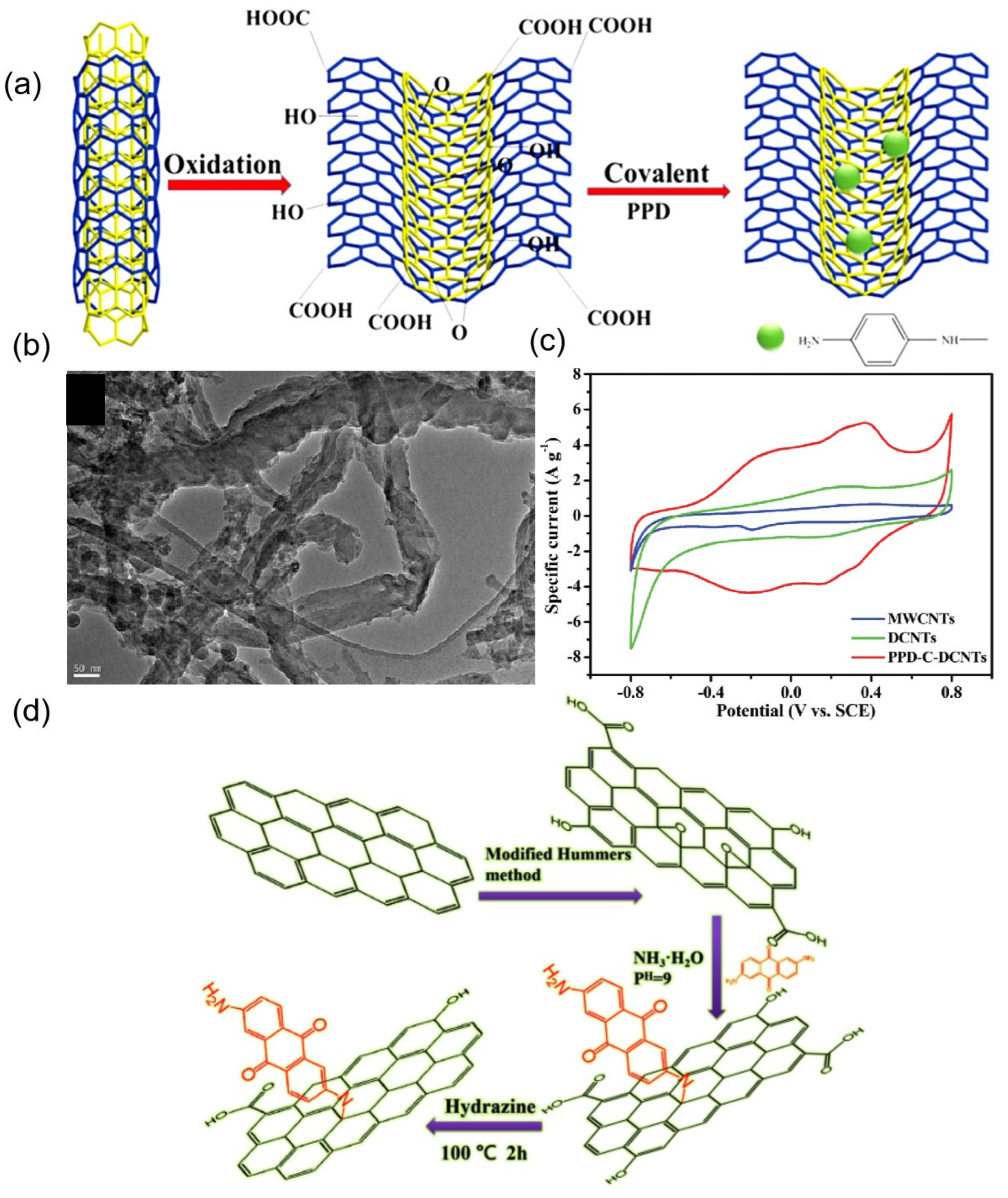
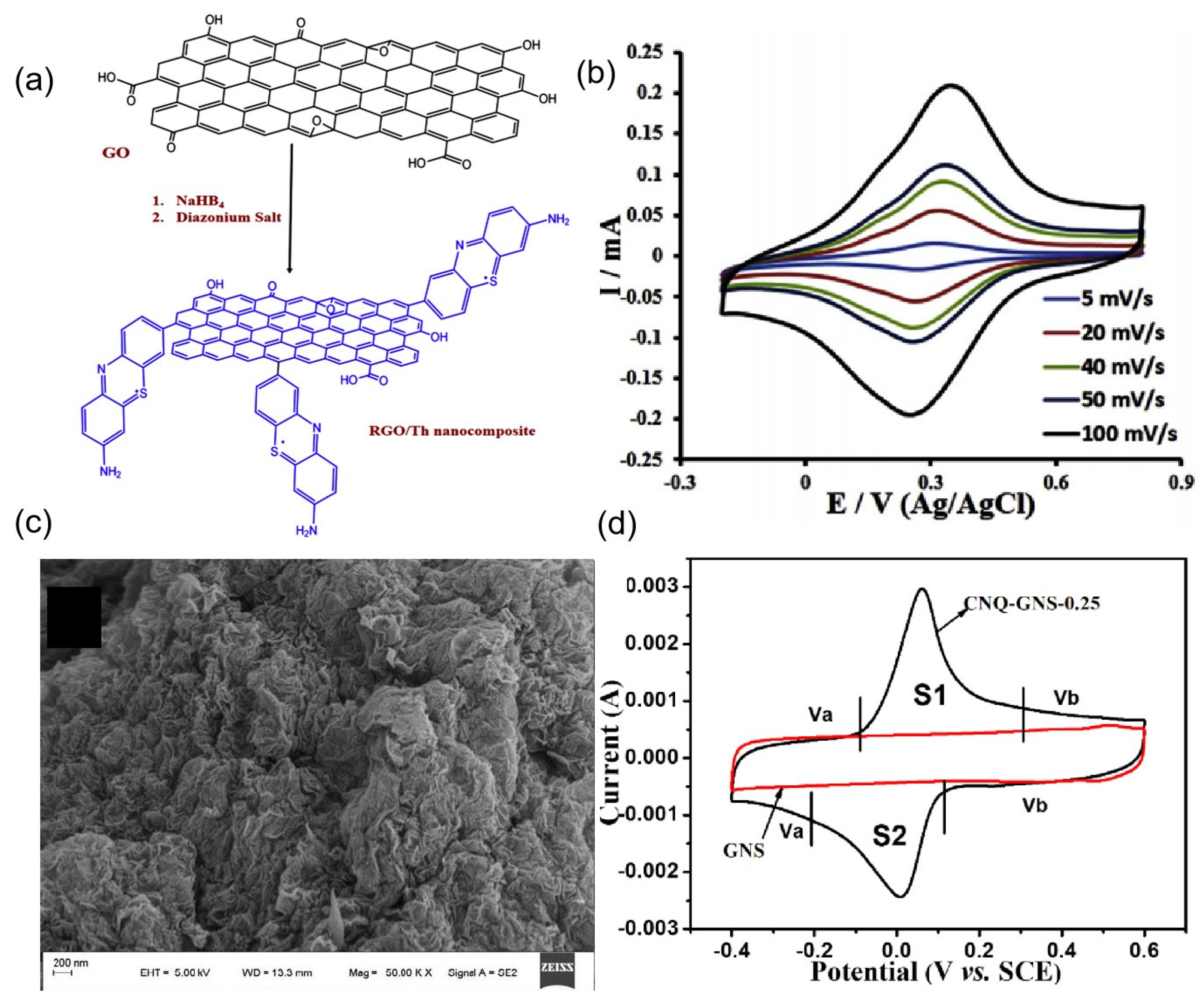



| Organic Small-Molecule Electrode (OME) | Organic Small Molecules | Conductive Carbon Substrate | Electrolyte | C (F g−1) | Emax (Wh kg−1) | Pmax (kW kg−1) | Ref. |
|---|---|---|---|---|---|---|---|
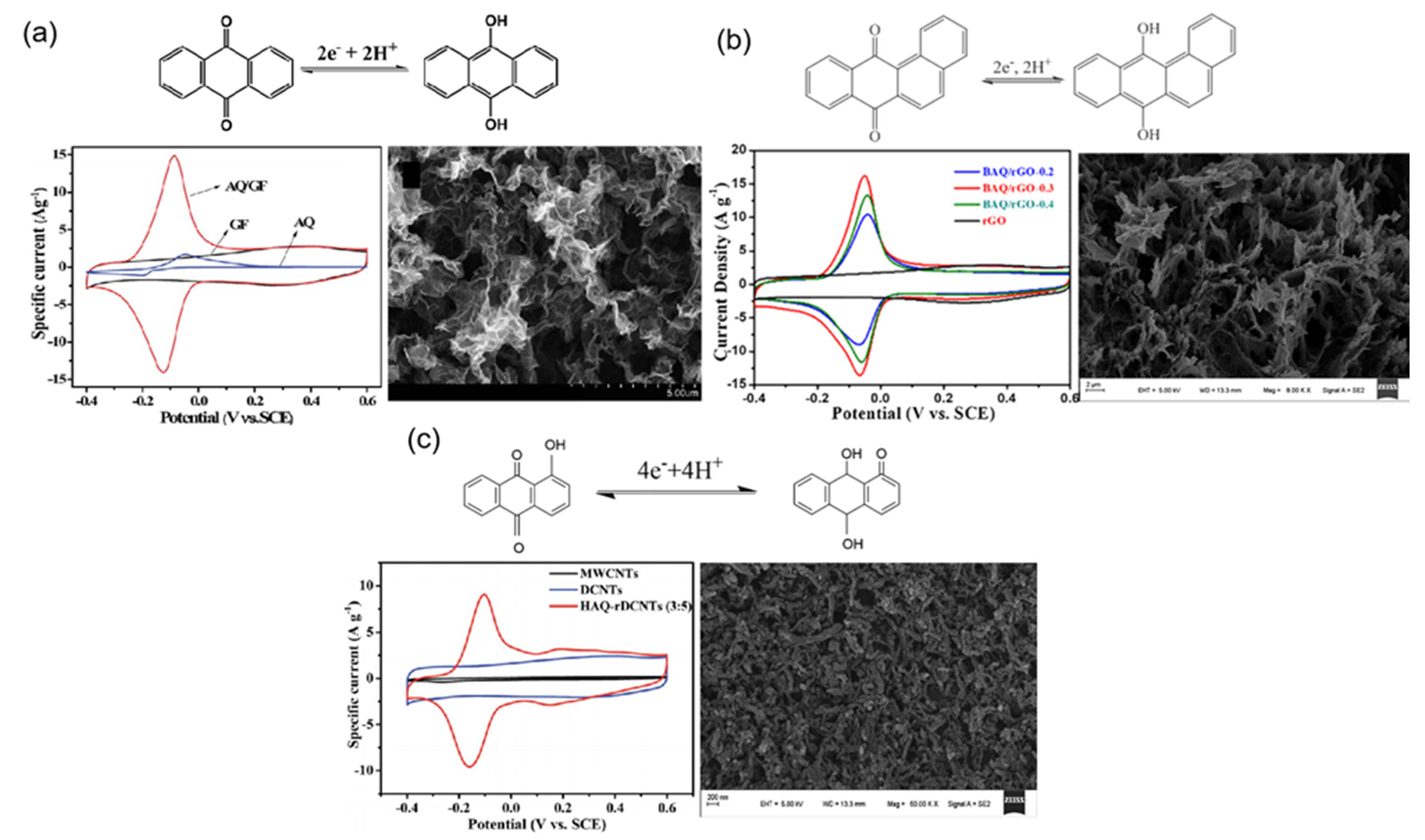
| AQ/GF | Anthraquinone | Graphene | 1 M H2SO4 | 396 at 1 A g−1 | 13.2 | 9.18 | [54] |
| BAQ/rGO | Benz[a]anthracene-7,12-quinone | Reduced graphene oxide | 1 M H2SO4 | 400 at 1 A g−1 | 30.33 | - | [55] |
| PT@rGO | 5,7,12,14-pentacenetetrone | Reduced graphene oxide | 1 M H2SO4 | 433.2 at 5 mV s−1 | 18.2 | 12.9 | [56] |
| HAQ-rDCNTs | 1-hydroxyanthrAquinone | Reduced graphene oxide | 1 M H2SO4 | 324 at 1 A g−1 | 12.3 | - | [57] |
| AZ–SGHs | Alizarin | Graphene hydrogels | 1 M H2SO4 | 350 at 1 A g−1 | 18.2 | - | [6] |
| rGO–π–IDT | Indanthrone | Reduced graphene oxide | 1 M H2SO4 | 535.5 at 1 A g−1 | 31.3 Wh L−1 | 38.55 kW L−1 | [58] |
| PY-OLC | 4,5-pyrenedione | Carbon onion | 1 M H2SO4 | 264 at 1.3 A g−1 | - | - | [59] |
| PYT-NH2/rGO | 2-aminopyrene-3,4,9,10-tetraone | Reduced graphene oxide | 1 M H2SO4 | 326 at 0.5 A g−1 | 15.4 | 6.1 | [60] |
| DGM4 | N-(4- aminophenyl)-3-oxobutanamide | Reduced graphene oxide | 1 M H2SO4 | 267.4 at 0.5 A g−1 | - | - | [61] |
| 2-NTQ-RGO | 2-amino-3-chloro-1,4-naphthoquinone | Reduced graphene oxide | 1 M H2SO4 | 453 at 1 A g−1 | 23.4 | - | [36] |
| PTCDA/rGO | 3,4,9,10-perylene-tetracarboxylicacid-dianhydride | Reduced graphene oxide | 1 M Li2SO4 | 242.9 at 2 A g−1 | 19.7 | 45 | [62] |
| Arg/GO | Arginine amino acid | Graphene oxide | 6 M KOH | 295 at 1 A g−1 | 50 | 2.2 | [63] |
| PTCA/rGO1 | 3,4,9,10- perylenetetracarboxylic acid | Reduced graphene oxide | 1 M H2SO4 | 422.7 at 5 mV s−1 | 14 | 4.9 | [64] |
| FG | Adenine | Graphene oxide | 0.5 M H2SO4 | 333 at 1 mV s−1 | 64.4 | 3.0 | [65] |
| APGHs | 2-aminopyridine | Graphene oxide | 6 M KOH | 266.7 at 0.3 A g−1 | 9.3 | 2.4 | [66] |
| 7-AirGO | 7-aminoindole | Reduced graphene oxide | 2 M KOH | 425.7 at 0.5 A g−1 | 350 | 10.5 | [67] |
| BDTD-rGO | Benzo [1,2-b:4,5-b’] dithiophene-4,8-dione | Reduced graphene oxide | 1 M H2SO4 | 360 at 1 A g−1 | 9.5 | [68] | |
| TGA | Thiosalicylic acid | Graphene aerogel | [Bmim][Tf2N] IL | - | 115 | 11.6 | [69] |
| Organic Small-Molecule Electrode (OME) | Organic Small Molecules | Conductive Carbon Substrate | Preparation Strategy | Electrolyte | C (F g−1) | Emax (Wh kg−1) | Pmax (kW kg−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| RGO@PPD | p-phenylenediamine | Reduced graphene oxide film | Epoxide Ring Opening | Zn(Ac)2 | - | 1.1 mWh cm−2 | 0.8 mW cm−2 | [73] |
| PPD-C-DCNT | p-phenylenediamine | dissected carbon nanotubes | Epoxide Ring Opening | 1 M H2SO4 | 388 at 1 A g−1 | 19.1 | 16 | [74] |
| DQ-RGO | 2,6-diaminoaquinone | Graphene oxide | Epoxide Ring Opening | 1 M H2SO4 | 332 at 1 A g−1 | 14.2 | - | [75] |
| Th/RGO | thionine | Reduced graphene oxide | Diazotisation | 0.5 M H2SO4 | 1255 at 0.5 A g−1 | - | - | [76] |
| adenine@rGO | adenine | Reduced graphene oxide | Diazotisation | 0.5 M H2SO4 | 700 at 0.5 A g−1 | - | - | [77] |
| BP-phenazin-Fe-700 | dipyrido[3,2-a:2′,3′-c]phenazin-11-amine (phenazin) | Commercial carbon blacks | Diazotisation | 0.5 M H2SO4 | 287 at 1 A g−1 | - | - | [78] |
| CNQ-GNS | 2-amino-3-chloro-1,4-naphthoquinone | Graphene nanosheets | Diazotisation | 1 M H2SO4 | 364.2 at 1.3 A g−1 | 19.1 | - | [79] |
| DAAQ-PGN | 2,6-diaminoanthraquinone | Graphene sheets | Diazotisation | 1 M H2SO4 | 522 at 5 mV s−1 | 19.8 | - | [79] |
| RGO-THBA | 3,4,5-trihydroxybenzamide | Graphene hydrogels | Acylation | 1 M H2SO4 | 390.6 at 5 mV s−1 | 14 | 4.9 | [80] |
| RGO/DAP | 2,6-diaminopyridine | Reduced graphene oxide | Acylation | 2 M KOH | 337.6 at 0.5 A g−1 | 14.6 | - | [81] |
| PPD-BC | p-phenylenediamine | Carbon spheres | Acylation | 2 M KOH | 451 at 2 mV s−1 | 94 | - | [82] |
| FGO-Ap/Cr | 5-aminopyridine-2-carboxylic acid Cr (Ⅲ) complex | Graphene oxide | Acylation | 3 M KNO3 | 461.5 at 1 A g−1 | 523.3 | 98.1 | [83] |
| HRFG | 3,4- dihydroxybenzaldehyde | Graphene | Cycloaddition | 2 M H2SO4 | 320 at 1 A g−1 | 15.4 | 6.1 | [84] |
| f2-RGO | pseudocyclic iodoxoborole | Reduced graphene oxide | Cycloaddition | 2 M KOH | 297 at 1 A g−1 | 6.7 | - | [85] |
| NPFG | pentafluoropyridine | Reduced graphene oxide | Doping of Heteroatoms | 6 M KOH | 319 at 0.5 A g−1 | 38 | 0.72 | [86] |
| G&GMH-CFA | caffeic acid | Graphene nanomesh hydrogel | π–π Interaction | 3 M H2SO4 | 482.6 at 1 A g−1 | 26.4 | 35 | [25] |
| DHFO/rGO | 2,7-dihydroxy9-fluorenone | Reduced graphene oxide | π–π Interaction | 1 M H2SO4 | 412.3 at 5 mV s−1 | 28.6 | 2.8 | [87] |
| QD/rGO | 2,8-quinolinediol | Reduced graphene oxide | π–π Interaction | 1 M H2SO4 | 371 at 5 mV s−1 | 28 | 5.7 | [88] |
| rGO-NDI | naphthalene diimine | Reduced graphene oxide | π–π Interaction | 1 M H2SO4 | 354 at 5 mV s−1 | 26.3 | 26.5 | [89] |
| RGO films | melamine | Graphene oxide | Hydrogen Bond | H2SO4-PVA gel | 197.3 F cm−3 at 500 mA cm−3 | - | - | [90] |
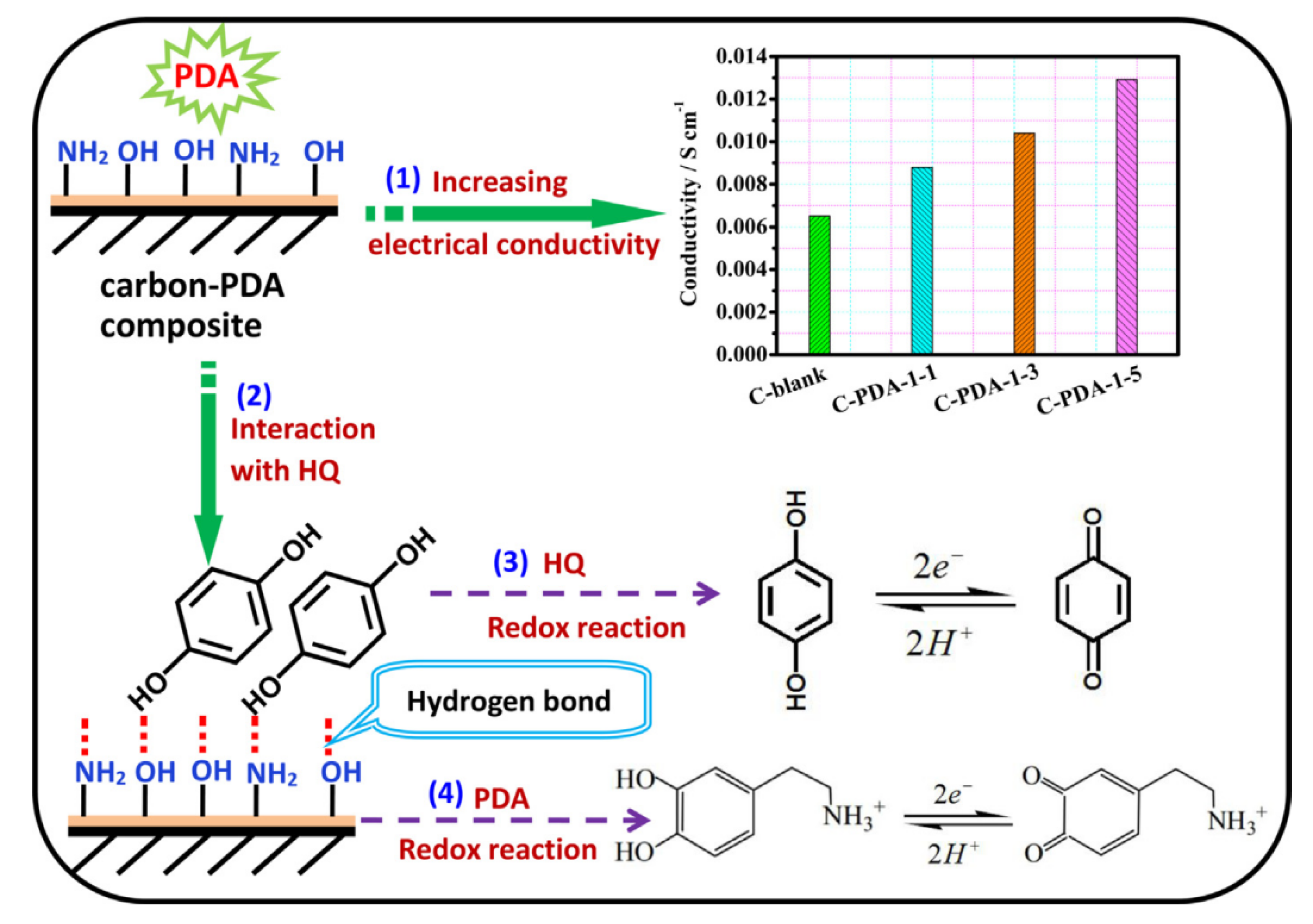
| C-PDA-HQ | hydroquinone | Polydopamine modified activated carbon | Hydrogen Bond | 1 M H2SO4 | 557 at 1 A g−1 | 19.4 | 25 | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wei, Q.; An, N.; Meng, C.; Hu, Z. Organic Small-Molecule Electrodes: Emerging Organic Composite Materials in Supercapacitors for Efficient Energy Storage. Molecules 2022, 27, 7692. https://doi.org/10.3390/molecules27227692
He Y, Wei Q, An N, Meng C, Hu Z. Organic Small-Molecule Electrodes: Emerging Organic Composite Materials in Supercapacitors for Efficient Energy Storage. Molecules. 2022; 27(22):7692. https://doi.org/10.3390/molecules27227692
Chicago/Turabian StyleHe, Yuanyuan, Qiaoqiao Wei, Ning An, Congcong Meng, and Zhongai Hu. 2022. "Organic Small-Molecule Electrodes: Emerging Organic Composite Materials in Supercapacitors for Efficient Energy Storage" Molecules 27, no. 22: 7692. https://doi.org/10.3390/molecules27227692
APA StyleHe, Y., Wei, Q., An, N., Meng, C., & Hu, Z. (2022). Organic Small-Molecule Electrodes: Emerging Organic Composite Materials in Supercapacitors for Efficient Energy Storage. Molecules, 27(22), 7692. https://doi.org/10.3390/molecules27227692