The Effects of Pepper (Zanthoxylum bungeanum) from Different Production Areas on the Volatile Flavor Compounds of Fried Pepper Oils Based on HS-SPME–GC–MS and Multivariate Statistical Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. The GC–MS Test Analysis of Pepper Oil Samples
2.2. Sensory Analysis of Pepper Oil Samples from Different Production Areas
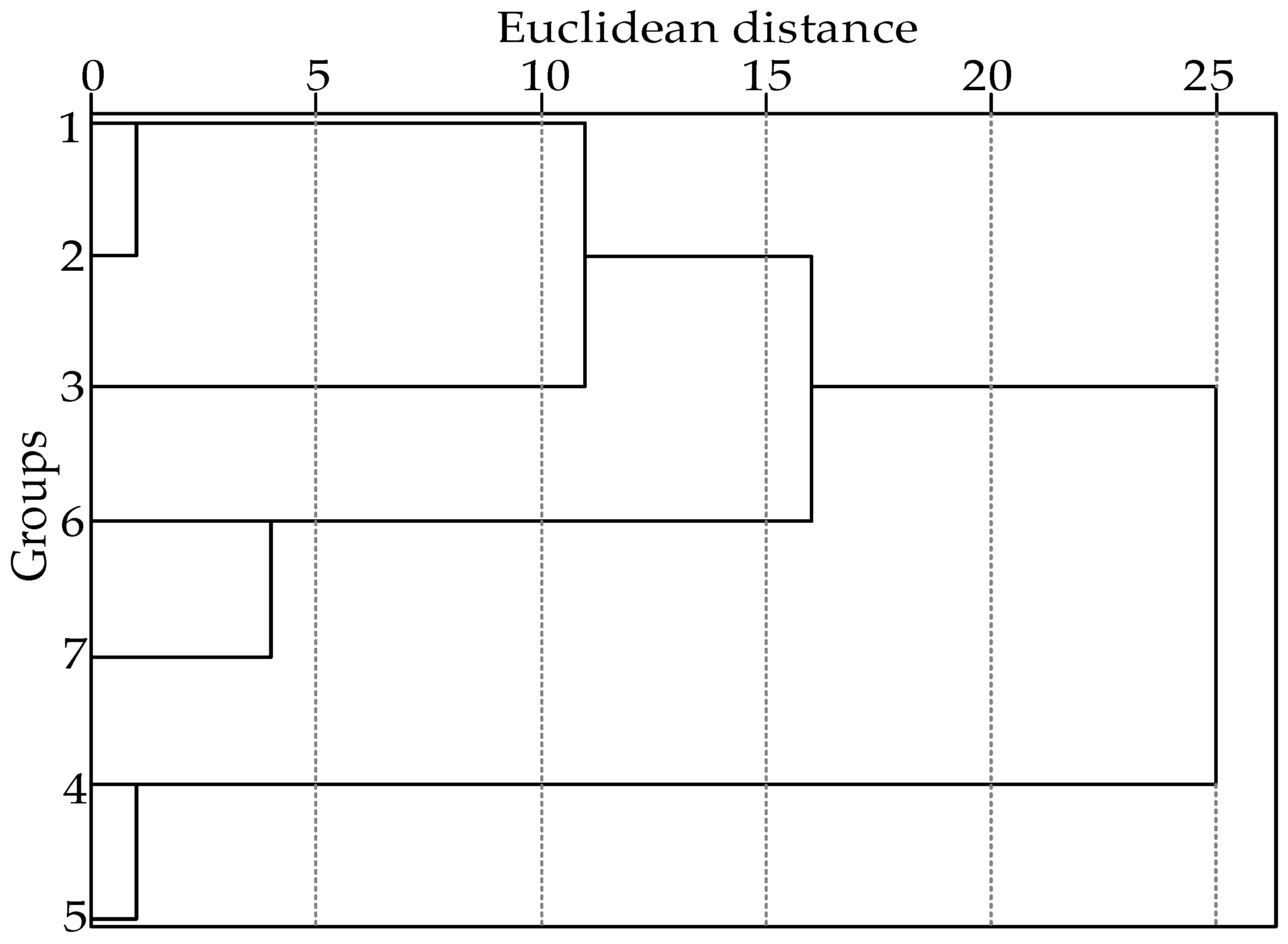
2.3. Cluster Analysis of Volatile Components in Pepper Oil
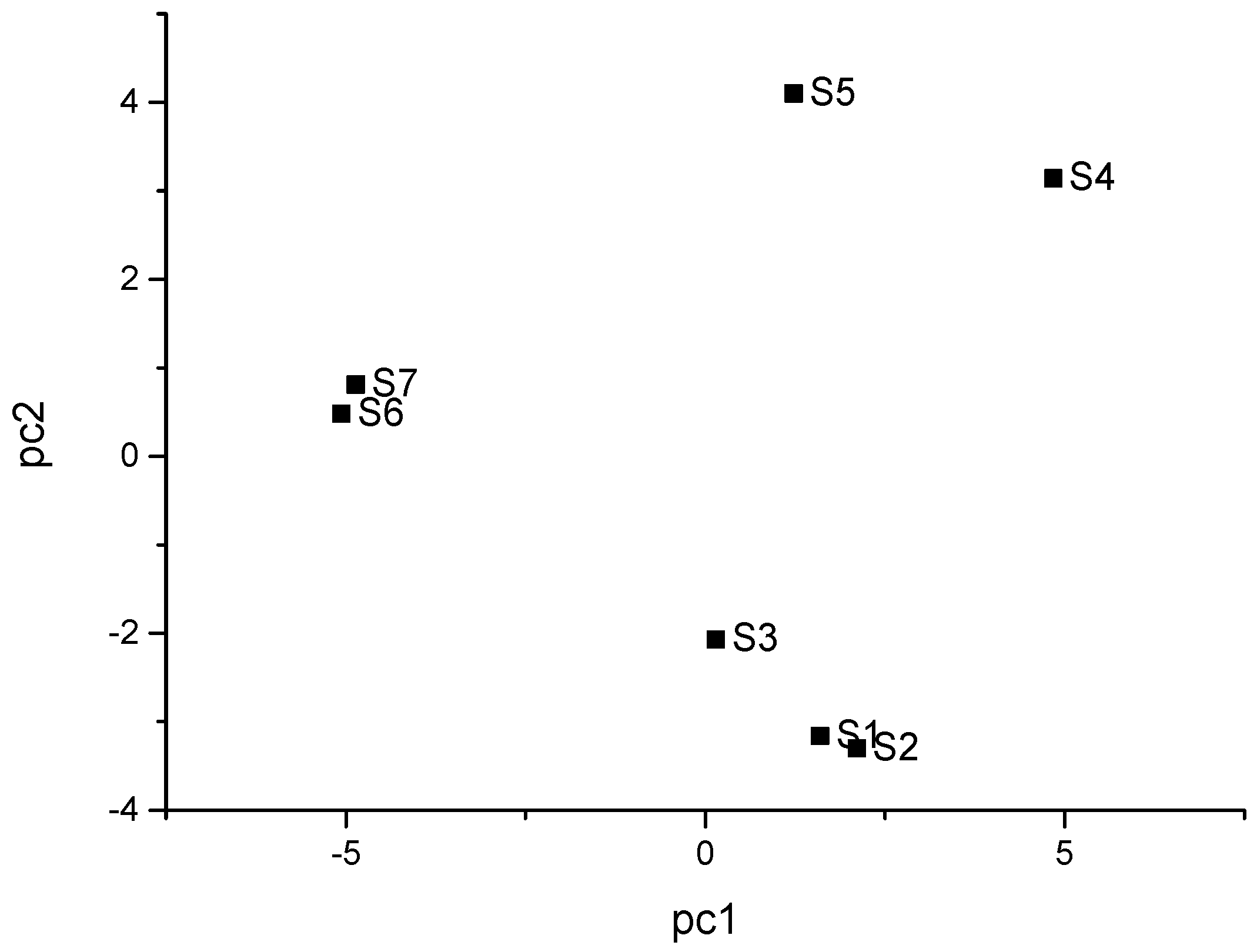
2.4. Principal Component Analysis of Pepper Oil
3. Materials and Methods
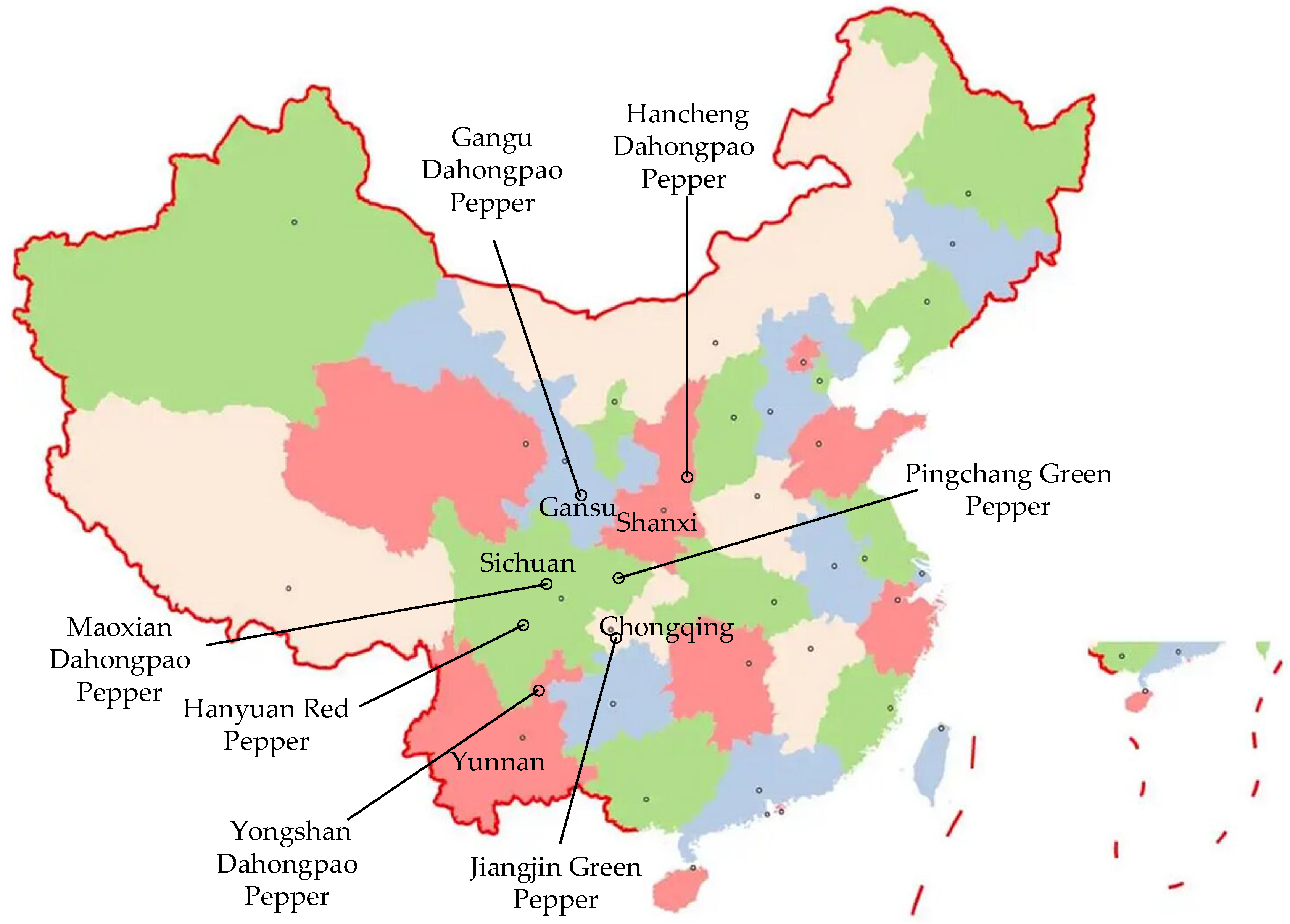
3.1. Materials and Instruments
3.2. Research Methods
3.2.1. Preparation of Pepper Oil [33]
3.2.2. Extraction of Volatile Flavor Substances
3.2.3. Test Conditions of GC-MS
3.2.4. Quantitative and Qualitative Methods
3.2.5. Sensory Evaluation
3.2.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ji, Y.; Li, S.M.; Ho, C.T. Chemical composition, sensory properties and application of Sichuan pepper (Zanthoxylum genus). Food Sci. Hum. Well. 2019, 8, 115–125. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China; Chemical Industry Press: Beijing, China, 2015; p. 169.
- Wei, S.; Zhang, H.; Wang, Y.; Wang, L.; Li, X.; Wang, Y.; Shi, Y. Ultrasonic nebulization extraction−heating gas flow transfer−headspace single drop microextraction of essential oil from pericarp of Zanthoxylum bungeanum Maxim. J. Chromatogr. A 2011, 1218, 4599–4605. [Google Scholar] [CrossRef]
- Jing, N.N.; Wang, M.Y.; Gao, M.L.; Zhong, Z.X.; Ma, Y.P.; Wei, A.Z. Color sensory characteristics, nutritional components and antioxidant capacity of Zanthoxylum bungeanum Maxim. as affected by different drying methods. Ind. Crops Prod. 2021, 160, 113167. [Google Scholar] [CrossRef]
- Hwang, K.A.; Kwon, J.E.; Noh, Y.H.; Park, B.K.; Jeong, Y.J.; Lee, S.M.; Kang, S.C. Effects of Zanthoxylum piperitum ethanol extract on osteoarthritis inflammation and pain. Biomed. Pharmacother. 2018, 105, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, X.; Hou, L.X.; Wei, A.Z. Extraction solvent affects the antioxidant, antimicrobial, cholinesterase and HepG2 human hepatocellular carcinoma cell inhibitory activities of Zanthoxylum bungeanum pericarps and the major chemical components. Ind. Crops Prod. 2019, 142, 111872. [Google Scholar] [CrossRef]
- Zhuo, Z.H.; Xu, D.P.; Pu, B.; Wang, R.L.; Ye, M. Predicting distribution of Zanthoxylum bungeanum Maxim. in China. BMC Ecol. 2020, 20, 366–375. [Google Scholar] [CrossRef]
- Liu, Y.L.; Li, J.; Wang, G.P.; Sun, G.H. Comparative Study on Flavor and Overall Quality of Zanthoxylum bungeanum Seed Oil and Zanthoxylum bungeanum Oil. Food Sci. 2021, 42, 195–201. [Google Scholar]
- Zeng, M.; Wang, J.; Zhang, M.; Chen, J.; He, Z.; Qin, F.; Chen, J. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chem. 2018, 239, 111–118. [Google Scholar] [CrossRef]
- Epifano, F.; Curini, M.; Carla, M.M.; Genovese, S. Searching for Novel Cancer Chemopreventive Plants and their Products: The Genus Zanthoxylum. Curr. Drug Targets 2011, 12, 1895–1902. [Google Scholar] [CrossRef]
- Yu, L.; Wu, W.; Pan, Y.; Wang, W.; Sun, L.; Liu, Y.; Li, D. Quality evaluation of different varieties of Zanthoxylum bungeanum Maxim. peels based on phenolic profiles, bioactivity, and HPLC fingerprint. J. Food Sci. 2020, 85, 1090–1097. [Google Scholar] [CrossRef]
- Xiao, P.G. New Annals of Traditional Chinese Medicine; Chemical Industry Press: Beijing, China, 2002; pp. 253–259. [Google Scholar]
- Wang, S.; Xie, J.C.; Yang, W.; Sun, B.G. Preparative separation and purification of alkylamides from Zanthoxylum bungeanum maxim by high-speed counter-current chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2640–2652. [Google Scholar] [CrossRef]
- Gao, X.J.; Gao, H.Y.; Zhao, L.; Shi, B.L.; Zhang, L.L.; Wang, H.Y.; Zhong, K. Analysis of Key Aroma Components in Fried Zanthoxylum bungeanum Oil from Different Production Areas by Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry Combined with Odor Activity Value. Food Sci. 2022, 43, 208–214. [Google Scholar]
- Zhao, Z.F.; Zhu, R.X.; Zhong, K.; He, Q.; Luo, A.M.; Gao, H. Characterization and Comparison of the Pungent Components in Commercial Zanthoxylum bungeanum oil and Zanthoxylum schinifolium oil. J. Food Sci. 2013, 78, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Sun, F.Y.; Wang, D.; Sun, B.G.; Zhang, Y.Y. Identification of key aroma-active compounds of fried zanthoxylum bungeanum oil by gradient dilution method combined with gas chromatography-olfactory-mass spectrometry. Food Ferment. Ind. 2017, 43, 191–198. [Google Scholar]
- Liu, Y.P.; Li, Q.R.; Yang, W.X.; Sun, B.G.; Zhou, Y.; Zheng, Y.; Huang, M.Q.; Yang, W.J. Characterization of the potent odorants in Zanthoxylum armatum DC Prodr. pericarp oil by application of gas chromatography-mass spectrometry-olfactometry and odor activity value. Food Chem. 2020, 319, 126564. [Google Scholar] [CrossRef]
- Sun, J.; Sun, B.G.; Ren, F.Z.; Chen, H.T.; Zhang, N.; Zhang, Y.Y. Characterization of Key Odorants in Hanyuan and Hancheng Fried Pepper (Zanthoxylum bungeanum) Oil. J. Agric. Food Chem. 2020, 68, 6403–6411. [Google Scholar] [CrossRef]
- Ni, R.J.; Zhan, P.; Tian, H.L. Analysis of the effect of frying time on volatile substances of Zanthoxylum bungeanum seasoning oil based on GC-IMS and multivariate statistical method. Food Sci. 2021, 18, 1–12. [Google Scholar]
- Zhang, N.; Sun, B.G.; Mao, X.Y.; Chen, H.T.; Zhang, Y.Y. Flavor formation in frying process of green onion (Allium fistulosum L.) deep−fried oil. Food Res. Int. 2019, 121, 296–306. [Google Scholar] [CrossRef]
- Lin, X.Y. The application of camphor leaf extract in cosmetics. Flavour Fragr. Cosmet. 2011, 2, 44–48. [Google Scholar]
- Hu, W.J.; Gao, H.D.; Jiang, X.M.; Yang, H.K. Analysis of the composition and content of leaf essential oils of camphor, naprocampus and isocamphora chemotypes. J. Cent. South Univ. For. Technol. 2012, 32, 186–194. [Google Scholar]
- Li, Z.Y.; Mao, D.S.; Xu, S.J.; Liu, Q. Analysis of volatile components in perilla oil by full two-dimensional gas chromatography time of flight mass spectrometry. Flavour Fragr. Cosmet. 2011, 6, 1–7. [Google Scholar]
- Zhang, S.P.; Gu, F.L.; Wu, G.P.; Wang, Q.H. Analysis of chemical constituents of essential oils from pepper fruit and pepper leaves. Chin. J. Trop. Crops 2014, 2, 387–395. [Google Scholar]
- Gao, T.T.; Sun, J.W.; Yang, K.Y.; Liu, Y.P.; Sun, B.G. Analysis of volatile components in fresh hawthorn pulp by SDE-GC-MS. J. Food Sci. Technol. 2015, 33, 22–27. [Google Scholar]
- Sun, B.G.; He, J. Fragrance Chemistry and Technology; Chemical Industry Press: Beijing, China, 2004. [Google Scholar]
- Zhao, Z.F.; Zhu, R.X.; Gao, H.; He, Q.; He, X.Y.; Gu, X.Q.; Yan, Z.N. Comparison of the Pungent Components in Fresh and Dried Zanthoxylum bungeanum Oil. Chin. Condiment. 2014, 39, 33–35. [Google Scholar]
- Fan, L.H.; Huang, Y.L.; Zhao, R.; Fan, W.X.; Zhang, M.M.; Zhang, H.Y.; Yan, X.J.; Li, L.; Wu, C.J. Geographical-origin discrimination and volatile oil quantitative analysis of Zanthoxylum bungeanum Maxim. with a portable near-infrared spectrometer. Anal. Methods 2019, 11, 5301–5310. [Google Scholar] [CrossRef]
- Stevanovic, T.; Garneau, F.X.; Jean, F.I.; Gagnon, H.L.; Vilotic, D.; Petrovic, S.; Ruzic, N.; Pichette, A. The essential oil composition of Pinus mugo turra from Serbia. Flavour Frag. J. 2005, 20, 96–97. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhao, J.W.; Xu, F.; Wu, X.Y.; Hu, W.X.; Chang, Y.F.; Zhang, L.L.; Chen, J.; Liu, C.H. GC-MS, GC-O and OAV analyses of key aroma compounds in Jiaozi Steamed Bread. Grain Oil Sci. Technol. 2020, 3, 9–17. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.Y.; Zhang, D.Q.; Shen, Q.W.; Pan, T.; Hui, T.; Ma, J.R. Characterization of Key Aroma Compounds in Beijing Roasted Duck by Gas Chromatography-Olfactometry-Mass Spectrometry, Odor-Activity Values, and Aroma-Recombination Experiments. J. Agr. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef]
- Zhu, X.L.; Gao, Y.; Chen, Z.Y.; Su, Q.D. Development of a chromatographic fingerprint of tobacco flavor by use of GC and GC -MS. Chromatographia 2009, 69, 735–742. [Google Scholar] [CrossRef]
- Sun, F.Y.; Wang, D.; Chen, H.T.; Sun, B.G.; Zhang, Y.Y. Comparative analysis of volatile flavor compounds in fried Zanthoxylum bungeanum oil by SPME and SAFE. Fine Chem. 2016, 33, 49–54+61. [Google Scholar]
- Meil, M.C.; Carrb, T.; Civille, G.V. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 1991; Volume 107, pp. 598–605. [Google Scholar]
- Liu, Y.; Ding, X.; Zheng, L.X.; Zhang, Y.; Zhou, H.L. Herbal drink formulation optimization of Trollius chinensis Bunge by sensory fuzzy comprehensive evaluation. Acta Sci. Pol. Technol. Aliment. 2020, 19, 185–194. [Google Scholar] [PubMed]
- Zhu, Y.Z.; Ji, W.; Ji, H.W.; Gao, J.; Liu, S.C.; Ruan, C.W. Optimization of vacuum frying process of papaya chips based on fuzzy mathematical evaluation method. Food Ind. Sci. Technol. 2017, 38, 183–188. [Google Scholar]
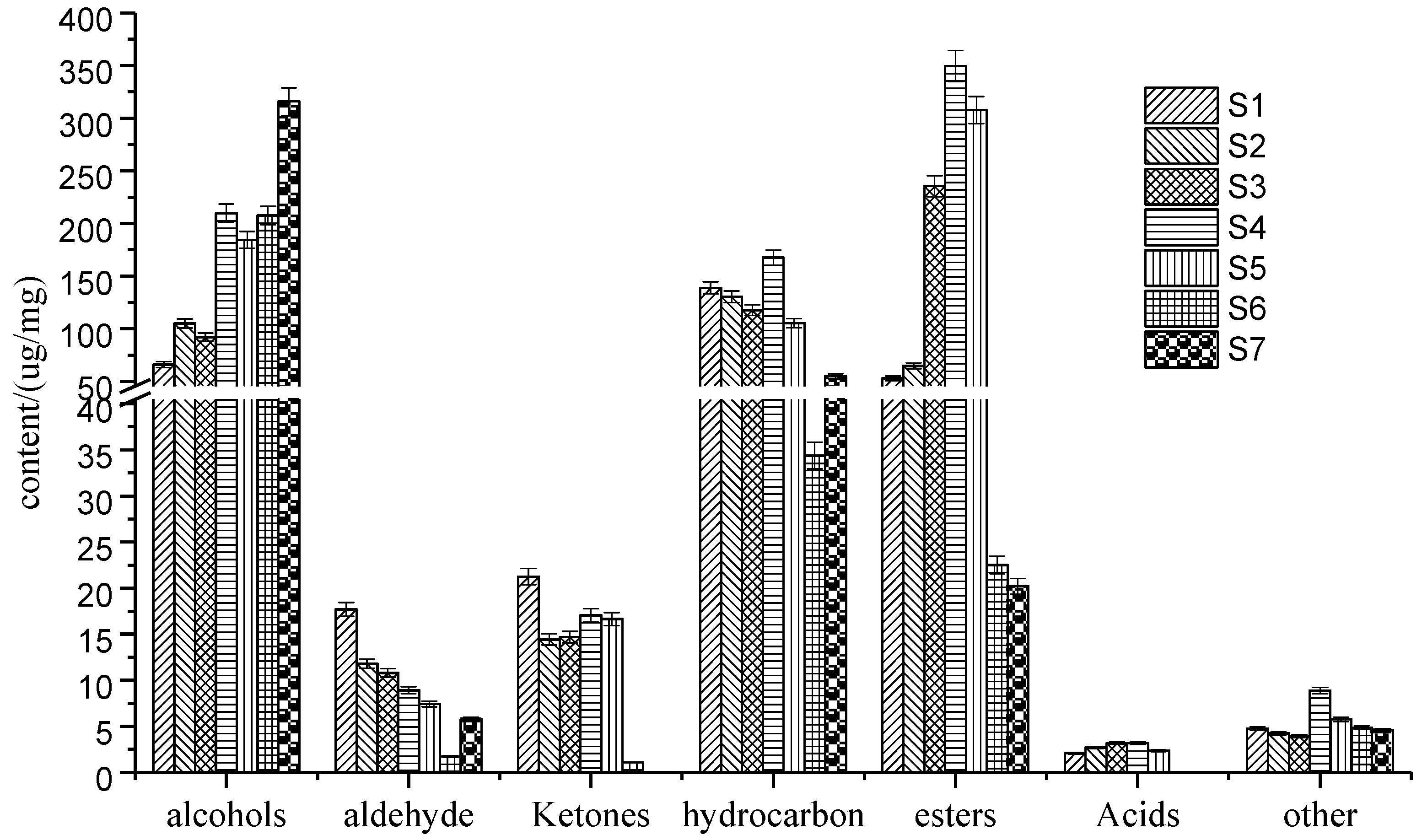

| Retention Time (min) | Compound Name | RI/Literature Value | Identification Method | Compound Content (ug/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | ||||
| Alcohols (15 kinds) | ||||||||||
| 17.069 | α-Terpineol | 1654/1662 | MS/RI | 12.43 ± 0.52 | 14.81 ± 0.62 | 7.52 ± 0.32 | - | - | 4.48 ± 0.19 | 5.12 ± 0.22 |
| 11.988 | Cis-4-Butynol | 1455/1450 | MS/RI | - | - | 1.61 ± 0.07 | 4.57 ± 0.19 | 4.1 ± 0.17 | 2.03 ± 0.09 | - |
| 13.39 | 5-Isopropyl-2-Methylbicyclo-3,1-Hexane-2-Ol | 1472/1470 | MS/RI | 3.61 ± 0.15 | 5.55 ± 0.23 | 3.66 ± 0.15 | 2.84 ± 0.12 | 2.45 ± 0.1 | 1.36 ± 0.04 | 3.03 ± 0.13 |
| 10.433 | Eucalyptol | 1227/1220 | MS/RI | 18.1 ± 0.76 | 18.38 ± 0.77 | 15.46 ± 0.65 | 23.54 ± 0.99 | - | - | - |
| 16.548 | Terpinen-4-Ol | 1607/1612 | MS/RI | 4.27 ± 0.18 | 4.84 ± 0.2 | 4.03 ± 0.17 | 5.59 ± 0.24 | 5.35 ± 0.23 | 1.61 ± 0.07 | 2.4 ± 0.1 |
| 13.543 | Linalool | 1545/1556 | MS/RI | 24.04 ± 1.01 | 55.62 ± 2.34 | 58.36 ± 2.45 | 115.64 ± 4.8 | 130.36 ± 5.4 | 198.24 ± 8.3 | 303.59 ± 12.8 |
| 18.071 | γ- Terpineol | 1667/1669 | MS/RI | - | - | - | 40.74 ± 1.71 | 34.18 ± 1.44 | - | - |
| 15.883 | 2-Methyl-6-Heptene-3-Ol | 1576/- | MS | 0.62 ± 0.03 | 0.65 ± 0.03 | - | - | - | - | - |
| 27.321 | Neroli Tert Alcohol | 2038/2046 | MS/RI | 0.41 ± 0.02 | - | - | - | - | - | - |
| 16.188 | Myrcenol | 1593/- | MS | 0.68 ± 0.03 | 0.69 ± 0.03 | - | - | - | - | - |
| 18.2 | (1R,5S)-Rel- Carvacrol | 1851/1858 | MS/RI | 0.41 ± 0.02 | 2.05 ± 0.09 | - | - | - | - | - |
| 17.061 | Trans-4-Platycladusorientalis Alcohol | 1638/1631 | MS/RI | - | - | - | 11.34 ± 0.48 | 8.1 ± 0.34 | - | - |
| 18.777 | Trans Carvitol | 1858/1860 | MS/RI | 1.56 ± 0.07 | 2.44 ± 0.10 | 1.57 ± 0.07 | 1.96 ± 0.08 | - | - | - |
| 29.654 | Trans-Menthyl-2,8-Diene-1-Alcohol | 1662/1670 | MS/RI | - | - | - | 3.45 ± 0.15 | - | - | - |
| 28.531 | Geraniol | 1847/1841 | MS/RI | - | - | - | - | - | 1.31 ± 0.06 | 1.65 ± 0.07 |
| Aldehydes (10 kinds) | ||||||||||
| 21.109 | Trans -2,4- Decadienal | 1823/1826 | MS/RI | - | 4 ± 0.17 | 1.42 ± 0.06 | 3.86 ± 0.25 | 1.86 ± 0.08 | - | - |
| 22.119 | 2,4- Decadienal | 1765/- | MS | 3.12 ± 0.13 | 2.86 ± 0.13 | 2.54 ± 0.09 | 6.94 ± 0.29 | 5.58 ± 0.23 | 1.73 ± 0.07 | 1.94 ± 0.08 |
| 22.127 | (E,E)-2,4-Dodecadienal | 1803/- | MS | 5.7 ± 0.24 | - | 4.52 ± 0.19 | - | - | - | - |
| 9.695 | Trans-2,4-Heptadiene -Aldehyde | 1389/1386 | MS/RI | 0.6 ± 0.03 | 0.7 ± 0.03 | - | - | - | - | - |
| 19.682 | Trans-2-Decenylaldehyde | 1634/1630 | MS/RI | 0.77 ± 0.03 | 0.52 ± 0.02 | - | - | - | - | - |
| 7.98 | Trans-2-Heptenal | 1312/1319 | MS/RI | 1.27 ± 0.05 | 1.71 ± 0.07 | 1.37 ± 0.06 | 3.24 ± 0.15 | 2.16 ± 0.07 | - | - |
| 15.659 | Citronellal | 1480/1478 | MS/RI | 0.66 ± 0.03 | - | - | - | - | - | - |
| 21.117 | (E,E)-2,4- Decadienal | 1756/1750 | MS/RI | 1.56 ± 0.07 | 1.53 ± 0.06 | - | 1.98 ± 0.08 | - | - | - |
| 8.854 | n-Hexaldehyde | 1335/1337 | MS/RI | 0.49 ± 0.02 | 0.65 ± 0.03 | - | - | - | - | - |
| 14.697 | Nonanal | 1452/1453 | MS/RI | 4.03 ± 0.17 | 2.7 ± 0.11 | 3.5 ± 0.15 | - | - | - | - |
| Ketones (5 kinds) | ||||||||||
| 16.869 | 4-(1- Methyl Ethyl)-2-Cyclohexene-1-Ketone | 1705/- | MS | 5.54 ± 0.23 | 7.42 ± 0.31 | 3.46 ± 0.15 | 4.26 ± 0.18 | 3.33 ± 0.14 | - | - |
| 15.242 | 2,3-Dihydro-3,5-Dihydroxy-6-Methyl-4h-Pyran-4-Ketone | 1692/- | MS | 1.47 ± 0.06 | 4.74 ± 0.20 | 2.39 ± 0.1 | 3.21 ± 0.14 | 5.51 ± 0.23 | - | - |
| 19.354 | Piperonone | 1721/1743 | MS/RI | 10.53 ± 0.44 | 7.62 ± 0.33 | 6.5 ± 0.27 | 9.58 ± 0.4 | 7.81 ± 0.33 | 1.1 ± 0.05 | - |
| 18.953 | Carvone | 1708/1702 | MS/RI | 1.43 ± 0.06 | - | 0.99 ± 0.04 | - | - | - | - |
| 31.946 | Pyranone | 2249/2240 | MS/RI | 0.71 ± 0.03 | 1.09 ± 0.05 | - | - | - | - | - |
| Hydrocarbons (34 kinds) | ||||||||||
| 9.062 | Myrcene | 1136/1143 | MS/RI | 20.54 ± 0.86 | 17.29 ± 0.73 | 12.95 ± 0.54 | 18.22 ± 0.77 | 10.94 ± 0.46 | 3.03 ± 0.13 | 4.63 ± 0.19 |
| 7.107 | α-Phellandrene | 1087/1086 | MS/RI | 4.76 ± 0.20 | 4.37 ± 0.18 | 5.96 ± 0.25 | 6.37 ± 0.23 | - | - | - |
| 11.138 | Ocimene | 1216/- | MS | 6.44 ± 0.27 | 4.64 ± 0.2 | 3.68 ± 0.16 | - | - | - | - |
| 10.329 | Limonene | 1193/1196 | MS/RI | 38.59 ± 1.62 | 34.27 ± 1.44 | - | 57.89 ± 2.43 | 42.87 ± 1.8 | - | - |
| 8.501 | Sabinene | 1132/1130 | MS/RI | 13.31 ± 0.56 | 12.12 ± 0.51 | 12.96 ± 0.54 | 19.51 ± 0.82 | 10.36 ± 0.44 | 7.98 ± 0.34 | 13.45 ± 0.57 |
| 11.154 | (E)-3,7- Dimethyl -1,3,6-Octriene | 1241/1250 | MS/RI | - | - | 4.98 ± 0.21 | 4.92 ± 0.21 | 3.09 ± 0.13 | - | - |
| 9.88 | α- Terpinene | 1188/1194 | MS/RI | - | - | - | 5.56 ± 0.23 | 3.37 ± 0.14 | - | - |
| 11.579 | γ-Terpinene | 1251/1250 | MS/RI | - | - | - | 11.4 ± 0.48 | 7.06 ± 0.3 | - | - |
| 9.463 | Phellandrene | 1164/1160 | MS/RI | - | - | 1.04 ± 0.04 | 1.47 ± 0.06 | - | - | - |
| 29.269 | γ- Cadinene | 1758/1765 | MS/RI | - | - | - | 1.83 ± 0.08 | 1.62 ± 0.07 | - | - |
| 12.917 | Terpinolene | 1283/1271 | MS/RI | 2.9 ± 0.12 | 3.41 ± 0.14 | - | - | - | - | - |
| 25.069 | Elemene | 1583/1580 | MS/RI | 6.15 ± 0.26 | 4.66 ± 0.2 | - | - | - | - | - |
| 25.975 | 1- Caryophyllene | 1596/1604 | MS/RI | 5.05 ± 0.21 | 4.69 ± 0.2 | 2.35 ± 0.1 | 5.68 ± 0.24 | 5.85 ± 0.25 | 2.64 ± 0.11 | 3.29 ± 0.14 |
| 27.089 | α- Caryophyllene | 1708/1706 | MS/RI | 1.42 ± 0.06 | 1.36 ± 0.06 | 1 ± 0.04 | 2.99 ± 0.13 | 3.44 ± 0.14 | 1.74 ± 0.07 | 2.15 ± 0.09 |
| 25.569 | β- Elemene | 1587/1580 | MS/RI | - | - | 1.94 ± 0.08 | 4.59 ± 0.19 | 3.79 ± 0.16 | - | 1.94 ± 0.08 |
| 10.721 | Trans -α- Ocimene | 1205/1218 | MS/RI | 13.78 ± 0.58 | 6.06 ± 0.25 | 7.81 ± 0.33 | 6.72 ± 0.28 | 3.52 ± 0.15 | 1.57 ± 0.07 | 2.71 ± 0.11 |
| 7.299 | α- Pinene | 1024/1028 | MS/RI | - | 2.94 ± 0.12 | 1.52 ± 0.06 | 2.7 ± 0.11 | - | 2 ± 0.08 | - |
| 23.506 | Camphene | 1495/1503 | MS/RI | 20.63 ± 0.87 | 17.62 ± 0.74 | 15.07 ± 0.63 | - | - | - | - |
| 10.168 | 1-Methyl-4-(1- Methyl ethyl)- Benzene | 1190/- | MS | 1.5 ± 0.06 | 1.58 ± 0.07 | 0.89 ± 0.04 | 1.7 ± 0.07 | - | - | - |
| 26.471 | γ- Elemene | 1602/1613 | MS/RI | - | - | - | 1.37 ± 0.06 | 1.42 ± 0.06 | - | - |
| 7.123 | α-Limonene Thuene | 1093/- | MS | - | - | - | 9.23 ± 0.39 | 4.93 ± 0.21 | - | - |
| 14.705 | Alloocimenols | 1339/1334 | MS/RI | - | - | - | 2.1 ± 0.09 | - | - | - |
| 22.84 | Germacrene-D | 1482/1479 | MS/RI | 0.9 ± 0.04 | 0.93 ± 0.04 | - | - | - | - | - |
| 27.081 | (+)-β- Selinene | 1716/1729 | MS/RI | - | - | 1.16 ± 0.05 | - | - | - | - |
| 21.638 | α- Piper Eggplant Olene | 1447/1446 | MS/RI | 0.51 ± 0.02 | 0.5 ± 0.02 | - | - | - | - | - |
| 11.138 | Trans -β- Basilene | 1237/1230 | MS/RI | - | 9.02 ± 0.38 | 4.98 ± 0.21 | 4.92 ± 0.21 | 3.09 ± 0.13 | - | - |
| 14.697 | (E,E)-2,6- Dimethyl -2,4,6-Octadiene | 1323/- | MS | - | - | 1.45 ± 0.06 | - | - | - | - |
| 26.463 | 3-Vinyl-2,5-Dimethyl-1,4-Hexadiene | 1599/1601 | MS/RI | 0.68 ± 0.03 | - | 2.58 ± 0.11 | - | - | - | - |
| 25.974 | Trans -β- Caryophyllene | 1592/1595 | MS/RI | - | - | - | 4.96 ± 0.21 | - | - | - |
| 28.579 | α-Claurolene | 1728/1719 | MS/RI | 0.72 ± 0.03 | 0.6 ± 0.03 | - | - | - | - | - |
| 20.195 | Vinyl Cyclohexene Dioxide | 1428/- | MS | 1.11 ± 0.05 | 1.1 ± 0.05 | - | - | - | - | - |
| 21.422 | 3-Carene | 1443/1450 | MS/RI | - | 3.33 ± 0.14 | - | - | - | - | - |
| 10.459 | M-Isopropyl Toluene | 1201/1206 | MS/RI | - | - | 35.53 ± 1.49 | - | - | 17 ± 0.71 | 26.81 ± 1.13 |
| 28.026 | Caryophyllene Oxide | 1939/1935 | MS/RI | - | - | - | 4.53 ± 0.19 | 5.75 ± 0.24 | - | - |
| Esters (11 kinds) | ||||||||||
| 24.828 | Geranyl Acetate | 1752/1746 | MS/RI | - | - | 2.83 ± 0.12 | 5.02 ± 0.21 | 3.44 ± 0.14 | - | - |
| 18.079 | Linalyl Acetate | 1546/1548 | MS/RI | 27.09 ± 1.14 | 28.72 ± 1.21 | 26.63 ± 1.12 | 298.2 ± 12.52 | 273.8 ± 11.5 | - | - |
| 24.115 | Neryl Acetate | 1728/1733 | MS/RI | 4.03 ± 0.17 | 2.35 ± 0.1 | 2.55 ± 0.11 | 3.96 ± 0.17 | 3.07 ± 0.13 | - | - |
| 23.69 | Citronellyl Acetate | 1657/1655 | MS/RI | 3.9 ± 0.16 | 2.75 ± 0.12 | 2.15 ± 0.09 | 3.43 ± 0.14 | - | - | - |
| 23.506 | Terpinyl Acetate | 1641/1652 | MS/RI | - | - | - | 28.57 ± 1.2 | 18.38 ± 0.77 | - | - |
| 23.89 | Citronellyl Butyrate | 1581/- | MS | - | - | 2.38 ± 0.1 | - | 1.98 ± 0.08 | - | - |
| 31.321 | 4-Terpineol Acetate | 1823/- | MS | - | - | - | - | - | 6.75 ± 0.28 | 7.21 ± 0.3 |
| 21.422 | 3-Methyl-2-Cyclohexene-1-Ol-Acetate | 1556/1557 | MS/RI | - | - | - | 4.65 ± 0.2 | 3.12 ± 0.13 | - | - |
| 23.193 | Bornyl Acetate | 1568/1566 | MS/RI | 3.35 ± 0.14 | 3.6 ± 0.15 | 2.53 ± 0.11 | 5.79 ± 0.24 | 3.82 ± 0.16 | - | - |
| 19.49 | 1,5-Dimethyl-1-Vinyl-4-Hexyl-2-Aminobenzoate | 1552/- | MS | 14.61 ± 0.61 | 27.45 ± 1.15 | 196.43 ± 8.25 | - | - | 15.77 ± 0.66 | 12.99 ± 0.55 |
| 21.43 | 1-Isopropyl-4-Methylbicyclohexane-3-yl Acetate | 1753/1750 | MS/RI | 3.31 ± 0.14 | - | 2.71 ± 0.11 | - | - | - | - |
| Acids (2 kinds) | ||||||||||
| 8.966 | Caproic Acid | 1854/1843 | MS/RI | 0.57 ± 0.02 | 1.01 ± 0.04 | 0.85 ± 0.04 | - | - | - | - |
| 1.664 | Acetic Acid | 1490/1492 | MS/RI | 1.52 ± 0.06 | 1.69 ± 0.07 | 2.32 ± 0.1 | 3.18 ± 0.13 | 2.36 ± 0.1 | - | - |
| Others (4 kinds) | ||||||||||
| 12.244 | 2-Ethyl-5-Methyl-Tetrahydro-Furan | 1569/1568 | MS/RI | 0.28 ± 0.01 | 0.87 ± 0.04 | - | - | - | - | - |
| 28.026 | Anethole | 1812/1817 | MS/RI | - | 1.83 ± 0.08 | 1.24 ± 0.05 | - | - | 2.75 ± 0.12 | - |
| 20.74 | Anthracene Violetin | 1724/- | MS | 0.61 ± 0.03 | 0.75 ± 0.03 | - | - | - | - | - |
| 51.463 | Zanthoxylin | 2676/- | MS | 0.56 ± 0.02 | 0.79 ± 0.03 | - | 2.56 ± 0.08 | - | 2.11 ± 0.08 | 4.59 ± 0.19 |
| 24.115 | Neryl Acetate | 1728/1733 | MS/RI | 4.03 ± 0.17 | 2.35 ± 0.1 | 2.55 ± 0.11 | 3.96 ± 0.17 | 3.07 ± 0.13 | - | - |
| 23.69 | Citronellyl Acetate | 1657/1655 | MS/RI | 3.9 ± 0.16 | 2.75 ± 0.12 | 2.15 ± 0.09 | 3.43 ± 0.14 | - | - | - |
| 23.506 | Terpinyl Acetate | 1641/1652 | MS/RI | - | - | - | 28.57 ± 1.2 | 18.38 ± 0.77 | - | - |
| sum of identified compounds (kinds) | 54 | 53 | 47 | 49 | 37 | 19 | 16 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, W.; Tian, H.; Zhan, P. The Effects of Pepper (Zanthoxylum bungeanum) from Different Production Areas on the Volatile Flavor Compounds of Fried Pepper Oils Based on HS-SPME–GC–MS and Multivariate Statistical Method. Molecules 2022, 27, 7760. https://doi.org/10.3390/molecules27227760
Niu W, Tian H, Zhan P. The Effects of Pepper (Zanthoxylum bungeanum) from Different Production Areas on the Volatile Flavor Compounds of Fried Pepper Oils Based on HS-SPME–GC–MS and Multivariate Statistical Method. Molecules. 2022; 27(22):7760. https://doi.org/10.3390/molecules27227760
Chicago/Turabian StyleNiu, Wenjing, Honglei Tian, and Ping Zhan. 2022. "The Effects of Pepper (Zanthoxylum bungeanum) from Different Production Areas on the Volatile Flavor Compounds of Fried Pepper Oils Based on HS-SPME–GC–MS and Multivariate Statistical Method" Molecules 27, no. 22: 7760. https://doi.org/10.3390/molecules27227760
APA StyleNiu, W., Tian, H., & Zhan, P. (2022). The Effects of Pepper (Zanthoxylum bungeanum) from Different Production Areas on the Volatile Flavor Compounds of Fried Pepper Oils Based on HS-SPME–GC–MS and Multivariate Statistical Method. Molecules, 27(22), 7760. https://doi.org/10.3390/molecules27227760





